Abstract
Chronic myelogenous leukemia (CML) is a malignant myeloproliferative disease arising from a hematopoietic stem cell expressing the BCR/ABL fusion protein. Leukemic and dendritic cells (DCs) develop from the same transformed hematopoietic progenitors. How BCR/ABL interferes with the immunoregulatory function of DCs in vivo is unknown. We analyzed the function of BCR/ABL-expressing DCs in a retroviral-induced murine CML model using the glycoprotein of lymphocytic choriomeningitis virus as a model leukemia antigen. BCR/ABL-expressing DCs were found in bone marrow, thymus, spleen, lymph nodes, and blood of CML mice. They were characterized by a low maturation status and induced only limited expansion of naive and memory cytotoxic T lymphocytes (CTLs). In addition, immunization with in vitro–generated BCR/ABL-expressing DCs induced lower frequencies of specific CTLs than immunization with control DCs. BCR/ABL-expressing DCs preferentially homed to the thymus, whereas only few BCR/ABL-expressing DCs reached the spleen. Our results indicate that BCR/ABL-expressing DCs do not efficiently induce CML-specific T-cell responses resulting from low DC maturation and impaired homing to secondary lymphoid organs. In addition, BCR/ABL-expressing DCs in the thymus may contribute to CML-specific tolerance induction of specific CTLs.
Introduction
Chronic myelogenous leukemia (CML) is a malignant clonal myeloproliferative disease. The BCR/ABL fusion protein results from the reciprocal chromosomal translocation t(9;22) forming the Philadelphia chromosome (Ph). BCR/ABL is responsible for the malignant phenotype of leukemic cells in CML and increases cell proliferation, inhibits apoptotic processes, and alters cellular adhesion of myeloid cells.1,2 CML is characterized by an initial chronic phase with a massive expansion of all stages of the granulocyte cell lineage. Eventually, hematopoietic differentiation becomes arrested and CML progresses to blast crisis with immature blast cells accumulating in the periphery.1
Several studies have shown that cytotoxic T lymphocytes (CTLs) are involved in the immunosurveillance of CML. BCR/ABL is a leukemia-specific antigen, and CTLs specific for peptides derived from its sequence could recognize CML cells in vitro and in vivo.3,4 This suggests that there is efficient intracellular processing and presentation of BCR/ABL-derived peptides by CML cells. In addition, overexpressed self-proteins, such as proteinase-3, Wilms tumor 1 protein, and minor histocompatibility antigens, can act as leukemia-specific antigens for T cells.5
Dendritic cells (DCs) are professional antigen-presenting cells and are key mediators for the initiation and regulation of both innate and adaptive immune responses.6 DCs are a heterogeneous population that can be divided into myeloid and plasmacytoid DCs based on their origin, expression of surface markers, and function.7 As CML mainly affects cells of the myeloid lineage, it is probable that myeloid BCR/ABL-expressing DCs are circulating in CML patients. Indeed, BCR/ABL-expressing DCs could be detected in the peripheral blood of CML patients.7,8 However, CML patients in chronic phase had reduced numbers of circulating myeloid and plasmacytoid DCs compared with healthy persons.9,10 Contradictory data regarding the maturation status and function of BCR/ABL-expressing DCs have been published. BCR/ABL-expressing DCs had either a normal maturation status or lower expression of the costimulatory molecules CD80/CD83/CD40 compared with control DCs.7,8,11,12 In acute myeloid leukemia, the plasmacytoid DCs were immature and could not elicit the proliferation of naive CD4+ T cells.13 Moreover, in vitro–generated BCR/ABL-expressing DCs have been reported to be defective in antigen processing.7,11 In contrast, other studies suggested that BCR/ABL-expressing DCs are able to effectively stimulate the proliferation of allogeneic and autologous T cells.8,14 Similarly, vaccination with autologous, nonirradiated leukemic DCs induced antileukemic T-cell responses in some CML patients.15
The function of BCR/ABL-expressing DCs in vivo is unknown. Therefore, we analyzed the function of BCR/ABL-expressing DCs in a murine retroviral-induced bone marrow transduction and transplantation model.16 To study antigen-specific immune responses, we used H8 transgenic mice, ubiquitously expressing the glycoprotein gp33 of the lymphocytic choriomeningitis virus (LCMV), as bone marrow donor mice. In CML mice, a large fraction of DCs expressed BCR/ABL. BCR/ABL-expressing DCs displayed a low maturation status and were functionally impaired. Immunization with bone marrow–derived BCR/ABL-expressing DCs induced only limited CTL expansion. Interestingly, BCR/ABL-expressing DCs did not home to the spleen. They preferentially migrated to the thymus where they induced a deletion of antigen-specific CD8+ T cells. Therefore, the low maturation status and impaired homing of BCR/ABL-expressing DCs to the spleen result in defective CTL induction. Moreover, the preferential homing to the thymus may induce central tolerance to some leukemia antigens. In summary, functional deficiencies of BCR/ABL-expressing DCs contribute to escape of CML from immunosurveillance.
Methods
Mice
C57BL/6 mice were purchased from Harlan (Horst, The Netherlands). H8 transgenic mice,17 ubiquitously expressing amino acids 1 to 60 of the LCMV glycoprotein, p14 T-cell receptor transgenic mice specific for the LCMV-gp3318 (line 318, ∼ 60% specific CD8+ T cells; line 327, ∼ 90% specific CD8+ T cells), and p14 (line 327) × RAG1−/− × CD45.1+, were obtained from the Institute for Laboratory Animals (Zurich, Switzerland). CD45.1+ mice were obtained from C. Mueller (Berne, Switzerland). Animal experiments were performed with sex- and age-matched mice. They were approved by the Experimental Animal Committee of the canton of Berne and were performed according to Swiss laws of animal protection.
Virus, virus detection, peptides, and retroviral vectors
LCMV, strain WE, was provided by R.M. Zinkernagel (Zurich, Switzerland) and propagated on L929 fibroblasts. The LCMV glycoprotein, amino acids 33 to 41 (gp33; KAVYNFATM) and LCMV nucleoprotein, amino acids 396 to 404 (np396; FQPQNGQFI), were purchased from NeoMPS (Strasbourg, France). The retroviral vectors pMSCV-p210 BCR/ABL-IRES-GFP (MSCV, mouse stem cell virus; IRES, internal ribosomal entry site; GFP, green fluorescent protein) and pMSCV-IRES-GFP (empty vector, control) and the packaging vector pIK6 were a gift from J. Schwaller (Basel, Switzerland).19-21
51Cr-release assay
Spleens were isolated and analyzed after 5 days of in vitro restimulation with irradiated (10 Gy) gp33-pulsed naive C57BL/6 splenocytes in a standard 51Cr-release assay, as described previously.22
Cells and retroviral particle production
Retroviral particles were generated by transient cotransfection of 293 T cells with the respective MSCV vector and the packaging vector pIK6, using Superfect transfection reagent (QIAGEN, Basel, Switzerland) according to the manufacturer's protocol. Forty-eight hours after transfection, virus-containing supernatant was harvested, filter-sterilized, and stored at −70°C. For determination of retroviral titers, BA/F3 cells were infected with different amounts of retroviral supernatant using polybrene transfection reagent (Sigma-Aldrich, St Louis, MO). After 48 hours, retroviral titers were determined by enumerating GFP+ cells by fluorescence-activated cell sorter (FACS) analysis.
