Abstract
The maturation of dendritic cells (DCs) in situ by danger signals plays a central role in linking innate and adaptive immunity. We previously demonstrated that the activation of invariant natural killer T (iNKT) cells by administration of α-galactosylceramide (α-GalCer)–loaded tumor cells can act as a cellular adjuvant through the DC maturation. In the current study, we used allogeneic fibroblasts loaded with α-GalCer and transfected with antigen-encoding mRNA, thus combining the adjuvant effects of iNKT-cell activation with delivery of antigen to DCs in vivo. We found that these cells produce antigen protein and activate NK and iNKT cells. When injected into major histocompatibility complex (MHC)–mismatched mice, they elicited antigen-specific T-cell responses and provided tumor protection, suggesting that these immune responses depend on host DCs. In addition, antigen-expressing fibroblasts loaded with α-GalCer lead to a more potent T-cell response than those expressing NK cell ligands. Thus, glycolipid-loaded, mRNA-transfected allogeneic fibroblasts act as cellular vectors to provide iNKT-cell activation, leading to DC maturation and T-cell immunity. By harnessing the innate immune system and generating an adaptive immune response to a variety of antigens, this unique tool could prove clinically beneficial in the development of immunotherapies against malignant and infectious diseases.
Introduction
Dendritic cells (DCs) play a pivotal role in determining the character and magnitude of an immune response. Because of this, many studies have developed methods to manipulate DCs to either augment the subsequent cellular immune response (to treat infectious diseases or cancer) or mute the immune response (to treat autoimmune diseases).1,2 In the context of cancer therapy, 2 approaches are the most promising: ex vivo generation of antigen-loaded mature DCs (ex vivo DC strategy), and targeted delivery of antigen to in vivo DCs followed by subsequent DC maturation (in vivo DC strategy). Previous studies based on tumor antigen-loaded DCs have produced encouraging results for some types of cancer. Antigen loaded onto DCs can take several forms: peptides,3 whole proteins,4 dying tumor cells,5-7 leukemic DCs,8 tumor cell–derived mRNA,9 and DNA.10,11 Short peptides corresponding to epitopes presented by major histocompatibility complex (MHC) class I or II molecules is a popular antigenic form for loading onto ex vivo DCs. However, these epitopes can only be used in an MHC-restricted manner. A method that breaks free of MHC class restriction is the use of antigenic epitope-specific mRNA generated from autologous tumor tissue. This approach has the advantage of inducing antigen-specific T-cell responses against a variety of tumor antigens. DCs transfected with antigen mRNA have already been shown to be effective in generating a robust cytotoxic T lymphocyte (CTL) response and antitumor immunity in both mice9,12 and humans.13-16
Once DCs are transfected with mRNA, the tumor antigens produced are presented as endogenous antigens of the DCs. For enhancing the antigen presentation on MHC class I, antigen must have access to the cytoplasm of the DC after the transfection of mRNA. Several groups have attempted to improve MHC class I antigen presentation by enhancing the delivery of viral and tumor proteins to the proteasome.17-19 One method of doing this is by fusing the antigen to ubiqutin, which leads to rapid cytoplasmic degradation and improved peptide delivery to the endoplasmic reticulum.18,20 A different approach attempts to enhance antigen presentation and antitumor immunity by administering DCs cotransfected with mRNA encoding tumor antigen and mRNA encoding several potent adjuvants, such as vascular endothelial growth factor receptor 2 (VEGFR-2),21 fibroblast activation protein (FAP),22 OX40 ligand,23 granulocyte-macrophage colony-stimulating factor (GM-CSF),24 or IL-12.25
Different from ex vivo DC strategy, the in vivo DC targeting approaches, such as dying cells,26-28 DEC205-conjugated antibody (Ab),29-31 and mannose receptor (MR)–conjugated Ab,32,33 have been used to drive in situ DCs to present antigen to T cells. Because it has been known that DCs induce tolerance to antigens they capture in steady-state conditions,1,29,34 DCs need a maturation stimulus to generate a cytolytic immune response. We and others have previously demonstrated that administration of α-galactosylceramide (α-GalCer) results in potent downstream activation of invariant NKT (iNKT) cells and maturation of DCs, cumulating in the establishment of antitumor immunity via primed NK cells35,36 and cytotoxic T cells.37-39 It has been reported that iNKT cells, known as type I NKT cells, comprise a unique population of T cells capable of regulating a broad range of immune responses, including autoimmunity, allergy, control of infection, and tumor rejection,40,41 although type II NKT cells have suppressive effects on tumor immunity.42 We have shown that when α-GalCer is given in conjunction with dying cells, in situ DCs take up antigen and are matured by activated iNKT cells to become potent stimulators of the cytolytic immune response.38,43 This concept has been explored with several strategies: tumor cells loaded with α-GalCer (tumor/Gal);44,45 combination therapy with α-GalCer, anti-DR5 Ab, and anti–4-1-BB Ab;46 combination therapy with α-GalCer and TLR-ligands;47 and α-GalCer–loaded, soluble CD1d-fused anti–HER2-scFv fusion protein.48 In our recent studies, we proved that syngeneic tumor/Gal activated iNKT cells and NK cells,40,44 which in turn attacked the tumor cells. Tumor cells killed by activated iNKT cells were then captured by endogenous DCs in a milieu of inflammatory cytokines, leading to the generation of tumor-specific cytotoxic T cells and subsequent antitumor immunity.45
Our current study modifies the strategy of tumor/Gal: instead of tumor cells as a source of antigen, we use allogeneic fibroblasts transfected with mRNA encoding tumor antigen. This approach could prove clinically useful in situations where access to autologous tumor is limited or response to a specific tumor antigen is desired.
Methods
Mice and cell lines
Pathogen-free C57BL/6 (B6) mice were purchased from CLEA Japan (Tokyo) at 6 to 8 weeks of age, and B6 CD4−/− and CD8−/− female mice were purchased from Jackson Laboratory (Bar Harbor, ME). OT-I T-cell receptor (TCR) transgenic mice were generously provided by Dr Heath (Walter and Eliza Hall Institute, Victoria, Australia), CD11c-DTR/GFP mice were a kind gift of Dr Littman (New York University, New York, NY) and were backcrossed 12 generations to C57BL/6 mice. Jα18−/− mice were generously provided by Dr Taniguchi (RIKEN) and were backcrossed more than 9 generations to C57BL/6 mice. These mice were maintained under specific pathogen–free conditions and studied in compliance with our institutional guidelines. B16, EL4, and EG7 cell lines were obtained from ATCC (Rockville, MD), and NIH3T3 cells were obtained from RIKEN Cell Bank. As previously described,44 for introduction of CD1d, pMX-mCD1d-IRES-GFP carrying mCD1d was retrovirally transduced into B16 melanoma or NIH3T3 cells, and cells were subsequently sorted based on the expression of green fluorescent protein (GFP) by the FACSVantage cell sorter.
Cell preparation
Bone marrow–derived DCs were generated from bone marrow progenitors as previously described.49 On day 6, α-GalCer (100 ng/mL) was added to DCs for 40 hours, and 100 ng/mL of lipopolysaccharide (LPS) was added for the last 16 hours. For loading of α-GalCer to other cell lines, fibroblasts (NIH3T3 or CD1dhi-NIH3T3) or tumor cells were cultured for 48 hours in the presence of 500 ng/mL of α-GalCer. These α-GalCer–loaded cells were washed 3 times before injection. CD1dhi-NIH3T3 was prepared as previously described.
CD70-NIH3T3, Rae1ϵ-NIH3T3, Rae1γ-NIH3T3, and Mult1-NIH3T3 were prepared as follows. Briefly, the mouse CD70 complementary (c) DNA, Rae1ϵ cDNA, Rae1γ cDNA, and Mult1 cDNA were cloned into the retroviral vector carrying pMX-ligand cDNA-IRES-GFP and infected into NIH3T3. The cells were subsequently sorted by expression of GFP.
Preparation of EGFP, OVA, and TRP-2 mRNA
Each full length of cDNA (EGFP, OVA, TRP-2) was subcloned using the pSP64 poly(A) vector (Promega, Madison, WI; Document S1 and Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). The vector carrying each cDNA was amplified and then linearized by cutting with the enzymes EcoRI (in the case of EGFP or OVA) or PvuII (in the case of TRP-2). After making capped-mRNA, Ribo m7G cap analog (Ambion, Austin, TX) was incorporated during the RiboMax transcription reaction for amplifying mRNA with RiboMax large-scale RNA production systems–SP6 (Promega).
Transfection of mRNAs
In vitro transcribed (IVT) RNAs were transfected into different cell lines with the TransMessenger transfection kit (QIAGEN, Valencia, CA) following the manufacturer's protocols. Briefly, 2 × 105 cells were seeded one day before transfection on 60-mm tissue-culture Petri dishes. The next day, cells were washed with phosphate-buffered saline (PBS) 3 times and transfected with different amounts of IVT RNAs. The ratio of mRNA, enhancer solution, and transmessenger reagent was 1:2:4. The cells were transfected for different lengths of time and then either directly harvested or incubated for an additional 16 hours in RPMI 1640 containing 10% fetal bovine serum. In Figure 1D through F, (1°) refers to the different initial transfection times (2, 4, 8, or 16 hours), and (2°) refers to the additional period of incubation, either 0 or 16 hours. These cells were analyzed for EGFP by fluorescence-activated cell sorter (FACS) and a laser-scanning confocal microscope (TCS-SP2 Leica DMRE; Leica, Heidelberg, Germany), or measured for OVA Protein by enzyme-linked immunosorbent assay (ELISA; Morinaga Institute of Biological Science, Yokohama, Japan).
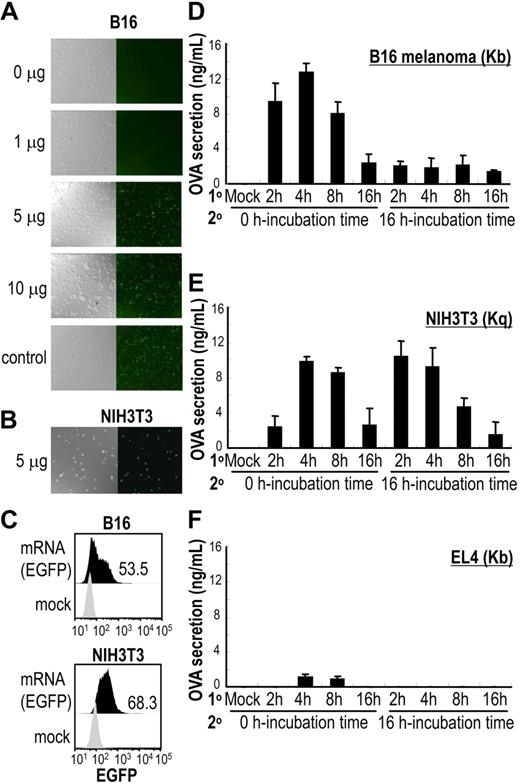
Determination of optimal conditions to introduce antigen-encoding mRNA into cells. (A,B) Graded doses of EGFP mRNA were transduced into B16 melanoma cells (H2-Kb) and 5 μg EGFP mRNA to NIH3T3 fibroblasts (H2-Kq). Levels of EGFP expression were evaluated by confocal microscopy (20×/0.7 NA oil objective). (C) A total of 5 μg EGFP mRNA–transduced B16 and NIH3T3 cells were evaluated by flow cytometry for expression of EGFP. The number shown represents the percent of EGFP+ cells. Data are representative of 3 independent experiments. (D-F) Whole OVA gene-carrying vector SP64 was linearized, and 5 μg of OVA mRNA was transduced to B16, NIH3T3, or EL4 cells for different incubation periods to determine optimal transduction time (incubation time, 1°). Levels of OVA protein from cell lysates were measured by an ELISA kit (Morinaga Institute of Biological Science) at 2 time points after transduction: 0 hours and 16 hours (incubation time, 2°). Data shown are means plus or minus SEM of 4 mice per group.
Determination of optimal conditions to introduce antigen-encoding mRNA into cells. (A,B) Graded doses of EGFP mRNA were transduced into B16 melanoma cells (H2-Kb) and 5 μg EGFP mRNA to NIH3T3 fibroblasts (H2-Kq). Levels of EGFP expression were evaluated by confocal microscopy (20×/0.7 NA oil objective). (C) A total of 5 μg EGFP mRNA–transduced B16 and NIH3T3 cells were evaluated by flow cytometry for expression of EGFP. The number shown represents the percent of EGFP+ cells. Data are representative of 3 independent experiments. (D-F) Whole OVA gene-carrying vector SP64 was linearized, and 5 μg of OVA mRNA was transduced to B16, NIH3T3, or EL4 cells for different incubation periods to determine optimal transduction time (incubation time, 1°). Levels of OVA protein from cell lysates were measured by an ELISA kit (Morinaga Institute of Biological Science) at 2 time points after transduction: 0 hours and 16 hours (incubation time, 2°). Data shown are means plus or minus SEM of 4 mice per group.
Real-time polymerase chain reaction assay
Total RNAs from different cell lines were isolated with either RNeasy kits (QIAGEN) or Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's protocols. In the case of the total RNA isolation from a small number of cells (<2 × 105 cells) with Trizol reagent, 5 μg of glycogen (Roche, Indianapolis, IN) was used for the coprecipitation. After the synthesis of cDNA from 1 μg of total RNAs, the quantification of mRNA expression was performed by real-time polymerase chain reaction (PCR) using Taqman probe primers (Applied Biosystems, Foster City, CA).
In vivo tumor studies
Mice were immunized intravenously with antigen-encoding mRNA–transfected NIH3T3 fibroblasts loaded with α-GalCer (5 × 105cells/mouse). In experiments evaluating the development of protective immunity to tumor challenge, immunized mice were challenged with tumor cells subcutaneously 2 weeks later and then the tumor size was measured. In some experiments, CD4−/− and CD8−/− mice were used as recipient mice.
Statistical analysis
Differences in the in vitro data were analyzed using the Mann-Whitney U test. To correct for multiple testing, the Bonferroni correction analysis was used. The conservative Bonferroni corrections were 0.05/2 = 0.025 in Figures 2 and 3, and 0.05/3 = 0.0167 in Figure 5. Consequently, P values less than .025 for data in Figures 2 and 3 and P values less than .017 for data in Figure 5 were considered statistically significant.
Results
Determination of optimal conditions to introduce antigen-encoding mRNA into allogeneic cells
We determined optimal transfection conditions for introducing mRNA into cells by a chemical method using lipofection (Figure S1).50,51 To determine the dose-dependent transduction rate of antigen-encoding mRNA into cells, the expression of EGFP mRNA transcribed in vitro from the linearized EGFP carrying SP64 vector was evaluated. The expression of EGFP in transfected B16 melanoma cells (H2-Kb) or NIH3T3 fibroblasts (H2-Kq) was analyzed by fluorescent microscopy at graded doses of mRNA (Figure 1A). By comparing different doses of mRNA transfections, we determined that 5 μg of EGFP mRNA was sufficient to express EGFP in B16 cells (Figure 1A) and NIH3T3 cells (Figure 1B). Both cell types sufficiently expressed EGFP at 4 hours, with EGFP expression continuing for at least 12 hours (data not shown). When analyzed by FACS, the efficiency of EGFP mRNA transduction to B16 melanoma cells or NIH3T3 fibroblast cell lines was almost equivalent (Figure 1C), and was much better than the transduction rate to EL4 thymoma cells (H2-Kb; less than 5%; data not shown).
Next, the time of maximal protein production after mRNA transduction was determined. OVA mRNA was transfected into the cell lines B16, EL4, or NIH3T3. The amount of OVA protein produced by 5 μg of OVA mRNA-transduced B16, EL4, or NIH3T3 cells (B16-ova, EL4-ova or NIH3T3-ova, respectively) was measured by ELISA after cell lysis. We evaluated the transfection time (2-16 hours) and found that both B16-ova and NIH3T3-ova produced OVA protein optimally after 4 hours of transfection (Figure 1D-F; 1° line on axis).
We then analyzed whether the cells continued to produce OVA protein after a transfection by measuring the amount of OVA protein. As shown on the 2° line of Figure 1D through F, expression of OVA protein by OVA mRNA–transfected NIH3T3 was similar to that of the B16 transfectant, but continued to be expressed when measured 16 hours later. The OVA mRNA–transfected EL4 cell line produced little OVA protein (Figure 1F) due to low levels of transfection. Therefore, we selected NIH3T3 fibroblasts for our experiments.
Transduction of the CD1d gene into cell lines lacking costimulatory molecules
We previously showed that even CD1d-expressing tumor cells lacking costimulatory molecules were able to present α-GalCer to primary iNKT cells.44 Here, we verified that NIH3T3 fibroblasts and B16 melanoma cells did not express CD40, CD70, CD86, and MHC class II (data not shown). We analyzed parental cell lines (NIH3T3 and B16) for the levels of CD1d expression and established stable variants that had been transduced with a retrovirus expressing high levels of murine CD1d as previously reported (Figure 2A,B).44,45 The stable CD1dhi cell lines were selected by sorting with a FACSVantage cell sorter to a purity greater than 98% (Figure 2B left). Parental B16 melanoma cells and NIH3T3 cells expressed lower levels of CD1d than bone marrow–derived DCs (Figure 2A). We found the highest expression of CD1d on CD1dhi-NIH3T3 cells compared with other cell lines and DCs by real-time PCR. This finding was verified by FACS (Figure 2B right).
Antigen-presenting activity in mice immunized with mRNA-transfected cells loaded with α-GalCer. (A) We examined CD1d RNA expression levels in B16 cells, NIH3T3 cells, and murine bone marrow–derived DCs (mBM-DCs) as well as B16 and NIH3T3 cells after retrovirus-mediated transfer of a murine CD1d gene (CD1dhi-B16, CD1d hi-NIH3T3) by real-time PCR. (B) Because the retroviral vector contained both murine CD1d and GFP genes, the stable CD1dhi cell lines were selected to a purity of more than 98% on a FACSVantage cell sorter (left panel). The CD1d expression of parental cell lines or CD1d transfectants was analyzed by FACS (right panel). (C) After a 4-hour transduction, the expression of OVA protein was measured from cell lysates by ELISA. (D) The antigen-presenting activity was evaluated by coculturing OVA-transgeneic CD8+ T cells (OT-I cells) with each type of transfectant for 48 hours. The supernatants were collected and IFN-γ secretion was measured by ELISA. In this experiment, the B16 cell line was pretreated with IFN-γ for 12 hours to enhance MHC class I expression before coculture. (E) C57BL/6 mice were given 2 × 106 OT-I cells and then immunized 24 hours later with OVA mRNA-transfectants loaded with or without α-GalCer. Absolute numbers of OT-I cells in the spleen were measured 3 days later. α-GalCer–loaded CD1dhi-B16 transfected with OVA mRNA (CD1dhi-B16/Gal-ova) was administered to mice as a positive control. Data are representative of 2 independent experiments with 2 mice per group. Data are means plus or minus SEM of 4 mice per group. *P < .025 (CD1dhi-NIH3T3-ova vs CD1dhi-NIH3T3/Gal-ova, CD1dhi-B16-ova vs CD1dhi-B16/Gal-ova).
Antigen-presenting activity in mice immunized with mRNA-transfected cells loaded with α-GalCer. (A) We examined CD1d RNA expression levels in B16 cells, NIH3T3 cells, and murine bone marrow–derived DCs (mBM-DCs) as well as B16 and NIH3T3 cells after retrovirus-mediated transfer of a murine CD1d gene (CD1dhi-B16, CD1d hi-NIH3T3) by real-time PCR. (B) Because the retroviral vector contained both murine CD1d and GFP genes, the stable CD1dhi cell lines were selected to a purity of more than 98% on a FACSVantage cell sorter (left panel). The CD1d expression of parental cell lines or CD1d transfectants was analyzed by FACS (right panel). (C) After a 4-hour transduction, the expression of OVA protein was measured from cell lysates by ELISA. (D) The antigen-presenting activity was evaluated by coculturing OVA-transgeneic CD8+ T cells (OT-I cells) with each type of transfectant for 48 hours. The supernatants were collected and IFN-γ secretion was measured by ELISA. In this experiment, the B16 cell line was pretreated with IFN-γ for 12 hours to enhance MHC class I expression before coculture. (E) C57BL/6 mice were given 2 × 106 OT-I cells and then immunized 24 hours later with OVA mRNA-transfectants loaded with or without α-GalCer. Absolute numbers of OT-I cells in the spleen were measured 3 days later. α-GalCer–loaded CD1dhi-B16 transfected with OVA mRNA (CD1dhi-B16/Gal-ova) was administered to mice as a positive control. Data are representative of 2 independent experiments with 2 mice per group. Data are means plus or minus SEM of 4 mice per group. *P < .025 (CD1dhi-NIH3T3-ova vs CD1dhi-NIH3T3/Gal-ova, CD1dhi-B16-ova vs CD1dhi-B16/Gal-ova).
Direct antigen-presenting activity of mRNA-transduced cell lines to T cells in vitro
Established cell lines expressing either high or low levels of CD1d were transfected with OVA mRNA, and cell lysates were analyzed for levels of OVA expression. Parental B16 and CD1dhi-B16 transfectants demonstrated an almost equivalent amount of OVA production as NIH3T3 or CD1dhi-NIH3T3 transfectants (Figure 2C). We verified that not only cell lines, but also transfectants, including EGFP-NIH3T3 or CD1dhi-NIH3T3, were able to be stably transduced with OVA mRNA.
The direct presentation activity of each transfectant as antigen-presenting cells was monitored. To analyze OVA-specific T-cell responses in vitro, a class I highly expressing B16 cell line was established by exposure to recombinant IFN-γ for 12 hours (data not shown).52 Parental cells or transfected cells were cocultured with OVA-specific TCR transgeneic CD8+ T cells (OT-I cells) for 48 hours, and IFN-γ levels were measured from the supernatants. IFN-γ secretion was elevated in response to the supernatant from OVA mRNA–transfected B16 cells (B16-ova), but not OVA mRNA–transfected NIH3T3 (NIH3T3-ova; Figure 2D), even though these 2 cell lines secreted similar levels of OVA protein (Figure 2C). This indicates that OVA peptide was expressed in the context of MHC class I molecules, which are the same for OT-I and B16 (Kb), but mismatched on NIH3T3 cells (Kq).
In vivo cross-presentation of OVA antigen in mRNA-transfected cells with or without NKT-cell help
As shown in Figure 2D, in spite of equal levels of OVA secretion, OT-I cells did not recognize the peptide antigen on the MHC class I–mismatched NIH3T3. We tested whether this observation held true in vivo. To monitor the in vivo antigen-presenting capacity of transfectant fibroblasts, OVA mRNA transfectants were loaded with or without α-GalCer and given to mice that had received an injection of OT-I cells. The absolute number of expanded OT-I cells in immunized mice was analyzed 3 days later. As shown in Figure 2E, mice given α-GalCer–loaded, OVA mRNA–transfected CD1dhi-NIH3T3 (CD1dhi-NIH3T3/Gal-ova) showed the OT-I cell proliferation more than mice given OVA mRNA–transfected CD1dhi-NIH3T3 (CD1dhi-NIH3T3-ova). The number of OT-I cells in mice given CD1dhi-NIH3T3/Gal-ova was equivalent to those found in mice given tumor/Gal (ie, CD1dhi-B16/Gal-ova; Figure 2E). Thus, CD1dhi-NIH3T3-ova was unable to stimulate OT-I cells in vitro due to MHC class I mismatch (Figure 2D); however, CD1dhi-NIH3T3/Gal-ova were able to generate OT-I cellular proliferation in vivo. This proliferation despite MHC class I mismatch suggests cross-presentation by endogenous DCs in the allogeneic hosts.
α-GalCer–loaded fibroblasts activate allogeneic NK and iNKT cells in vivo
We wanted to measure the capacity of allogeneic cells with or without α-GalCer to stimulate the innate immune system in vivo. NK cell responses were analyzed by flow cytometry for the expression of CD69 and IFN-γ 16 hours after immunization. NK cells up-regulated CD69 and secreted IFN-γ in mice given CD1dhi-NIH3T3/Gal. Only a weak allogeneic response was seen in NK cells from mice injected with NIH3T3- or CD1dhi-NIH3T3–injected mice (Figure 3A).
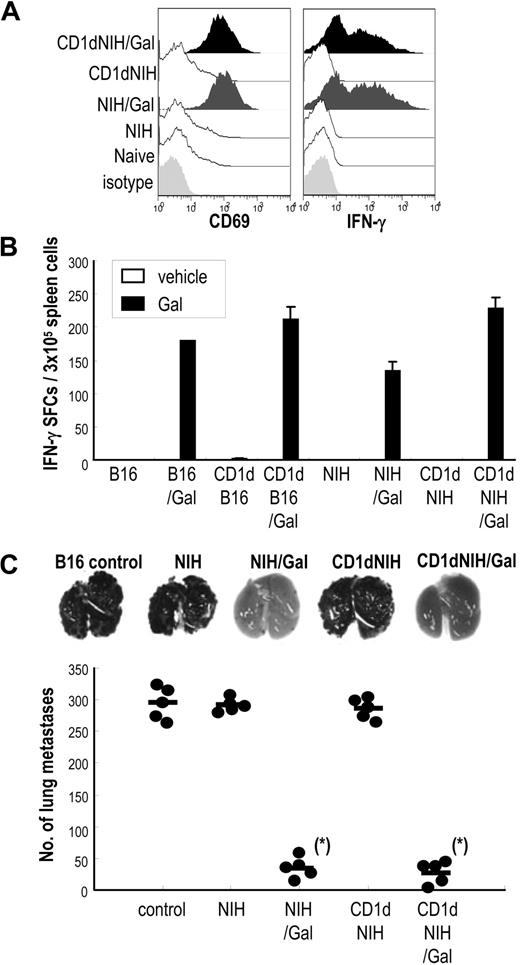
iNKT and NK cell–mediated antitumor effects in mice immunized with α-GalCer–loaded allogeneic fibroblasts. (A) C57BL/6 mice were immunized with α-GalCer–loaded, mRNA-transfected allogeneic fibroblasts, and spleen cells were collected 16 hours after immunization. Cells were stained with CD3–fluorescein isothiocyanate (FITC) and NK1.1-allophycocyanin and either CD69–phycoerythrin (PE) or intracellular IFN-γ–PE and evaluated by FACS (Document S1). Data of CD69 and IFN-γ shown have been gated on CD3−NK1.1+ cells. (B) To evaluate the function of iNKT cells, mice were injected intravenously with either 5 × 105 parental CD1dhi-NIH3T3/Gal cells or CD1dhi-B16/Gal cells. Spleens were removed 2 days later, and suspended cells were restimulated with or without α-GalCer (100 ng/mL) for 16 hours in IFN-γ ELISPOT assay plates (Document S1).53,54 Data are means from 3 mice per group. (C) Antitumor immunity generated by innate lymphocytes in response to injected allogeneic fibroblasts was evaluated using a B16 lung metastasis model. Mice were injected with 2 × 105 B16 melanoma cells intravenously, then 3 hours later given 5 × 105 NIH3T3, NIH3T3/Gal, CD1dhi-NIH3T3, or CD1dhi-NIH3T3/Gal. The number of lung metastases was counted 14 days later through the stereomicroscope (Leica MZ7.5) (n = 5 per group). Similar results were obtained in 2 independent experiments. *P < .025 (NIH3T3/Gal, CD1dhi-NIH3T3/Gal vs other groups; ie, NIH3T3, CD1dhi-NIH3T3, and control).
iNKT and NK cell–mediated antitumor effects in mice immunized with α-GalCer–loaded allogeneic fibroblasts. (A) C57BL/6 mice were immunized with α-GalCer–loaded, mRNA-transfected allogeneic fibroblasts, and spleen cells were collected 16 hours after immunization. Cells were stained with CD3–fluorescein isothiocyanate (FITC) and NK1.1-allophycocyanin and either CD69–phycoerythrin (PE) or intracellular IFN-γ–PE and evaluated by FACS (Document S1). Data of CD69 and IFN-γ shown have been gated on CD3−NK1.1+ cells. (B) To evaluate the function of iNKT cells, mice were injected intravenously with either 5 × 105 parental CD1dhi-NIH3T3/Gal cells or CD1dhi-B16/Gal cells. Spleens were removed 2 days later, and suspended cells were restimulated with or without α-GalCer (100 ng/mL) for 16 hours in IFN-γ ELISPOT assay plates (Document S1).53,54 Data are means from 3 mice per group. (C) Antitumor immunity generated by innate lymphocytes in response to injected allogeneic fibroblasts was evaluated using a B16 lung metastasis model. Mice were injected with 2 × 105 B16 melanoma cells intravenously, then 3 hours later given 5 × 105 NIH3T3, NIH3T3/Gal, CD1dhi-NIH3T3, or CD1dhi-NIH3T3/Gal. The number of lung metastases was counted 14 days later through the stereomicroscope (Leica MZ7.5) (n = 5 per group). Similar results were obtained in 2 independent experiments. *P < .025 (NIH3T3/Gal, CD1dhi-NIH3T3/Gal vs other groups; ie, NIH3T3, CD1dhi-NIH3T3, and control).
We also analyzed iNKT-cell activation in mice by injecting either parental, CD1d-transfected NIH3T3, or B16 cells with or without α-GalCer. We restimulated spleen cells in an IFN-γ enzyme-linked immunospot (ELISPOT) assay with or without 100 ng/mL α-GalCer (filled bar and open bar in Figure 3B).44,53,54 The number of IFN-γ–producing spots in NIH3T3/Gal- or CD1dhi-NIH3T3/Gal–injected mice were similar to B16/Gal or CD1dhi-B16/Gal, respectively (Figure 3B). These data further indicate that CD1dhi-NIH3T3/Gal as well as CD1dhi-B16/Gal act as antigen-presenting cells for innate iNKT-cell and NK cell responses in vivo.
Innate lymphocyte-mediated antitumor effects generated by α-GalCer–loaded allogeneic fibroblasts
To assess antitumor effects by innate immune cells in response to injected NIH3T3 in an MHC-mismatched manner, we used a lung metastasis model in which mice were given B16 melanoma cells intravenously and injected 3 hours later with NIH3T3, NIH3T3/Gal, CD1dhi-NIH3T3, or CD1dhi-NIH3T3/Gal.44,53,54 As we have previously shown, resistance to the establishment of lung metastases does not require T cells, but mainly depends on NK and iNKT cells.44,53 Mice given allogeneic fibroblasts without α-GalCer readily developed lung metastases (Figure 3C second and fourth columns). However, this did not occur in mice given NIH3T3/Gal or CD1dhi-NIH3T3/Gal (Figure 3C third and last columns). Jα18−/− mice, which do not have iNKT cells, did not demonstrate this resistance to tumor metastases (data not shown). These results indicate that the activation of innate lymphocytes by NIH3T3/Gal or CD1dhi-NIH3T3/Gal in vivo is sufficient to block the establishment of lung metastases.
A crucial role of in vivo DC maturation in response to α-GalCer–loaded allogenic fibroblasts
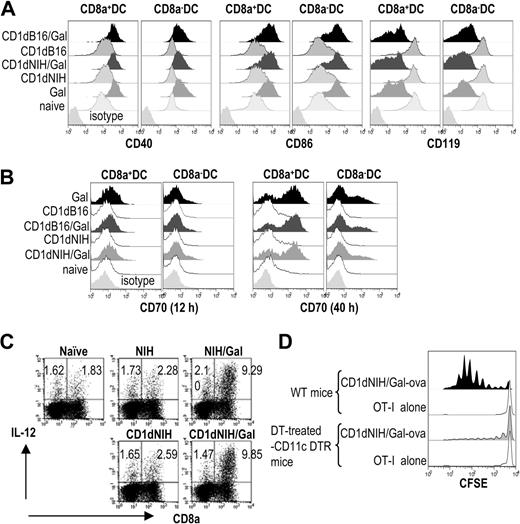
In our previous studies, we injected mice with α-GalCer–loaded tumor cells and demonstrated that host DCs needed to undergo maturation after capturing antigen to generate T-cell responses.45 In the current study, as shown in Figure 3A,B, NIH3T3/Gal activated innate lymphocytes. We then tested whether in vivo DC maturation occurred after injecting mice with NIH3T3 or CD1dhi-NIH3T3 loaded with or without α-GalCer. At 12 hours after injection, spleen cells were collected and analyzed for expression of DC surface markers CD40, CD86, and CD119 by flow cytometry. We found an up-regulation of CD40 and CD86 and a down-regulation of CD119, changes consistent with DC maturation (Figure 4A).38 As shown in Figure 4A, increased expression of CD86 on both CD8α+ and CD8α− subsets of DCs was similar to that seen in mice immunized with CD1dhi-B16/Gal or free α-GalCer. Because of a recent report that CD70 was expressed on DCs after intravenous immunization with free α-GalCer,55 we analyzed the CD70 expression as well. We found levels of CD70 did not increase 12 hours after injection of CD1dhi-B16/Gal or CD1dhi-NIH3T3/Gal, but did increase after 40 hours (Figure 4B). In the later phase, we also found that CD8a+ DCs expressed more CD70 than CD8a− DCs (Figure 4B). A manifestation of functional DC maturation generally involves the secretion of IL-12. DCs from mice immunized with NIH3T3/Gal or CD1dhi-NIH3T3/Gal intravenously secreted high levels of IL-12, but not DCs from animals immunized with NIH3T3 cells or CD1dhi-NIH3T3 (Figure 4C). These indications of DC maturation, alterations in cell-surface markers and secretion of IL-12, were ablated in Jα18-deficient mice (data not shown), indicating iNKT cells were necessary for DC maturation. From these data, it appears that DCs begin to mature soon after injection of allogeneic fibroblasts loaded with α-GalCer. The glycolipid loaded on fibroblasts not only directly, but also indirectly after captured by host DCs activate iNKT cells, which in turn mature the DCs.38,39
In vivo DC maturation in response to α-GalCer–loaded allogeneic fibroblasts. (A) Several DC maturation markers were evaluated 12 hours after intravenous injection of NIH3T3 or CD1dhi-NIH3T3 cells with or without α-GalCer. (B) Expression of CD70 on DCs was analyzed 12 hours and 40 hours after immunization. (C) IL-12 production from CD11c+ DCs in vivo was evaluated by intracellular staining 4 hours after giving mice CD1dhi-NIH3T3/Gal or NIH3T3 cells as previously reported.43,54 (D) To determine whether the T-cell response seen in mice immunized with CD1dhi-NIH3T3/Gal-ova is dependent on DCs, 2 × 106 CFSE-labeled OT-I T cells were transferred before immunization in wild-type (WT) mice or diphtheria toxin (DT)–treated CD11c-DTR mice (Document S1). OT-I proliferation was evaluated by dilution of CFSE-labeled cells 3 days later. Data are representative of 2 independent experiments with 2 mice in each group.
In vivo DC maturation in response to α-GalCer–loaded allogeneic fibroblasts. (A) Several DC maturation markers were evaluated 12 hours after intravenous injection of NIH3T3 or CD1dhi-NIH3T3 cells with or without α-GalCer. (B) Expression of CD70 on DCs was analyzed 12 hours and 40 hours after immunization. (C) IL-12 production from CD11c+ DCs in vivo was evaluated by intracellular staining 4 hours after giving mice CD1dhi-NIH3T3/Gal or NIH3T3 cells as previously reported.43,54 (D) To determine whether the T-cell response seen in mice immunized with CD1dhi-NIH3T3/Gal-ova is dependent on DCs, 2 × 106 CFSE-labeled OT-I T cells were transferred before immunization in wild-type (WT) mice or diphtheria toxin (DT)–treated CD11c-DTR mice (Document S1). OT-I proliferation was evaluated by dilution of CFSE-labeled cells 3 days later. Data are representative of 2 independent experiments with 2 mice in each group.
In situ DCs are essential for eliciting adaptive immunity in CD1dhi-NIH3T3/Gal-ova–injected mice
In Figure 2E, immunized mice demonstrated that OT-I cell proliferation to class I–mismatched CD1dhi-NIH3T3/Gal-ova. To determine whether host DCs were involved in the presentation of OVA antigen to OT-I cells, we used CD11c-diphtheria toxin receptor (DTR) transgenic (CD11c-DTR/GFP) mice treated with diphtheria toxin (DT) to ablate host CD11c+ DCs in vivo.56 In these mice, there was little proliferation of OT-I cells, demonstrating the role of DCs in the cross-presentation of antigens in mice given CD1dhi-NIH3T3/Gal-ova cells (Figure 4D).
Immunization of C57BL/6 mice with CD1dhi-NIH3T3/Gal-ova leads to a strong adaptive immune response
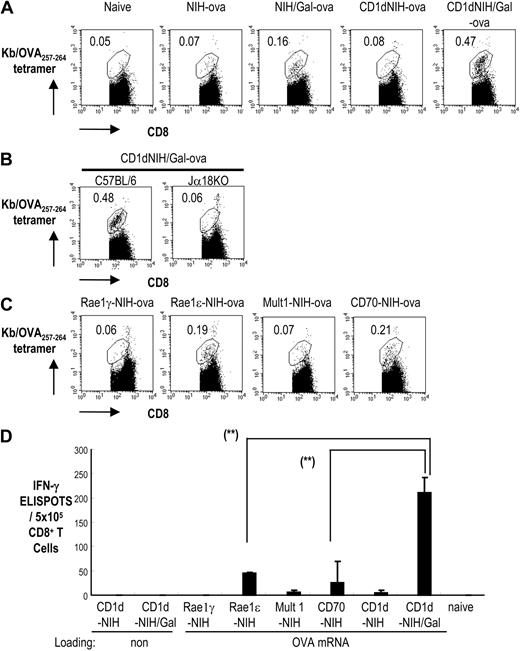
Once our method of generating an immune response to mRNA-transfected fibroblasts was established using mice injected with transgenic OT-I cells, it is more important to purse the immune response generated in wild-type mice. We then investigated the importance of iNKT-cell activation and CD1d-expressing fibroblasts in the induction of OVA-specific T-cell responses in wild-type mice using OVA mRNA–transfected fibroblasts loaded with α-GalCer. To study this, we immunized mice with variations of parental or CD1dhi-NIH3T3 cells transfected with OVA mRNA: NIH3T3-ova, NIH3T3/Gal-ova, CD1dhi-NIH3T3-ova, and CD1dhi-NIH3T3/Gal-ova. After 7 days, we collected spleen cells and analyzed the number of CD8+ T cells specific to the OVA peptide SIINFEKL by staining with the Kb/OVA257-264 tetramer. As shown in Figure 5A, the number of OVA-tetramer–positive cells increased in mice given NIH3T3/Gal-ova or CD1dhi-NIH3T3/Gal-ova, but not in mice given NIH3T3-ova or CD1dhi-NIH3T3-ova. These did not occur when Jα18-deficient mice were used as recipients (Figure 5B). In addition, C57BL/6 mice immunized with CD1dhi-NIH3T3/Gal-ova generated a higher number of OVA257-264 peptide–specific T cells than mice given NIH3T3/Gal-ova.
Evaluation of the effects of NK- and NKT-cell ligands on T-cell priming to NIH3T3 cells transfected with OVA antigen mRNA. (A) C57BL/6 mice were immunized with NIH3T3-ova, CD1dhi-NIH3T3-ova, NIH3T3/Gal-ova, or CD1dhi-NIH3T3/Gal-ova. Spleen cells were collected one week later to test for the development of OVA-specific T-cell immunity using Kb/OVA257-264 tetramer-PE and CD8-FITC. The number shown indicates the percentage of Kb/OVA257-264 tetramer–positive cells of the total CD8+ T cells. (B) C57BL/6 mice and Jα18−/− mice were immunized with CD1dhi-NIH3T3/Gal-ova. Spleen cells were analyzed as shown in panel A. The number shown indicates the percentage of Kb/OVA257-264 tetramer–positive cells of the total CD8+ T cells. (C) Mice were immunized with Rae-1ϵ-NIH3T3-ova, Rae-1γ-NIH3T3-ova, Mult1-NIH3T3-ova, or CD70-NIH3T3-ova. Spleen cells were analyzed as shown in panel A. (D) Spleen cells from different groups of immunized mice were collected one week after immunization. CD8+ T cells were positively selected from the spleens of naive or immunized mice and cocultured with OVA257-264 peptide-pulsed CD11c+ cells for 36 hours. IFN-γ secretion from CD8+ T cells in response to OVA257-264 peptide was evaluated by ELISPOT (Document S1). All data are means plus or minus SEM from 3 independent experiments with 2 mice per group. **P < .001 (indicated in panels: Rae-1ϵ-NIH3T3-ova and CD70-NIH3T3-ova vs CD1dhi-NIH3T3/Gal-ova).
Evaluation of the effects of NK- and NKT-cell ligands on T-cell priming to NIH3T3 cells transfected with OVA antigen mRNA. (A) C57BL/6 mice were immunized with NIH3T3-ova, CD1dhi-NIH3T3-ova, NIH3T3/Gal-ova, or CD1dhi-NIH3T3/Gal-ova. Spleen cells were collected one week later to test for the development of OVA-specific T-cell immunity using Kb/OVA257-264 tetramer-PE and CD8-FITC. The number shown indicates the percentage of Kb/OVA257-264 tetramer–positive cells of the total CD8+ T cells. (B) C57BL/6 mice and Jα18−/− mice were immunized with CD1dhi-NIH3T3/Gal-ova. Spleen cells were analyzed as shown in panel A. The number shown indicates the percentage of Kb/OVA257-264 tetramer–positive cells of the total CD8+ T cells. (C) Mice were immunized with Rae-1ϵ-NIH3T3-ova, Rae-1γ-NIH3T3-ova, Mult1-NIH3T3-ova, or CD70-NIH3T3-ova. Spleen cells were analyzed as shown in panel A. (D) Spleen cells from different groups of immunized mice were collected one week after immunization. CD8+ T cells were positively selected from the spleens of naive or immunized mice and cocultured with OVA257-264 peptide-pulsed CD11c+ cells for 36 hours. IFN-γ secretion from CD8+ T cells in response to OVA257-264 peptide was evaluated by ELISPOT (Document S1). All data are means plus or minus SEM from 3 independent experiments with 2 mice per group. **P < .001 (indicated in panels: Rae-1ϵ-NIH3T3-ova and CD70-NIH3T3-ova vs CD1dhi-NIH3T3/Gal-ova).
We then compared the magnitude of T-cell responses after priming with the iNKT-cell ligand α-GalCer versus ligands of NK cells, such as retinoic acid early inducible-1ϵ (Rae1ϵ), Rae1γ, CD70, and murine UL16-binding protein-like transcript 1 (Mult1). The NK cell ligands were cloned using an EGFP-carrying retrovirus vector. Coexpression of each molecule and EGFP was verified by FACS analysis (data not shown). T-cell proliferation was evaluated with tetramer staining one week after immunization with CD70-NIH3T3-ova, Rae1ϵ-NIH3T3-ova, Mult1-NIH3T3-ova, or Rae1γ-NIH3T3-ova. As is shown in Figure 5C, the Rae1ϵ-NIH3T3-ova– and CD70-NIH3T3-ova–immunized groups demonstrated Kb/OVA257-264 tetramer–positive cell proliferation, but the other NK ligand–immunized groups did not. T-cell responses specific for OVA were also tested using IFN-γ ELISPOT. The number of IFN-γ–producing T-cell responses was much higher in mice given CD1dhi-NIH3T3/Gal-ova than in mice given Rae1ϵ-NIH3T3-ova, Mult1-NIH3T3-ova, CD70-NIH3T3-ova, or CD1dhi-NIH3T3-ova (Figure 5D). Therefore, α-GalCer–loaded, antigen-carrying fibroblasts lead to a stronger immune response by linking innate and adaptive immunity in naive mice.
Vaccination with CD1dhi-NIH3T3/Gal-ova induces antitumor T-cell immunity
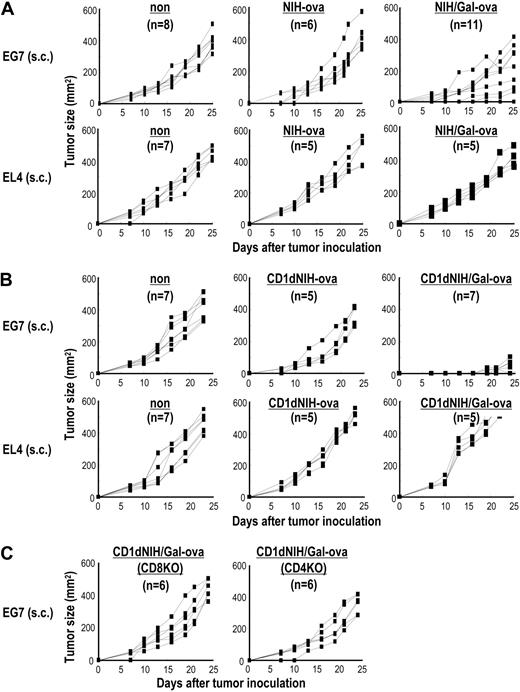
To evaluate whether the T-cell response in mice immunized with CD1dhi-NIH3T3/Gal-ova can lead to antitumor immunity, mice were challenged subcutaneously with 2 × 105 EL4 thymoma or OVA-expressing EL4 (EG7) 2 weeks after immunization intravenously with 5 × 105 NIH3T3-ova, CD1dhi-NIH3T3-ova, NIH3T3/Gal-ova, or CD1dhi-NIH3T3/Gal-ova (Figure 6). Antitumor effects in mice given CD1dhi-NIH3T3/Gal-ova were shown against EG7, but not EL4, indicating a tumor-specific immune response (Figure 6B). In 11 mice immunized with NIH3T3/Gal-ova, 3 mice demonstrated inhibition of tumor growth, but in 8 mice, the vaccination failed to provide the protective effect (Figure 6A top right). These results indicated that CD1d expression is for adaptive immunity rather than innate immunity, probably due to the α-GalCer–loading capacity. Mice given NIH3T3-ova (Figure 6A), CD1dhi-NIH3T3-ova (Figure 6B), or CD1dhi-NIH3T3/Gal (data not shown) developed EL4 and EG7 tumors. Protection against tumor development after subcutaneous inoculation requires CD4+ and CD8+ T-cell responses (Figure 6C). We also tested if the CD1dhi-NIH3T3/Gal-ova cells would provide the same protection from tumor development after irradiation with 30 Gy, and we found similar results in groups of mice receiving irradiated cells (data not shown).
Immunization with CD1dhi-NIH3T3/Gal-ova confers protection against tumor development in mice. C57BL/6, CD4−/−, or CD8−/− mice were given 5 × 105 NIH3T3-ova, NIH3T3/Gal-ova, CD1dhi-NIH3T3-ova, or CD1dhi-NIH3T3/Gal-ova intravenously. At 2 weeks later, the vaccinated mice were injected subcutaneously with 2 × 105 of either EL4 or EG7 cells. Tumor size was measured at the indicated time points (n = 5-11 per group). Similar results were obtained in 2 independent experiments.
Immunization with CD1dhi-NIH3T3/Gal-ova confers protection against tumor development in mice. C57BL/6, CD4−/−, or CD8−/− mice were given 5 × 105 NIH3T3-ova, NIH3T3/Gal-ova, CD1dhi-NIH3T3-ova, or CD1dhi-NIH3T3/Gal-ova intravenously. At 2 weeks later, the vaccinated mice were injected subcutaneously with 2 × 105 of either EL4 or EG7 cells. Tumor size was measured at the indicated time points (n = 5-11 per group). Similar results were obtained in 2 independent experiments.
Antitumor effects driven by adaptive immunity in response to α-GalCer–loaded, trp2 mRNA–transfected NIH3T3 cells
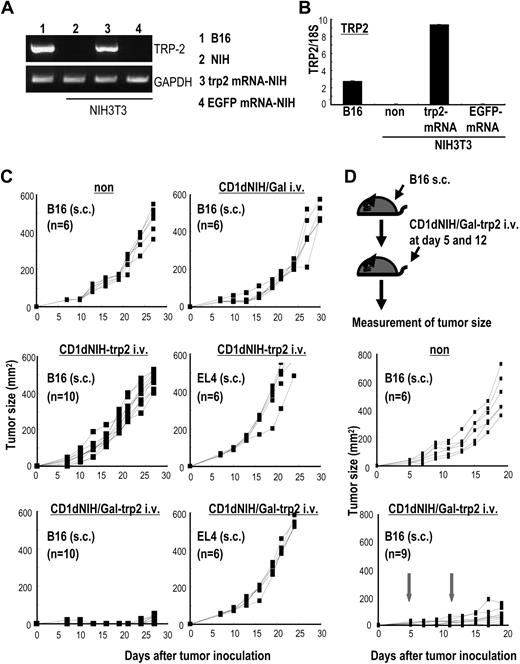
We demonstrated the link between innate and adaptive immunity by immunizing with α-GalCer–loaded, mRNA-transduced allogeneic cell lines in OVA models (Figure 6). We then applied this concept to real tumor models by immunizing mice with CD1dhi-NIH3T3/Gal cells transduced with mRNA encoding the melanocyte differentiation antigen, tyrosinase-relating protein 2 (trp2). Trp2 expression in NIH3T3-trp2 was verified by reverse trancription (RT)–PCR (Figure 7A), quantified by real-time PCR (Figure 7B), and shown to be nearly 3 times that of trp2 endogenously expressed on B16 melanoma cells.
Vaccination with irradiated CD1dhi-NIH3T3/Gal-trp2 induces protection from melanoma. mRNA from the melanocyte differentiation antigen, trp2, was transduced into NIH3T3 cells. Trp2 expression on B16 cells and trp2 mRNA–transduced NIH3T3 cells was evaluated by (A) RT-PCR and (B) real-time PCR. (C) WT mice were immunized with 5 × 105 CD1dhi-NIH3T3/Gal, CD1dhi-NIH3T3-trp2, and CD1dhi-NIH3T3/Gal-trp2 intravenously. At 2 weeks later, the mice were challenged subcutaneously with 5 × 104 B16 or 2 × 105 EL4 cells. Tumor size in each group was measured at the indicated time points (n = 6-8 per group). Similar results were obtained in 2 independent experiments. (D) Antitumor responses to established tumor were assessed. C57BL/6 mice were injected 5 × 104B16 subcutaneously (top panel). The mice were given 5 × 105 CD1dhi-NIH3T3/Gal-trp2 intravenously at 5 days and again at 12 days. Tumor size was evaluated at the indicated time points (n = 6-10 per group). ↓ indicates the treatment with CD1dhi-NIH3T3/Gal-trp2.
Vaccination with irradiated CD1dhi-NIH3T3/Gal-trp2 induces protection from melanoma. mRNA from the melanocyte differentiation antigen, trp2, was transduced into NIH3T3 cells. Trp2 expression on B16 cells and trp2 mRNA–transduced NIH3T3 cells was evaluated by (A) RT-PCR and (B) real-time PCR. (C) WT mice were immunized with 5 × 105 CD1dhi-NIH3T3/Gal, CD1dhi-NIH3T3-trp2, and CD1dhi-NIH3T3/Gal-trp2 intravenously. At 2 weeks later, the mice were challenged subcutaneously with 5 × 104 B16 or 2 × 105 EL4 cells. Tumor size in each group was measured at the indicated time points (n = 6-8 per group). Similar results were obtained in 2 independent experiments. (D) Antitumor responses to established tumor were assessed. C57BL/6 mice were injected 5 × 104B16 subcutaneously (top panel). The mice were given 5 × 105 CD1dhi-NIH3T3/Gal-trp2 intravenously at 5 days and again at 12 days. Tumor size was evaluated at the indicated time points (n = 6-10 per group). ↓ indicates the treatment with CD1dhi-NIH3T3/Gal-trp2.
Adaptive antitumor responses to injected trp2-encoding mRNA–transfected CD1dhi-NIH3T3/Gal were assessed. Mice were immunized intravenously with CD1dhi-NIH3T3/Gal-trp2, CD1dhi-NIH3T3/Gal, or CD1dhi-NIH3T3-trp2. When the mice were given subcutaneous challenge of B16 melanoma cells 2 weeks later to assess antitumor protection, growth of B16 tumor cells was inhibited in mice that received CD1dhi-NIH3T3/Gal-trp2 (Figure 7C bottom left), but not in mice receiving CD1dhi-NIH3T3-trp2 (Figure 7C middle left) or CD1dhi-NIH3T3/Gal (Figure 7C top right). None of the groups of immunized mice demonstrated any antitumor immunity against EL4 thymoma cells.
We then assessed the effects of immunization with trp2-encoding mRNA–transfected CD1dhi-NIH3T3/Gal on established tumors. As shown in top of Figure 7D, 9 mice were injected with B16 cells subcutaneously. The mice were then injected (intravenously) with 5 × 105 CD1dhi-NIH3T3/Gal-trp2 cells on days 5 and 12, and tumor size was evaluated. Inhibition of tumor growth was apparently seen in immunized mice until day 20 (Figure 7D), although no mouse demonstrated complete rejection of the tumor.
Discussion
In this study, we have established an antitumor vaccination strategy in which allogeneic fibroblasts are loaded with α-GalCer and transduced with mRNA encoding tumor antigen as a cellular vector. The use of antigen-derived mRNA is beneficial in some instances because mRNA can be derived from tumor cell lines or tumors from third-party patients without the need for human leukocyte antigen (HLA) matching. The advantage of our strategy over previous approaches using tumor-derived mRNA-transduced DCs is the addition of iNKT-cell help, which acts to mature DCs in situ and drives an effective immunogenic immune response.
Instead of transferring DCs that have been transduced with antigen-encoding mRNA, our strategy uses in vivo DCs to capture mRNA-derived antigen proteins in the presence of activated innate lymphocytes, which resulted in DC maturation. As shown in Figure 2D, mRNA-transfected, MHC-mismatched NIH3T3 cells did not present antigen directly to T cells due to the MHC disparity, but MHC-matched B16 cells did. However, mice given OVA-specific OT-I cells followed by immunization with OVA mRNA–transfected NIH3T3/Gal cells demonstrated enhanced proliferation of OT-I cells, indicating that host DCs process antigen from mRNA-transfected cells and cross-prime onto MHC class I molecules (Figures 2E,4D).
α-GalCer works as an immunologic agent by activating iNKT cells, which in turn mature DCs.38,39 The matured DCs then prime T cells in the context of inflammation, thus generating cytotoxic T cells as opposed to tolerized T cells. The use of allogeneic cells, which are easily killed when transferred in vivo, may lead to an enhanced NK as well as iNKT-cell response. However, as is shown in Figure 3, administration of allogeneic NIH3T3 fibroblasts or CD1dhi-NIH3T3 cells alone did not lead to the strong activation of NK or iNKT cells; that is, simple alloantigens did not strongly activate NK or iNKT cells, but α-GalCer loading onto fibroblasts caused both NK and iNKT cells to respond with the secretion of IFN-γ.
Several studies have shown that tumor cells transfected with NK-cell ligands lead to NK cell– and/or CD8+ T cell–mediated tumor rejection57-61 due to constitutive cell-surface expression of NKG2D on almost all NK cells and activated CD8+ T cells. Therefore, we evaluated the ability of known NKG2D ligands, such as Rae-1ϵ, Rae-1γ, and Mult1 as well as CD70, to act as adjuvants for the generation of T-cell immunity. To compare iNKT cell–mediated T-cell immunity with NK cell–mediated immunity, we established 3 groups of NKG2D transfectants and one CD70 transfectant (Figure 5). When NK cell ligand transfectants expressing OVA were given, small numbers of tetramer-positive cells were shown in mice given Rae1ϵ-NIH3T3-ova and CD70-NIH3T3-ova, but none were seen in mice given Rae1γ-NIH3T3-ova or Mult1-NIH3T3-ova.62 In papers observing the efficacy of NKG2D ligands as adjuvants, CD8+ T-cell responses in mice given Rae-1γ–transfected cells were generated (0.25%) only after boosting with additional Rae-1γ–transfected cells.59 These results suggest that targeted activation of iNKT cells leads to a more powerful immunogenic response than activation of NK cells under the same conditions.
Recently, it has been shown that iNKT-cell activation leads to up-regulation of CD70 on DCs and is a key component for the induction of T-cell immunity.55,63 Taraban et al showed that CD70-CD27 interaction between DCs and T cells follows CD40-CD40L coupling between DCs and iNKT cells.55,63 The importance of this interaction was demonstrated in studies where CD70-CD27 interactions were blocked, abolishing the ability of iNKT cells to generate a cytotoxic T-cell response.55,63 As is shown in Figure 4A and B, we immunized mice and measured expression levels of costimulatory molecules after 12 hours (to allow for up-regulation of CD40, CD86, and down regulation of CD119) and 40 hours (to allow for up-regulation of CD70; Figure 4B). In the current study, we found that T-cell priming in situ can be broken down into 2 phases: an initial phase of NKT-cell activation and functional maturation of DCs through CD40-CD40L interaction, and a second phase in which naive T cells are cross-primed by endogenous mature DCs through CD70-CD70L interaction. In particular, CD8a+ DCs expressed more CD70 and secreted more IL-12 than CD8a− DCs, thus suggesting an important role for these cells in this type of immunotherapy (Figure 4).
In terms of adjuvant effect, some Toll-like receptor (TLR) agonists for TLR3, TLR7, and TLR9 have begun for patients with several types of cancer in clinical studies.64-66 Imiquimod, a Food and Drug Administration (FDA)–approved topically applied TLR7 agonist, especially has shown clinical efficacy against basal cell carcinoma and has been used in combination with NY-ESO-1 protein in patients with malignant melanoma.64 Allogeneic cells carrying antigen and transduced with the gene encoding GM-CSFs have been shown to be safe in trials using GM-CSF gene–transduced irradiated cancer vaccine (GVAX) therapy.67,68 In the current study, we demonstrated that low concentrations of antigen together with the glycolipid-loaded, allogeneic fibroblast effectively matured in situ DCs and led to a protective cytotoxic T-cell response.
Immunologically, CD1d expression level may be one of the key factors linking innate and adaptive immunity.41 α-GalCer–loaded, nontransfected NIH3T3 cells activate NK and iNKT cells, blocking tumor establishment in the lung in mice injected with B16 melanoma (Figure 3C). Nevertheless, mice given NIH3T3/Gal-ova exhibited weaker T-cell responses (Figure 5A) and only partial antitumor effects (Figure 6) compared with mice given CD1dhi-NIH3T3/Gal-ova. To ease clinical application of this method, further investigations into enhancing the use of parental NIH3T3 cells could eliminate the need to transfect cells to overexpress CD1d. Possible alterations in the current method are an increase in the number of vaccinations or the amount of protein, α-GalCer, or cell dose used.
The combination strategy of mRNA-encoded antigen and iNKT-cell ligand packaged in allogeneic cells offers the advantage of supplying antigen to host DCs in the context of activated iNKT cells. Our strategy replicates the cascade of events occurring in vivo that lead to an effective adaptive immune response. This approach could be translated into clinical trials with the intended outcome of a better antitumor response and fewer side effects compared with conventional cancer therapies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Ms H. Fujimoto, Y. Hachiman, Y. Kurosawa, and M. Fukui for providing technical assistance and Dr K. Bickham for critical reading of the manuscript. We also thank Dr Y. Ishii (Yokohama RIKEN) for providing α-GalCer.
This work is supported by grants from the Ministry of Education, Science, Sports, and Culture of Japan (to K.S.) and from Mitsubishi Pharma Research Foundation (Osaka, Japan) and Takeda Science Foundation (Osaka, Japan; to S.F.).
Authorship
Contribution: S.F. and K.S. designed and performed research, analyzed data, and wrote the paper; and A.G. performed research and analyzed data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shin-ichiro Fujii, Research Unit for Cellular Immunotherapy, RCAI, RIKEN, Yokohama, Kanagawa, 230-0045, Japan; e-mail: fujiis@rcai.riken.jp.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal