Abstract
We previously reported the inhibitory action of interleukin-6 (IL-6) on B lymphopoiesis with SHIP−/− mice and showed that IL-6 biases lineage commitment toward myeloid cell fates in vitro and in vivo. Because elevated IL-6 is a feature of chronic inflammatory diseases, we applied an animal model of systemic lupus erythematosus (SLE) to determine whether IL-6 has similar effects on hematopoiesis. We found that IL-6 levels were elevated in the B6.Sle1.Yaa mice, and the increase was accompanied by losses of CD19+ B cells and more primitive B-lymphoid progenitors in bone marrow. Both the CD19+ B-cell population and their progenitors recovered in an IL-6−/− background. The uncommitted progenitors, containing precursors for both lymphoid and myeloid fates, expressed IL-6 receptor-α chain and responded to IL-6 by phosphorylation of STAT3. IL-6 stimulation caused uncommitted progenitors to express the Id1 transcription factor, which is known to inhibit lymphopoiesis and elevate myelopoiesis, and its expression was MAPK dependent. We conclude that chronic inflammatory conditions accompanied by increased IL-6 production bias uncommitted progenitors to a myeloid fate by inducing Id1 expression.
Introduction
All hematopoietic cells develop from pluripotent hematopoietic stem cells (HSCs). Hematopoietic development proceeds in stages of decreasing lineage choices and decreasing capacity for self-renewal of the progeny. HSCs feature a lack of all lineage markers (Lin−) but express high levels of the receptor tyrosine kinase c-Kit (c-Kithi) and the surface protein Sca-1 (termed the LSK fraction). HSCs give rise to multipotent progenitors (MPPs), marked by a loss of self-renewal capacity, the ability to form either myeloid or lymphoid cells, and up-regulation of the Flk2/Flt3 receptor tyrosine kinase.1 The earliest lymphoid-biased progenitors (ELPs) represent a subset of MPPs that can be resolved from all other progenitors on the basis of recombination activating gene 1 expression.2 Lymphoid progenitors that develop from MPPs have reduced levels of c-Kit, display surface interleukin-7 receptor α (IL-7Rα), and have other properties that define a population called common lymphoid progenitors (CLPs).3
Hematopoiesis is normally a well-controlled system designed to replenish blood cells at a constant rate, such that the balance of lymphoid and myeloid cells is maintained. However, the rate of output of particular blood cell types can be altered by conditions such as leukemia, radiation, or acute inflammation.4-11 One condition termed “emergency hematopoiesis,”12,13 is characterized by elevated production of myeloid cells, occurring during acute infections or acute allergic responses. The mechanism by which hematopoiesis is altered to elevate production of myeloid cells is not known. The consequence of emergency hematopoiesis is to increase circulating monocytes, granulocytes, and neutrophils, presumably present to fight infection.
Interleukin-6 (IL-6) is a prominent cytokine produced during infectious disease (reviewed in Pritts et al14 ) and is needed in the systemic acute-phase reaction. Consistent with this, IL-6−/− mice are prone to numerous viral and bacterial diseases (reviewed in Bluethmann et al15 ) and fail to show accumulation of myeloid cells in inflamed organs, including lung,16 the coronary artery in atherosclerosis,17 and skin wounds.18 The loss of the Ship gene produces an emergency hematopoiesis phenotype similar to acute infection, whereby myeloid cell production is elevated at the expense of lymphoid cell production.19,20 Our earlier work established that the changes in hematopoiesis of SHIP−/− mice are caused by IL-6 because the increased myeloid output from uncommitted progenitors in this model was blocked by neutralizing antibodies to IL-6,20 and CD19+ B lymphopoiesis was restored in SHIP−/−IL-6−/− double-deficient mice.19 Remarkably, IL-6 is able to support emergency granulopoiesis in animals that lack granulocyte colony-stimulating factor and granulocyte-macrophage colony stimulating factor,21 2 critical cytokines necessary for myelopoiesis.22 These findings indicate that IL-6 plays a key role in emergency granulopoiesis that accompanies acute infections.
Similar to acute infectious diseases, chronic inflammatory disorders show an elevation in myeloid cells that cause pathology by release of digestive and degradative enzymes.23-27 Removal of myeloid cells by various strategies can improve the clinical outcome in several autoimmune diseases.28,29 However, the mechanism by which myeloid cell numbers increase during chronic inflammatory diseases is not understood.
We have studied the development of lymphoid and myeloid cells with the use of the autoimmune-prone B6.Sle1.Yaa model of systemic lupus erythematosus (SLE). The Sle1 region, derived from chromosome 1 of NZM2410 lupus-prone mice, contains at least 3 lupus susceptibility genes30 and is associated with loss of tolerance toward nuclear antigens.31 The Y autoimmune accelerator gene (Yaa gene) is a single locus derived from the Y chromosome of BXSB lupus-prone mice32 that can accelerate disease in combination with several other lupus susceptibility loci, including Sle1.33 The Yaa gene was recently shown to be a translocation of a portion of the X chromosome that includes Toll-like receptor 7 (TLR7).33,34 The translocation causes TLR7 to be overexpressed in males having the gene. Animals with both the Sle1 region and Yaa translocation on the C57Bl/6 background (B6.Sle1.Yaa mice) have a highly penetrant SLE with approximately 60% rate of mortality at 9 months of age.30,35
We discovered the inhibitory action of IL-6 on B lymphopoiesis with SHIP−/− mice.19,20 We found that IL-6 biased the lineage choice of progenitors having both lymphoid and myeloid potential such that the myeloid lineage fates were the only available option. The precise progenitor cell population affected by IL-6 was not clear from these experiments, but it was contained within the LSK population of very primitive progenitors. Indeed, our studies showed that the IL-6 target was a cell type more primitive than the ELPs. On the basis of this work, we propose that IL-6 is one mediator of emergency hematopoiesis that serves to elevate myeloid production at the expense of lymphoid production in the setting of chronic inflammation that accompanies autoimmunity.
To test this hypothesis, we examined IL-6 levels and hematopoietic output in the B6.Sle1.Yaa autoimmune-prone strain. We report that, like SHIP−/− animals, B6.Sle1.Yaa mice have high circulating levels of IL-6 that coincide with development of severe SLE. IL-6 production was accompanied by a loss in CD19+ B cells and a more primitive B-lymphoid progenitor, the CLPs, in bone marrow. Both the CLPs and CD19+ cells ware normal in B6.Sle1.Yaa mice with the IL-6−/− background. We also found that the uncommitted progenitors expressed IL-6 receptor α (IL-6Rα) and responded to IL-6 by phosphorylation of STAT3. Furthermore, IL-6 may bias uncommitted progenitors toward a myeloid fate by inducing Id1 expression. This phenomenon partially depends on Erk, one of the MAP kinase modules. We conclude that IL-6 is a major mediator of emergency hematopoiesis in this autoimmune disease model, in part because it can alter lineage choices among very primitive cells within bone marrow.
Methods
Mice
B6.Sle1 mice were obtained from Dr Edward Wakeland (University of Texas, Southwestern Medical Center, Dallas, TX). B6.Yaa mice and IL-6−/− mice on the C57Bl/6 background were purchased from The Jackson Laboratory (Bar Harbor, ME). B6.Sle1.Yaa and B6.Sle.1.Yaa.IL-6−/− mice were obtained by interbreeding. The mice were analyzed at 20 to 26 weeks of age unless otherwise indicated. The boundaries of the Sle1 interval were defined by NZW alleles at the D1mit17 and D1mit47 loci.36 Genotyping for IL-6 was conducted with the following primers: P1 (common, sense): CCA, CAT, CCA, GTT, GCC, TTC, TTG, G; P2 (wild-type, antisense): TTC, TCA, TTT, CCA, CGA, TTT, CCC, AG; Neo (mutant, antisense): CCG, GAG, AAC, CTG, CGT, GCA, ATC, C. B6.Id1GFP/GFP mice were maintained as described.37 All studies were approved by the institutional animal care and use committee of the Oklahoma Medical Research Foundation.
Antibodies and reagents
Anti-CD3 (145-2C11), anti–Mac-1/CD11b (M1/70), anti–CD16/32 (2.4G2), anti-B220/CD45R (RA3/6B2), anti-Gr1/Ly6G (RB6-8C5), and anti–TER-119/Ly76 were used as purified antibodies. Fluorochrome- or biotin-conjugated antibodies (anti-CD3, anti-CD4 [GK1.5], anti-CD8 [53-6.7], anti-CD19 [1D3], anti–CD127/IL-7Rα [SB/199], anti-B220, anti–Mac-1, anti–Sca-1/Ly-6A/E [D7], anti–Kit/CD117 [2B8], anti-Gr1, anti-Flt3/CD135 [A2F10], anti-NK1.1 [PK136], and anti–TER-119) were purchased from BD PharMingen (San Diego, CA). Phycoerythrin–Cy5.5 and APC Alexa Fluor 750–conjugated streptavidin was purchased from Invitrogen (Carlsbad, CA). Phycoerythrin anti–IL-6Rα/CD126 and anti–phospho-Stat3 (PY705) were purchased from BD Biosciences (San Jose, CA). Dead cells were eliminated by staining with 7-amino-actinomycin D (BD Biosciences) in the final step. Recombinant mouse IL-6, IL-7, stem cell factor (SCF), and Flt3 ligand were purchased from R&D Systems (Minneapolis, MN).
Flow cytometry for surface antigen and intracellular staining
Staining for surface antigen was as described before.19,20 Briefly, flushed bone marrow cells were lineage depleted by incubating with anti–Mac-1, anti-B220, anti-CD3, anti-Gr1, and anti–TER-119 followed by goat anti–rat IgG magnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany). Cells were stained with fluorochrome-conjugated antibodies to Sca-1, c-Kit, and a mixture of lineage markers (NK1.1, CD3, CD8, CD19, B220, Mac-1, Gr1, TER-119) and Flt3. Stained cells were sorted with the MoFlo cell sorter (Dako, Fort Collins, CO). For intracellular phospho-STAT3 measurements, the sorted cells were stimulated with mouse recombinant IL-6 (100 ng/mL), fixed, permeabilized, and stained with phycoerythrin anti–phospho-Stat3. Stained cells were analyzed by LSR-II (BD Biosciences, San Jose, CA). Collected data were analyzed with FlowJo software (TreeStar, Ashland, OR) or FACSDiva software (BD Biosciences).
Stromal cell–free, serum-free culture
Stromal cell–free, serum-free culture of progenitor cells was described previously.19,38 Sorted cells were cultured at 5000 cells/well with X-VIVO 15 medium (Lonza, Walkersville, MD) containing 1% detoxified BSA (StemCell Technologies, Vancouver, BC), 50 μM mercaptoethanol, 2 mM l-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, 2 ng/mL IL-7, 100 ng/mL Flt3 ligand, and 20 ng/mL SCF, with or without IL-6 (10 ng/mL). For inhibitor treatment, U0126 (10 μM; Calbiochem, Dermstad, Germany) was used along with DMSO.
Enzyme-linked immunoabsorbent assay
Serum IL-6 levels were determined in mouse serum samples by enzyme-linked immunoabsorbent assay (ELISA) kit from eBioscience (San Diego, CA) according to the manufacturer's protocol.
Real-time polymerase chain reaction
Sorted LSK cells were cultured in the stromal cell-free, serum-free culture with or without IL-6 and U0126. RNA was isolated used by RNeasy Mini Kit (QIAGEN, Valencia, CA), and the cDNA was prepared by using the RT2 First Strand Kit according to the manufacturer's protocol (SABiosciences, Frederick, MD). The relative Id1 mRNA level was analyzed by real-time polymerase chain reaction (PCR) with SYBR Green/ROX qPCR Master Mix Kit (SABiosciences) in the ABI PRISM 7500 (Applied Biosystems, Foster City, CA). Primers for Id1 and 18S rRNA were purchased from SABiosciences, and PCR cycles were performed according to the manufacturer's instructions. mRNA level of Id1 was determined by the comparative Ct method relative to the housekeeping gene 18S rRNA.
Results
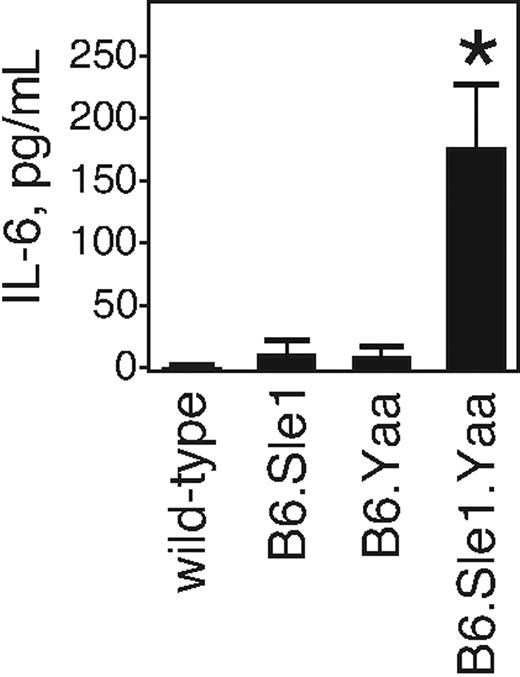
C57Bl/6 mice congenic for the Sle1 locus and the Yaa gene develop SLE with high penetrance.30,35 Because this is a chronic autoimmune disease, and becauseIL-6 accompanies many such diseases, we tested whether IL-6 levels were elevated in animals having this genetic combination. We collected serum samples from 20- to 26-week-old wild-type C57Bl/6 mice, mice with only one genetic locus (Sle1 or Yaa), or those having both loci. Sera were tested for IL-6 levels by a capture ELISA. We found that wild-type mice had no detectable IL-6 (Figure 1). Animals with only a single lupus-conferring locus (Sle1 or Yaa) had low but detectable IL-6 levels. However, animals with both loci did not show detectable IL-6 at 2 months of age. IL-6 at levels higher than wild-type became detectable by 5 months and continued to rise until reaching approximately 200 pg/mL IL-6 in their sera at approximately 6 months of age. This level is approximately the same as we find in SHIP-deficient mice (K.M. and K.M.C., unpublished observations, June 2008). Thus, animals having the B6.Sle1.Yaa genetic combination display elevated serum IL-6.
Serum IL-6 levels are elevated in B6.Sle1.Yaa mice. The serum IL-6 levels of wild-type, B6.Sle1, B6.Yaa, and B6.Sle1.Yaa mice at the ages of 20 to 26 weeks are shown. The serum was obtained by cardiac puncture, and the levels of IL-6 were determined by ELISA. Results from 5 mice from each animal strain are shown as picograms per milliliter (mean ± SD). *P < .006 compared with the wild-type, B6.Sle1, and B6.Yaa mice.
Serum IL-6 levels are elevated in B6.Sle1.Yaa mice. The serum IL-6 levels of wild-type, B6.Sle1, B6.Yaa, and B6.Sle1.Yaa mice at the ages of 20 to 26 weeks are shown. The serum was obtained by cardiac puncture, and the levels of IL-6 were determined by ELISA. Results from 5 mice from each animal strain are shown as picograms per milliliter (mean ± SD). *P < .006 compared with the wild-type, B6.Sle1, and B6.Yaa mice.
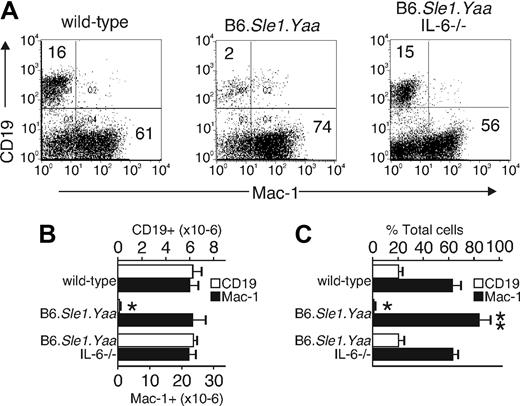
Because IL-6 levels are increased in this model similar to SHIP−/− mice, we explored changes in CD19-expressing B cells and Mac-1–expressing myeloid cells. With the use of 20- to 26-week-old animals, we found that 16% of total bone marrow cells in wild-type animals expressed CD19, whereas 61% expressed Mac-1 (Figure 2).19,20 The total number of bone marrow cells in each bone was decreased by 20% in B6.Sle1.Yaa mice relative to that of wild-type mice. Therefore, the total number of Mac-1–expressing cells per bone in the B6.Sle1.Yaa was approximately equal to the total number in wild type. In contrast, the B6.Sle1.Yaa mice had a near-complete loss of B-lymphoid cells. The loss of development of B cells correlated with the age-dependent increase of IL-6, such that no differences were observed before 4 months, and the hematopoietic developmental differences were concomitant with the increase in IL-6. These features are similar to those in SHIP-deficient mice, whereby IL-6 is likewise elevated and is causally related to the loss in B-lymphoid cells.19,20 To test the relation between IL-6 and B-cell development, we mated in the lupus-prone B6.Sle1.Yaa mice with IL-6−/− mice to generate B6.Sle1.Yaa mice having or lacking the IL-6 gene. When bone marrow of these strains was evaluated for levels of CD19- and Mac-1–expressing cells, we found that B-lymphoid and myeloid cells were normal when IL-6 was deficient (Figure 2). These data indicate that, like SHIP-deficient animals, the increased IL-6 levels in B6.Sle1.Yaa mice caused a block in B lymphopoiesis.
Elevated IL-6 levels inhibit B lymphopoiesis and increase myelopoiesis in B6.Sle1.Yaa mice. Bone marrow cells from wild-type, B6.Sle1.Yaa, and B6.Sle1.Yaa.IL-6−/− mice were analyzed by flow cytometry. (A) B lymphocytes and myeloid cells from the bone marrow of each animal strain were detected with anti-CD19 and anti–Mac-1 antibodies. The results are representative of 5 separate animals. The percentage of cells in the quadrants is indicated. Numbers in the top left corner show the percentage of CD19+ cells in total bone marrow cells; numbers in the bottom right show Mac-1+ cells in total bone marrow cells. (B,C) The cell numbers and percentages of CD19+ B lymphocytes and Mac-1+ myeloid cells in each animal strain are shown. The data were obtained from 5 mice per group and are shown as an average (mean ± SD). Cell numbers are shown as the number from one femur and one tibia. *P < .001, **P < .05 compared with the wild-type, B6.Sle1.Yaa.IL-6−/− mice.
Elevated IL-6 levels inhibit B lymphopoiesis and increase myelopoiesis in B6.Sle1.Yaa mice. Bone marrow cells from wild-type, B6.Sle1.Yaa, and B6.Sle1.Yaa.IL-6−/− mice were analyzed by flow cytometry. (A) B lymphocytes and myeloid cells from the bone marrow of each animal strain were detected with anti-CD19 and anti–Mac-1 antibodies. The results are representative of 5 separate animals. The percentage of cells in the quadrants is indicated. Numbers in the top left corner show the percentage of CD19+ cells in total bone marrow cells; numbers in the bottom right show Mac-1+ cells in total bone marrow cells. (B,C) The cell numbers and percentages of CD19+ B lymphocytes and Mac-1+ myeloid cells in each animal strain are shown. The data were obtained from 5 mice per group and are shown as an average (mean ± SD). Cell numbers are shown as the number from one femur and one tibia. *P < .001, **P < .05 compared with the wild-type, B6.Sle1.Yaa.IL-6−/− mice.
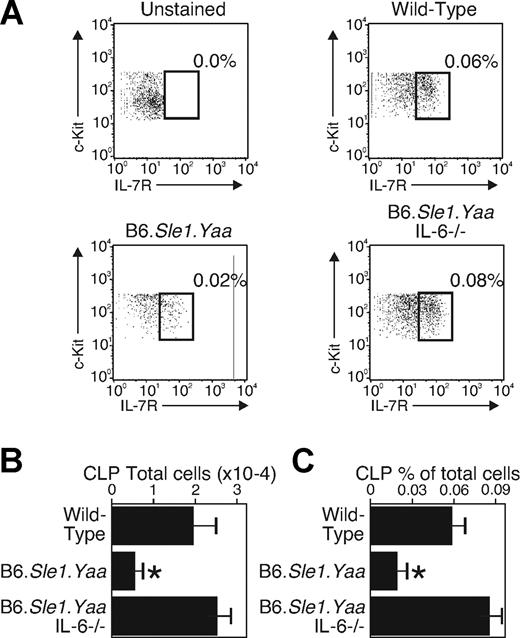
Our earlier work showed that mice with high IL-6 levels lacked CLPs and ELPs, the earliest progenitors committed to lymphoid products. We interpreted these findings to indicate that the IL-6 targets a bone marrow progenitor more primitive than ELPs, directing them toward myelopoiesis. To more precisely identify the IL-6 target population in B6.Sle1.Yaa strain mice marrow, cells were stained with antibodies to lineage markers (CD3, CD8, Mac-1, Gr-1, B220, CD19, Ter119, NK1.1) to exclude maturing cells from the analysis. These lineage-negative cells were electronically gated and analyzed for levels of c-Kit, Flt3, and IL-7Rα by staining with antibodies to these proteins. We did not include the Sca-1 marker because earlier studies established that Sca-1 expression is elevated on progenitors on their exposure to proinflammatory conditions.39 Of the lineage-negative cells, we gated c-Kitlo, Flt3+ cells and assessed levels of IL-7Rα. Previous work has characterized CLPs in this IL-7Rα+ fraction.3 With the use of this gating strategy, we found that the percentages and the total numbers of IL-7Rα–expressing CLPs were reduced by two-thirds in the B6.Sle1.Yaa bone marrow (Figure 3). These data are consistent with our earlier work showing an early and primitive progenitor as the target for IL-6.19,20 Furthermore, the IL-7Rα–expressing population was completely normal when the lupus-prone B6.Sle1.Yaa animals had an IL-6–deficient background. Thus, like in SHIP−/− mice, the IL-6 target cells are primitive and uncommitted progenitors that arise before the CLPs. Furthermore, the defect in B lymphopoiesis in the lupus-prone animals is entirely due to the elevation in IL-6, as is also the case in SHIP−/− mice.19
Progenitors that arise before CLPs are the IL-6 targets. Bone marrow cells from wild-type, B6.Sle1.Yaa, and B6.Sle1.Yaa.IL-6−/− mice were selected as Lin− (NK1.1, CD3, CD8, CD19, B220, Mac-1, Gr1, TER-119), c-Kitlo, and Flt3+. The selected population was divided according to surface expression of IL-7Rα. IL-7Rα+ cells were considered as CLPs. (A) CLPs from the bone marrow of each strain are shown; unstained shows the cells not stained with anti–IL-7Rα. The results are representative 5 separate animals. The percentage of total cells meeting CLP criteria is indicated. The squares show IL-7+ CLP cells; the numbers next to them show the percentage of CLPs in total bone marrow. (B,C) Cell numbers and percentages of CLPs in each strain are shown. The data were obtained from 5 mice and are shown as and average (mean ± SD). Cell numbers are shown as number from one femur and one tibia. *P < .001 compared with the wild-type and B6.Sle1.Yaa.IL-6−/− mice.
Progenitors that arise before CLPs are the IL-6 targets. Bone marrow cells from wild-type, B6.Sle1.Yaa, and B6.Sle1.Yaa.IL-6−/− mice were selected as Lin− (NK1.1, CD3, CD8, CD19, B220, Mac-1, Gr1, TER-119), c-Kitlo, and Flt3+. The selected population was divided according to surface expression of IL-7Rα. IL-7Rα+ cells were considered as CLPs. (A) CLPs from the bone marrow of each strain are shown; unstained shows the cells not stained with anti–IL-7Rα. The results are representative 5 separate animals. The percentage of total cells meeting CLP criteria is indicated. The squares show IL-7+ CLP cells; the numbers next to them show the percentage of CLPs in total bone marrow. (B,C) Cell numbers and percentages of CLPs in each strain are shown. The data were obtained from 5 mice and are shown as and average (mean ± SD). Cell numbers are shown as number from one femur and one tibia. *P < .001 compared with the wild-type and B6.Sle1.Yaa.IL-6−/− mice.
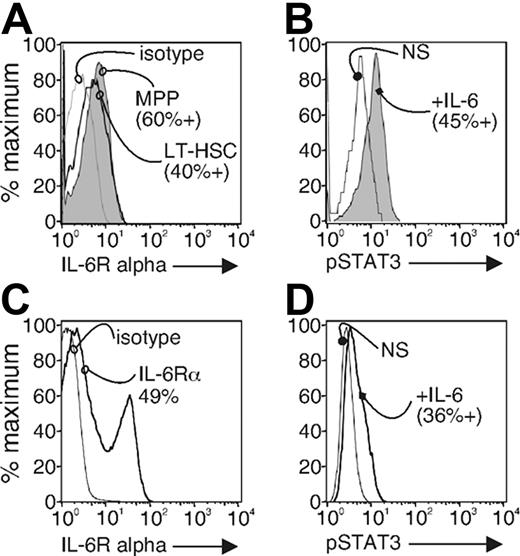
We do not know what cells within the LSK fraction are targeted by IL-6. We hypothesize that the IL-6 target is a primitive progenitor because it has both lymphoid and myeloid potential.19 The most primitive cells in hematopoiesis are present in the LSK population. This fraction contains long- and short-term hematopoietic stem cells, multipotent progenitors, and early lymphoid-committed progenitors among other cells. Our findings predict that the population affected by the proinflammatory conditions and altering its lineage choices should expresses IL-6 receptors and respond directly to exogenous IL-6. Alternatively, it is possible that stromal and other IL-6R+ cells influence hematopoiesis indirectly. It is not known whether IL-6Rα is expressed within the complex mixture of cells of the LSK population of hematopoietic progenitors. To test IL-6Rα expression in the target population itself, we stained total bone marrow cells from wild-type mice with lineage markers as above as well as with antibodies to the c-Kit, Sca-1, and Flt3 receptor. We sorted the LSK fraction and stained them with antibodies to IL-6Rα (Figure 4A). Splenocytes were used for positive control for IL-6Rα staining (Figure 4C). We measured IL-6Rα expression on the MPPs, defined by cells expressing the Flt3, and long-term (LT)–HSCs defined by cells that lack Flt3 expression.40 We found that IL-6Rα was present on 60% of the Flt3+ MPPs and short-term HSC fraction of the LSK and by 40% of the Flt3− long-term HSCs fraction (Figure 4A). These data indicate that IL-6Rα expression might be a useful parameter for resolving cell in this LSK population, and some of them could be direct cytokine targets in autoimmune mice.
The LSK fraction expresses IL-6 receptor and responds to IL-6 by phosphorylation of STAT-3. (A) Sorted LSK fraction from wild-type bone marrow cells was stained with anti–IL-6Rα or isotype control antibody. LT-HSC and MPP populations were resolved by expression level of Flt3. Stained cells were analyzed by flow cytometry. The results are representative of 3 separate experiments. Splenocyte cells were used as a positive control for IL-6Rα staining (C). (B) The sorted LSK fraction from wild-type bone marrow cells were left unstimulated (NS) or were stimulated with IL-6 (100 ng/mL) for 15 minutes (+IL-6). The cells were fixed, permeabilized, and stained with anti–phospho-STAT3. Stained cells were analyzed by flow cytometry. The results are representative of 3 separate experiments. Splenocyte cells were used as a positive control for phospho-STAT3 staining (D).
The LSK fraction expresses IL-6 receptor and responds to IL-6 by phosphorylation of STAT-3. (A) Sorted LSK fraction from wild-type bone marrow cells was stained with anti–IL-6Rα or isotype control antibody. LT-HSC and MPP populations were resolved by expression level of Flt3. Stained cells were analyzed by flow cytometry. The results are representative of 3 separate experiments. Splenocyte cells were used as a positive control for IL-6Rα staining (C). (B) The sorted LSK fraction from wild-type bone marrow cells were left unstimulated (NS) or were stimulated with IL-6 (100 ng/mL) for 15 minutes (+IL-6). The cells were fixed, permeabilized, and stained with anti–phospho-STAT3. Stained cells were analyzed by flow cytometry. The results are representative of 3 separate experiments. Splenocyte cells were used as a positive control for phospho-STAT3 staining (D).
To test whether any LSK are able to respond in this way to IL-6, we sorted and exposed them to 100 ng/mL IL-6. The cells were fixed, permeabilized with methanol, and stained with antibodies to tyrosine 705–phosphorylated STAT3, a downstream signaling protein of the IL-6 receptor. Tyrosine-705 site of STAT3 is phosphorylated in IL-6–stimulated cells and is necessary for STAT dimerization (reviewed in Levy and Darnell41 ). Splenocytes were used for positive control for phosphorylated STAT3 staining (Figure 4D). We found that at least 45% of the IL-6–stimulated LSK fraction responded to exogenous IL-6 by phosphorylation of STAT3 (Figure 4B). This percentage is similar to the LSK fraction that expresses the IL-6Rα. Thus, at least subsets of MPPs and HSCs fractions express IL-6Rα and are able to directly respond to IL-6. The findings are consistent with our hypothesis that the cell affected by IL-6 is a primitive progenitor with multiple lineage potentials.
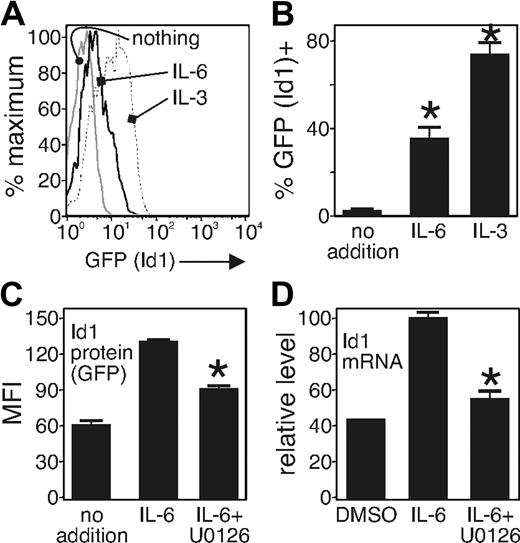
Earlier studies showed that expression of the inhibitory Id transcription factors block E2A function, which is needed for lymphopoiesis.42 Furthermore, Id1 is up-regulated by C/EBPb,43 a transcription factor activated by IL-6.44 Thus, one possibility is that IL-6 stimulates the activity of C/EBPb to up-regulate Id1 expression and block E2A function. To test the notion that IL-6 induces Id1 expression, we used Id1/GFP knockin mice in which a segment encoding GFP has been used to replace one allele of the Id1 gene.37 For these experiments, we applied an in vitro culture system that supports lymphopoiesis,38 and one in which the developmental block caused by IL-6 is apparent.19,20 We incubated sorted and purified LSK cells from Id-1/GFP knockin mice in serum-free, stromal-free culture conditions38 with (10 ng/mL) or without IL-6. IL-3 was used for positive control becauseIL-3 is a potent inducer of Id-1 expression,45 and it has an important role in producing basophils and mast cells during parasitic infections.46 We found that at least 30% of cells cultured with IL-6 showed Id1/GFP expression after 24 hours, whereas there was little Id-1/GFP expression in cells in the absence of IL-6 (Figure 5A,B). We also investigated the signal transduction pathway by which Id1 expression is induced by IL-6 in the LSK population. We found that the MAP kinase inhibitor U0126 (10 μM) blocked 50% of Id1/GFP expression induced by IL-6 (Figure 5C). We confirmed the observation that Id1 protein expression was increased in cells exposed to IL-6 by applying a real-time PCR measurement of Id1 mRNA in the same cell population from wild-type mice. For these experiments, we cultured sorted LSK population from wild-type mice and cultured the purified cells in the same media as above and treated IL-6 and/or U0126 for 12 hours. After stimulation, we collected RNA from each sample and analyzed gene expression by real-time PCR. We found that the Id1 mRNA was decreased when the cells were incubated with U0126 and IL-6 (Figure 5D), relative to the Id1 mRNA in the IL-6–only cultures. These findings confirm our hypothesis that IL-6 does indeed increase expression of Id1 in the LSK population, and its expression is partially MAPK dependent.
IL-6 induces MAP kinase–dependent Id1 expression in primitive progenitors. Sorted LSK fraction from Id1GFP/GFP mice was cultured in serum-free, stromal cell–free conditions as described19,38 and with added carrier (media) or IL-6 or IL-3 as indicated. Id1 expression was analyzed by GFP expression level using flow cytometry. (A) Id1 expression is shown 24 hours after treatment with or without IL-6 (10 ng/mL) or IL-3 (10 ng/mL). The results are representative of 3 separate experiments. (B) The relative ratio of GFP+ to GFP− cells is shown. *P < .005 compared with the control. (C) Sorted cells were placed in serum-free stroma cell–free system with IL-6 10 ng/mL and treated with carrier (DMSO) or MAPK inhibitor (U0126) 10 μM. Id1 expression was determined by flow cytometry after 24 hours of culture. The data were obtained from 3 different experiments and are shown as an average (mean ± SD). *P < .005 compared with the control. (D) The relative expression of Id1 mRNA is shown. The purified LSK cells were cultured in serum-free, stroma-cell free system with IL-6 (10 ng/mL) and treated with control (DMSO) or MAPK inhibitor (U0126) 10 μM, as in panel C. The total RNA was extracted from each sample after 12 hours of incubation, and real-time PCR was conducted. Id1 expression was normalized by 18S rRNA expression.
IL-6 induces MAP kinase–dependent Id1 expression in primitive progenitors. Sorted LSK fraction from Id1GFP/GFP mice was cultured in serum-free, stromal cell–free conditions as described19,38 and with added carrier (media) or IL-6 or IL-3 as indicated. Id1 expression was analyzed by GFP expression level using flow cytometry. (A) Id1 expression is shown 24 hours after treatment with or without IL-6 (10 ng/mL) or IL-3 (10 ng/mL). The results are representative of 3 separate experiments. (B) The relative ratio of GFP+ to GFP− cells is shown. *P < .005 compared with the control. (C) Sorted cells were placed in serum-free stroma cell–free system with IL-6 10 ng/mL and treated with carrier (DMSO) or MAPK inhibitor (U0126) 10 μM. Id1 expression was determined by flow cytometry after 24 hours of culture. The data were obtained from 3 different experiments and are shown as an average (mean ± SD). *P < .005 compared with the control. (D) The relative expression of Id1 mRNA is shown. The purified LSK cells were cultured in serum-free, stroma-cell free system with IL-6 (10 ng/mL) and treated with control (DMSO) or MAPK inhibitor (U0126) 10 μM, as in panel C. The total RNA was extracted from each sample after 12 hours of incubation, and real-time PCR was conducted. Id1 expression was normalized by 18S rRNA expression.
Discussion
Chronic autoimmune diseases are associated with increased numbers of activated macrophages and neutrophils in inflamed tissues. The mechanism by which these increases come about is not clear, but similar features of peripheral myeloid cell production occur during emergency hematopoiesis. We hypothesized that such changes might at least in part be due to elevated production of IL-6, which alters hematopoiesis such that the lymphoid option is closed to primitive progenitors.
To test this possibility, we examined chronic autoimmune disease–prone B6.Sle1.Yaa mice that develop a form of SLE. We found that, like animals lacking the inositol phosphatase SHIP, serum IL-6 levels were increased, bone marrow production of CD19+ lymphoid cells was decreased, and the percentage of cells developing along the myeloid lineage was increased. The target of IL-6 in B6.Sle1.Yaa mice was an early progenitor, present within the LSK fraction. Subsets of cells within the LSK fraction expressed the IL-6 receptor and responded to IL-6 by increased phosphorylation of STAT3. Deletion of the IL-6 gene in the SLE-prone animals allowed production of CD19+ cells and CLPs. We also found that IL-6 increased expression of Id1, a transcription factor known to suppress lymphopoiesis in HSCs.45 The Id1 expression caused by IL-6 depended at least in part on the MAP kinase Erk, because Erk inhibition reduced the expression of Id1 protein and mRNA. We conclude that increased production of proinflammatory cytokines such as IL-6 during chronic autoimmunity or acute infectious disease can suppress B-cell production from bone marrow by causing Id1 expression in primitive progenitors. The increased Id1 expression biases the progenitor output toward myeloid pathways.
Although an increase of IL-6 level accompanies many proinflammatory diseases, the effect of IL-6 on hematopoiesis has not been studied. Nonetheless, a decrease of peripheral lymphoid cells is observed in many proinflammatory diseases, including SLE.47 The mechanism by which IL-6 increases in these proinflammatory diseases is not well understood. Although IL-6 can be produced by several kinds of cells, peritoneal macrophages appear to be a particularly rich source. Peritoneal macrophages of NZBxNZW F1, a murine SLE model, were shown to be a main source of IL-6 that accompanies the onset of lupus and may be responsible for pathogenic IgG production.48 The trigger for IL-6 production could be an immune complex consisting of autoimmune antibodies produced by B cells and possibly exacerbated by increased IL-6, because IL-6 supports IgG production from B cells.49
In emergency hematopoiesis, infection results in the immediate increase of myeloid-derived cells from the bone marrow.50 However, the developmental decrease of CLPs we show here and earlier19,20 is distinct from CLP mobilization caused by TNFα51 because we have shown that IL-6 causes a dramatic loss of these progenitors. A developmental reprogramming strategy may be a more efficient and long-lasting means to elevate myeloid cell production than mobilization, but it might be associated with more pathology to the host because it increases production of the effector cells.
The increased expression of Id1 mRNA and protein is particularly interesting and offers a mechanism for the effects of IL-6 on hematopoiesis. Id transcription family members (Id1-4) bind E-box transcription family members such as E2A but prevent their ability to activate transcription.42 Because E2A is required for B lymphopoiesis,52 Id1 transgenic animals fail to develop B cells,53 similar to the phenotype we describe for B6.Sle1.Yaa in this report. Id1 expression is induced by C/EBPb,43 a transcription factor whose activity is stimulated by IL-644 and is required for emergency hematopoiesis.54 Id1 and Id2 are induced in progenitor cells when incubated with cytokines that stimulate a myeloid fate.55 Other than Id-1 and C/EBPb, PU.1 is also involved in myelopoiesis induced by IL-6.56 We measured PU.1 levels by PCR but found that the changes paralleled the changes in hematopoiesis (Koji Nakamura and K.M.C., unpublished data, September 2004). Thus, we think that increased PU.1 expression is a result, not a cause, of the changes in hematopoiesis caused by IL-6. The IL-6–induced Id1 expression that occurs in chronic inflammation represents a potential cause of the IL-6–induced changes in hematopoiesis.
Only half of the cells within the LSK fraction of uncommitted progenitors expressed IL-6 receptors and were able to respond to IL-6. Nevertheless, the developmental bias toward myelopoiesis is more complete such that production of CD19+ cells in bone marrow is nearly eliminated when IL-6 is high. It may be that the IL-6 receptor is developmentally regulated, and all cells within the LSK fraction must at least briefly express the IL-6 receptor. Passing through this stage would render all developing progenitors sensitive to the suppressive effects of IL-6. Alternatively, IL-6 can signal to cells that lack the IL-6 receptor by dimerizing with soluble IL-6 receptor through the ubiquitous gp130 signaling subunit (reviewed in Knupfer and Preiss57 ).
Anti–IL-6R mAb treatment has been successfully applied to several autoimmune diseases, including Castleman disease58 Crohn disease,59 and rheumatoid arthritis60 ; moreover SLE is now being considered.61 In animal models of rheumatoid arthritis, anti–IL-6R antibodies reduced numbers of neutrophils and other cells of the myeloid lineage in inflamed joints.62 Similarly, the humanized version of anti–IL-6R reduced cartilage turnover and decreased CRP levels in patients with juvenile rheumatoid arthritis63 and in adults with active arthritis.64 It is remarkable that elevated IL-6 occurring as a consequence of overexpression of TLR7 and the presence of the Sle1 locus sets off a sequelae of events that target developing progenitors, biasing their output away from lymphoid cell products to favor myeloid cell products. The elevations in myeloid cell output caused by closing the lymphoid option almost certainly contributes to the pathology in autoimmune disease because these cells secrete degradative enzymes that damage the tissues harboring the activated myeloid cells.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Paul Kincade for critical reading of the manuscript. We also thank Christina Lowrence and the flow cytometry facility at the Oklahoma Medical Research Foundation for technical support.
Authorship
Contribution: K.M. performed and helped design all the experiments and analyzed all the data; A.M. assisted with the flow cytometry, serum cytokine measurements, and animal caretaking; B.N.T.-W. performed the preliminary work on B6.Sle1.Yaa, including flow cytometry analysis of marrow and serum cytokine measurements; X.-H.S. contributed the B6.Id1GFP animals and critically read the manuscript; A.D.F. contributed the B6.Sle1.Yaa animals, analyzed the data, and critically read the manuscript; and K.M.C. designed all the experiments, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: K. Mark Coggeshall, Oklahoma Medical Research Foundation, Program in Immunobiology and Cancer, 825 NE 13th St, Oklahoma City, OK 73104; e-mail: mark-coggeshall@omrf.ouhsc.edu.