CML model
H8 bone marrow donor mice were pretreated with 150 mg/kg 5-fluorouracil intraperitoneally (Sigma-Aldrich) dissolved in phosphate-buffered saline (PBS; Biochron AG, Berne, Switzerland) 6 days before bone marrow harvest. Two days before bone marrow transplantation, femurs and tibias were collected from 5-fluorouracil–treated mice and flushed with RPMI 10% fetal calf serum (FCS). Erythrocytes were removed by Puregene red blood cell lysis solution (Bioconcept, Basel, Switzerland), and the bone marrow cells were incubated in transplantation media (RPMI 10% FCS with 6 ng/mL recombinant murine interleukin-3 [IL-3], BD Biosciences, San Jose, CA; 10 ng/mL recombinant murine stem cell factor, Biocoba, Reinach, Switzerland; and 10 ng/mL recombinant human IL-6, BD Biosciences) in bacteriologic Petri dishes for 24 hours at 37°C in an atmosphere with 5% CO2. A total of 4 × 106 bone marrow cells were transfected twice on 2 consecutive days with BCR/ABL-GFP-containing retroviral particles with polybrene (6.7 μg/mL) and N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid 0.01 M through spin infection (90 minutes/300g/30°C). A total of 105 transduced bone marrow cells were injected intravenously into previously irradiated (4.5 Gy) syngeneic recipient mice.
Blood smears and cytospins
For cytospins, the erythrocytes were removed with red blood cell lysis solution from 100 μL blood of CML mice or C57BL/6 control mice. Then, the cells were resuspended in 50 μL PBS/0.5% human serum albumin (Stem Cell Laboratory, Inselspital, Berne, Switzerland), loaded on a funnel, and spun down to a microscope slide (20g for 15 minutes). Air-dried and unfixed peripheral blood smears and cytospins were stained with a Pappenheim staining (Merck Grogg Chemie, Berne, Switzerland) according to the manufacturer's protocol. The images were captured by a Nikon Eclipse 800 (Tokyo, Japan).
DC generation
H8 bone marrow cells were spin infected as described in “CML model” with retroviral particles containing BCR/ABL-GFP or empty GFP vector as a control. A total of 2 × 106 transduced bone marrow cells were cultivated in Petri dishes for 10 days in DC medium (RPMI 10% FCS, 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mM l-glutamine, 50 μM β-mercaptoethanol; (Sigma-Aldrich) and 10% supernatant of a granulocyte-macrophage colony-stimulating factor [GM-CSF] hybridoma, provided by T. Rolink, (University of Basel, Basel, Switzerland). DCs were matured in tissue culture dishes for one or 2 days in the presence of lipopolysaccharide (LPS, 10 μg/mL; Sigma-Aldrich) or 10% supernatant of an αCD40 hybridoma (provided by T. Rolink) in DC medium containing 5% GM-CSF supernatant. DCs were collected from the supernatant and sorted by flow cytometry (FACSVantage, BD Biosciences) for living BCR/ABL-GFP+ and GFP+ DCs. To generate nontransduced DCs from C57BL/6 mice, bone marrow was harvested, cultivated for 12 days in DC medium, and maturated for 1 day with LPS. For immunization, nontransduced DCs were pulsed with LCMV-gp33 (10−7 M) and -np396 (10−6 M) peptides at 37°C for 90 minutes and washed 3 times with Hank balanced salt solution (Sigma-Aldrich). A total of 2 × 105 pulsed DCs were injected intravenously into recipient mice.
Antibodies and flow cytometry
αCD8-phycoerythrin (PE), αCD4-biotin, αB220-biotin, αI-Ab-major histocompatibility complex (MHC) class II-biotin and phycoerythrin-cyanin5 (PE-Cy5), αCD11c-biotin, -PE and PE-Cy7, αCD86-PE, αCD80-PE, αCD70-PE, αCD83-PE, αCXCR4-PE, αCD44-biotin, α-interferon (IFN)-γ-fluorescein isothiocyanate, streptavidin-PE and allophycocyanin (APC), αCD45.1-APC and PE-Cy7, αCD45.2-PE, αCCR7-PE-Cy7, αCD19-biotin, αCD3-biotin, αNK1.1-biotin, rat IgG2b isotype-PE, rat IgG2b isotype-PE-Cy5, Golden Syrian hamster IgG isotype-PE, rat IgG2a isotype-APC, and αVLA-4-PE were purchased from eBioscience (San Diego, CA). αGR-1-PE, αCD8-PerCP-Cy5.5, and αCD162-PE were purchased from BD Biosciences PharMingen (San Diego, CA). MHC class I (H-2Db) tetramer-PE complexed with gp33 was purchased from Beckman Coulter (Fullerton, CA) and used according to the manufacturer's protocol.
For intracellular staining, splenocytes were restimulated with LCMV-gp33 peptide (10−6 M per well) or np396 (10−6 M per well) in the presence of 25 U/mL recombinant mouse IL-2 and 5 μg/mL brefeldin A (Sigma-Aldrich) for 5 hours. Cells were stained for surface molecules and fixed with 4% paraformaldehyde (Sigma-Aldrich) in PBS. Then, cell membranes were permeabilized with Perm-buffer (PBS, 2% FCS, 5 mM ethylenediaminetetraacetic acid, 0.1% saponin, 0.1% NaN3; Sigma-Aldrich) and stained with αIFNγ-fluorescein isothiocyanate. Relative fluorescence intensities were measured on a BD LSRII flow cytometer (BD Biosciences) and analyzed using FlowJo software (TreeStar, Ashland, OR).
Proliferation assays
Memory p14 T cells were generated as described previously.23 Naive and memory p14 splenocytes were purified for CD8+ T cells by magnetic cell sorting (MACS; Miltenyi Biotec, Bergisch Gladbach, Germany). A total of 2 × 105 purified CD8+ T cells were restimulated for 4 days with 2 × 105, 1 × 105, 0.4 × 105, or 0.2 × 105 in vitro–generated, LPS-matured, and FACS-sorted irradiated GFP+ BCR/ABL-expressing or control DCs (empty GFP vector–transduced). Alternatively, CD11c+ DCs from the spleen of CML mice and naive H8 mice were isolated with MACS. DCs from CML mice were sorted by flow cytometry for living BCR/ABL-GFP+ DCs. A total of 2 × 105 purified CD8+ T cells were restimulated for 4 days with 0.4 × 105 or 0.2 × 105 purified DCs from CML or H8 mice. 3H-Thymidine (GE Healthcare, Little Chalfont, United Kingdom) was added for the last 14 hours. Then, cells were collected with a Packard Filtermate 196-Harvester (PerkinElmer Life and Analytical Sciences, Boston, MA) on UniFilter-96, GF/C plates (PerkinElmer Life and Analytical Sciences), and the incorporation of radioactivity was measured after adding Microscint 0 (PerkinElmer Life and Analytical Sciences) on a Top Count microplate scintillation counter (PerkinElmer Life and Analytical Sciences).
Thymus homing
CD45.1+ mice were immunized intravenously with 4 × 106 in vitro–generated, LPS-matured, and FACS-sorted GFP+ BCR/ABL-expressing and control DCs (empty GFP vector–transduced). Eighteen hours later, thymus and spleen were collected and the number of CD11c+CD45.1−GFP+ DCs was determined by flow cytometry.
Deletion of antigen-specific CD8+ T cells
C57BL/6 mice (CD45.2+) were lethally irradiated on day 0 (2 × 6.5 Gy). Bone marrow cells were isolated from p14 (327) × RAG−/− × CD45.1+ and C57BL/6 (CD45.2+) mice. Mature lymphocytes and natural killer (NK) cells were removed by staining with αCD19-biotin, αCD3-biotin, and αNK1.1-biotin followed by antibiotin MACS. A total of 4 × 106 remaining p14 (327) × RAG−/− × CD45.1+ and CD45.2+ cells were injected intravenously into each irradiated recipient mouse. On days 20 and 21, bone marrow chimeric mice were immunized with 2.5 to 3 × 106 in vitro–generated, LPS-matured, and FACS-sorted GFP+ BCR/ABL-expressing or control DCs (empty GFP vector–transduced) or left untreated. On day 23, thymi were collected, and the frequency of CD45.1+CD8+ and CD45.2+CD8+ T cells was determined by flow cytometry.
Statistical analysis
Statistical significance was determined using the unpaired Student t test (2-tailed). P values less than .05 were considered significant.
Results
BCR/ABL-expressing DCs in CML
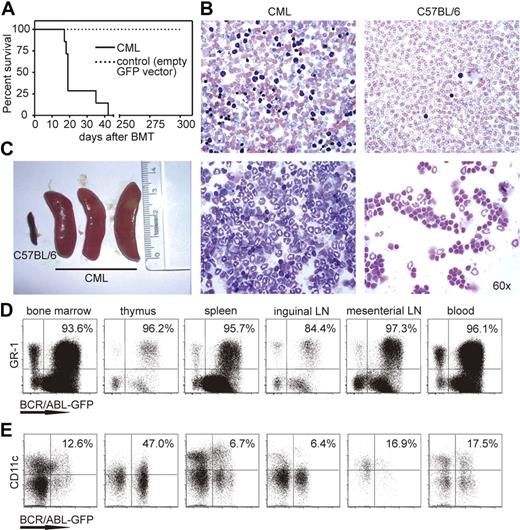
Bone marrow cells of H8 transgenic donor mice were transduced with retroviral particles expressing BCR/ABL-GFP and injected into sublethally irradiated (4.5 Gy) C57BL/6 recipient mice. In previous studies, recipient mice were lethally irradiated.16,24 This eliminates most lymphocytes, destroys the architecture of secondary lymphoid organs, and prevents the induction of a leukemia-specific immune response by the host. In contrast, sublethally irradiated mice (4.5 Gy) have a largely intact immune system from the recipient mouse with leukemia cells originated from donor bone marrow (S.M., unpublished results, 2008). CML developed in approximately 2 weeks, and animals died within 40 days (Figure 1A). Microscopy of blood smears and cytospin preparations showed an increased number of segmented granulocytes in the circulation (Figure 1B), and CML mice developed splenomegaly (Figure 1C), pulmonary hemorrhage, and granulocyte infiltrations in different organs. Control C57BL/6 mice receiving donor bone marrow transduced with an empty GFP vector did not develop CML. The frequency of GFP-transduced cells in these mice in the blood was low with 6.6% plus or minus 3.5% 19 days after transplantation and further decreased to 2.6% plus or minus 2.4% 34 days after transplantation.
CML model and BCR/ABL-expressing granulocytes and DCs in different organs. (A) Survival of CML (—) and control mice ( , empty GFP vector–transduced bone marrow) is shown in Kaplan-Meier plots. (B) Blood smear (top) and cytospin analysis (bottom) of CML mice and naive C57BL/6 mice. One representative Pappenheim staining of 10 is shown (original magnification ×60) included in “Methods.” (C) Spleen size of naive C57BL/6 mice and CML mice. (C,D) Bone marrow, thymus, spleen, inguinal and mesenterial lymph nodes (LN), and blood were isolated from CML mice. Samples were analyzed for the presence of BCR/ABL-GFP–expressing GR-1+ granulocytes (D) and CD11c+ DCs (E) by flow cytometry. Numbers indicate the percentage of BCR/ABL-expressing cells of total GR-1+ or CD11c+ cells. One representative FACS plot of 4 independent experiments is shown.
, empty GFP vector–transduced bone marrow) is shown in Kaplan-Meier plots. (B) Blood smear (top) and cytospin analysis (bottom) of CML mice and naive C57BL/6 mice. One representative Pappenheim staining of 10 is shown (original magnification ×60) included in “Methods.” (C) Spleen size of naive C57BL/6 mice and CML mice. (C,D) Bone marrow, thymus, spleen, inguinal and mesenterial lymph nodes (LN), and blood were isolated from CML mice. Samples were analyzed for the presence of BCR/ABL-GFP–expressing GR-1+ granulocytes (D) and CD11c+ DCs (E) by flow cytometry. Numbers indicate the percentage of BCR/ABL-expressing cells of total GR-1+ or CD11c+ cells. One representative FACS plot of 4 independent experiments is shown.
CML model and BCR/ABL-expressing granulocytes and DCs in different organs. (A) Survival of CML (—) and control mice ( , empty GFP vector–transduced bone marrow) is shown in Kaplan-Meier plots. (B) Blood smear (top) and cytospin analysis (bottom) of CML mice and naive C57BL/6 mice. One representative Pappenheim staining of 10 is shown (original magnification ×60) included in “Methods.” (C) Spleen size of naive C57BL/6 mice and CML mice. (C,D) Bone marrow, thymus, spleen, inguinal and mesenterial lymph nodes (LN), and blood were isolated from CML mice. Samples were analyzed for the presence of BCR/ABL-GFP–expressing GR-1+ granulocytes (D) and CD11c+ DCs (E) by flow cytometry. Numbers indicate the percentage of BCR/ABL-expressing cells of total GR-1+ or CD11c+ cells. One representative FACS plot of 4 independent experiments is shown.
, empty GFP vector–transduced bone marrow) is shown in Kaplan-Meier plots. (B) Blood smear (top) and cytospin analysis (bottom) of CML mice and naive C57BL/6 mice. One representative Pappenheim staining of 10 is shown (original magnification ×60) included in “Methods.” (C) Spleen size of naive C57BL/6 mice and CML mice. (C,D) Bone marrow, thymus, spleen, inguinal and mesenterial lymph nodes (LN), and blood were isolated from CML mice. Samples were analyzed for the presence of BCR/ABL-GFP–expressing GR-1+ granulocytes (D) and CD11c+ DCs (E) by flow cytometry. Numbers indicate the percentage of BCR/ABL-expressing cells of total GR-1+ or CD11c+ cells. One representative FACS plot of 4 independent experiments is shown.
Bone marrow, thymus, spleen, inguinal and mesenterial lymph nodes, and blood of CML mice were analyzed for the presence of BCR/ABL-GFP–expressing cells. BCR/ABL-expressing GR-1+ granulocytes were found in all organs analyzed, confirming the development of CML (Figure 1D). A preferential accumulation of high numbers of granulocytes was found in spleen and blood. Interestingly, BCR/ABL-expressing DCs were also present in all organs (Figure 1E). BCR/ABL expression was detected in approximately 50% of DCs in the thymus, in 6% of DCs in spleen and inguinal lymph nodes, and in 12% to 20% of DCs in bone marrow, blood, and mesenterial lymph nodes. In addition, BCR/ABL expression was also found in few CD8+ and CD4+ T cells, B cells, and NK cells (data not shown). These data suggest that CML in mice as well as CML in human patients developed from a pluripotent stem cell with the ability to differentiate to various cell lineages. However, the preferential differentiation to the myeloid lineage resulted in large granulocyte counts and a high percentage of BCR/ABL-expressing DCs.
BCR/ABL-expressing DCs in CML display a low maturation status
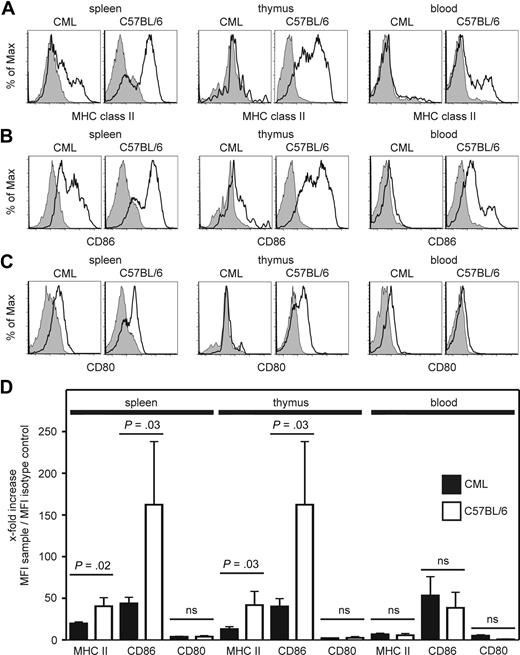
DCs have several functions in innate and adaptive immunity, such as activating NK cells, priming T-cell responses, or polarizing immune responses to either T helper 1- or 2-type reactions.6 To fulfill all these functions, DCs must undergo terminal differentiation and maturation.6 To determine the maturation status of BCR/ABL-expressing DCs in CML mice, we analyzed the expression of MHC class II molecules and the costimulatory molecule CD86 and CD80 on DCs in spleen, thymus, blood, inguinal and mesenterial lymph nodes, and bone marrow (Figure 2; and data not shown). We compared the fluorescence shifts of MHC class II molecules, CD86 and CD80 staining vs isotype staining. The x-fold increase in mean fluorescence intensity was calculated (Figure 2D). The expression of MHC class II molecules and CD86, but not CD80, on BCR/ABL-expressing DCs in the spleen and thymus was significantly reduced compared with DCs isolated from naive C57BL/6 mice (Figure 2D). Therefore, BCR/ABL-expressing DCs display a low maturation status in vivo. The expression level of MHC class II molecules and CD86 on DCs isolated from blood of naive C57BL/6 mice and from CML mice was very low, indicating that most DCs circulating in blood are phenotypically immature DCs independent of the expression of BCR/ABL.
BCR/ABL-expressing DCs have a low maturation status in vivo. (A-C) Spleen, thymus, and blood of CML and naive C57BL/6 mice were isolated. The expression of MHC class II molecules (A), CD86 (B), and CD80 (C) on BCR/ABL-GFP-expressing CD11c+ DCs in CML mice and on CD11c+ DCs from naive C57BL/6 mice (open histograms) was compared with isotype control stainings (filled histograms). One representative FACS plot of 3 independent experiments is shown. (D) Fold increase of mean fluorescence intensity of stainings of CML mice compared with naive C57BL/6 mice. Pooled data from 3 independent experiments with a total of 7 CML mice and 3 C57BL/6 mice are shown; ns indicates not significant.
BCR/ABL-expressing DCs have a low maturation status in vivo. (A-C) Spleen, thymus, and blood of CML and naive C57BL/6 mice were isolated. The expression of MHC class II molecules (A), CD86 (B), and CD80 (C) on BCR/ABL-GFP-expressing CD11c+ DCs in CML mice and on CD11c+ DCs from naive C57BL/6 mice (open histograms) was compared with isotype control stainings (filled histograms). One representative FACS plot of 3 independent experiments is shown. (D) Fold increase of mean fluorescence intensity of stainings of CML mice compared with naive C57BL/6 mice. Pooled data from 3 independent experiments with a total of 7 CML mice and 3 C57BL/6 mice are shown; ns indicates not significant.
BCR/ABL-expressing DCs in CML are functionally impaired
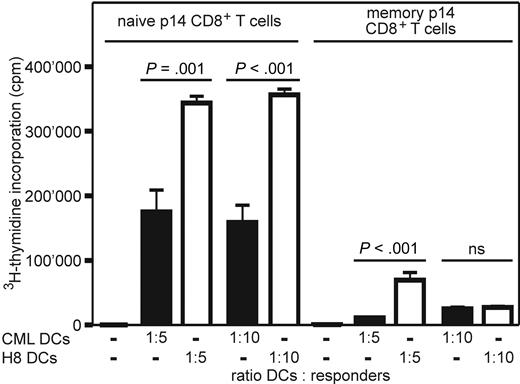
To determine the capacity of BCR/ABL-expressing DCs of CML mice to induce proliferation of antigen-specific naive and memory CD8+ T cells, BCR/ABL-expressing DCs were isolated from CML mice. 3H-Thymidine incorporation of naive and memory p14 CD8+ T cells was measured after restimulation with titrated numbers of isolated BCR/ABL-expressing DCs and DCs from naive H8 transgenic mice. The expansion of naive and memory p14 CD8+ T cells after stimulation with BCR/ABL-expressing DCs was significantly lower compared with DCs isolated from H8 transgenic mice (Figure 3). Therefore, DCs isolated from CML mice are functionally impaired.
CML DCs induce limited expansion of naive and memory p14 CD8+ T cells ex vivo. Naive and memory p14 CD8+ T cells were restimulated in vitro for 4 days with titrated numbers of irradiated DCs (CD11c+) isolated from the spleen of CML mice (■) or naive H8 mice (□). 3H-Thymidine incorporation was measured during the last 14 hours of culture. Results are mean plus or minus SEM of 3 samples per group. Pooled data from 2 independent experiments are shown; ns indicates not significant.
CML DCs induce limited expansion of naive and memory p14 CD8+ T cells ex vivo. Naive and memory p14 CD8+ T cells were restimulated in vitro for 4 days with titrated numbers of irradiated DCs (CD11c+) isolated from the spleen of CML mice (■) or naive H8 mice (□). 3H-Thymidine incorporation was measured during the last 14 hours of culture. Results are mean plus or minus SEM of 3 samples per group. Pooled data from 2 independent experiments are shown; ns indicates not significant.
In vitro–generated BCR/ABL-expressing DCs display low maturation and are functionally impaired
To analyze the effect of BCR/ABL on the maturation of DCs and the functional consequences thereof, we generated BCR/ABL-expressing and control DCs from H8 bone marrow cells in vitro. Control DCs were transduced with an empty GFP vector. Immature DCs transduced with BCR/ABL-GFP or control DCs were phenotypically similar when analyzed after 10 days of in vitro culture in DC medium (Figure 4A). In contrast, similar to BCR/ABL-expressing DCs in vivo, expression of MHC class II molecules and CD86 was reduced on in vitro–generated and matured BCR/ABL-expressing DCs. This was independent of the maturation protocol used. After maturation with LPS, BCR/ABL-expressing DCs expressed significantly lower levels of MHC class II molecules and the costimulatory molecules CD86, but similar levels of CD80 and CD70 compared with control DCs (Figure 4B,D). BCR/ABL-expressing DCs matured with αCD40 antibody expressed lower levels of MHC class II molecules and CD86 compared with control DCs (Figure 4C). In contrast, expression of CD83 on BCR/ABL-expressing DCs was higher than in control DCs independently of the maturation status (Figure 4B,D).
In vitro–generated BCR/ABL-expressing DCs have a low maturation status. (A-D) DCs were generated in vitro from BCR/ABL-GFP or empty vector GFP–transduced H8 bone marrow cells. Immature (A), LPS-matured (B), and αCD40-maturated (C) BCR/ABL-expressing and control DCs were analyzed for MHC class IIhigh molecules, CD86, CD80, CD83, and CD70 expression by flow cytometry. Dot plots are gated on GFP-expressing cells. Numbers indicate the percentage of CD11c+ DCs expressing the stained molecule. (D) Summary of data in panel B of 3 independent experiments is shown as percentage (mean ± SEM) of MHC class IIhigh, CD86+, CD80+, CD83+, or CD70+ cells within total GFP+CD11c+ cells: (■) represents BCR/ABL-expressing DCs; (□), empty GFP vector–transduced control DCs, LPS-matured; ns indicates not significant.
In vitro–generated BCR/ABL-expressing DCs have a low maturation status. (A-D) DCs were generated in vitro from BCR/ABL-GFP or empty vector GFP–transduced H8 bone marrow cells. Immature (A), LPS-matured (B), and αCD40-maturated (C) BCR/ABL-expressing and control DCs were analyzed for MHC class IIhigh molecules, CD86, CD80, CD83, and CD70 expression by flow cytometry. Dot plots are gated on GFP-expressing cells. Numbers indicate the percentage of CD11c+ DCs expressing the stained molecule. (D) Summary of data in panel B of 3 independent experiments is shown as percentage (mean ± SEM) of MHC class IIhigh, CD86+, CD80+, CD83+, or CD70+ cells within total GFP+CD11c+ cells: (■) represents BCR/ABL-expressing DCs; (□), empty GFP vector–transduced control DCs, LPS-matured; ns indicates not significant.
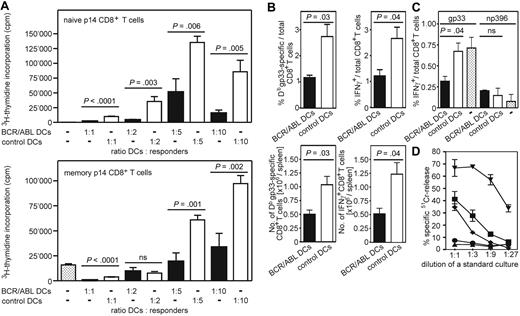
We next tested the ability of in vitro–generated BCR/ABL-expressing DCs to induce proliferation of antigen-specific naive CD8+ T cells and to reexpand antigen-specific memory CTLs in vivo. These from H8 bone marrow cells in vitro–generated DCs expressed LCMV-gp33 on MHC class I molecules, which served as model leukemia antigen to stimulate transgenic gp33-specific p14 CD8+ T cells. GFP+ DCs were sorted by FACS from in vitro–generated and LPS-matured BCR/ABL-expressing and control DCs (empty GFP vector–transduced). Stimulation with titrated numbers of BCR/ABL-expressing DCs induced only a very limited expansion of naive or memory p14 CD8+ T cells (Figure 5A). In contrast, control DCs efficiently stimulated proliferation of naive and memory p14 CD8+ T cells. Therefore, DCs isolated from CML mice and in vitro–generated BCR/ABL-expressing bone marrow derived DCs are phenotypically and functionally comparable.
Primary and secondary immune responses are reduced after immunization with BCR/ABL-expressing DCs. (A) Naive and memory p14 CD8+ T cells were restimulated in vitro for 4 days with titrated numbers of LPS-matured BCR/ABL-expressing (■) or control DCs (□, empty GFP vector–transduced) generated from H8 bone marrow cells. 3H-Thymidine incorporation was measured during the last 14 hours of culture. Results are mean plus or minus SEM of 3 to 4 samples per group. Pooled data from 2 independent experiments are shown. (B) Naive C57BL/6 mice were immunized intravenously with 2 × 105 LPS-maturated BCR/ABL-expressing (■) or control DCs (□, empty GFP vector–transduced). Ten days later, the frequency and absolute number of gp33-specific CD8+ T cells in the spleen were determined by tetramer staining and by intracellular IFN-γ staining after in vitro restimulation with gp33. Results are mean plus or minus SEM of 4 mice per group. One representative experiment of 3 is shown. (C) C57BL/6 mice previously immunized with 2 × 105 LPS-maturated BCR/ABL-expressing (■) or control DCs (□, empty GFP vector–transduced) and naive C57BL/6 mice (□ dotted) were immunized 14 days later with gp33- and np396-pulsed DCs generated from C57BL/6 mice. Ten days later, the frequency of gp33- and np396-specific CD8+ T cells was determined by intracellular IFN-γ staining after in vitro restimulation with gp33 and np396. (D) Splenocytes were isolated 10 days after challenge immunization and analyzed in a standard 51Cr-release assay. ● represents mice primarily immunized with BCR/ABL-expressing DCs; ■, mice with control DCs (empty GFP vector–transduced); ♦, mice receiving only challenge immunization; ▾, LCMV-immune mice; and ▴, naive C57BL/6 mice. Symbols represent 51Cr release of gp33-pulsed target cells. Specific 51Cr release of unpulsed target cells was less than 10%. CTL activity is given as mean plus or minus SEM of 4 mice per group, except for LCMV-immune and naive C57BL/6 mice; ns indicates not significant.
Primary and secondary immune responses are reduced after immunization with BCR/ABL-expressing DCs. (A) Naive and memory p14 CD8+ T cells were restimulated in vitro for 4 days with titrated numbers of LPS-matured BCR/ABL-expressing (■) or control DCs (□, empty GFP vector–transduced) generated from H8 bone marrow cells. 3H-Thymidine incorporation was measured during the last 14 hours of culture. Results are mean plus or minus SEM of 3 to 4 samples per group. Pooled data from 2 independent experiments are shown. (B) Naive C57BL/6 mice were immunized intravenously with 2 × 105 LPS-maturated BCR/ABL-expressing (■) or control DCs (□, empty GFP vector–transduced). Ten days later, the frequency and absolute number of gp33-specific CD8+ T cells in the spleen were determined by tetramer staining and by intracellular IFN-γ staining after in vitro restimulation with gp33. Results are mean plus or minus SEM of 4 mice per group. One representative experiment of 3 is shown. (C) C57BL/6 mice previously immunized with 2 × 105 LPS-maturated BCR/ABL-expressing (■) or control DCs (□, empty GFP vector–transduced) and naive C57BL/6 mice (□ dotted) were immunized 14 days later with gp33- and np396-pulsed DCs generated from C57BL/6 mice. Ten days later, the frequency of gp33- and np396-specific CD8+ T cells was determined by intracellular IFN-γ staining after in vitro restimulation with gp33 and np396. (D) Splenocytes were isolated 10 days after challenge immunization and analyzed in a standard 51Cr-release assay. ● represents mice primarily immunized with BCR/ABL-expressing DCs; ■, mice with control DCs (empty GFP vector–transduced); ♦, mice receiving only challenge immunization; ▾, LCMV-immune mice; and ▴, naive C57BL/6 mice. Symbols represent 51Cr release of gp33-pulsed target cells. Specific 51Cr release of unpulsed target cells was less than 10%. CTL activity is given as mean plus or minus SEM of 4 mice per group, except for LCMV-immune and naive C57BL/6 mice; ns indicates not significant.
To determine whether the reduced maturation of BCR/ABL-expressing DCs affects the induction of antigen-specific primary CTL responses in vivo, naive C57BL/6 mice were immunized with LPS-matured BCR/ABL-expressing and control DCs. Ten days later, gp33-specific IFNγ-secreting and tetramer-positive CD8+ T cells were assessed in the spleen. Both the frequency and absolute number of antigen-specific CD8+ T cells in mice immunized with BCR/ABL-expressing DCs were 2-fold lower compared with immunization with control DCs (Figure 5B).
Secondary expansion of CTLs in vivo was analyzed after immunization of naive C57BL/6 mice with BCR/ABL-expressing and control DCs. Fourteen days later, mice were rechallenged with nontransduced DCs generated from C57BL/6 bone marrow cells and pulsed with gp33 and a second viral peptide, LCMV-np396. Ten days later, the frequency of gp33- and np396-specific IFNγ-secreting CD8+ T cells was determined. Similarly to the initial immunization, the frequency of gp33-specific CTLs was 2-fold lower in mice pre-immunized with BCR/ABL-expressing DCs compared with mice initially immunized with control DCs or mice receiving only the challenge immunization (Figure 5C). In contrast, the frequency of np396-specific IFNγ-secreting CD8+ T cells was comparable in all groups (Figure 5C).
In the same experimental setting, CTLs were isolated 10 days after the challenge immunization to analyze functional differences in vitro. CTLs from mice pre-immunized with LPS-matured BCR/ABL-expressing DCs did not lyse peptide-pulsed target cells (Figure 5D). CTLs isolated from mice primarily immunized with LPS-matured control DCs, or from mice only receiving the challenge immunization, lysed peptide-pulsed target cells to a similar extent (Figure 5D). Thus, immunization with BCR/ABL-expressing DCs induces only a reduced frequency of specific CTLs in vivo compared with empty vector-transduced control DCs. A challenge immunization with nontransduced DCs did not improve the gp33-specific CTL frequency but induced a normal CTL response against a nonrelated control peptide.
Preferential homing of BCR/ABL-expressing DCs to the thymus
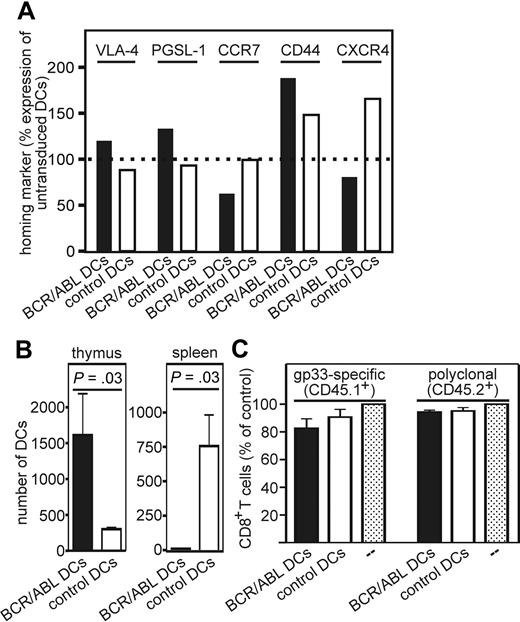
After capturing antigens in the periphery, DCs mature and migrate to secondary lymphoid organs.25 In addition, it has been shown that circulating DCs can be recruited to the thymus.26 We therefore analyzed the homing of BCR/ABL-expressing DCs to the spleen and thymus. First, the expression of the thymus homing markers VLA-4 (CD29) and P-selectin ligand (PSGL-1, CD162)26 as well as the spleen homing marker CCR7 was determined on in vitro–generated and LPS-matured BCR/ABL-expressing DCs, control DCs (empty GFP vector–transduced), and nontransduced DCs. In addition, expression of homing markers that are of relevance in the homing of BCR/ABL+ myeloid progenitor cells was analyzed (CD44 and CXCR4, receptor for SDF-1). The expression of VLA-4, PSGL-1, and CD44 was increased on BCR/ABL-expressing DCs compared with control DCs (Figure 6A, standardized to nontransduced DCs). In contrast, CCR7 and CXCR4 expression was decreased on BCR/ABL-expressing DCs compared with control DCs (Figure 6A). Thus, BCR/ABL-expressing DCs display an increased expression of homing markers for the thymus but a reduced expression of homing marker for the spleen and lymph nodes compared with control DCs.
BCR/ABL-expressing DCs preferentially home to the thymus. (A,B) From H8 bone marrow in vitro–generated and LPS-matured BCR/ABL-expressing and control DCs (empty GFP vector–transduced) were analyzed for VLA-4, PSGL-1, CCR7, CD44, and CXCR4 expression by flow cytometry. (A) The expression of the homing markers on BCR/ABL-expressing (■) and control DCs (□, empty vector–transduced) is displayed as relative change compared with nontransduced DCs (100%). One representative experiment of 2 is shown. (B) CD45.1+ recipient mice were immunized with 4 × 106 BCR/ABL-expressing or control DCs. Eighteen hours later, the number of CD11c+CD45.1−GFP+ cells in the thymus and spleen was analyzed by flow cytometry. The number of BCR/ABL-expressing (■) or control DCs (□, empty GFP vector–transduced) in the thymus and spleen was analyzed by flow cytometry. (C) Bone marrow chimeric mice were immunized on day 20 and 21 after bone marrow transplantation with BCR/ABL-expressing (■) or control DCs (□) or nonimmunized (□ dotted). On day 23, thymi were analyzed for the frequency of gp33-specific CD8+ T cells (CD45.1+) and polyclonal CD8+ T cells (CD45.2+). Data are shown as frequency of CD8+ T cells of CD45.1+ lymphocytes or CD8+ T cells of CD45.2+ lymphocytes in mice immunized with BCR/ABL-expressing or control DCs relative to nontreated control mice. Results are mean plus or minus SEM of 3 samples per group.
BCR/ABL-expressing DCs preferentially home to the thymus. (A,B) From H8 bone marrow in vitro–generated and LPS-matured BCR/ABL-expressing and control DCs (empty GFP vector–transduced) were analyzed for VLA-4, PSGL-1, CCR7, CD44, and CXCR4 expression by flow cytometry. (A) The expression of the homing markers on BCR/ABL-expressing (■) and control DCs (□, empty vector–transduced) is displayed as relative change compared with nontransduced DCs (100%). One representative experiment of 2 is shown. (B) CD45.1+ recipient mice were immunized with 4 × 106 BCR/ABL-expressing or control DCs. Eighteen hours later, the number of CD11c+CD45.1−GFP+ cells in the thymus and spleen was analyzed by flow cytometry. The number of BCR/ABL-expressing (■) or control DCs (□, empty GFP vector–transduced) in the thymus and spleen was analyzed by flow cytometry. (C) Bone marrow chimeric mice were immunized on day 20 and 21 after bone marrow transplantation with BCR/ABL-expressing (■) or control DCs (□) or nonimmunized (□ dotted). On day 23, thymi were analyzed for the frequency of gp33-specific CD8+ T cells (CD45.1+) and polyclonal CD8+ T cells (CD45.2+). Data are shown as frequency of CD8+ T cells of CD45.1+ lymphocytes or CD8+ T cells of CD45.2+ lymphocytes in mice immunized with BCR/ABL-expressing or control DCs relative to nontreated control mice. Results are mean plus or minus SEM of 3 samples per group.
To analyze the homing of transduced DCs in vivo, mice were immunized with LPS-matured BCR/ABL-expressing or control DCs and the presence of CD11c+CD45.1−GFP+ DCs was determined in the thymus and spleen. BCR/ABL-expressing DCs preferentially homed to the thymus, resulting in a 4-fold accumulation compared with control DCs (Figure 6B). In contrast, hardly any BCR/ABL-expressing DCs homed to the spleen (Figure 6B).
Functional consequences of the migration of BCR/ABL-expressing DCs to the thymus were analyzed in bone marrow chimeric mice. Lethally irradiated CD.45.2+ mice were reconstituted with bone marrow from p14 (327) xRAG1−/−xCD45.1+ mice (50%) and CD45.2+ mice (50%). Chimeric mice will generate gp33-specific CD8+CD45.1+ T cells and polyclonal CD8+CD45.2+ T cells. Twenty days after reconstitution, chimeric mice were immunized twice with LPS-matured BCR/ABL-expressing or control DCs. Thymi were collected 23 days after reconstitution (3 days after initial DC immunization). The frequency of the gp33-specific CD8+ T cells (CD45.1+) and polyclonal CD8+ T cells (CD45.2+) in immunized chimeric mice was determined and compared with nonimmunized chimeric mice (Figure 6C). The polyclonal CD8+ T-cell population in mice immunized with BCR/ABL-expressing or control DCs remained unchanged. In contrast, the frequency of gp33-specific CD8+ T cells was reduced in chimeric mice immunized with BCR/ABL-expressing DCs, without reaching statistical significance. This suggests that BCR/ABL-expressing DCs may be able to delete antigen-specific CD8+ T cells in the thymus.
Discussion
Although CTLs specific for BCR/ABL and other leukemia antigens can be detected in CML patients, eventually these CTLs fail to control the progression of the disease. Various mechanisms may explain the escape of CML from the immunosurveillance, including the expression of FasL during blast crisis,27 the deletion of high-avidity CTLs specific for a leukemia-associated self-antigen,28 as well as the development of functional blocks in the caspase activation pathway in acute myeloid leukemia cells.29
We now analyzed the role of BCR/ABL-expressing DCs in the induction of specific CTLs in a murine CML model. The murine CML model recapitulates cardinal features of human CML, such as markedly elevated leukocyte counts with granulocyte predominance, splenomegaly, multiple organ involvement, and the expression of BCR/ABL in affected tissues.16,24,30
So far, the role of BCR/ABL-expressing DCs in vivo is poorly defined. In our CML model, 6% to 50% of all DCs expressed BCR/ABL. We found that in vitro–generated BCR/ABL-expressing DCs as well as BCR/ABL-expressing DCs in CML mice had a lower maturation status compared with control DCs. In addition, the immunization with BCR/ABL-expressing DCs resulted in impaired CTL responses. Importantly, CTL priming was only defective for antigens that are selectively expressed on BCR/ABL-expressing DCs, including leukemia-specific antigens. The presence of a large proportion of normal non–BCR/ABL-expressing DCs explains why CML patients and mice with CML-like disease develop normal CTL responses to infectious pathogens.
Myeloid DCs isolated directly from CML patients expressed lower levels of CD83, CD80, and CD40 compared with DCs from healthy donors.8 This is comparable with our results in the murine CML model. Although the isolated human CML DCs efficiently stimulated alloreactive T cells in vitro and induced cytokine production, they failed to induce proliferation of autologous T cells.8 In contrast, DCs generated from human peripheral blood mononuclear cells (PBMCs)7 or bone marrow12 of CML patients can be matured in vitro to express costimulatory molecules comparable with those DCs generated from healthy donors. Similarly, in vitro–generated and maturated CML DCs from human PBMCs were able to induce CML-specific CTL responses in vitro and in vivo.15,31 The differences in maturation may be explained by different maturation protocols. Human CML DCs generated and matured with GM-CSF, IL-4, and tumor necrosis factor-α were similarly matured as normal DCs,7,12 whereas maturation with GM-CSF, IL-4, and LPS led to a reduced maturation status of CML DCs.11 Together, these results indicate that the maturation of DCs in vivo in human CML patients and in CML mice is reduced. However, depending on the maturation protocol used, mature human DCs can be generated in vitro and probably used for immunotherapy.7,12
BCR/ABL can interfere with the maturation of DCs via different mechanisms. First, cytokines produced by BCR/ABL-expressing cells may inhibit DC maturation. The maturation of DCs can be inhibited efficiently by transforming growth factor-β (TGF-β) or IL-10 through blockade of the nuclear factor-κB pathway.32 It has been demonstrated that BCR/ABL expression dramatically up-regulates TGF-β signaling in transduced cells in vitro.33 Genes of the TGF-β signaling pathway are significantly up-regulated in CD34+ bone marrow cells of untreated CML patients.34 In addition, human CML cells express IL-10 mRNA35 and produce high levels of IL-10 in vitro.36 Therefore, the secretion of TGF-β and/or IL-10 by leukemic cells may contribute to a reduced maturation status of BCR/ABL-expressing DCs. Second, BCR/ABL activates the signal transducer and activator of transcription (STAT) pathway.34,37 BCR/ABL constitutively activates STAT3 through the Janus kinase (JAK) and mitogen-activated protein-kinase pathway.38 Although signaling via JAK2/STAT3 is crucial for normal DC differentiation,39,40 constitutive activation of STAT3 inhibits DC maturation.41 Interestingly, IL-10 signals also through STAT3.42
Primary antigen-specific immune responses are induced exclusively in secondary lymphoid organs.43 During maturation, DCs acquire the capacity to migrate to secondary lymphoid organs and to prime naive T cells.25 Recently, it has been demonstrated that immature peptide-presenting DCs preferentially migrate to the thymus and induce clonal deletion of antigen-specific CD4+ T cells.26 The circulating DCs were recruited to the thymus through a 3-step adhesion cascade involving P-selectin, the integrin VLA-4, and chemoattractant signaling by pertussis toxin.26 We now found that, on BCR/ABL-expressing DCs, the expressions of the thymus homing molecules VLA-4 and PSGL-1 were higher whereas the expression of the spleen-homing molecule CCR7 was lower compared with control DCs. This resulted in an impaired homing of BCR/ABL-expressing DCs to the spleen. Instead, BCR/ABL-expressing DCs preferentially migrated to the thymus. Differences in the migration of DCs from CML patients vs DCs from healthy donors have been documented before in vitro. DCs generated from CD34+ PBMCs of CML patients had altered actin organization and reduced migratory ability to a chemokine gradient of MIP-1α.7 We documented differences in cell surface expression of PSGL-1, VLA-4 (β1-integrin), CD44, CXCR4, and CCR7. It has been shown that CD44 surface expression is regulated by BCR/ABL and is required for the homing of BCR/ABL-expressing leukemic cells.44 In contrast, other studies documented functional defects in β-1 integrins.45 The BCR/ABL oncogene product p210 interacts with a variety of cytoskeletal elements important for normal integrin signaling.45 Moreover, BCR/ABL alters the chemotactic response of myeloid cells to SDF-1, not by regulating the expression of its receptor CXCR-4 but probably by inhibition of receptor function.46 The observed change of expression of β1-integrins and P-selectin ligands in the present study may be a consequence of impaired DC maturation because this phenotype was also found in immature non–BCR/ABL-expressing DCs.26 In addition, BCR/ABL may contribute to an impaired homing by interfering with integrin signaling. In summary, besides the reduced expression of costimulatory molecules on BCR/ABL-expressing DCs, the impaired migration to the spleen explains the inefficient CTL induction in vivo.
Central tolerance is maintained through the clonal deletion of high affinity T cells specific for antigens presented in the thymus.47 Central tolerance was originally thought to be restricted to proteins that are expressed by thymic medullary epithelial cells, or thymic DCs, or to proteins that enter the thymus through blood circulation.48 However, it has been demonstrated that peptide-presenting DCs can home to the thymus and induce clonal deletion of antigen-specific T cells. Importantly, mainly immature DCs migrated to the thymus and accumulated in the medulla, in close proximity to endogenous thymic DCs and T cells.26 In our study, we documented that BCR/ABL-expressing DCs in the thymus deplete part of the antigen-specific CTLs. Our finding that the central depletion of specific p14 CD8+ T cells was limited to approximately 20% may reflect the fact that p14 CD8+ T cells are continuously generated in the bone marrow. In contrast, mice were immunized only twice with BCR/ABL-expressing DCs with relatively low numbers, which could be generated in vitro. However, a role of peripheral dendritic cells in the modulation of acquired thymic tolerance has been documented before.6,49
Taken together, BCR/ABL-expressing DCs do not efficiently induce specific CTL responses in secondary lymphoid organs but may present leukemia antigens in the thymus and induce central tolerance to these antigens. Both mechanisms may contribute to an impaired immunosurveillance of CML. In contrast, CML is very susceptible to CTL-mediated lysis after donor lymphocyte infusions.50 The transfer of donor lymphocytes that recognize minor histocompatibility antigens does not depend on BCR/ABL-expressing DCs because minor histocompatibility antigens are expressed on all DCs of the patients. Therefore, adoptive immunotherapy is one therapeutic strategy to circumvent the impaired immunosurveillance in CML patients.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Swiss National Science Foundation (Berne; grant 632-66 020), Oncosuisse (grants OCS-01 312-02-2003 and OCS-01 627-02-2005), and the Bernische Krebsliga (Berne, Switzerland).
Authorship
Contribution: S.M. designed and performed experiments, analyzed data, and wrote the manuscript; C.C., C.S., V.P., and M.S.M. performed experiments; and A.F.O. designed experiments, wrote the manuscript, and supervised the project.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Adrian F. Ochsenbein, Institute for Medical Oncology, Inselspital, CH-3010 Berne, Switzerland; e-mail: adrian.ochsenbein@insel.ch.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal