Abstract
Vascular endothelial growth factor-D (VEGF-D) has angiogenic and lymphangiogenic activity, but its biologic role has remained unclear because knockout mice showed no clear phenotype. Transgenic (TG) mice expressing the mature form of human VEGF-D (hVEGF-D) were produced by lentiviral (LV) transgenesis using the perivitelline injection method. Several viable founders showed a macroscopically normal phenotype and the transgene transmitted through germ line. Expression of hVEGF-D mRNA was high in skeletal muscles, skin, pancreas, heart, and spleen. A significant increase was found in capillary density of skeletal muscles and myocardium, whereas no changes were observed in lymphatic capillary density. After induction of hindlimb ischemia, the TG mice showed enhanced capacity for muscle regeneration. However, on aging the TG mice had significantly increased mortality from malignant tumors, of which half were breast adenocarcinomas characterized with the absence of periductal muscle cells. Some tumors metastasized into the lungs. In addition, lung and skin tumors were found, but no blood- or lymphatic vessel–derived malignancies were detected. We conclude that in mice hVEGF-D is an angiogenic factor associated with improved muscle regeneration after ischemic injury but also with increased incidence of tumor formation with a preference for mammary gland tumors.
Introduction
Vascular endothelial growth factors (VEGFs) are key mediators of blood and lymphatic vessel formation during embryonic development and in adults. Blood vessel formation occurs either by vasculogenesis, when a new network of capillaries is formed from endothelial precursor cells differentiating in situ, or by angiogenesis, when new capillaries invade the organ by forming vascular sprouts that originate from pre-existing vessels.1 The lymphatic system comprises a separate vascular system that also permeates most organs. Lymphatic vessels are essential for immune surveillance, tissue fluid homeostasis, and fat absorption. Defects in formation or function of lymphatic vessels cause lymphedema, and lack of lymphatic vessels result in fluid accumulation, which causes prenatal death.2 Lymphangiogenesis occurs either by local de novo differentiation of lymphatic endothelium from lymphangioblasts or by sprouting from preexisting lymphatic veins.3
Currently, 7 members have been identified in the VEFG family: VEGF-A, -B, -C, -D, -E (viral VEGF analogs), -F (snake venom VEGFs), and placental growth factor (PlGF). Of these, VEGF-E and -F represent exogenous VEGFs.4
Several VEGF receptors (VEGFRs) have been identified for VEGFs: VEGFR-1 (flt-1; fms-induced tyrosine kinase receptor), VEGFR-2 (KDR; kinase insert domain–containing receptor), and VEGFR-3 (flt-4) belong to the superfamily of receptor tyrosine kinases (RTKs). They share a common structure, yet they bind VEGFs in distinct affinities and specificities. In addition, Neuropilins 1 and 2 (Nrp-1, Nrp-2) bind some specific VEGFs. Nrps are non-RTKs that are believed to serve as coreceptors for certain VEGFs and their isoforms.5
VEGF-D (also known as c-fos–induced growth factor, FIGF) is a secreted growth factor consisting of a central VEGF-homology domain (VHD), receptor binding domains, and propeptides in both termini. VEGF-D is secreted into the extracellular space as a full-length VEGF-D homodimer. After secretion, proprotein convertases cleave the C- and N-terminal propeptides from the VHD to form the mature VEGF-D.6 The mature hVEGF-D binds to hVEGFR-2 and -3 with higher affinities than the full-length unprocessed hVEGF-D. Numerically, the mature hVEGF-D has approximately 290-fold higher affinity to hVEGFR-2 and approximately 40-fold higher affinity to hVEGFR-3 than the unprocessed full-length hVEGF-D, which mainly binds to hVEGFR-3.7 In this article hVEGF-D refers to mature hVEGF-DΔNΔC. Cellular effects of hVEGF-D depend on the receptor and target cell type. Vascular endothelial cells express VEGFR-2, and the expression is up-regulated inter alia by angiogenesis.8 hVEGF-D exerts angiogenic effects on binding to VEGFR-2, whereas when bound to VEGFR-3 on lymphatic endothelium it stimulates lymphangiogenesis. In addition to VEGFR-2 and -3, hVEGF-D is able to interact with Nrp-2, which has a role in lymphangiogenesis.5 VEGF-D probably possesses differing functions in different species; in mouse hVEGF-D binds to both VEGFR-2 and -3, whereas mVEGF-D binds only to VEGFR-3.9,10
We used lentiviral (LV) perivitelline injection technique for the generation of transgenic (TG) mice and followed them up to generation F3. The LV transgenesis method is still rarely used despite its versatility and advantages over traditional transgenesis methods.11 In this study, LV transgenesis was an effective method for producing TG offspring. We found that hVEGF-D TG mice showed no major changes in lymphatic capillary density. Instead, they showed enhanced angiogenesis as well as improved muscle regeneration after injury. Unexpectedly, the TG mice possessed an increased susceptibility to tumor formation.
Methods
Lentiviral vector construction
Vesicular stomatitis virus G-protein (VSV-G)–pseudotyped, third generation LVs were constructed to directly express the mature form of hVEGF-D under the human phosphoglyserate kinase (hPGK) promoter. Woodchuck hepatitis virus pre-element (wPRE) and central polypurine tract (cPPT) were used in the vector backbone. The virus production, concentration, and p24 assays were performed as described earlier.12 Titers of the concentrated LVs ranged from 8.5 × 108 to 1.9 × 109 TU/mL. A schematic representation of the LV vector is shown in Figure 1A.
Schematic presentation of the third generation lentiviral vector used in the transgenesis studies and Southern blot image of a copy number analysis. (A) CMV indicates cytomegalovirus promoter; Ψ, packaging signal; cPPT, central polypurine tract. The TG cassette contains the human phosphoglycerate kinase (hPGK) promoter, human VEGF-D cDNA truncated from C- and N-termini (hVEGF-D), and woodchuck hepatitis virus PRE element (wPRE). SIN indicates that the lentiviral vector is self-inactivating because of the deletion in U3 LTR to form ΔU3; U5, 5′ LTR; RRE, rev-responsive element. (B) Analysis of the 4 hVEGF-D TG founder mice (1, 2, 3, and 4) and 3 F1 mice (2a, 2b, and 2c) from founder number 2. The copy numbers of standards (left half of the image) are indicated (copies/15 μg of mouse tail genomic DNA). wPRE was used as a probe sequence. Molecular weight marker (MW) between the standard and sample lanes is 2.5 kb. A vertical line has been inserted to indicate a repositioned gel lane; 1, 1 to 5 copies; 2, 7 to 10 copies; 3, 5 to 7 copies; 2a, 5 to 7 copies; 2b, 7 to 10 copies; and 2c, 7 to 10 copies.
Schematic presentation of the third generation lentiviral vector used in the transgenesis studies and Southern blot image of a copy number analysis. (A) CMV indicates cytomegalovirus promoter; Ψ, packaging signal; cPPT, central polypurine tract. The TG cassette contains the human phosphoglycerate kinase (hPGK) promoter, human VEGF-D cDNA truncated from C- and N-termini (hVEGF-D), and woodchuck hepatitis virus PRE element (wPRE). SIN indicates that the lentiviral vector is self-inactivating because of the deletion in U3 LTR to form ΔU3; U5, 5′ LTR; RRE, rev-responsive element. (B) Analysis of the 4 hVEGF-D TG founder mice (1, 2, 3, and 4) and 3 F1 mice (2a, 2b, and 2c) from founder number 2. The copy numbers of standards (left half of the image) are indicated (copies/15 μg of mouse tail genomic DNA). wPRE was used as a probe sequence. Molecular weight marker (MW) between the standard and sample lanes is 2.5 kb. A vertical line has been inserted to indicate a repositioned gel lane; 1, 1 to 5 copies; 2, 7 to 10 copies; 3, 5 to 7 copies; 2a, 5 to 7 copies; 2b, 7 to 10 copies; and 2c, 7 to 10 copies.
Experimental animals
TG mice were produced by LV transgenesis with the use of the perivitelline injection method as described.11,13 Briefly, after breeding, fertilized 1-cell–stage oocytes were collected from oviducts of superovulated CD2F1 donor females weighing 9 to 12 g. Approximately 100 pL of 109 TU/mL hVEGFD-LV was injected into the perivitelline space of the oocytes. After overnight recovery and growth in vitro, the 2-cell–stage embryos were transferred to pseudopregnant CD2F1 female mice. Pups were weaned and genotyped at 3 weeks of age. The material for genotype polymerase chain reaction (PCR) was skin attained from earmarking. The genotyping was performed by PCR with the use of the following primers: forward primer, 5′-TTGCCAGCTCTACCACCAG-3′; reverse primer, 5′-TTCATTGCAACAGCCACCAC-3′. The primers were specific for hVEGF-D cDNA.
Animals used in the study were from F0 to F3 generations. TG-negative littermates (the same genetic and epigenetic background lacking hVEGF-D) were used as controls. All animal studies had the approval of the Experimental Animal Committee of Kuopio University.
Hindlimb ischemia
Hindlimb ischemia model has been described.14 Briefly, mice were anesthetized with Rompun-Ketalar mixture (10 mg/kg xylazine [Rompun], 80 mg/kg ketamine), and ligation of the arteria femoralis superficialis was performed. Animals were killed by CO2 inhalation 1, 2, and 3 weeks after the operation. The organs were perfused with 1 × PBS via left ventricle. After perfusion, samples from all major tissues were collected for molecular biological and histological analyses. Histologic samples were fixed in 4% PFA–15% sucrose for 4 hours, rinsed in 15% sucrose, processed, and embedded in paraffin. Slides (5 μm thick) were cut and routinely stained with hematoxylin-eosin (HE).
Copy number and integration site analyses
Mouse genomic DNA was digested with AflII or SacII restriction endonucleases for the copy number analysis and for the determination of the number of integration sites, respectively. Prehybridization of the membranes was performed before hybridization with 32P-CTP–labeled wPRE probe. For the copy number analysis, a standard curve was generated by adding the plasmid amount equal to copy numbers of 1, 5, 10, 20, and 30 into 15 μg of TG-negative mouse genomic DNA.
Reverse transcriptase–PCR
Expression of hVEGF-D mRNA in tissues of TG mice was analyzed by real-time reverse transcriptase–PCR (RT-PCR). Total RNAs of different tissues were extracted by RNeasy kit (QIAGEN, Valencia, CA) according to the manufacturer's instructions. cDNA synthesis was performed with the use of random hexamer primers and PCR by predesigned hVEGF-D primers and probe (Applied Biosystems, Foster City, CA). Normalization was performed with the use of mouse β-actin control kit (Applied Biosystems).
Enzyme-linked immunoabsorbent assays and serum clinical chemistry
hVEGF-D and mVEGF-A concentrations were analyzed with the use of Quantikine Immunoassays (Human VEGF-D or Mouse VEGF-A; R&D Systems, Minneapolis, MN). Immunoassays were performed from tissue lysates and from serum samples. Tissues were homogenized in T-PER buffer (Tissue Protein Extraction Reagent; Pierce Chemical, Rockford, IL), and lysates were prepared according to the manufacturer's instructions.
Mouse serum samples from TG-negative and hVEGF-D TG mice were analyzed with the use of standard clinical chemistry methods at the Department of Clinical Chemistry, Kuopio University Central Hospital.
Immunohistochemistry
Paraffin-embedded sections (5 μm thick) were immunostained with the following antibodies: CD31 (platelet endothelial cell adhesion molecule 1[PECAM-1], 1:50; BD Biosciences PharMingen, San Diego, CA), CD34 (1:20; Hycult Biotechnology, Uden, The Netherlands), LYVE-1 (1:1000, microwave pretreatment in citrate buffer; ReliaTech, Braunschweig, Germany), hVEGF-D (1:100, microwave pretreatment in citrate buffer; R&D Systems), VEGFR-2 (1:100; eBiosciences, San Diego, CA), VEGFR-3 (1:50; R&D Systems), cytokeratin (CK, 1:50; Affinity BioReagents, Golden, CO), cytokeratin 7 (CK7, 1:50, microwave pr-treatment in citrate buffer; Santa Cruz Biotechnology, Santa Cruz, CA). Immunohistochemical reactions were developed with the use of ABC Vectastain Elite staining kit (Vector Laboratories, Burlingame, CA) and DAB (Invitrogen Zymed, San Francisco, CA).
Light microscopic analysis
Histologic sections were assessed with an Olympus AX-70 microscope (Olympus, Tokyo, Japan) with AnalySIS software (Soft Imaging System, Muenster, Germany). Paraffin slices of all major organs (liver, spleen, pancreas, heart, lungs, salivary glands, testes/ovaries, aorta, limb skeletal muscles, small intestine, brain, and skin) of TG-positive and TG-negative mice were stained with HE, and their histologies were observed for major phenotypic changes. Hindlimb skeletal muscle (musculus rectus and musculus caput gastrocnemius) and myocardium slices were stained with CD34 immunohistochemistry and photographed under 20× objective.
Capillary densities (capillaries/myocyte in skeletal muscles, capillaries/mm2 in myocardium) were determined as described.14 Hindlimb skeletal muscles with ischemic injury were stained with HE. Areas of regeneration and different manifestations of ischemic injury were measured with the use of AnalySIS software (Soft Imaging System). In assessing capillary density and morphometry, 8 randomly selected fields were analyzed blindly.
Statistical methods
Statistical analyses were performed with GraphPad Prism version 4.00 (GraphPad Software, San Diego, CA). Results of the TG-positive and TG-negative groups were analyzed with the use of unpaired, 2-tailed t test with confidentiality of 95%, considering P values less than .05 as statistically significant.
Results
Lentiviral transgenesis
We produced hVEGF-D mice with the use of LV transgenesis. The amount of LV injected into one oocyte was 103 TU. Ninety-one percent of all injected embryos survived viral microinjection, and on the average 70% of the born founders were TG positive. Southern blot analysis was used to analyze copy numbers of the TG mice (Figure 1B). The copy numbers in all founders were between 1 and 10 copies/genome, showing a moderate variation between the mice. Either the same or a lower copy number was detected in F1 offspring. In consecutive generations the copy numbers remained similar to the copy numbers of F1 mice.
The number of chromosomal integrations was determined from F1, F2, and F3 TG mice. Results showed that the provirus was integrated into one chromosome in most mice, whereas in one F1 mouse and one F3 mouse, derived from the same founder, 2 chromosomal integration sites were found (data not shown). Thus, copy numbers of more than one in most mice are probably due to concatamerization of the provirus.
In F1 generations the litters were small (2-3 pups), but 90% to 100% of the pups were positive for the transgene. As the number of generation advanced, the litter sizes grew, but the number of TG-positive pups decreased. Nevertheless, the transgene was transmitted through the germ line from one generation to another.
hVEGF-D mRNA
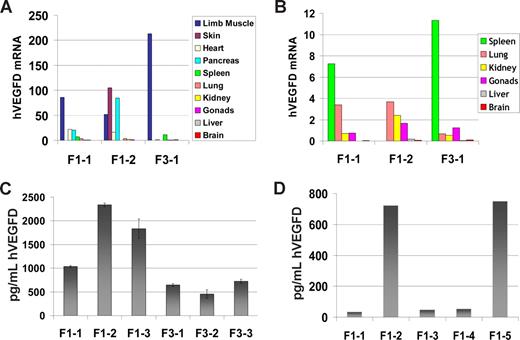
Expression levels of hVEGF-D mRNA varied between mice, as well as between tissues in each mouse. However, each RT-PCR run showed that the same tissues had highest hVEGF-D expressions in all generations. The tissues of high expression were skeletal muscle, skin, pancreas, and heart (Figure 2A). Most of the other tissues fell into the category of moderate expression (spleen, lung, kidney, and gonads; Figure 2B), whereas each analysis showed very low or no TG expression in liver and brain (Figure 2B). The expression was easily detectable in TG mice in all generations.
Human VEGF-D expression in TG mice. Results of one representative real-time RT-PCR run with 3 mice were chosen to show the general pattern of TG expression in different tissues. F1-1 and F1-2 are mice of generation F1; F3-1, -2 and -3 are TG mice of generation F3. (A) All analyzed tissues are included in the same histogram. Because the scale is wide, the tissues that showed moderate to very low TG expression are shown with a lower scale in panel B. (C,D) Representative ELISA assays for hVEGF-D protein content in TG mice. (C) hVEGF-D protein in tissue lysates of F1 and F3 TG mice. (D) hVEGF-D protein in plasma of 5 F1 mice. Error bars represent ± SD.
Human VEGF-D expression in TG mice. Results of one representative real-time RT-PCR run with 3 mice were chosen to show the general pattern of TG expression in different tissues. F1-1 and F1-2 are mice of generation F1; F3-1, -2 and -3 are TG mice of generation F3. (A) All analyzed tissues are included in the same histogram. Because the scale is wide, the tissues that showed moderate to very low TG expression are shown with a lower scale in panel B. (C,D) Representative ELISA assays for hVEGF-D protein content in TG mice. (C) hVEGF-D protein in tissue lysates of F1 and F3 TG mice. (D) hVEGF-D protein in plasma of 5 F1 mice. Error bars represent ± SD.
hVEGF-D protein
Protein expression of hVEGF-D in TG mice is shown in Figure 2C and D. Concentrations were measured from tissue lysates (Figure 2C) and serum (Figure 2D). No hVEGF-D was detected in TG-negative mouse samples. Similar to mRNA levels, the number of generation of the TG mice did not affect TG protein expression levels. The hVEGF-D concentration was slightly lower in the F3 mice than in the F1 mice (Figure 2C). However, the difference between the generations was not significant. In serum samples the hVEGF-D protein concentration varied between 20 and 700 pg/mL (Figure 2D).
The results of mVEGF-A enzyme-linked immunoabsorbent assays (ELISAs) showed that hVEGF-D did not affect endogenous expression of mVEGF-A in TG mice with various copy numbers (data not shown).
Clinical chemistry
Markers of lipid metabolism, liver and kidney function, and tissue damage were measured from serum of TG-positive and TG-negative mice. Blood for these measurements was collected on killing. The concentration of total serum cholesterol (Chole), high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglycerides (Trigly), aspartate aminotransferase (ASAT), creatinine (Crea), lactate dehydrogenase (LD), and creatine kinase (CK) were measured with the use of standard clinical chemistry methods. Results of clinical analyses are summarized in Table 1. No significant differences were observed between the groups.
Serum clinical analysis of hVEGFD TG mice
| Mouse group . | Chole, mmol/L . | HDL, mmol/L . | LDL, mmol/L . | Trigly, mmol/L . | ASAT, U/mL . | Crea, μmol/L . | LD, U/mL . | CK, U/mL . |
|---|---|---|---|---|---|---|---|---|
| Controls F1 | 2.0 ± 0.4 | 1.6 ± 0.3 | 0.13 ± 0.04 | 1.5 ± 0.2 | 1.00 ± 0.23 | 12.3 ± 1.5 | 2.5 ± 0.5 | 23.5 ± 5.2 |
| Controls F3 | 0.9 ± 0.1 | 0.7 ± 0.1 | 0.13 ± 0.02 | 1.2 ± 0.1 | 1.12 ± 0.35 | 12.3 ± 1.5 | 1.6 ± 0.3 | 16.2 ± 2.4 |
| Controls all | 1.4 ± 0.3 | 1.1 ± 0.2 | 0.13 ± 0.02 | 1.3 ± 0.1 | 1.08 ± 0.44 | 12.3 ± 0.1 | 2.3 ± 0.3 | 21.2 ± 3.1 |
| VEGF-D TG F1 | 1.9 ± 0.3 | 1.5 ± 0.4 | 0.24 ± 0.02 | 1.1 ± 0.3 | 0.61 ± 0.14 | 11.7 ± 0.7 | 1.5 ± 0.2 | 11.9 ± 3.1 |
| VEGF-D TG F3 | 1.3 ± 0.3 | 1.1 ± 0.2 | 0.10 ± 0.02 | 0.8 ± 0.2 | 0.37 ± 0.03 | 9.0 ± 1.2 | 0.7 ± 0.1 | 8.5 ± 1.9 |
| VEGF-D TG all | 1.5 ± 0.2 | 1.2 ± 0.2 | 0.15 ± 0.02 | 1.3 ± 0.2 | 0.47 ± 0.06 | 10.9 ± 0.7 | 1.2 ± 0.07 | 11.3 ± 1.6 |
| Mouse group . | Chole, mmol/L . | HDL, mmol/L . | LDL, mmol/L . | Trigly, mmol/L . | ASAT, U/mL . | Crea, μmol/L . | LD, U/mL . | CK, U/mL . |
|---|---|---|---|---|---|---|---|---|
| Controls F1 | 2.0 ± 0.4 | 1.6 ± 0.3 | 0.13 ± 0.04 | 1.5 ± 0.2 | 1.00 ± 0.23 | 12.3 ± 1.5 | 2.5 ± 0.5 | 23.5 ± 5.2 |
| Controls F3 | 0.9 ± 0.1 | 0.7 ± 0.1 | 0.13 ± 0.02 | 1.2 ± 0.1 | 1.12 ± 0.35 | 12.3 ± 1.5 | 1.6 ± 0.3 | 16.2 ± 2.4 |
| Controls all | 1.4 ± 0.3 | 1.1 ± 0.2 | 0.13 ± 0.02 | 1.3 ± 0.1 | 1.08 ± 0.44 | 12.3 ± 0.1 | 2.3 ± 0.3 | 21.2 ± 3.1 |
| VEGF-D TG F1 | 1.9 ± 0.3 | 1.5 ± 0.4 | 0.24 ± 0.02 | 1.1 ± 0.3 | 0.61 ± 0.14 | 11.7 ± 0.7 | 1.5 ± 0.2 | 11.9 ± 3.1 |
| VEGF-D TG F3 | 1.3 ± 0.3 | 1.1 ± 0.2 | 0.10 ± 0.02 | 0.8 ± 0.2 | 0.37 ± 0.03 | 9.0 ± 1.2 | 0.7 ± 0.1 | 8.5 ± 1.9 |
| VEGF-D TG all | 1.5 ± 0.2 | 1.2 ± 0.2 | 0.15 ± 0.02 | 1.3 ± 0.2 | 0.47 ± 0.06 | 10.9 ± 0.7 | 1.2 ± 0.07 | 11.3 ± 1.6 |
Chole indicates cholesterol; HDL, high-density lipoprotein; LDL, low-density lipoprotein; Trigly, triglycerides; ASAT, aspartate aminotransferase; Crea, creatinine; LD, lactate dehydrogenase; and CK, creatine kinase.
Vascularity
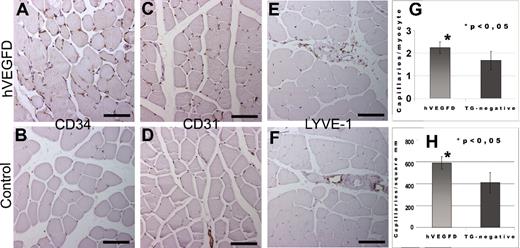
The effect of the transgene on vascular density was measured from intact hindlimb skeletal muscles and myocardium of F0 to F3 mice by CD34 immunohistochemistry. The angiogenic effect was also determined in mice aged 1 to 2 years to study whether the transgene was functional also in aged mice. A representative image of CD34 immunostained skeletal muscle is shown in Figure 3A and B. For methodologic comparison, we also show a representative image of a serial section immunostained for CD31 in both TG-positive and TG-negative mice (Figure 3C,D, respectively). Results show that constitutively expressed hVEGF-DΔNΔC is a potent angiogenic factor in mice. The effect of the transgene on the density of lymphatic capillaries was studied with LYVE-1 immunohistochemistry. These stainings showed a similar number of lymphatic capillaries in TG mice and control mice. Thus, the hVEGF-DΔNΔC transgene has no lymphangiogenic effect in the TG mice (Figure 3E,F). Difference in the capillary count of hindlimb skeletal muscles of nonoperated TG-negative and TG-positive mice was statistically significant (P < .05; Figure 3G). The number of capillaries in the myocardium was also significantly higher in the TG-positive mice than in the TG-negative littermates (Figure 3H). No major effect was found on lymphatic capillary density in skeletal muscles or other tissues. These results suggest that the constitutively expressed mature form of hVEGF-D is a highly potent angiogenic factor but not a lymphangiogenic factor in mice.
Blood and lymphatic capillary density in TG and control mice as shown by CD34, CD31, and LYVE-1 stainings. Images were taken from immunohistochemically stained 5-μm paraffin sections of mouse tissues and analyzed with Olympus Provis AX70 Microscope attached to Olympus ColorView 12 Camera. The software for imaging and analyses was AnalySIS (Soft Imaging System). (A) Representative figure from a TG mouse showed an increase in capillary density (CD). (B) In comparison, a normal capillary density was present in control mouse (CD34). (C) Representative figure from a TG mouse showed an increase in capillary density (CD31). (D) In comparison a normal capillary density was present in control mouse (CD31). (E) Lymphatic vessels were detected only in the interstitium accompanied by venules and arterioles in TG mice (LYVE-1). (F) Control muscles showed a similar pattern and number of lymphatic vessels (LYVE-1). Bars in panels A through F = 100 μm. (G) Capillaries/myocyte of nonoperated musculus rectus of the hVEGFD TG mice and the TG-negative controls. The mice were from generations F1, F2, and F3. (H) Capillary density in myocardium of the hVEGF-D TG-positive and the TG-negative littermates. The mice were from generations F1, F2, and F3. Error bars represent ± SD.
Blood and lymphatic capillary density in TG and control mice as shown by CD34, CD31, and LYVE-1 stainings. Images were taken from immunohistochemically stained 5-μm paraffin sections of mouse tissues and analyzed with Olympus Provis AX70 Microscope attached to Olympus ColorView 12 Camera. The software for imaging and analyses was AnalySIS (Soft Imaging System). (A) Representative figure from a TG mouse showed an increase in capillary density (CD). (B) In comparison, a normal capillary density was present in control mouse (CD34). (C) Representative figure from a TG mouse showed an increase in capillary density (CD31). (D) In comparison a normal capillary density was present in control mouse (CD31). (E) Lymphatic vessels were detected only in the interstitium accompanied by venules and arterioles in TG mice (LYVE-1). (F) Control muscles showed a similar pattern and number of lymphatic vessels (LYVE-1). Bars in panels A through F = 100 μm. (G) Capillaries/myocyte of nonoperated musculus rectus of the hVEGFD TG mice and the TG-negative controls. The mice were from generations F1, F2, and F3. (H) Capillary density in myocardium of the hVEGF-D TG-positive and the TG-negative littermates. The mice were from generations F1, F2, and F3. Error bars represent ± SD.
Regeneration after hindlimb ischemic injury
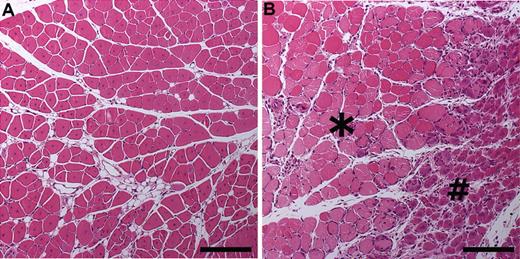
To study the effect of the transgene expression on mouse muscle biology in pathologic conditions, we induced hindlimb ischemia using a surgical operation. Signs of tissue damage and regeneration were studied by morphometrical measurements of the injured muscles and by measuring the areas of active regeneration. HE-stained musculus caput gastrocnemius of the TG mice and the TG-negative littermates 7 days after the ischemic injury are shown in Figure 4. When the ischemic area (apoptosis/necrosis, fibrosis, fat atrophy) was measured, 4% to 15% of the total muscle areas in the hVEGF-D TG mice was damaged, whereas the same range in the TG-negative mice was 35% to 89%. Signs of active regeneration were found in larger areas of ischemic limb muscles in the hVEGF-D TG mice compared with the TG-negative control mice (data not shown).
Morphologic characteristics in musculus caput gastrocnemius 7 days after hindlimb ischemia operation. Images were taken from HE-stained 5-μm paraffin sections of mouse tissues and analyzed with Olympus Provis AX70 Microscope attached to Olympus ColorView 12 Camera. The software for imaging and analyses was AnalySIS (Soft Imaging System). (A) Increased regeneration shown in a hVEGF-D TG mouse on day 7 after hindlimb ischemia operation. Whole area showed late regeneration features as eosinophilic cytoplasm and internalization of nuclei. Bar, 200 μm. (B) Control group showed large area of necrosis with pale flocculated cytoplasm (*). Early stage of regeneration is represented by basophilic small myocytes with internalized nuclei (#). Bar, 200 μm.
Morphologic characteristics in musculus caput gastrocnemius 7 days after hindlimb ischemia operation. Images were taken from HE-stained 5-μm paraffin sections of mouse tissues and analyzed with Olympus Provis AX70 Microscope attached to Olympus ColorView 12 Camera. The software for imaging and analyses was AnalySIS (Soft Imaging System). (A) Increased regeneration shown in a hVEGF-D TG mouse on day 7 after hindlimb ischemia operation. Whole area showed late regeneration features as eosinophilic cytoplasm and internalization of nuclei. Bar, 200 μm. (B) Control group showed large area of necrosis with pale flocculated cytoplasm (*). Early stage of regeneration is represented by basophilic small myocytes with internalized nuclei (#). Bar, 200 μm.
Spontaneous tumors
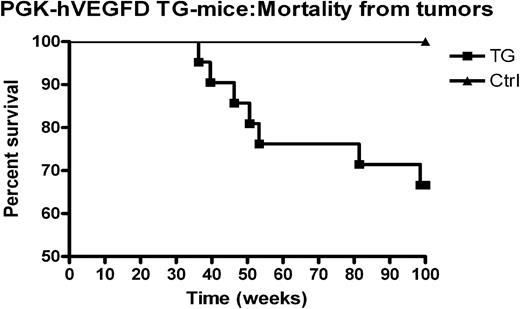
On aging the hVEGF-D TG mice showed high frequency of malignant tumors (Figure 5). Mice obtained from LV transgenesis with the same vector backbone and the same promoter but a different transgene (human Nrf2) have not developed any tumors (data not shown). In addition, the TG-negative littermates never developed tumors during the study. Mice with tumors originated from 2 different founders. This implies that the integration site is not involved in the tumor formation. Unexpectedly, half of the tumors were mammary adenocarcinomas, and one of the mice with mammary gland tumor was a male (aged 9 months). In addition, 2 lung adenocarcinomas and 2 skin carcinomas were found (Table 2). No lymphomas or other types of lymphatic malignancies were found in the TG mice.
Kaplan-Mayer survival curve showing tumor mortality of hVEGF-D TG mice and TG-negative controls. This curve presents all hVEGF-D TG mice that died of cancer, regardless of their original background or generation. There were 2 high-expressing founders and 1 founder with lower level of expression. First 4 points represent mice that died of mammary gland tumor before the age of 1 year. The TG-negative controls had no tumors in any generation during the follow-up.
Kaplan-Mayer survival curve showing tumor mortality of hVEGF-D TG mice and TG-negative controls. This curve presents all hVEGF-D TG mice that died of cancer, regardless of their original background or generation. There were 2 high-expressing founders and 1 founder with lower level of expression. First 4 points represent mice that died of mammary gland tumor before the age of 1 year. The TG-negative controls had no tumors in any generation during the follow-up.
Histopathologic and immunohistochemical characterization of tumors
| Organ and tumor type . | Histology . | Immunohistochemistry . | Metastases . | Generation, sex, comment . |
|---|---|---|---|---|
| Mammary gland | ||||
| Adenocarcinoma | Solid, comedo, trabecular growth pattern, single acinar/ductal structures, necrosis, grade 3 | hVEGF-D positive in ductal part (surface); CD31, CD34, LYVE-1 negative in tumor cells | − | F1, male, left cervical mammary gland |
| Adenocarcinoma | Solid, comedo growth pattern, necrosis | hVEGF-D positive in ductal part; CK7 positive; CD31, CD34, LYVE-1 negative in tumor cells; increased number of vessels in tumor; angiogenic features | − | F3, female, left inguinal mammary gland, lactating, skin ulcerated |
| Mammary gland preserved at the edge, grade 3 | ||||
| Adenocarcinoma | Tubular, nestal, papillar, and trabecular growth pattern; necrosis | hVEGF-D positive in ductal part (surface); CK7 positive; CD31, CD34, LYVE-1 negative in tumor cells; increased number of vessels in tumor; angiogenic features | Lung metastases | F1, female, right inguinal mammary gland |
| Mammary gland preserved at the edge, grade 3 | ||||
| Adenocarcinoma | Tubular growth pattern, in situ component,mammary gland largely preserved, grade 1 | hVEGF-D positive in ductal part (surface); CD31, CD34, LYVE-1 negative in tumor cells; increased amount of vessels in tumor; angiogenic features | − | F1, female, right abdominal mammary gland |
| Lung | ||||
| Adenocarcinoma | Multifocal, foci of bronchioloalveolar proliferation, grade 1 | hVEGF-D negative | − | F1, female |
| Papillary adenocarcinoma | Multifocal, papillary growth pattern, grade 1 | hVEGF-D positive in stroma of papilla stalk; CD31, CD34, LYVE-1 negative in tumor cells | − | F1, male |
| Skin | ||||
| Basal cell carcinoma | Predominantly solid growth of round basal cells, necrosis | hVEGF-D negative; CD31, CD34, LYVE-1 negative in tumor cells | − | F3, female |
| Anaplastic carcinoma | Solid infiltrative growth of spindle and round cells, necrosis, infiltrating soft tissues (muscle, bone) | hVEGF-D positive in some tumor cells; CD31, CD34, LYVE-1 negative in tumor cells; increased amount of vessels in tumor; angiogenesis features | − | F1, male |
| Organ and tumor type . | Histology . | Immunohistochemistry . | Metastases . | Generation, sex, comment . |
|---|---|---|---|---|
| Mammary gland | ||||
| Adenocarcinoma | Solid, comedo, trabecular growth pattern, single acinar/ductal structures, necrosis, grade 3 | hVEGF-D positive in ductal part (surface); CD31, CD34, LYVE-1 negative in tumor cells | − | F1, male, left cervical mammary gland |
| Adenocarcinoma | Solid, comedo growth pattern, necrosis | hVEGF-D positive in ductal part; CK7 positive; CD31, CD34, LYVE-1 negative in tumor cells; increased number of vessels in tumor; angiogenic features | − | F3, female, left inguinal mammary gland, lactating, skin ulcerated |
| Mammary gland preserved at the edge, grade 3 | ||||
| Adenocarcinoma | Tubular, nestal, papillar, and trabecular growth pattern; necrosis | hVEGF-D positive in ductal part (surface); CK7 positive; CD31, CD34, LYVE-1 negative in tumor cells; increased number of vessels in tumor; angiogenic features | Lung metastases | F1, female, right inguinal mammary gland |
| Mammary gland preserved at the edge, grade 3 | ||||
| Adenocarcinoma | Tubular growth pattern, in situ component,mammary gland largely preserved, grade 1 | hVEGF-D positive in ductal part (surface); CD31, CD34, LYVE-1 negative in tumor cells; increased amount of vessels in tumor; angiogenic features | − | F1, female, right abdominal mammary gland |
| Lung | ||||
| Adenocarcinoma | Multifocal, foci of bronchioloalveolar proliferation, grade 1 | hVEGF-D negative | − | F1, female |
| Papillary adenocarcinoma | Multifocal, papillary growth pattern, grade 1 | hVEGF-D positive in stroma of papilla stalk; CD31, CD34, LYVE-1 negative in tumor cells | − | F1, male |
| Skin | ||||
| Basal cell carcinoma | Predominantly solid growth of round basal cells, necrosis | hVEGF-D negative; CD31, CD34, LYVE-1 negative in tumor cells | − | F3, female |
| Anaplastic carcinoma | Solid infiltrative growth of spindle and round cells, necrosis, infiltrating soft tissues (muscle, bone) | hVEGF-D positive in some tumor cells; CD31, CD34, LYVE-1 negative in tumor cells; increased amount of vessels in tumor; angiogenesis features | − | F1, male |
Characterization of tumors
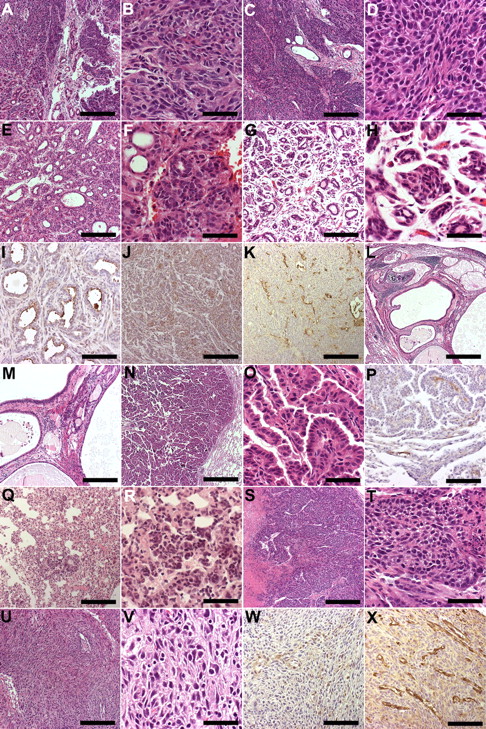
Most of the mammary gland tumors were poorly differentiated (grade 3) with a solid and comedo growth pattern. One of the mice with tumors was lactating when the tumor was noticed. In one of the mice the mammary gland tumor metastasized into the lungs. hVEGF-D was expressed in the surface layer of the ductal component, but not in the solid parts of the tumor. Active angiogenesis was detected in most tumors. Well-differentiated multifocal adenocarcinomas in the lungs, as well as skin tumors (basal cell carcinoma and anaplastic carcinoma) were found in some mice. In the lung tumor, the papillary and stroma of the papilla stalk were hVEGF-D positive. Anaplastic skin carcinoma showed focal hVEGF-D positivity and intratumoral angiogenesis. A representative panel of the immunostained tumor samples is shown in Figure 6. Detailed characterization of the tumors is presented in Table 2. All tumors showed detectable amounts of hVEGF-D mRNA with high or moderate expression levels.
Representative histology of tumors in hVEGF-D TG mice. Images were taken from HE-stained or immunohistochemically stained paraffin sections of mouse tissues and analyzed with Olympus Provis AX70 Microscope attached to Olympus ColorView 12 Camera. The software for imaging and analyses was AnalySIS (Soft Imaging System). (A) Mammary adenocarcinoma with a predominantly solid growth pattern showed focal ducts. Bar, 200 μm. The objective lens used was UPlanApo 4×/0.16 ∞/− (Olympus). (B) Higher magnification of the solid part of mammary carcinoma shown in panel A with atypical cells and a few mitotic figures. Bar, 50 μm. The objective lens used was UPlanApo 20×/0.70 (Olympus). (C) Mammary adenocarcinoma with a solid growth pattern displaying only single remnants of ducts. Bar, 200 μm. The objective lens used was UPlanApo 4×/0.16 ∞/− (Olympus). (D) Higher magnification of the solid part of mammary carcinoma shown in panel C with atypical cells and a few mitotic figures. Bar, 50 μm. The objective lens used was UPlanApo 20×/0.70 (Olympus). (E) Mammary adenocarcinoma showing a tubular growth pattern. Bar. 200 μm. The objective lens used was UPlanApo 4×/0.16 ∞/− (Olympus). (F) Higher magnification of the carcinoma shown in panel E showing both tubular and solid areas. Bar, 50 μm. The objective lens used was UPlanApo 20×/0.70 (Olympus). (G) Well-differentiated mammary adenocarcinoma showing a tubular growth pattern. Bar, 200 μm. The objective lens used was UPlanApo 4×/0.16 ∞/− (Olympus). (H) Higher magnification of the carcinoma shown in panel G. Bar, 50 μm. (I) Immunostaining for hVEGF-D was positive in the surface of ductular structures. Picture from carcinoma is shown in panels E and F. Bar, 100 μm. The objective lens used was UPlanApo 10×/0.4 Ph1 ∞/0.17 (Olympus). (J) Solid parts of tumor shown in panels E and F showed CK7 immunopositivity. Bar, 200 μm. The objective lens used was UPlanApo 4×/0.16 ∞/− (Olympus). (K) Increased number of vessels with angiogenic features in mammary adenocarcinoma shown in panels C and D. Bar, 200 μm. The objective lens used was UPlanApo 4×/0.16 ∞/− (Olympus). (L) Lung with metastases of mammary adenocarcinoma. Original tumor shown in panels E and F. Bar, 50 μm. The objective lens used was UPlanApo 20×/0.70 (Olympus). (M) Higher magnification of the lung metastasis shown in panel L. Note dilated ducts. Bar, 200 μm. The objective lens used was UPlanApo 4×/0.16 ∞/− (Olympus). (N) Lung papillary adenocarcinoma. Bar, 50 μm. (O) Higher magnification of the adenocarcinoma papillae in tumor shown in panel N. Bar, 50 μm. The objective lens used was UPlanApo 20×/0.70 (Olympus). (P) Positive immunostaining for hVEGF-D in papilla stalk. Note positivity also in vessels at the border of the tumor. Bar, 100 μm. The objective lens used was UPlanApo 10×/0.4 Ph1 ∞/0.17 (Olympus). (Q) Multifocal foci of bronchioloalveolar proliferation in lung adenocarcinoma. Bar, 200 μm. The objective lens used was UPlanApo 4×/0.16 ∞/− (Olympus). (R) Higher magnification of the bronchioloalveolar proliferation in adenocarcinoma shown in panel R. Bar, 50 μm. The objective lens used was UPlanApo 20×/0.70 (Olympus). (S) Solid growth in skin basal cell carcinoma. Note necrosis. Bar, 200 μm. The objective lens used was UPlanApo 4×/0.16 ∞/− (Olympus). (T) Higher magnification of the basal cell carcinoma. Note mitosis. Bar, 50 μm. The objective lens used was UPlanApo 20×/0.70 (Olympus). (U) Skin anaplastic carcinoma consists of spindle cells. Bar, 200 μm. The objective lens used was UPlanApo 4×/0.16 ∞/− (Olympus). (V) Higher magnification of the anaplastic carcinoma. Note atypia. Bar, 50 μm. The objective lens used UPlanApo 20×/0.70 (Olympus). (W) Some tumor cells were positive for hVEGF-D immunostaining in an anaplastic carcinoma. Bar, 100 μm. The objective lens used was UPlanApo 10×/0.4 Ph1 ∞/0.17 (Olympus). (X) Increased number of vessels with angiogenic features in an anaplastic carcinoma shown in panels U and V. Bar, 100 μm. The objective lens used was UPlanApo 10×/0.4 Ph1 ∞/0.17 (Olympus).
Representative histology of tumors in hVEGF-D TG mice. Images were taken from HE-stained or immunohistochemically stained paraffin sections of mouse tissues and analyzed with Olympus Provis AX70 Microscope attached to Olympus ColorView 12 Camera. The software for imaging and analyses was AnalySIS (Soft Imaging System). (A) Mammary adenocarcinoma with a predominantly solid growth pattern showed focal ducts. Bar, 200 μm. The objective lens used was UPlanApo 4×/0.16 ∞/− (Olympus). (B) Higher magnification of the solid part of mammary carcinoma shown in panel A with atypical cells and a few mitotic figures. Bar, 50 μm. The objective lens used was UPlanApo 20×/0.70 (Olympus). (C) Mammary adenocarcinoma with a solid growth pattern displaying only single remnants of ducts. Bar, 200 μm. The objective lens used was UPlanApo 4×/0.16 ∞/− (Olympus). (D) Higher magnification of the solid part of mammary carcinoma shown in panel C with atypical cells and a few mitotic figures. Bar, 50 μm. The objective lens used was UPlanApo 20×/0.70 (Olympus). (E) Mammary adenocarcinoma showing a tubular growth pattern. Bar. 200 μm. The objective lens used was UPlanApo 4×/0.16 ∞/− (Olympus). (F) Higher magnification of the carcinoma shown in panel E showing both tubular and solid areas. Bar, 50 μm. The objective lens used was UPlanApo 20×/0.70 (Olympus). (G) Well-differentiated mammary adenocarcinoma showing a tubular growth pattern. Bar, 200 μm. The objective lens used was UPlanApo 4×/0.16 ∞/− (Olympus). (H) Higher magnification of the carcinoma shown in panel G. Bar, 50 μm. (I) Immunostaining for hVEGF-D was positive in the surface of ductular structures. Picture from carcinoma is shown in panels E and F. Bar, 100 μm. The objective lens used was UPlanApo 10×/0.4 Ph1 ∞/0.17 (Olympus). (J) Solid parts of tumor shown in panels E and F showed CK7 immunopositivity. Bar, 200 μm. The objective lens used was UPlanApo 4×/0.16 ∞/− (Olympus). (K) Increased number of vessels with angiogenic features in mammary adenocarcinoma shown in panels C and D. Bar, 200 μm. The objective lens used was UPlanApo 4×/0.16 ∞/− (Olympus). (L) Lung with metastases of mammary adenocarcinoma. Original tumor shown in panels E and F. Bar, 50 μm. The objective lens used was UPlanApo 20×/0.70 (Olympus). (M) Higher magnification of the lung metastasis shown in panel L. Note dilated ducts. Bar, 200 μm. The objective lens used was UPlanApo 4×/0.16 ∞/− (Olympus). (N) Lung papillary adenocarcinoma. Bar, 50 μm. (O) Higher magnification of the adenocarcinoma papillae in tumor shown in panel N. Bar, 50 μm. The objective lens used was UPlanApo 20×/0.70 (Olympus). (P) Positive immunostaining for hVEGF-D in papilla stalk. Note positivity also in vessels at the border of the tumor. Bar, 100 μm. The objective lens used was UPlanApo 10×/0.4 Ph1 ∞/0.17 (Olympus). (Q) Multifocal foci of bronchioloalveolar proliferation in lung adenocarcinoma. Bar, 200 μm. The objective lens used was UPlanApo 4×/0.16 ∞/− (Olympus). (R) Higher magnification of the bronchioloalveolar proliferation in adenocarcinoma shown in panel R. Bar, 50 μm. The objective lens used was UPlanApo 20×/0.70 (Olympus). (S) Solid growth in skin basal cell carcinoma. Note necrosis. Bar, 200 μm. The objective lens used was UPlanApo 4×/0.16 ∞/− (Olympus). (T) Higher magnification of the basal cell carcinoma. Note mitosis. Bar, 50 μm. The objective lens used was UPlanApo 20×/0.70 (Olympus). (U) Skin anaplastic carcinoma consists of spindle cells. Bar, 200 μm. The objective lens used was UPlanApo 4×/0.16 ∞/− (Olympus). (V) Higher magnification of the anaplastic carcinoma. Note atypia. Bar, 50 μm. The objective lens used UPlanApo 20×/0.70 (Olympus). (W) Some tumor cells were positive for hVEGF-D immunostaining in an anaplastic carcinoma. Bar, 100 μm. The objective lens used was UPlanApo 10×/0.4 Ph1 ∞/0.17 (Olympus). (X) Increased number of vessels with angiogenic features in an anaplastic carcinoma shown in panels U and V. Bar, 100 μm. The objective lens used was UPlanApo 10×/0.4 Ph1 ∞/0.17 (Olympus).
Discussion
The high success rate of the LV transgenesis is most likely due to the LV perivitelline injection method, compared with the traditional transgenesis method based on plasmid microinjection into the oocyte pronucleus. In contrast to the perivitelline injection, in the traditional plasmid microinjection method the oocyte membrane must be pierced to bring the plasmid into the pronucleus. In an alternative LV transgenesis method, the zona pellucida must be removed, whereas in the LV perivitelline injection technique it is left in place to cover the oocyte during migration through the oviduct into the uterus and during implantation of the embryo. Thus, the LV perivitelline injection TG method results in a significantly higher number of implanted embryos and transgenic founders.11 Another advantage of the perivitelline injection method over the zona removal is that the fertilized oocytes are transduced at the 1-cell stage. Thus, the transgene is incorporated into each cell of the developing embryo, and mosaicism is usually avoided.
To our knowledge all published studies of LV transgenesis to date were performed only up to the F1 generation. Therefore, we also wanted to study whether the transgene still was functional in the TG mice of consecutive generations and in TG mice aged from 1 to 2 years. Our results suggest that the hPGK-driven transgenes were integrated into genomic sites, where they most probably were not subjected to methylation,15 and that the hPGK promoter can be used to achieve constitutive long-term expression of transgenes. Our results also suggest that LV transgenesis leads to a long-term and effective expression of transgenes in successive generations.
All TG mice with constitutive hVEGF-D expression showed normal growth, were fertile, and appeared generally healthy with no obvious macroscopic phenotype. This implies that constitutive hVEGF-D expression is well tolerated during embryonic development.
Overexpression of VEGFR-2–specific ligands promotes angiogenesis and lymphatic vessel enlargement but no lymphatic vessel sprouting.16 Therefore, we hypothesized that the hVEGF-D TG mice could have a proangiogenic phenotype. Indeed, increased blood capillary density was found in skeletal muscles and myocardium of the TG-positive mice compared with the TG-negative littermates. Moreover, after ischemic hindlimb injury, muscle regeneration was faster, and injured areas were significantly smaller, suggesting that hVEGF-D overexpression improves healing capacity of muscle tissue. These results are consistent with our previous findings that intramuscular and intracardiac adenoviral hVEGF-D gene transfers increased vascularity and improved blood flow in transduced tissues.14,17,18 Thus, mature hVEGF-D is primarily a proangiogenic growth factor, whether used as a transgene in mice, or applied with the use of local gene therapy in larger animals, implying that the effects are mostly mediated by VEGFR-2.
Until now, only mice with skin-specific overexpression of full-length hVEGF-D have been generated, whereby the human keratin 14 (hK14) promoter was used to drive the expression of the transgene.9 The hK14 promoter directs the TG expression into the basal cells of the epidermis. These mice showed strong and selective hyperplasia of skin lymphatic vessels but not of skin blood vessels. Furthermore, the lymphatic capillaries in the skin were dilated and 3-fold larger than those of the wild-type mice. However, in the hK14–hVEGF-D study,9 a different form of hVEGF-D was used than in the present study. The processed form of hVEGF-D–hVEGF-DΔNΔC (formed by proteolytical procession of N- and C-terminal ends of the hVEGF-D prepropeptide) almost exclusively binds to VEGFR-2, leading to angiogenesis.6,7,17 The unprocessed or full-length hVEGF-D, which was used as the transgene in the hK14–hVEGF-D study,9 equally stimulates phosphorylation of both VEGFR-2 and VEGFR-3.6
In the present study we have produced TG mice by using a LV that, when integrated into the host cell genome, directly produces the hVEGF-DΔNΔC and does not increase the amount of full-length, unprocessed hVEGF-D. In addition, the difference in the VEGFR binding capabilities between human and mouse further explains the angiogenic phenotype of the TG mice. In mouse, both proteolytically processed and unprocessed mVEGF-D almost solely bind to mVEGFR-3, leading to enhanced lymphangiogenesis.6,7,10 In addition, it has been shown that the hVEGF-DΔNΔC in mouse binds to the angiogenic mVEGFR-2 and not the lymphangiogenic mVEGFR-3.10,17 These findings most probably explain the different phenotypes regarding the blood and lymphatic vasculature in the 2 studies, as well as offer a logical explanation for the absence of lymphangiogenesis in the present study.
The role of VEGF-D in vivo has also been studied by creating VEGF-D knockout (KO) mice.19 These mice showed a normal phenotype, including normal lymphatic vasculature, and the researchers thus concluded that either the mVEGF-D does not have a major role in lymphangiogenesis during the embryonic development or that it is compensated by VEGF-C or some other, yet unknown, VEGFR-3–activating agents.19 Because mVEGF-D is capable of binding only to mVEGFR-3, it is not surprising that these KO mice did not have an angiogenic phenotype.
In addition to increased capillary density and improved muscle regeneration after injury, the phenotype of hVEGF-D TG mice also included a tendency to form tumors. In addition, half of the tumors were primary mammary gland adenocarcinomas. In the present study, we observed hVEGF-D expression in the well-differentiated ductal part of the mammary adenocarcinomas, in lung papillary adenocarcinomas, and in skin anaplastic carcinomas, suggesting a pathogenetic role of hVEGF-D in tumorigenesis. However, blood and lymphatic vessel–derived malignancies, such as hemangioma, lymphangioma, angiosarcoma, and lymphangiosarcoma, were not found. There was no expression of CD31, CD34, or LYVE-1 outside the vasculature. Nevertheless, we observed intratumoral angiogenesis, but not lymphangiogenesis, in the samples of mammary adenocarcinomas and skin anaplastic carcinomas. The mouse strain used in this study (CD2F1) is not known for spontaneous tumor formation. However, LVs could be connected to tumorigenesis. Therefore, we compared the hVEGFD TG mice with other LV-produced TG mice, namely hPGK-GFP and hPGK-hNrf2. In these TG mice, no malignancies or early deaths have been recorded (data not shown).
In line with our findings, tumor angiogenesis has been shown to be induced by hypoxia, estrogens, VEGF-A, and VEGF-D in primary breast carcinomas.20,21 Because myoepithelia plays an important role in mammary gland tumorigenesis, one possible mechanistic explanation could be the overexpression of VEGF-D in these cells. In previous studies, the role of VEGF-D was seen mainly in the context of lymph node metastasis and intratumoral lymphangiogenesis.20,22 Nevertheless, in breast cancer cultures, an autocrine role of hVEGF-D and other members of the VEGF family has been implicated.22,23
In conclusion, the hVEGF-D TG mice showed a significantly increased angiogenesis in skeletal muscles and myocardium, and less muscle damage after hindlimb ischemic injury compared with the TG-negative controls. However, the incidence of tumor formation was significantly increased in the TG mice, with a preference for mammary gland adenocarcinoma formation.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the technical staff of the Department of Biotechnology and Molecular Medicine for their expert contribution to the study.
This work was supported by the Academy of Finland, Finnish Funding Agency for Technology and Innovation, Kuopio University Foundation, European Union (Lymphangiogenomics: ISLH-2007-00284/Ha-7), and Leducq Foundation/Fondation Leducq (06 CVD 04).
Authorship
Contribution: A.-M.K. designed, coordinated and performed research, analyzed data, and wrote the paper; A.K. performed histologic analyses, analyzed data, contributed to the animal work, and prepared the images; J.H. performed transgeneses; I.K. analyzed all tumor data and reported the results of the analyzed tumors; S.E.H. performed each hindlimb ischemia surgical operation; A.S. performed most of the additional immunohistochemistry; M.H.D. and E.H. contributed to molecular biology analyses; H.P. set up analysis for expression levels; P.I.M. originally cloned the LV backbone vector; M.P.T. performed ELISA assays; and S.Y.-H. contributed to conception and design of the research, critically revised the manuscript for important intellectual content, and supervised the research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Seppo Ylä-Herttuala, University of Kuopio, A.I.Virtanen Institute, Department of Biotechnology and Molecular Medicine, Neulaniementie 2, 70210 Kuopio, Finland; e-mail: seppo.ylaherttuala@uku.fi.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal