Abstract
ABT-737 and its orally active analog, ABT-263, are rationally designed inhibitors of BCL2 and BCL-XL. ABT-263 shows promising activity in early phase 1 clinical trials in B-cell malignancies, particularly chronic lymphocytic leukemia (CLL). In vitro, peripheral blood CLL cells are extremely sensitive to ABT-737 (EC50 ∼7 nM), with rapid induction of apoptosis in all 60 patients tested, independent of parameters associated with disease progression and chemotherapy resistance. In contrast to data from cell lines, ABT-737–induced apoptosis in CLL cells was largely MCL1-independent. Because CLL cells within lymph nodes are more resistant to apoptosis than those in peripheral blood, CLL cells were cultured on CD154-expressing fibroblasts in the presence of interleukin-4 (IL-4) to mimic the lymph node microenvironment. CLL cells thus cultured developed an approximately 1000-fold resistance to ABT-737 within 24 hours. Investigations of the underlying mechanism revealed that this resistance occurred upstream of mitochondrial perturbation and involved de novo synthesis of the antiapoptotic proteins BCL-XL and BCL2A1, which were responsible for resistance to low and high ABT-737 concentrations, respectively. Our data indicate that after therapy with ABT-737–related inhibitors, resistant CLL cells might develop in lymph nodes in vivo and that treatment strategies targeting multiple BCL2 antiapoptotic members simultaneously may have synergistic activity.
Introduction
Clinically, chronic lymphocytic leukemia (CLL) is heterogeneous; some patients live for many years, whereas others die with rapidly progressive, chemotherapy-resistant disease. Biologic factors such as an unmutated IGHV gene segment usage, deletion of 17p, p53 mutation, or high CD38 expression have been associated with poor prognosis and disease progression.1 CLL was initially thought to represent the gradual accumulation of B cells that failed to die.2 Recently, a more dynamic picture has emerged, with proliferation in the “proliferation centers” in certain microenvironments of lymph nodes and bone marrow as well as apoptosis in the peripheral blood.3 Most CLL cells require prosurvival signaling from other cells, including “nurse-like cell” populations in the peripheral blood, to maintain viability.4 CLL cells in the peripheral blood are often chemosensitive, whereas those in specific microenvironments, such as lymph nodes and bone marrow, are more chemoresistant.5,6 Therefore, a major challenge for treating CLL is the elimination of leukemic cells in bone marrow and lymph nodes.
Targeting the apoptotic pathways may provide a new therapeutic approach.7 Apoptosis can be activated either by ligation of cell surface death receptors or by perturbation of mitochondria, resulting in release of cytochrome c and caspase activation. Loss of cytochrome c is regulated by BCL2 family proteins, which comprise both pro- and antiapoptotic members.8 The multidomain proapoptotic BCL2 family members, BAX and BAK, oligomerize during apoptosis, forming pores in the outer mitochondrial membrane through which cytochrome c may be released. BAX and BAK are kept in check by the antiapoptotic BCL2 proteins, namely BCL2, BCL-XL, BCL-w, MCL1, and BCL2A1 (Bfl-1/A1), which either directly sequester BAX and BAK, or inhibit the proapoptotic BH3-only proteins. BH3-only proteins serve as stress sensors and are essential for initiation of cell death, acting upstream of BAX and BAK. Once induced, BH3-only proteins insert their BH3 domain into the hydrophobic cleft of antiapoptotic BCL2 proteins, thereby initiating BAX and BAK activation.8,9 BH3-only proteins, such as BIM, PUMA, and BID, bind to all antiapoptotic BCL2 family members, whereas others bind more selectively to certain subsets of antiapoptotic proteins.10,11
Deregulation of pro- and antiapoptotic BCL2 proteins plays an important role in the pathogenesis and progression of cancer, as shown for follicular lymphoma that overexpress BCL2 due to a t(14;18)(q32;q21) translocation.12 CLL cells express high BCL2 levels and exhibit high BCL2/BAX ratios. Thus, BCL2 is an attractive target for the development of new treatments. ABT-737 is a rationally designed small molecule that binds with high affinity into the hydrophobic cleft of BCL2, BCL-XL, or BCL-w and induces apoptosis in a BAX and BAK dependent manner.13 ABT-737 binds only weakly to MCL1 or BCL2A1, and resistance to ABT-737 has been linked to high MCL1 expression.14-19 The microenvironment of CLL cells changes the expression of BCL2 proteins. Thus, CLL cells residing in lymph nodes compared with those in peripheral blood, show high expression of antiapoptotic BCL2 proteins and low NOXA expression.20 Similar changes are found by culturing CLL cells on CD154/CD40L-expressing fibroblasts, suggesting this system mimics the lymph node environment.20,21 Engagement of CD40 on B cells by CD154 occurs in vivo in secondary lymphoid tissues during T- and B-cell interactions, resulting in nuclear factor (NF)–κB activation. Among the NF-κB targets activated upon CD40 ligation are BCL-XL and BCL2A122 ; consequently, these proteins, which are undetectable in peripheral blood CLL cells, may be up-regulated in “proliferation centers” within lymph nodes.
In agreement with recent studies,13,23 we also found that CLL cells are exquisitely sensitive to ABT-737–induced apoptosis (EC50 = 7 nM at 4 hours).24 Furthermore, we found that ABT-737 induces rapid apoptosis by activation of the intrinsic pathway with activation of BAX and BAK, loss of mitochondrial membrane potential, release of mitochondrial cytochrome c, activation of caspase-9 as the apical caspase, and induction of ultrastructural changes including nuclear condensation.24 In the present study, ligation of CD40 on peripheral blood CLL cells resulted in approximately 1000-fold resistance to ABT-737.
Methods
Clinical data and cell culture
Peripheral blood samples from CLL patients were collected after patient informed consent was obtained in accordance with the Declaration of Helsinki and ethical committee approval from the regional National Health Service (NHS) Research Ethics Committee was received. Lymphocytes were purified and cultured in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS) and 5 mM l-glutamine (all from Life Technologies, Paisley, United Kingdom). DNA was extracted from peripheral blood lymphocytes using DNeasy tissue kit (Qiagen, Crawley, United Kingdom) according to the manufacturer's instructions. To detect TP53 mutations, polymerase chain reaction (PCR) was performed as described.25 Amplification and analysis of IGHV rearrangements was performed according to BIOMED-2 protocols.26 Interphase fluorescence in situ hybridization (FISH) was performed for detection of 13q14, 11q22, 17p13, and trisomy 12 as described.27 For culture of CLL cells, parental or CD154-expressing mouse fibroblast L cells were irradiated with 75 Gray and afterward seeded in 24-well plates (3 × 105 cells/well). Freshly isolated CLL cells were cultured at 1.5 × 106 cells/well on the L cells, and 10 ng/mL IL-4 (R&D Systems, Abingdon, United Kingdom) was added. CLL cells were removed from the L cells by gentle washing with RPMI before treatment.
Reagents
IL-4 and interferon γ (IFNγ) were from R&D Systems; in addition, cycloheximide (Sigma-Aldrich, Poole, United Kingdom), seliciclib (Calbiochem, Nottingham, United Kingdom), and trichostatin A (TSA; Sigma-Aldrich) were used. Dehydroxymethylepoxyquinomicin (DHMEQ) was from Dr K. Umezawa (Tokyo, Japan).28 The BCL2A1 overexpression vector was from Dr I. Berberich (Wuerzburg, Germany). ABT-737 was from Abbott Laboratories (Abbott Park, IL).
Immunohistochemistry
Lymph node sections were obtained from 6 individual CLL patients with informed consent in accordance with the Declaration of Helsinki and ethical committee approval from the regional NHS Research Ethics Committee. Staining for BCL2A1 used an anti-BCL2A1 antibody (Ab) (1: 50) provided by Dr J. Borst. As control, nonimmunized rabbit serum was used. Staining was visualized using StrepABComplex/HRP Duet (Dako UK, Ely, United Kingdom).
Overexpression of BCL2A1 and down-regulation of BCL2A1 and BCL-XL
For transfection of siRNA in CLL cells, Amaxa Human B-cell Nucleofection Kit was used (Amaxa, Cologne, Germany). Transfection efficiency, which was assessed by transfection with 2 μg pMaxGFP and fluorescence-activated cell sorting (FACS) analysis after 3 hours, varied significantly between individual patients (mean transfection efficiency 37.7%, SD 10.7 for n = 44). For knockdown experiments, only cells with transfection efficiency greater than 35% were used. To knock down BCL2A1, we used the following oligos: sense (GUUUGAAGACGGCAUCAU) and antisense (AAUGAUGCCGUCUUCAAAC) (Ambion, Warrington, United Kingdom) and an untargeted oligo as control (Ambion ID 4611). To knock down BCL-X, we used a combination of 3 different siRNAs: sense-1 (CAGCUUGGAUGGCCACUUAUU), antisense-1 (UAAGUGGCCAUCCAAGCUGUU), sense-2 (ACAAGGAGAUGCAGGUAUUUU), antisense-2 (AAUACCUGCAUCUCCUUGUUU), sense-3 (CCUACAAGCUUUCCCAGAAUU), antisense-3 (UUCUGGGAAAGCUUGUAGGUU) (Ambion). For transfection, 10 × 106 CLL cells mixed with Amaxa solution (100 μL) and 2 μg of siRNA were nucleofected using program X-5. Directly after nucleofection, CLL cells were cultured on CD154-expressing L cells in the presence of 20% FCS and IL-4 (10 ng/mL) for 16 hours. Viability of CLL cells after transfection was assessed by phosphatidylserine externalization and was greater than 90%. Knockdown of BCL2A1 and BCL-XL was controlled by Western blotting. To express BCL2A1, 3.5 × 106 Jurkat E6.1 cells were transfected with 2 μg yellow fluorescent protein (pYFP)-BCL2A1 or empty vector using Amaxa program C-16 and KitV (Amaxa AG). Transfection efficiency was approximately 20%. After 16 hours, BCL2A1 expression was confirmed by Western blotting. Transfected cells were incubated with different concentrations of ABT-737 for an additional 4 hours before staining with annexin V/allophycocyanin (APC; Invitrogen, Paisley, United Kingdom).
Western blotting and immunoprecipitation
Western blot analysis was performed using rabbit anti-MCL1 Ab (Santa Cruz Biotechnologies, Santa Cruz, CA), mouse anti-BCL2 Ab (Dako UK), rabbit anti-BCL-XL Ab (BD Bioscience, San Diego, CA), mouse anti-α-tubulin Ab (Calbiochem), rabbit anti-BAX and anti-BAK Abs (Upstate Biotechnology, Lake Placid, NY), rabbit anti-BIM Ab (Calbiochem), rabbit anti-PUMA antibody (ProSci, Poway, CA), mouse anti-NOXA Ab (Calbiochem), and mouse anti-cytochrome c Ab (BD Bioscience). Rabbit and rat anti-BCL2A1 Abs were provided by Drs J. Borst and D. Huang, respectively. Enhanced chemiluminescence (GE Healthcare, Little Chalfont, United Kingdom) was used for detection. To quantify levels of BCL2 proteins, ImageQuant TL (GE Healthcare) was used.
For immunoprecipitation (IP), 3 × 108 CLL cells either were treated with ABT-737 (10 nM) for 2 hours or were cultured on CD154-expressing L cells in the presence of IL-4 (10 ng/mL) for 4, 8, or 16 hours. After removal from CD154-expressing cells, CLL cells were washed and lysed in buffer containing 0.5% Triton X-100, 20 mM TrisHCl (pH 8), 150 mM NaCl, and Protease Inhibitor Cocktail (Roche Diagnostics, Basel, Switzerland). For IP upon exposure to ABT-737, cells were lysed in buffer containing 1% CHAPS, 20 mM Tris HCl (pH 8), 150 mM NaCl, and protease inhibitor cocktail. Hamster anti-BCL2 Ab (BD Bioscience), rabbit anti-BCL-XL Ab (BD Biosciences), rabbit anti-BAK Ab (Upstate Biotechnology), rabbit anti-BCL2A1 Ab, and rabbit anti-MCL1 Ab (Epitomics, Burlingame, CA) were crosslinked with ProtA/G-dynabeads using 20 mM dimethylpimelinediimidate (Fluka Biochemika, Switzerland). Crosslinked antibodies were incubated with 500 μg protein for 12 hours at 4°C. Beads were washed with lysis buffer before elution in sodium dodecyl sulfate (SDS) loading dye and Western blotting.
Determination of apoptosis
Apoptosis was assessed by phosphatidylserine (PS) externalization or loss of mitochondrial membrane potential as described.24 To assess cytochrome c release, 10 × 106 cells were washed in cold PBS and resuspended in 100 μL mitochondrial isolation buffer (250 mM sucrose, 20 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), pH 7.4, 5 mM MgCl2, and 10 mM KCl) containing 0.05% digitonin, prepared from a 1% stock digitonin dispersion. Cells were left on ice for 10 minutes followed by centrifugation at 16 000g for 3 minutes. Subsequently, supernatant and pellets were analyzed by Western blotting. Activation of BAX and BAK was assessed as described.24
Statistical analysis
Statistical analysis was done using GraphPad Prism (GraphPad Software, La Jolla, CA). For Figure 1, the individual EC50 values were determined and statistical analysis carried out using an unpaired t test or one-way analysis of variance (ANOVA). In Figure 6C,D and Figure 7B,D, one-way ANOVA was initially performed, and if P was less than .05, it was followed by Bonferroni (Figure 6C) or Dunnett multiple comparison test (Figures 6D and 7B,D) with the parental group as control.
ABT-737 induces efficient and rapid apoptosis in CLL independent of prognostic markers. (A) PBMCs derived from 60 patients with CLL were exposed for 4 hours to 7 different concentrations of ABT-737 (0.1-100 nM). Apoptosis was assessed by externalization of PS and Annexin V/FITC binding. Each line represents one patient. The EC50 value was plotted against (B) IGHV status (M = mutated, U = unmutated), (C) p53 mutational status (U = unmutated, M = mutated), (D) genomic aberrations, including deletions (del) in 13q14, 11q23, and 17p13 as well as trisomy 12 (t12) detected using FISH, (E) the Binet stage of the disease at diagnosis, (F) whether the patient had received prior treatment or not, and (G) whether the patient was resistant to fludarabine (Y) or not (N). Statistics were done with GraphPad Prism. To determine P values, the unpaired t test was used in panels B, C, F, and G, and one-way ANOVA in panels D and E.
ABT-737 induces efficient and rapid apoptosis in CLL independent of prognostic markers. (A) PBMCs derived from 60 patients with CLL were exposed for 4 hours to 7 different concentrations of ABT-737 (0.1-100 nM). Apoptosis was assessed by externalization of PS and Annexin V/FITC binding. Each line represents one patient. The EC50 value was plotted against (B) IGHV status (M = mutated, U = unmutated), (C) p53 mutational status (U = unmutated, M = mutated), (D) genomic aberrations, including deletions (del) in 13q14, 11q23, and 17p13 as well as trisomy 12 (t12) detected using FISH, (E) the Binet stage of the disease at diagnosis, (F) whether the patient had received prior treatment or not, and (G) whether the patient was resistant to fludarabine (Y) or not (N). Statistics were done with GraphPad Prism. To determine P values, the unpaired t test was used in panels B, C, F, and G, and one-way ANOVA in panels D and E.
Results
CLL cells are uniformly sensitive to ABT-737
ABT-737 caused rapid, concentration-dependent induction of apoptosis in CLL cells from all 60 patients, (EC50 = 7 nM at 4 hours; Figure 1A). Sensitivity to ABT-737, as assessed by the individual EC50, was independent of the common clinical and prognostic parameters used to assess CLL, including the stage of disease at diagnosis, IGHV- and TP53-mutation status, interphase FISH abnormalities, previous treatments, and in vivo resistance toward fludarabine (Figure 1B-G). These data demonstrate that all CLL cells were exquisitely sensitive to ABT-737 irrespective of genetic abnormalities or prior treatment, so extending our findings and those of others.23,24
To investigate the possible contribution of different BCL2 family members to ABT-737–induced apoptosis, we analyzed their expression levels. BCL2, MCL1, BAX, BAK, and BIM were expressed in all CLL patients analyzed, in agreement with a previous study, although some interindividual variation was observed (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).29 No protein expression of BCL-XL or BCL2A1 was detected in the samples analyzed (data not shown). No correlation was observed between expression of BCL2 proteins and ABT-737–induced apoptosis (Figure S1). Thus, even cells from patients with high MCL1 levels were sensitive to ABT-737.
Up-regulation of MCL1 does not block ABT-737–induced apoptosis in CLL cells
As CLL cells in lymph nodes express higher MCL1 levels20 and elevated MCL1 expression is responsible for resistance to ABT-737 in several cancer cell lines,14-19 we examined whether increased MCL1 expression conferred resistance. To increase MCL1 levels, freshly isolated CLL cells were incubated with IL-4, IFNγ, or a combination for 4 to 20 hours before treatment with ABT-737. Treatment with both cytokines increased MCL1 expression, without affecting BCL2 levels or inducing BCL-XL or BCL2A1 expression (Figure 2A,B and data not shown), and caused a decrease in spontaneous apoptosis (Figure 2C,D). Despite MCL1 induction by both IL-4 and IFNγ, sensitivity to ABT-737 was only marginally decreased, and this inhibition was readily overcome by increasing ABT-737 concentrations (Figure 2C,D). Thus, MCL1 levels in CLL do not pose a major impediment to ABT-737 activity.
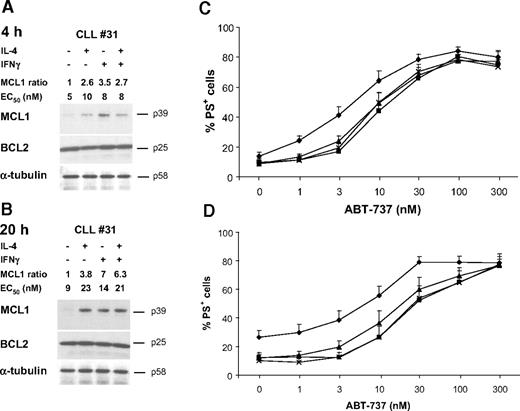
Up-regulation of MCL1 is not sufficient to induce resistance to ABT-737. CLL cells were pretreated with either 50 ng/mL IL-4 or IFNγ for 4 hours (A,C) or 20 hours (B,D). (A,B) After cytokine treatment, proteins were prepared and analyzed by Western blotting for expression of MCL1, BCL2, or α-tubulin as a loading control. Data from cells of one representative patient typical of 9 examined are shown. MCL1 levels of all 9 patients were quantified by densitometric analysis of Western blots, and values shown are the ratio of MCL1 in treated compared with control cells. The EC50 of ABT-737 at 4 hours is also shown. (C,D) After cytokine treatment (control ♦, IL-4 ▲, IFNγ ■, IL-4 and IFNγ ×), CLL cells were exposed for 4 hours to ABT-737 and apoptosis assessed by PS externalization. Data are the mean plus SEM (n = 9).
Up-regulation of MCL1 is not sufficient to induce resistance to ABT-737. CLL cells were pretreated with either 50 ng/mL IL-4 or IFNγ for 4 hours (A,C) or 20 hours (B,D). (A,B) After cytokine treatment, proteins were prepared and analyzed by Western blotting for expression of MCL1, BCL2, or α-tubulin as a loading control. Data from cells of one representative patient typical of 9 examined are shown. MCL1 levels of all 9 patients were quantified by densitometric analysis of Western blots, and values shown are the ratio of MCL1 in treated compared with control cells. The EC50 of ABT-737 at 4 hours is also shown. (C,D) After cytokine treatment (control ♦, IL-4 ▲, IFNγ ■, IL-4 and IFNγ ×), CLL cells were exposed for 4 hours to ABT-737 and apoptosis assessed by PS externalization. Data are the mean plus SEM (n = 9).
Stimulation with CD154 and IL-4 induces approximately 1000-fold resistance to ABT-737
The microenvironment is critical for signaling the maintenance and growth of CLL cells not only by direct cell contact with CD4+ T cells, stromal cells, and dendritic cells, but also by cytokines and chemokines.30 The microenvironment may be partially mimicked in vitro using CLL cells cultured on CD154-expressing L cells in the presence of IL-4.20 Sensitivity of CLL cells to ABT-737 was not significantly altered upon culture on parental L cells for up to 6 days (Figure 3A). However, cells cultured for 16 to 24 hours on CD154-expressing L cells became approximately 1000-fold more resistant to ABT-737 (EC50 > 10 μM at 4 hours; Figure 3B and data not shown). This resistance occurred before induction of proliferation, since CLL cells did not proliferate until 6 days of culture.
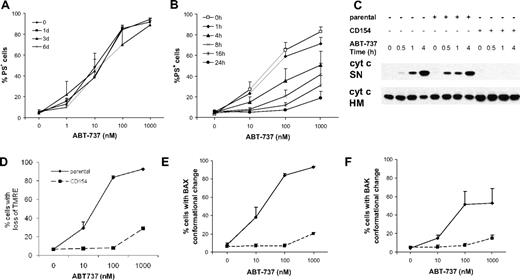
CLL cells exposed to CD154 develop a rapid and sustained resistance to ABT-737 upstream of mitochondrial perturbation. (A,B) CLL cells were cultured on parental (A) or CD154-expressing L cells (B) in the presence of IL-4 (10 ng/mL) for 1, 3, or 6 days in (A) and 1 to 24 hours in (B) before addition of different concentrations of ABT-737 for 4 hours in the absence of L cells. Apoptosis was assessed by PS externalization. Treatment with 10 μM ABT-737 did not result in more than 40% apoptosis upon 24 hours of culture on CD154-expressing cells (data not shown). (C) Freshly isolated CLL cells or CLL cells cultured on either parental or CD154-expressing L cells in the presence of IL-4 (10 ng/mL) for 1 day were exposed to ABT-737 (10 nM) for up to 4 hours, in the absence of L cells and IL-4. Release of cytochrome c from mitochondrial heavy membrane fraction (HM) to the supernatant (SN) was assessed by digitonin lysis and Western blotting. (D-F) CLL cells were cultured on either parental (solid lines) or CD154-expressing L cells (dotted lines) in the presence of IL-4 (10 ng/mL) for 1 day before exposure to ABT-737 for additional 4 hours, in the absence of L cells and IL-4. (D) CLL cells were stained with TMRE (20 nM) to assess the mitochondrial membrane potential (Ψm) by flow cytometry. (E,F) Conformational change of BAX (E) or BAK (F) was detected using conformational specific antibodies and flow cytometric analysis. Data are the mean plus SEM (n = 4).
CLL cells exposed to CD154 develop a rapid and sustained resistance to ABT-737 upstream of mitochondrial perturbation. (A,B) CLL cells were cultured on parental (A) or CD154-expressing L cells (B) in the presence of IL-4 (10 ng/mL) for 1, 3, or 6 days in (A) and 1 to 24 hours in (B) before addition of different concentrations of ABT-737 for 4 hours in the absence of L cells. Apoptosis was assessed by PS externalization. Treatment with 10 μM ABT-737 did not result in more than 40% apoptosis upon 24 hours of culture on CD154-expressing cells (data not shown). (C) Freshly isolated CLL cells or CLL cells cultured on either parental or CD154-expressing L cells in the presence of IL-4 (10 ng/mL) for 1 day were exposed to ABT-737 (10 nM) for up to 4 hours, in the absence of L cells and IL-4. Release of cytochrome c from mitochondrial heavy membrane fraction (HM) to the supernatant (SN) was assessed by digitonin lysis and Western blotting. (D-F) CLL cells were cultured on either parental (solid lines) or CD154-expressing L cells (dotted lines) in the presence of IL-4 (10 ng/mL) for 1 day before exposure to ABT-737 for additional 4 hours, in the absence of L cells and IL-4. (D) CLL cells were stained with TMRE (20 nM) to assess the mitochondrial membrane potential (Ψm) by flow cytometry. (E,F) Conformational change of BAX (E) or BAK (F) was detected using conformational specific antibodies and flow cytometric analysis. Data are the mean plus SEM (n = 4).
Resistance to ABT-737 occurs upstream of mitochondrial perturbation
Next, we examined which step in the apoptosis signaling cascade induced by ABT-737 was inhibited upon CD154 stimulation. After exposure to ABT-737, CLL cells cultured on CD154-expressing L cells compared with those on parental L cells were phosphatidylserine negative, retained cytochrome c in the mitochondria, and had an intact mitochondrial membrane potential (Figure 3B-D). Furthermore, ABT-737-induced BAX and BAK conformational change was abrogated in CLL cells cultured on CD154-expressing cells but not on parental cells (Figure 3E,F). Thus, resistance to ABT-737 occurred upstream of mitochondrial perturbation and BAX/BAK activation, suggesting that the resistance involves either BH3-only proteins or antiapoptotic BCL2 family members. Therefore, we analyzed BCL2 family proteins after culture. No marked changes in the levels of BAX, BAK (data not shown), BCL2, or MCL1 were observed, whereas increased expression of BCL2A1 and BCL-XL was observed (Figure 4A), the latter in agreement with a previous report.21 Induction of BCL2A1 mRNA and protein levels occurred as early as 1 or 4 hours of culture, respectively (Figure 4A and data not shown) and correlated with the resistance (Figure 3B). Thus, we speculate that induction of BCL2A1 and/or BCL-XL in CLL cells might be responsible for ABT-737 resistance. To test whether our model system recapitulates the in vivo situation, we analyzed BCL2A1 expression in lymph nodes using immunohistochemistry. CLL lymph nodes contained BCL2A1-expressing cells scattered within the tissue (Figure 4B), indicating that our in vitro model reflects certain cells in lymph nodes, possibly those in “proliferation centers” or in close proximity to CD154-expressing T cells.
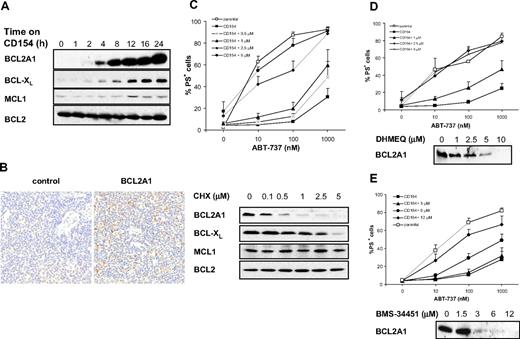
Resistance to ABT-737 correlates with NF-κB–mediated up-regulation of BCL2A1 and BCL-XL. (A) Expression of BCL2 proteins was assessed by Western blotting after 0 to 24 hours of culture on CD154-expressing cells. (B) Expression of BCL2A1 was assessed by immunohistochemistry on lymph node sections from CLL patients. (C) CLL cells were cultured on CD154-expressing cells for 24 hours and simultaneously exposed to different concentrations of cycloheximide (CHX). Apoptosis was assessed by PS externalization and annexin V/FITC binding (top panel, n = 3). Expression of BCL2 proteins was assessed by Western blotting (bottom panel). (D,E) CLL cells were cultured on CD154-expressing cells for 24 hours and simultaneously exposed to different concentrations of DHMEQ (D) or BMS-34451 (E). Apoptosis was assessed by PS externalization and AnnexinV/FITC binding (top panel, n = 3). Expression of BCL2A1 was assessed by Western blotting (bottom panel).
Resistance to ABT-737 correlates with NF-κB–mediated up-regulation of BCL2A1 and BCL-XL. (A) Expression of BCL2 proteins was assessed by Western blotting after 0 to 24 hours of culture on CD154-expressing cells. (B) Expression of BCL2A1 was assessed by immunohistochemistry on lymph node sections from CLL patients. (C) CLL cells were cultured on CD154-expressing cells for 24 hours and simultaneously exposed to different concentrations of cycloheximide (CHX). Apoptosis was assessed by PS externalization and annexin V/FITC binding (top panel, n = 3). Expression of BCL2 proteins was assessed by Western blotting (bottom panel). (D,E) CLL cells were cultured on CD154-expressing cells for 24 hours and simultaneously exposed to different concentrations of DHMEQ (D) or BMS-34451 (E). Apoptosis was assessed by PS externalization and AnnexinV/FITC binding (top panel, n = 3). Expression of BCL2A1 was assessed by Western blotting (bottom panel).
Resistance to ABT-737 is mediated by de novo protein synthesis
As CD154 signaling involves NF-κB activation and induction of its target genes, including BCL2A1 and BCL-XL,22 we examined whether resistance to ABT-737 required de novo protein synthesis. De novo protein synthesis was clearly required as the resistance was reversed by cycloheximide (Figure 4C). Moderate cycloheximide concentrations (0.5-1 μM) resulted in loss of BCL2A1 and resensitization to high ABT-737 concentrations, whereas higher cycloheximide concentrations (2.5-5 μM) induced concurrent loss of BCL2A1 and BCL-XL, and complete resensitization to ABT-737–induced apoptosis (Figure 4C). To investigate the contribution of NF-κB signaling to the resistance, we used 2 NF-κB inhibitors: DHMEQ, which inhibits NF-κB nuclear translocation, and BMS-345541, a highly selective IκB kinase inhibitor.31,32 Both agents reversed the resistance in a concentration-dependent manner and also caused a concentration-dependent decrease of BCL2A1 (Figure 4D,E). Taken together, our results support the hypothesis that the resistance to ABT-737 of CLL cells stimulated by CD154 is mediated by up-regulation of NF-κB target genes, including BCL2A1 and BCL-XL.
Newly synthesized BCL-XL and BCL2A1 are not bound by BH3-only proteins
Because new protein synthesis of BCL-XL and BCL2A1 upon culture on CD154-expressing cells changes the balance of pro- and antiapoptotic BCL2 proteins, we asked whether the binding pattern of BH3-only proteins to antiapoptotic BCL2 proteins changed upon exposure to CD154. Therefore, we performed IP of the antiapoptotic BCL2 proteins after 0 to 16 hours of exposure to CD154. Firstly, culture on CD154-expressing cells did not change the levels of BIM or PUMA (Figure 5A). In agreement with a previous study in CLL,23 BIM was constitutively bound to BCL2 (Figure 5A). We also found that PUMAα was constitutively bound to BCL2 (Figure 5A). Despite up-regulation of BCL2A1 and BCL-XL upon exposure to CD154, BIM and PUMAα remained bound to BCL2, indicating that once bound to BCL2, they did not translocate to other BCL2 proteins. NOXA was also transiently up-regulated upon exposure to CD154 (Figure 5A) in contrast to the down-regulation observed previously after 3 days of culture in a similar system.20 These differences are likely due to the early times used in the present study. Up-regulated NOXA bound to MCL1 but not BCL2A1, indicating that NOXA might neutralize the antiapoptotic activity of MCL1 (Figure 5A). Thus, in CLL the BH3-only proteins were bound to BCL2 and MCL1, while upon induction on CD154-expressing cells no binding partners for newly synthesized BCL2A1 and BCL-XL were detected.
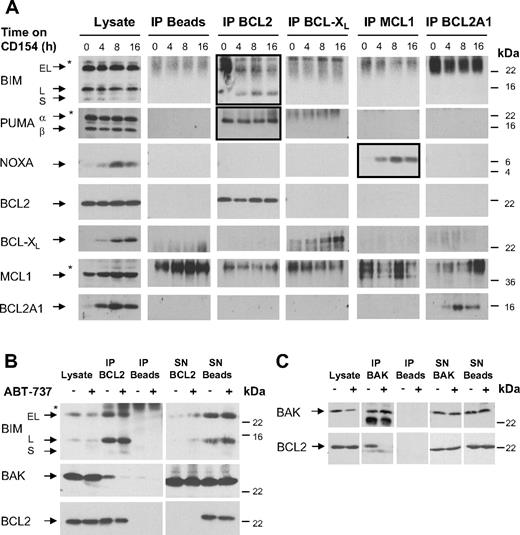
BIM and PUMA are sequestered by BCL2, whereas newly synthesized BCL-XL and BCL2A1 are not bound by BH3-only proteins. (A) The binding pattern of antiapoptotic BCL2 proteins to BH3-only proteins was investigated upon 0 to 16 hours of culture of CLL cells on CD154-expressing cells. BCL-XL, BCL2A1, and NOXA are up-regulated at 4 to 16 hours, while levels of BCL2, MCL1, BIM, and PUMA are not altered (Lysate). IP of BCL2, BCL-XL, MCL1, and BCL2A1 was done in lysis buffer containing 0.5% Triton X-100; however, to exclude the influence of detergent, IPs were repeated in CHAPS-containing lysis buffer with similar results (data not shown). Upon culture on CD154-expressing cells BIM and PUMAα were bound to BCL2 for up to 16 hours of culture, and up-regulated NOXA was bound by MCL1. In contrast, no binding of BH3-only proteins to BCL-XL or BCL2A1 was detected (*nonspecific band). (B,C) Freshly isolated CLL cells were treated with 10 nM ABT-737 for 2 hours. After treatment, cells were lysed in CHAPS-containing lysis buffer. (B) IP of BCL2 revealed that upon treatment with ABT-737 the majority of BIM remained bound to BCL2 and only little BIM appeared in the supernatant (SN) of the IP. An interaction of BCL2 and BAK was found in 5 of 8 patients examined. Upon treatment with ABT-737, BAK was displaced from BCL2. (C) The reverse IP of BAK confirmed displacement of BAK from BCL2 by ABT-737.
BIM and PUMA are sequestered by BCL2, whereas newly synthesized BCL-XL and BCL2A1 are not bound by BH3-only proteins. (A) The binding pattern of antiapoptotic BCL2 proteins to BH3-only proteins was investigated upon 0 to 16 hours of culture of CLL cells on CD154-expressing cells. BCL-XL, BCL2A1, and NOXA are up-regulated at 4 to 16 hours, while levels of BCL2, MCL1, BIM, and PUMA are not altered (Lysate). IP of BCL2, BCL-XL, MCL1, and BCL2A1 was done in lysis buffer containing 0.5% Triton X-100; however, to exclude the influence of detergent, IPs were repeated in CHAPS-containing lysis buffer with similar results (data not shown). Upon culture on CD154-expressing cells BIM and PUMAα were bound to BCL2 for up to 16 hours of culture, and up-regulated NOXA was bound by MCL1. In contrast, no binding of BH3-only proteins to BCL-XL or BCL2A1 was detected (*nonspecific band). (B,C) Freshly isolated CLL cells were treated with 10 nM ABT-737 for 2 hours. After treatment, cells were lysed in CHAPS-containing lysis buffer. (B) IP of BCL2 revealed that upon treatment with ABT-737 the majority of BIM remained bound to BCL2 and only little BIM appeared in the supernatant (SN) of the IP. An interaction of BCL2 and BAK was found in 5 of 8 patients examined. Upon treatment with ABT-737, BAK was displaced from BCL2. (C) The reverse IP of BAK confirmed displacement of BAK from BCL2 by ABT-737.
Next, we asked whether ABT-737 could displace binding partners from BCL2. Upon exposure of freshly isolated CLL cells to ABT-737 (10 nM), BIM was not significantly displaced from BCL2. Although slightly more BIM was detected in the supernatant of the IP, the amount of BIM bound to BCL2 did not markedly decrease. In addition to BIM and PUMAα, a small amount of BAK was also sequestered by BCL2, as evident by co-IP of BAK with BCL2 in the absence of a significant decrease of BAK in the supernatant of the BCL2-IP (Figure 5B,C). These results are in line with a previous report showing interaction of BCL2 with BAK.33 BAX was mainly cytoplasmic in CLL, and no interaction with BCL2 was detected (data not shown). Notably, BAK was displaced from BCL2 by ABT-737 (Figure 5B,C). These results indicate that besides displacing BIM from BCL2 as described previously,23 ABT-737 might act by displacing BAK from BCL2 in CLL cells.
BCL2A1 and BCL-XL are critical resistance factors for ABT-737 in CLL cells
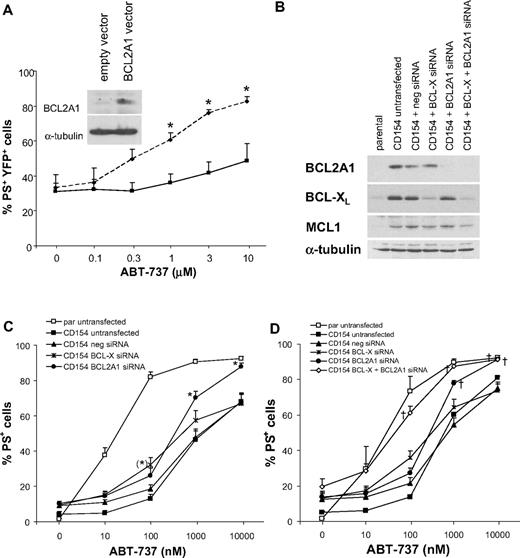
To investigate the importance of elevated BCL2A1 in ABT-737 resistance, we overexpressed BCL2A1 in Jurkat T cells, chosen because they are sensitive to ABT-737, have no detectable endogenous BCL2A1 expression, and are more amenable to transfection than CLL cells. BCL2A1 overexpression decreased ABT-737–induced apoptosis compared with Jurkat cells transfected with empty vector alone (Figure 6A). To assess the importance of BCL2A1 and BCL-XL in the resistance of CLL cells, we used siRNA in CLL cells cultured on CD154-expressing cells. The siRNA against BCL2A1 and BCL-XL inhibited BCL2A1 or BCL-XL protein expression, respectively, without affecting expression of other BCL2 proteins (Figure 6B). BCL2A1 knockdown resulted in significant sensitization of CLL cells to ABT-737 at 1000 and 10 000 nM compared with the negative control siRNA (Figure 6C). In contrast, BCL-XL knockdown resulted in only minor sensitization to lower ABT-737 concentrations (100 nM). Thus, BCL2A1 may contribute to resistance at high ABT-737 concentrations, while BCL-XL appears to make only a minor contribution to ABT-737 resistance. However, single knockdown of BCL2A1 or BCL-XL failed to fully restore sensitivity to ABT-737. To examine whether BCL2A1 and BCL-XL might synergize in conferring resistance, we performed simultaneous knockdown of both proteins. For unclear reasons, combined knockdown of BCL2A1 and BCL-XL was only successful in 3 of 11 patients, which showed comparable down-regulation of both BCL2A1 and BCL-XL as the single knockdowns (Figure 6B). Intriguingly, in these patients the combined knockdown completely restored CLL sensitivity on CD154-expressing cells (Figure 6D). In the other 8 of 11 patients, combined knockdown of BCL2A1 and BCL-XL did not inhibit BCL2A1 expression as efficiently as the single knockdown, and this inefficient knockdown did not fully restore the sensitivity of CLL cells (Figure S2). Thus, concurrent up-regulation of BCL2A1 and BCL-XL in CLL cells can synergize to induce resistance to ABT-737. Furthermore, expression of BCL2A1 and BCL-XL is sufficient to induce approximately 1000-fold resistance to ABT-737.
Identification of BCL2A1 and BCL-XL as resistance factors for ABT-737. (A) Jurkat T cells were transiently transfected with pYFP-BCL2A1 (solid line) or empty vector control (dotted line) using Amaxa program C-16. After transfection for 16 hours, cells were exposed to different ABT-737 concentrations for 4 hours and apoptosis was assessed by PS externalization in the YFP-positive population. Data are mean plus SEM of 5 independent experiments. Statistics were done using t test (*P < .05). Inset shows expression of BCL2A1 in the total population by Western blotting in one representative experiment. (B-D) CLL cells were transfected with siRNA against BCL-X, BCL2A1, or negative control siRNA using Amaxa program X-5. Directly after nucleofection, cells were cultured on CD154-expressing cells in the presence of IL-4 (10 ng/mL) for 16 hours. (B) Knockdown of BCL-XL and BCL2A1 was confirmed by Western blotting. (C) After knockdown of BCL-XL or BCL2A1, CLL cells were removed from CD154-expressing cells and exposed to different concentrations of ABT-737 for 4 hours. Apoptosis was assessed by PS externalization and AnnexinV/FITC binding (n = 15). Statistics were done using one-way ANOVA followed by Bonferroni multiple comparison test. The BCL2A1 siRNA showed significant difference to both CD154 untransfected as well as CD154 negative siRNA group (*P < .01), while BCL-X siRNA showed significant difference only compared with CD154 untransfected group (*P < .01). (D) After combined knockdown of BCL-XL and BCL2A1, CLL cells were removed from CD154-expressing cells and exposed to different concentrations of ABT-737 for 4 hours. Apoptosis was assessed by PS externalization and annexin V/FITC binding (n = 3). In cells from another 8 patients, the combined knockdown was inefficient compared with the single knockdowns (Figure S2). Statistics were done using one-way ANOVA followed by Dunnett multiple comparison test using parental untransfected as control group. Reversal of resistance as indicated by no significant difference to control group is indicated with † (P > .05).
Identification of BCL2A1 and BCL-XL as resistance factors for ABT-737. (A) Jurkat T cells were transiently transfected with pYFP-BCL2A1 (solid line) or empty vector control (dotted line) using Amaxa program C-16. After transfection for 16 hours, cells were exposed to different ABT-737 concentrations for 4 hours and apoptosis was assessed by PS externalization in the YFP-positive population. Data are mean plus SEM of 5 independent experiments. Statistics were done using t test (*P < .05). Inset shows expression of BCL2A1 in the total population by Western blotting in one representative experiment. (B-D) CLL cells were transfected with siRNA against BCL-X, BCL2A1, or negative control siRNA using Amaxa program X-5. Directly after nucleofection, cells were cultured on CD154-expressing cells in the presence of IL-4 (10 ng/mL) for 16 hours. (B) Knockdown of BCL-XL and BCL2A1 was confirmed by Western blotting. (C) After knockdown of BCL-XL or BCL2A1, CLL cells were removed from CD154-expressing cells and exposed to different concentrations of ABT-737 for 4 hours. Apoptosis was assessed by PS externalization and AnnexinV/FITC binding (n = 15). Statistics were done using one-way ANOVA followed by Bonferroni multiple comparison test. The BCL2A1 siRNA showed significant difference to both CD154 untransfected as well as CD154 negative siRNA group (*P < .01), while BCL-X siRNA showed significant difference only compared with CD154 untransfected group (*P < .01). (D) After combined knockdown of BCL-XL and BCL2A1, CLL cells were removed from CD154-expressing cells and exposed to different concentrations of ABT-737 for 4 hours. Apoptosis was assessed by PS externalization and annexin V/FITC binding (n = 3). In cells from another 8 patients, the combined knockdown was inefficient compared with the single knockdowns (Figure S2). Statistics were done using one-way ANOVA followed by Dunnett multiple comparison test using parental untransfected as control group. Reversal of resistance as indicated by no significant difference to control group is indicated with † (P > .05).
Resistance to ABT-737 may be overcome by combination treatments
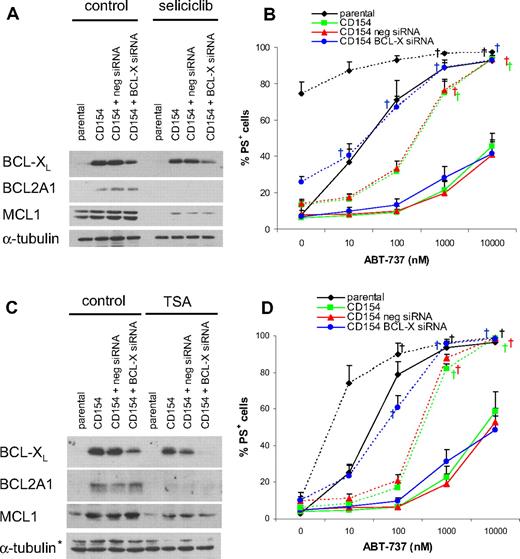
Next, we asked whether combination treatments could overcome the resistance induced by CD154. As ABT-737 synergizes with many agents, we initially examined whether combination with fludarabine or anti-CD20 antibody (rituximab), drugs commonly used in treating CLL, might circumvent the resistance. However, combination with these drugs or tumor necrosis factor (TNF)–related apoptosis inducing ligand (TRAIL) failed to circumvent the resistance (Figure S3A). Next we examined if ABT-737 would synergize with seliciclib, a cyclin-dependent kinase inhibitor, trichostatin A (TSA), a histone deacetylase (HDAC) inhibitor, or proteasome inhibitors. Our rationale to use seliciclib was based on studies showing that it caused a rapid decrease in MCL1 and sensitized cells to ABT-737–induced apoptosis.19,34 As both MCL1 and BCL2A1 have short half-lives,35,36 we rationalized that seliciclib may also cause an analogous decrease in BCL2A1. TSA was used because HDAC inhibitors down-regulate many genes, including antiapoptotic BCL2 proteins.37,38 Proteasome inhibitors synergize with ABT-737 in a preclinical B-cell lymphoma model.39 Combination with seliciclib, TSA, or proteasome inhibitors resulted in a marked but incomplete reversal of the resistance (Figure 7 and Figure S3B-D). Notably, exposure to these agents occurred 1 to 3 days after induction of resistance, but they still sensitized to ABT-737, suggesting that in a clinical setting resistance to ABT-737 may be overcome.
Knockdown of BCL-XL in combination with agents that suppress BCL2A1 completely resensitizes CLL cells to ABT-737. CLL cells were transfected with negative control siRNA or BCL-X siRNA and immediately after nucleofection cultured on parental or CD154-expressing cells in the presence of IL-4 (10 ng/mL) for 24 hours. (A,B) After removal from parental or CD154-expressing cells, CLL cells were exposed to 50 μM seliciclib for 20 hours, and protein expression was analyzed by Western blotting (A). Seliciclib treatment was followed by exposure to different concentrations of ABT-737 for 4 hours, and apoptosis was assessed as PS externalization and Annexin V/FITC binding (solid lines indicate control; dotted lines, seliciclib; B, n = 4). (C,D) After removal from CD154-expressing cells, CLL cells were exposed to 500 nM TSA for 20 hours, and protein expression was analyzed by Western blotting (C; *nonspecific band). TSA treatment was followed by exposure to different concentrations of ABT-737 for 4 hours, and apoptosis was assessed as PS externalization and Annexin V/FITC binding (solid lines indicate control; dotted lines, TSA; D, n = 3). (B,D) Statistics were done using one-way ANOVA followed by Dunnett multiple comparison test using parental as the control group. Reversal of resistance as indicated by no significant difference to the control group is indicated with † (P > .05).
Knockdown of BCL-XL in combination with agents that suppress BCL2A1 completely resensitizes CLL cells to ABT-737. CLL cells were transfected with negative control siRNA or BCL-X siRNA and immediately after nucleofection cultured on parental or CD154-expressing cells in the presence of IL-4 (10 ng/mL) for 24 hours. (A,B) After removal from parental or CD154-expressing cells, CLL cells were exposed to 50 μM seliciclib for 20 hours, and protein expression was analyzed by Western blotting (A). Seliciclib treatment was followed by exposure to different concentrations of ABT-737 for 4 hours, and apoptosis was assessed as PS externalization and Annexin V/FITC binding (solid lines indicate control; dotted lines, seliciclib; B, n = 4). (C,D) After removal from CD154-expressing cells, CLL cells were exposed to 500 nM TSA for 20 hours, and protein expression was analyzed by Western blotting (C; *nonspecific band). TSA treatment was followed by exposure to different concentrations of ABT-737 for 4 hours, and apoptosis was assessed as PS externalization and Annexin V/FITC binding (solid lines indicate control; dotted lines, TSA; D, n = 3). (B,D) Statistics were done using one-way ANOVA followed by Dunnett multiple comparison test using parental as the control group. Reversal of resistance as indicated by no significant difference to the control group is indicated with † (P > .05).
Next, we investigated the influence of TSA and seliciclib on the expression of BCL2 proteins. Exposure to seliciclib resulted in the loss of BCL2A1 and MCL1, whereas exposure to TSA induced the loss of BCL2A1 expression without major effects on MCL1 levels (Figure 7A,C). Because neither of these agents resulted in loss of BCL-XL, we asked whether additional BCL-XL knockdown would further sensitize to ABT-737. Thus, we combined a BCL-XL siRNA with exposure to seliciclib or TSA. TSA (500 nM) alone was not toxic, whereas seliciclib (50 μM) alone induced apoptosis in cells cultured on parental L cells (Figure 7B,D). However, CLL cells cultured on CD154-expressing cells were resistant to seliciclib (Figure 7B). TSA induced apoptosis synergistically with ABT-737 in CLL cells cultured on parental cells. Both seliciclib and TSA alone resensitized CLL cells exposed to CD154 to high but not low ABT-737 concentrations (Figure 7B,D). Knockdown of BCL-XL alone had only minor effects on ABT-737 sensitivity. Combination of either seliciclib or TSA with BCL-XL siRNA resulted in a complete resensitization to all ABT-737 concentrations, whereas the negative control siRNA had no effect (Figure 7B.D).
Discussion
In this study, we highlight the potential use of a BCL2 antagonist for treating CLL. ABT-737 induced rapid apoptosis in cells from 60 CLL patients, independent of their prognostic markers or clinical parameters, indicating such treatment may be promising even in patients with the worst prognosis. Our data support the evaluation of ABT-263, an orally active analog of ABT-737, for CLL treatment.40
Accumulating data suggest the microenvironment is critical for CLL survival and, thus, has to be considered for the development of successful new treatment strategies.5,6 In this respect, special attention has been paid to cellular changes occurring in bone marrow and lymph nodes, where CLL cells interact with both T cells and stromal cells, resulting in an alteration in the balance between pro- and antiapoptotic BCL2 proteins and a stimulation of proliferation.20,21 In the present study, we simulated the lymph node environment in vitro by culturing CLL cells on CD154-expressing fibroblasts. Our studies demonstrated approximately 1000-fold resistance to ABT-737 within 24 hours of culture, indicating that in certain microenvironments, resistant cells may survive exposure to ABT-737. Although this model system is used to mimic the microenvironment within lymph nodes, we cannot exclude that the sustained NF-κB activation may exaggerate the precise in vivo microenvironment. Nevertheless, BCL2A1 staining in some cells in the lymph nodes of CLL patients (Figure 4B) supports a report that NF-κB activation occurs within lymph nodes,41 because BCL2A1 is a NF-κB regulated gene.22 Taken together our data predicts that after initial treatment with ABT-263,40 most circulating CLL cells would be killed together with a reduction in overall tumor mass in lymph nodes, but survival of those cells expressing high levels of antiapoptotic proteins, such as BCL2A1. Thus, CLL cells in a protected niche, where they receive survival signals from the microenvironment,4-6 may become resistant to the initial chemotherapy, thereby providing a reservoir of tumor cells that may give rise to a population of drug-resistant cells. However, when the resistant CLL cells exit their protected niche and reenter the circulation they may lose their survival signals resulting in a down-regulation of antiapoptotic BCL2 proteins and a restoration of sensitivity to ABT-737 or ABT-263, so limiting the potential resistance we have observed.
The approximately 1000-fold decrease in sensitivity may be explained partly by the exquisite sensitivity of freshly isolated CLL cells to ABT-737. After culture on CD154-expressing cells for 24 hours, ABT-737 had an EC50 more than 10 μM after 4 hours and approximately 7.5 μM after 24 hours of treatment. Thus, even with the 1000-fold resistance, these cells exhibit a similar sensitivity as many cells considered ABT-737 sensitive.13,18 Nevertheless, this potential resistance may be important clinically, and, for proof of principle, we have shown that this resistance can be overcome using different combinations. In line with our data, a recent report showed synergistic activity of a proteasome inhibitor with ABT-737 in a preclinical B-cell lymphoma model.39
BCL-XL and BCL2A1 protein expression is very low or absent in circulating CLL cells but is up-regulated at the mRNA level in lymph nodes of CLL patients.20 BCL2A1 mRNA expression in CLL has been linked to chemoresistance and to suppression of spontaneous apoptosis,42-44 supporting the function of BCL2A1 as an important antiapoptotic protein in malignant B cells. Our studies strongly suggest that BCL2A1 and BCL-XL up-regulation is responsible for ABT-737 resistance after CD154 stimulation. Firstly, resistance of CLL cells occurred upstream of BAX and BAK activation, strongly indicating alterations within the BCL2 family (Figure 3). Secondly, the resistance involved both NF-κB activation and de novo protein synthesis, suggesting antiapoptotic NF-κB target genes were responsible (Figure 4). Thirdly, among the antiapoptotic BCL2 family members, only BCL2A1 and BCL-XL were rapidly induced upon CD154 stimulation (Figure 4A). Fourthly, combined knockdown of BCL2A1 and BCL-XL upon culture on CD154-expressing cells resulted in complete resensitization to ABT-737 (Figure 6). Fifthly, knockdown of BCL-XL in combination with agents that inhibit BCL2A1 expression completely reversed the resistance (Figure 7).
Closer inspection of the data raises novel insights into the roles of BCL2A1 and BCL-XL in conferring ABT-737 resistance in CLL. BCL2A1 knockdown alone only partially reversed the resistance and surprisingly, this reversal was only significant at high (1000-10 000 nM) but not low ABT-737 (10-100 nM) concentrations (Figure 6C). However, the complete reversal of resistance observed in cells that showed a good combined knockdown of both BCL-XL and BCL2A1 (Figure 6B,D) supports the hypothesis that newly synthesized BCL-XL and BCL2A1 confer resistance to low and high ABT-737 concentrations, respectively. Support for this hypothesis was provided by the reversal of resistance obtained by combining seliciclib or TSA with BCL-XL siRNA (Figure 7). Exposure to seliciclib or TSA alone, which both decreased BCL2A1 sensitized only to high ABT-737 concentrations, whereas combination with BCL-XL siRNA completely reversed resistance at all ABT-737 concentrations (Figure 7). Why do these newly synthesized proteins confer such extensive resistance to CLL cells? Newly synthesized BCL-XL and BCL2A1, whether present in the cytosol or mitochondria, were not bound to the major BH3-only proteins (Figure 5A), and thus, we speculate they may act as a sink for ABT-737 or for BH3-containing proteins, such as BIM, PUMA, or BAK, displaced from BCL2 by ABT-737 (Figure S4). Our preliminary experiments suggest that the newly synthesized BCL-XL might bind BAK and this binding may be disrupted by ABT-737. By contrast, high BCL2 levels in CLL cells are probably incapable of sequestering significant amounts of ABT-737, as the BH3 binding pocket is already occupied by BIM and PUMA (Figure 5).23 Newly synthesized BCL-XL should sequester low concentrations of ABT-737 or ABT-263 (Figure S4B) because of its high affinity for these molecules, whereas BCL2A1 has a much lower affinity and therefore would not sequester or neutralize such low concentrations.40 Furthermore, the exquisite sensitivity of CLL cells to ABT-737 also dictates the relative importance of different antiapoptotic proteins in the resistance pattern observed. Only antiapoptotic proteins with a high affinity for ABT-737 (Ki ∼1 nM),40 namely BCL2 and BCL-XL, will be important in conferring resistance at low nanomolar ABT-737 concentrations. In contrast, BCL2A1 and MCL1, with a low affinity for ABT-737, may only bind high ABT-737 concentrations and will be ineffective at protecting against low concentrations (Figure S4C).
This insight may explain the apparent discrepancy of our results with CLL cells compared with published data from cell lines, where resistance has occurred at micromolar ABT-737 concentrations and often been attributed to high MCL1 levels. In several cell lines, MCL1 knockdown conferred ABT-737 sensitivity, while high MCL1 expression was associated with resistance.14-19 However, our data with cytokines (Figure 2) suggest that ABT-737–induced apoptosis in CLL cells is largely MCL1-independent. Another plausible reason for the MCL1 independence of ABT-737–induced apoptosis in CLL cells is that MCL1 is expressed at 4- to 14-fold lower levels than BCL2 in CLL cells.23 Furthermore, resistant CLL cells were resensitized to ABT-737 by a decrease of BCL2A1 and BCL-XL, in the presence of TSA and siRNA for BCL-XL, with only modest effects on MCL1 (Figure 7C,D). In this regard, CLL cells express similar levels of MCL1 but higher BCL2 levels than many cell lines (unpublished data). Furthermore, endogenous MCL1 in CLL cells can bind BH3-only proteins, such as NOXA (Figure 5A). The relative MCL1-independent induction of apoptosis by ABT-737 in CLL may be largely due to their exquisite sensitivity to ABT-737 because MCL1, due to its low affinity, will not bind low ABT-737 concentrations. In contrast, in studies where MCL1 is a resistance factor for ABT-737,14-19 the cells are less sensitive to ABT-737 invariably requiring micromolar concentrations often for 24 to 48 hours. At these high concentrations, MCL1 may bind to ABT-737 or to BH3-containing proteins displaced from other antiapoptotic BCL2 family proteins. Why is BCL2A1 but not MCL1 involved in ABT-737 resistance upon CD154 exposure? A possible explanation may be provided by our data showing that upon CD154-exposure, NOXA binds to MCL1 but not to BCL2A1 (Figure 5A). This leaves BCL2A1 free to bind directly to ABT-737 or to BH3-containing proteins. To our knowledge no binding partners of endogenous BCL2A1 have been described but overexpressed BCL2A1 interacts with BAK and tBID but not with BAX, whereas in another study it interacts with both BAK and BAX.45-48 BCL2A1 also binds to BH3-peptides from BIM, BID, PUMA, and possibly NOXA.10,19 Thus, although the exact function of BCL2A1 is unclear, there are indications supporting a model in which BCL2A1 sequesters either BIM (Figure S4C Model A) or BAK (Figure S4C Model B) displaced from BCL2 by high ABT-737 concentrations.
Our studies have demonstrated a dramatic 1000-fold resistance to ABT-737 that developed in CLL cells within 24 hours after culture on CD154-expressing cells due to up-regulation of BCL2A1 and BCL-XL.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Jannie Borst (The Netherlands Cancer Institute, Amsterdam), Dr David Huang (Walter and Eliza Hall Institute, Melbourne, Australia) and Dr I. Berberich (University of Wuerzburg, Wuerzburg, Germany) for reagents and Dr Saul Rosenberg (Abbott Laboratories, Abbott Park, IL) for ABT-737. We thank Prof Reiner Siebert (University Hospital Schleswig-Holstein, Kiel, Germany) for interphase FISH analysis, Dr Reg Davies for assistance with RT-PCR for BCL2A1, Dr Satoshi Inoue for help establishing the siRNA for BCL2A1, and Richard and Jenny Edwards for help with the immunohistochemistry of BCL2A1. We thank Dr Gary Willars for advice on the statistical analysis.
This work was supported by the MRC London, United Kingdom, and Leukemia Research Fund, London, United Kingdom.
Authorship
Contribution: M.V. designed and performed many experiments, optimized CLL transfection, analyzed data, and contributed to the manuscript; M.B. designed and performed many experiments and optimized CD154 cultures; A.M. and R.J.W. were involved in sample acquisition and analysis of CLL prognostic markers; X.-M.S. carried out assays of cytochrome c release; M.J.S.D. provided clinical input, discussed experiments, and contributed to the manuscript; and G.M.C. designed experiments, analyzed data, and contributed to the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gerald M. Cohen, MRC Toxicology Unit, Hodgkin Bldg, University of Leicester, PO Box 138, Lancaster Rd, Leicester, LE1 9HN, United Kingdom; e-mail: gmc2@le.ac.uk.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal