Abstract
The number of neutrophils in the blood is tightly regulated to ensure adequate protection against microbial pathogens while minimizing damage to host tissue. Neutrophil homeostasis in the blood is achieved through a balance of neutrophil production, release from the bone marrow, and clearance from the circulation. Accumulating evidence suggests that signaling by CXCL12, through its major receptor CXCR4, plays a key role in maintaining neutrophil homeostasis. Herein, we generated mice with a myeloid lineage–restricted deletion of CXCR4 to define the mechanisms by which CXCR4 signals regulate this process. We show that CXCR4 negatively regulates neutrophil release from the bone marrow in a cell-autonomous fashion. However, CXCR4 is dispensable for neutrophil clearance from the circulation. Neutrophil mobilization responses to granulocyte colony-stimulating factor (G-CSF), CXCL2, or Listeria monocytogenes infection are absent or impaired, suggesting that disruption of CXCR4 signaling may be a common step mediating neutrophil release. Collectively, these data suggest that CXCR4 signaling maintains neutrophil homeostasis in the blood under both basal and stress granulopoiesis conditions primarily by regulating neutrophil release from the bone marrow.
Introduction
Neutrophils play an essential role in the innate immune response, as they are required to effectively protect the host against a variety of bacterial and fungal pathogens. Under basal conditions, the great majority of neutrophils reside in the bone marrow. In response to infection or other stresses, this pool of neutrophils can be mobilized into the blood, providing the host with a mechanism to rapidly increase neutrophil delivery to sites of infection. It is essential that neutrophil numbers in the blood be tightly regulated. Persistent neutropenia is associated with profound immunodeficiency, whereas excessive neutrophil infiltration and activation contributes to tissue damage in certain inflammatory disorders, such as rheumatoid arthritis. Neutrophil homeostasis is maintained through a balance of neutrophil production, release from the bone marrow, and clearance from the circulation.1 Contributing to the complexity of this process, neutrophils have the shortest survival of any circulating cell, with reported half-lives of 8 to 16 hours under basal conditions.2-7 Despite its importance, the mechanisms regulating neutrophil number in the blood are incompletely understood
Accumulating evidence suggests that the chemokine CXCL12 (stromal derived factor-1, SDF-1), through interaction with its major receptor CXCR4, plays a key role in controlling neutrophil homeostasis.8 Mice deficient for CXCL12 or CXCR4 die perinatally, but the fetal circulation is characterized by elevated numbers of neutrophils and a failure to establish bone marrow myelopoiesis.9-12 In CXCR4−/− fetal liver chimeras, mature neutrophils and granulocytic precursors are increased in the blood, whereas the number of mature neutrophils in the bone marrow is reduced.13,14 Humans and mice treated with AMD3100, a selective antagonist of CXCR4, or CXCR4-blocking antibodies display a rapid mobilization of neutrophils into the blood.15-17 Truncation mutations of CXCR4 that cause increased receptor signaling are responsible for most cases of WHIM (warts, hypogammaglobulinemia, infections, myelokathexis) syndrome, which is characterized by abnormal retention of neutrophils in the bone marrow.18-20 Importantly, CXCR4 is expressed by neutrophils as well as most other hematopoietic cells, and CXCL12 is constitutively expressed at high levels in the bone marrow stroma.21,22 Together, these data support a model in which CXCL12 signaling through CXCR4 provides a key retention signal for neutrophils in the bone marrow and therefore negatively regulates their release.
As noted previously, peripheral blood neutrophil counts can increase rapidly in response to infection or other stress. A wide variety of infectious agents, bacterial products, cytokines, and chemokines is thought to contribute to this “emergency” or “stress” granulopoiesis response.23,24 The downstream signals that regulate this response are relatively undefined, but recent evidence suggests that CXCR4 may play a role. Treatment with granulocyte colony-stimulating factor (G-CSF), a key cytokine in the stress granulopoiesis response, results in a decrease in CXCL12 expression in the bone marrow and the down-regulation of CXCR4 expression on neutrophils.25-28 These observations suggest the hypothesis that disruption of CXCR4 signaling is a key step mediating neutrophil release by G-CSF. Whether disruption of CXCL12/CXCR4 signaling is a common mechanism by which other mobilizing agents increase neutrophil counts in the blood is unknown.
Neutrophil homeostasis in the blood is determined, in part, by the rate of clearance from the circulation. Neutrophils are cleared primarily in the liver, spleen, or bone marrow, where apoptotic or aged neutrophils are thought to be phagocytosed by macrophages.17,29,30 Recent studies suggest that CXCR4 may play a role in the clearance of aged, senescent neutrophils, particularly at bone marrow sites. CXCR4 expression increases on neutrophils as they age, and blocking antibodies to CXCR4 impede neutrophil homing to the bone marrow.17,31 Thus, CXCR4 may have a dual role in regulating neutrophil homeostasis by acting as a signal clearing senescent neutrophils from the blood in addition to regulating neutrophil release.
The broad expression of CXCR4 on hematopoietic cells complicates the analysis of this gene during granulopoiesis. For example, CXCR4−/− fetal liver chimeras display defects in multiple hematopoietic lineages, including hematopoietic stem cells.13,14,32 Therefore, in the present study we use transgenic mice carrying a myeloid-specific deletion of CXCR4 to further define the role of CXCR4 in basal and stress granulopoiesis. Using this system, we show that CXCR4 is dispensable for neutrophil clearance from the blood. In contrast, CXCR4 is required for neutrophil mobilization from the bone marrow in response to G-CSF, CXCL2 (growth regulated gene β, GROβ), or Listeria monocytogenes infection. These results suggest that CXCR4 signaling is an essential regulator of neutrophil homeostasis under both basal and stress granulopoiesis conditions.
Methods
Mice
The LysMCre and CXCR4+/− mice12,33 were obtained from The Jackson Laboratory (Bar Harbor, ME) and conditional CXCR4 (CXCR4flox/flox)34 mice were obtained from Dr Dan R. Littman (New York University, New York, NY). All mice were inbred onto a C57BL/6 background at least 10 generations. Mice were genotyped by polymerase chain reaction (PCR) as described.12,33,34 Congenic wild-type C57BL/6 mice (B6.SJL-Ptprc* Pep3b BoyJ; The Jackson Laboratory) that have the Ly 5.1 gene were used to facilitate analysis of chimeric mice. Sex- and age-matched mice between 6 and 16 weeks of age were used in accordance with the guidelines of the Washington University Animal Studies Committee.
CXCR4 genotyping
Peripheral blood neutrophils (Gr-1brightSSChi) from CXCR4flox/− or LysMCre/+CXCR4flox/− mice were isolated using a MoFlo high-speed cell sorter (Dako, Carpinteria, CA). Genomic DNA was prepared using the ArchivePure DNA kit (5Prime, Gaithersburg, MD). The presence of either a floxed or deleted CXCR4 allele was revealed by PCR amplification using the listed primers: floxed: 5′-CCACCCAGGACAGTGTGACTCTAA-3′ and 5′-GATGGGATTTCTGTATGAGGATTAGC-3′; deleted: 5′-TCTAACGTCCCAGATCCACC-3′ and 5′-AACCAAACAAACCATCACACAG-3′.
Blood, bone marrow, or spleen analysis
Blood, bone marrow, and spleen cells were harvested from mice using standard techniques, and the number of nucleated cells in these tissues was quantified using a Hemavet automated cell counter (CDC Technologies, Oxford, CT). In some cases, manual leukocyte differentials were performed on Wright-stained blood smears (minimum 100 cells) or bone marrow cytospins (minimum 300 cells). As reported previously,26 the percentage of total body neutrophils in the blood was estimated using the neutrophil distribution index (NDI), which is calculated by dividing the number of neutrophils in the blood by the number in the blood and bone marrow. Blood and bone marrow neutrophils were calculated assuming a blood volume of 1.8 mL and a whole femur equivalent to 6% of the total bone marrow.35
Flow cytometry
Cells from bone marrow, blood, or spleen were depleted of red cells by hypotonic lysis, resuspended in phosphate-buffered saline (PBS) supplemented with 0.2% bovine serum albumin (BSA) and 0.1% sodium azide (FACS buffer), incubated for 10 minutes with anti-CD16/32 (Fc-block; BD Biosciences, San Diego, CA), and stained for 20 to 30 minutes at 4°C with one or more of the following antibodies as described in the text: allophycocyanin (APC)–conjugated anti-Ly6G (Gr-1; Invitrogen, Carlsbad, CA), biotinylated anti-CXCR4 (eBioscience, San Diego, CA), biotinylated anti-CD115 (GM-CSFR; eBioscience), PerCP-Cy5.5–conjugated anti-CD3e (BD Biosciences), PerCP-Cy5.5–conjugated anti-Ly5.1 (CD45.1; BD Biosciences), fluorescein (FITC)–conjugated anti-Ly5.2 (CD45.2; eBioscience), Alexa 488–conjugated anti-F4/80 (Invitrogen), and APC–Alexa 750–conjugated anti-CD45R (B220; Invitrogen). Biotinylated antibodies were detected with phycoerythrin (PE)–conjugated streptavidin (eBioscience). Isotype-matched antibodies and unstained cells were used as negative controls. Mature neutrophils were gated as Gr-1hiSSChi cells, and in some experiments F4/80 or CD115 was used to further exclude blood monocytes. Data were collected on a FACScan 5-color, 2-laser flow cytometer (BD Biosciences and Cytek Development, Fremont, CA) using CellQuest software (BD Biosciences) and analyzed with the FlowJo software package (TreeStar, Ashland, OR).
Colony-forming cell assay
Blood (20 μL), nucleated spleen cells (105), or bone marrow cells (2 × 104) were plated in 2.5 mL methylcellulose media supplemented with a cocktail of recombinant cytokines (MethoCult 3434; StemCell Technologies, Vancouver, BC) or 10 ng/mL recombinant human G-CSF (M3231; StemCell Technologies). Cultures were plated in duplicate and placed in a humidified chamber with 6% CO2 at 37°C. Colonies containing at least 50 cells were counted on days 7 to 10 of culture.
Bone marrow transplantation
Wild-type (Ly5.1) or LysMCre/+CXCR4flox/− (Ly5.2) bone marrow cells were harvested. A total of 2 million bone marrow cells were mixed at a 1:1 ratio and injected retroorbitally into lethally irradiated wild-type mice (Ly5.1). Recipient mice were conditioned with 1000 cGy from a 137Cesium source at a rate of approximately 95 cGy/minute before transplantation. Prophylactic antibiotics (trimethoprim-sulfamethoxazole; Alpharma, East Bridgewater, NJ) were given during the initial 2 weeks after transplantation. Mice were analyzed 8 to 10 weeks after transplantation.
BrdU labeling
Bromodeoxyuridine (BrdU, 10 mg/mL solution in 1× Dulbecco PBS; Sigma-Aldrich, St Louis, MO) was given by a single intraperitoneal injection at a dose of 1 to 2 mg/mouse. The percentage of BrdU+/Gr-1+ cells was determined by staining with APC-conjugated Gr-1 antibody followed by fixation, permeabilization, and intracellular staining with a FITC-conjugated anti-BrdU antibody using reagents from the BrdU Flow kit (BD Biosciences). The half-life (t1/2) of neutrophils in the blood was calculated using the formula N = N0e−λt where N0 = the peak number of labeled cells, N = the number of cells at time t, and λ = the decay constant.
Adoptive transfer experiments
Bone marrow cells were harvested, red blood cells removed by hypotonic lysis at room temperature for 2 minutes, and 8 to 10 × 106 cells injected intravenously into unconditioned wild-type (Ly5.1) recipients. Bone marrow cells were harvested 1.5 to 2.5 hours after infusion, and donor neutrophils were identified based on Gr-1 and Ly5.2 expression. The absolute number of donor neutrophils in the bone marrow was calculated assuming a whole femur equivalent to 6% of the total bone marrow.35 This number was divided by the total number of donor neutrophils that were infused to yield the percentage of donor neutrophils that had homed to the bone marrow.
Neutrophil mobilization
G-CSF.
Recombinant human G-CSF, a generous gift from Amgen (Thousand Oaks, CA), was diluted in PBS with 0.1% low-endotoxin BSA (Sigma-Aldrich) and administered by twice daily subcutaneous injection at a dose of 125 μg/kg per day for 5 days. Mice were analyzed 3 to 4 hours after the final injection on day 5. Some cohorts of mice were given a single subcutaneous injection of G-CSF (125 μg/kg), and peripheral blood was analyzed at the indicated times.
GROβ.
Human GROβ, a generous gift from Genzyme (Cambridge, MA), was reconstituted in sterile PBS and administered as a single subcutaneous injection at a dose of 100 μg/kg. Peripheral blood was analyzed at the indicated times.
L monocytogenes infection
Mice were infected intraperitoneally with a dose of 5 × 105 colony-forming units (CFU) of L monocytogenes strain EGD. Peritoneal cells were obtained by lavage with 10 mL cold PBS, nucleated cells counted, and manual leukocyte differential counts (200 cells) performed on Wright-stained cytospins. At 72 hours, L monocytogenes was quantified by homogenizing the spleens and livers in 10 mL PBS with 0.05% Triton X-100 and plating serial dilutions on LB-agar.
Statistical analysis
Statistical significance was assessed using a 2-tailed Student t test assuming equal variance for comparison of 2 groups, 1-way ANOVA with Bonferroni posttesting for experiments with more than 2 groups, or, for time-course experiments, a 2-way ANOVA with Bonferroni posttesting at individual time points. All data are presented as the mean plus or minus SEM.
Results
CXCR4 is selectively deleted in myeloid cells of LysMCre/+ CXCR4flox/− (MKO) mice
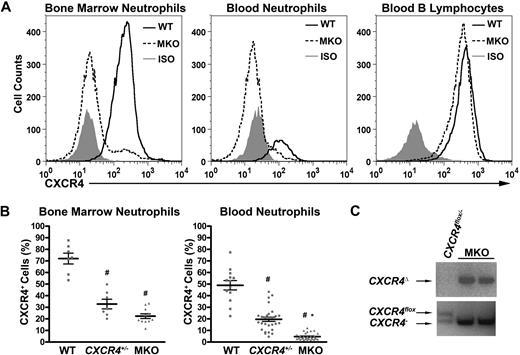
Mice expressing Cre-recombinase under the control of the myeloid lysozyme M promoter (LysMCre) were crossed with CXCR4+/− and CXCR4flox/flox mice to generate myeloid-restricted knockout (MKO) mice with the genotype LysMCre/+CXCR4flox/−. Efficient deletion of CXCR4 in mature neutrophils (Gr-1brightSSChi) of MKO mice in both the blood and bone marrow was observed by flow cytometry. Whereas CXCR4 expression was easily detectable in wild-type neutrophils in the blood, no expression was detected in MKO neutrophils (Figure 1A,B). CXCR4+/− mice had intermediate levels of CXCR4 expression on their neutrophils. There was some residual expression of CXCR4 in MKO bone marrow neutrophils, possibly representing less mature myeloid cells that had not yet undergone Cre-mediated excision of CXCR4. As expected, expression of CXCR4 was normal to slightly reduced in B lymphocytes and T lymphocytes from MKO mice compared with wild-type mice (Figure 1A and data not shown). PCR analysis of genomic DNA isolated from MKO blood neutrophils confirmed complete Cre-mediated excision of the coding region of exon 2 of the CXCR4 gene (Figure 1C). These data show that CXCR4 is efficiently and specifically deleted in myeloid cells in MKO mice.
CXCR4 is efficiently deleted in neutrophils from LysMCre/+ CXCR4flox/− (MKO) mice. (A) Representative histograms showing cell-surface CXCR4 expression in the mature neutrophil (Gr-1brightSSChi) population from bone marrow or peripheral blood or the peripheral blood B-lymphocyte (B-220+) population in wild-type (WT) or MKO mice. The isotype control (ISO) is shown in gray. (B) Cell-surface CXCR4 expression in the mature neutrophil population in the bone marrow or peripheral blood. Data represent the mean ± SEM. #P < .05 compared with wild-type mice; *P < .05 compared with CXCR4+/− mice. (C) Genomic DNA was isolated from MKO or control (CXCR4flox/− without LysMCre) blood neutrophils and the CXCR4 gene amplified using primers that specifically detected the deleted (CXCR4Δ), floxed (CXCR4flox), or null (CXCR4−) CXCR4 alleles.
CXCR4 is efficiently deleted in neutrophils from LysMCre/+ CXCR4flox/− (MKO) mice. (A) Representative histograms showing cell-surface CXCR4 expression in the mature neutrophil (Gr-1brightSSChi) population from bone marrow or peripheral blood or the peripheral blood B-lymphocyte (B-220+) population in wild-type (WT) or MKO mice. The isotype control (ISO) is shown in gray. (B) Cell-surface CXCR4 expression in the mature neutrophil population in the bone marrow or peripheral blood. Data represent the mean ± SEM. #P < .05 compared with wild-type mice; *P < .05 compared with CXCR4+/− mice. (C) Genomic DNA was isolated from MKO or control (CXCR4flox/− without LysMCre) blood neutrophils and the CXCR4 gene amplified using primers that specifically detected the deleted (CXCR4Δ), floxed (CXCR4flox), or null (CXCR4−) CXCR4 alleles.
Loss of CXCR4 results in the redistribution of neutrophils from the bone marrow to blood
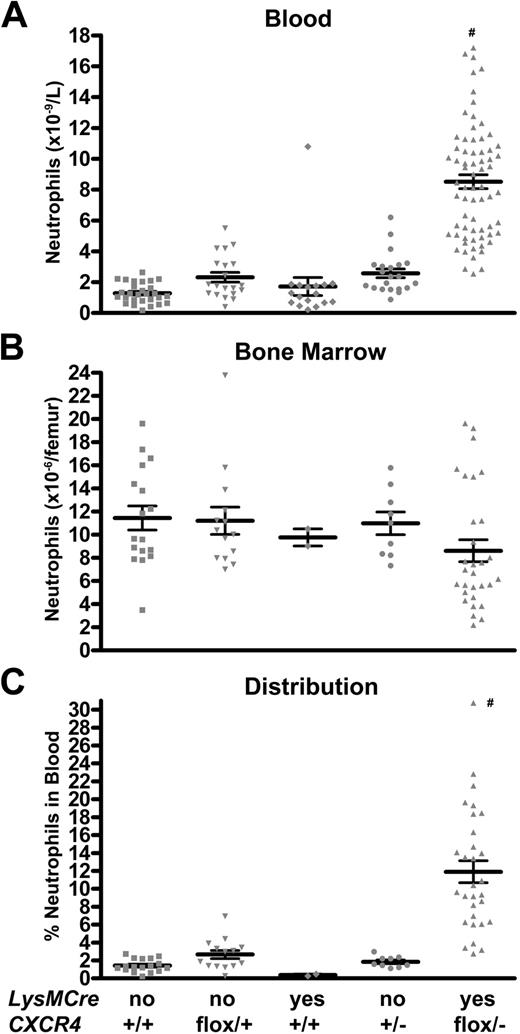
To assess the effect of the loss of CXCR4 on basal granulopoiesis, MKO or control mice housed in standard pathogen-free conditions were analyzed. We initially analyzed 4 separate control groups: wild-type mice, CXCR4flox/+ mice, LysMCre/+CXCR4+/+ mice, and CXCR4+/− mice. The wild-type, CXCR4flox/+, and LysMCre/+CXCR4+/+ mice had similar phenotypes for all assays and were subsequently pooled as control mice (Figure 2 and data not shown). MKO mice displayed a marked isolated neutrophilia in the peripheral blood (Figure 2A). The number of Gr-1brightSSChi cells (neutrophils) in the blood was 1.7 plus or minus 0.2 ×109/L in control mice versus 8.5 plus or minus 0.4 × 109/L in MKO mice (P < .001). This increase in circulating neutrophils was confirmed by manual inspection and differential counts of blood smears (absolute neutrophil count: 1.6 ± 0.4 × 109/L [control mice] vs 5.3 ± 0.7 × 109/L [MKO mice]; P < .001). Interestingly, no increase in immature myeloid cells in the blood was observed (data not shown). MKO mice displayed normal numbers of circulating B lymphocytes and T lymphocytes (data not shown). They also had normal numbers of circulating and splenic hematopoietic progenitor cells as measured by colony-forming assays (data not shown).
Basal granulopoiesis in MKO mice is characterized by a shift of neutrophils from the bone marrow to the blood. (A,B) The number of mature neutrophils (Gr-1brightSSChi) in the blood and bone marrow was quantified by flow cytometry in mice of the indicated genotype. (C) The neutrophil distribution index (NDI) was calculated to estimate the percentage of total body neutrophils in the blood, using the following formula: NDI = blood neutrophils/(blood + bone marrow neutrophils). Data represent the mean ± SEM. #P < .05 compared with all other groups.
Basal granulopoiesis in MKO mice is characterized by a shift of neutrophils from the bone marrow to the blood. (A,B) The number of mature neutrophils (Gr-1brightSSChi) in the blood and bone marrow was quantified by flow cytometry in mice of the indicated genotype. (C) The neutrophil distribution index (NDI) was calculated to estimate the percentage of total body neutrophils in the blood, using the following formula: NDI = blood neutrophils/(blood + bone marrow neutrophils). Data represent the mean ± SEM. #P < .05 compared with all other groups.
In the bone marrow of MKO mice, the number of Gr-1brightSSChi cells was reduced to 71.8% plus or minus 2.9% of control mice (Figure 2B). Although the Gr-1brightSSChi cell population reliably measures mature neutrophils in the blood, this population is more heterogeneous in the bone marrow with approximately 20% immature myeloid cells.36 Thus, to confirm these findings, we also performed manual leukocyte differentials of bone marrow cells (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Consistent with the flow cytometry data, a trend to decreased band and segmented neutrophils in the bone marrow of MKO mice was observed (control, 6.3 ± 0.9 × 106/femur vs MKO, 4.2 ± 1.0 × 106/femur; P = .14). Of note, the frequency of granulocytic precursors and number and cytokine responsiveness of myeloid progenitors were comparable in MKO and control mice (Figures S1,S2), suggesting that neutrophil differentiation was normal.
Neutrophil trafficking from the bone marrow was estimated by determining the percentage of neutrophils in the blood versus the total number of neutrophils in the bone marrow and blood.26 Consistent with previous reports, in control mice, only 1.9% plus or minus 0.2% of neutrophils are in the blood. Strikingly, in MKO mice, this percentage increased to 11.9% plus or minus 1.2% (Figure 2C; P < .001). There also was a significant increase in splenic neutrophils in MKO mice (number of neutrophils per spleen: 4.3 ± 1.3 × 106 [control] vs 13.3 ± 1.8 × 106 [MKO]; P < .01). Collectively, these data suggest that the loss of CXCR4 results in the redistribution of neutrophils from the bone marrow to the blood and spleen.
Loss of CXCR4 results in premature release of neutrophils from the bone marrow but normal clearance from the blood
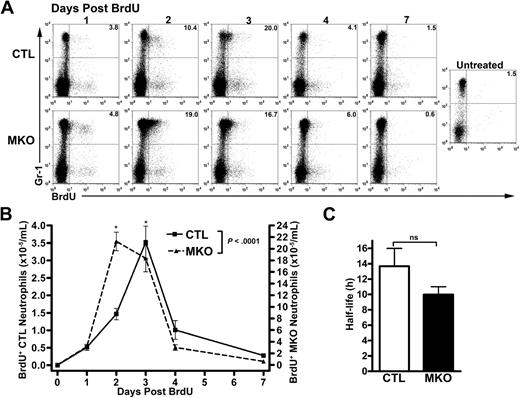
An increase in circulating neutrophil number could be caused by increased production of neutrophils in the bone marrow, increased release into the blood, decreased clearance from the blood, or some combination thereof. To investigate differences in kinetics of neutrophil release into the blood and the potential difference in neutrophil survival, control or MKO mice were given a single injection of bromodeoxyuridine (BrdU) to label newly synthesized neutrophils. The fate of blood neutrophils pulse-labeled with BrdU in vivo was determined by flow cytometry for Gr-1+BrdU+ cells (Figure 3A). In the bone marrow, a similar percentage of myeloid cells was labeled with BrdU, suggesting that loss of CXCR4 does not affect granulocytic cell proliferation (data not shown). Consistent with a role for CXCR4 in regulating neutrophil release, the transit time for labeled neutrophils to appear in the circulation was significantly reduced in MKO mice. In control mice, the peak number of labeled neutrophils in the blood occurred 72 hours after BrdU administration, whereas in the majority of MKO mice, the peak was at 46 hours (Figure 3B). However, there was no significant difference in the disappearance of labeled cells from the blood. Consistent with previous studies in mice and humans, the calculated half-life of control neutrophils was 13.7 plus or minus 2.3 hours. A similar calculated half-life was observed for MKO neutrophils (10.0 ± 1.0 hours, Figure 3C). Taken together, these data show that neutrophils lacking CXCR4 have accelerated release from the bone marrow but normal clearance from the blood.
Blood neutrophil half-life in MKO mice is normal. BrdU (2 mg) was administered to control (CTL) or MKO mice by a single intraperitoneal injection. Peripheral blood was obtained at the indicated time points and the number of BrdU+ Gr-1bright cells determined by flow cytometry. (A) Representative dot plots showing BrdU staining in the Gr-1bright (mature neutrophil) population. The numbers shown indicate the percentage of Gr-1bright cells that were BrdU+. (B) The absolute number of BrdU+ Gr-1bright neutrophils in the blood is shown. (C) Neutrophil half-life (t1/2) in the blood was calculated according to the formulas t1/2 = ln 2/λ and nt = n0e−λt where n0 is the number at a given time, nt is the number t hours later, and λ is the decay constant. The data shown represent the mean ± SEM of n = 10 to 11 mice in each group. *P < .05 compared with control mice at the same time point. ns indicates not significant.
Blood neutrophil half-life in MKO mice is normal. BrdU (2 mg) was administered to control (CTL) or MKO mice by a single intraperitoneal injection. Peripheral blood was obtained at the indicated time points and the number of BrdU+ Gr-1bright cells determined by flow cytometry. (A) Representative dot plots showing BrdU staining in the Gr-1bright (mature neutrophil) population. The numbers shown indicate the percentage of Gr-1bright cells that were BrdU+. (B) The absolute number of BrdU+ Gr-1bright neutrophils in the blood is shown. (C) Neutrophil half-life (t1/2) in the blood was calculated according to the formulas t1/2 = ln 2/λ and nt = n0e−λt where n0 is the number at a given time, nt is the number t hours later, and λ is the decay constant. The data shown represent the mean ± SEM of n = 10 to 11 mice in each group. *P < .05 compared with control mice at the same time point. ns indicates not significant.
CXCR4 directs homing of neutrophils to the bone marrow
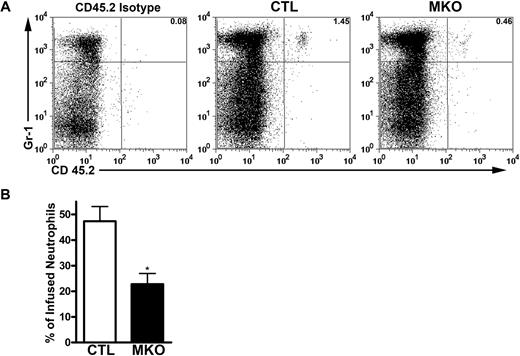
Previous studies suggested that aged neutrophils preferentially home to, and are cleared in, the bone marrow in a CXCR4-dependent fashion.17,31 To test this possibility, we measured the trafficking of neutrophils to the bone marrow after adoptive transfer of control or MKO neutrophils into wild-type recipient mice. Donor neutrophils from control mice were clearly detectable in recipient bone marrow 1.5 to 2.5 hours after transfer. However, homing of MKO neutrophils to the bone marrow was significantly reduced, although not eliminated completely (Figure 4). These data confirm that CXCR4 contributes to neutrophil homing to the bone marrow. However, given the normal circulating half-life of endogenous MKO neutrophils, it appears that the bone marrow is not an essential site of neutrophil clearance.
MKO neutrophils have impaired homing to the bone marrow. Bone marrow cells (8-10 × 106) from control (CTL) or MKO mice carrying the Ly 5.2 allele were adoptively transferred to WT recipients carrying the Ly 5.1 allele, enabling detection of infused neutrophils in the bone marrow by flow cytometry using an allele-specific CD45.2 antibody. (A) Representative dot plots showing donor neutrophils as the percentage of total bone marrow neutrophils 1.5 to 2.5 hours after infusion. (B) The percentage of transferred control or MKO neutrophils present in the bone marrow of recipient mice. Data represent the mean ± SEM of n = 8 to 12 recipients for each genotype from 3 separate experiments. *P < .05 compared with control neutrophils.
MKO neutrophils have impaired homing to the bone marrow. Bone marrow cells (8-10 × 106) from control (CTL) or MKO mice carrying the Ly 5.2 allele were adoptively transferred to WT recipients carrying the Ly 5.1 allele, enabling detection of infused neutrophils in the bone marrow by flow cytometry using an allele-specific CD45.2 antibody. (A) Representative dot plots showing donor neutrophils as the percentage of total bone marrow neutrophils 1.5 to 2.5 hours after infusion. (B) The percentage of transferred control or MKO neutrophils present in the bone marrow of recipient mice. Data represent the mean ± SEM of n = 8 to 12 recipients for each genotype from 3 separate experiments. *P < .05 compared with control neutrophils.
CXCR4 acts in a cell-autonomous fashion to retain neutrophils in the bone marrow
Recent studies suggest that loss of adhesion molecules on neutrophils may result in neutrophilia in a noncell-autonomous fashion through disruption of a homeostatic feedback loop.37,38 To determine whether CXCR4 acts in a cell-autonomous fashion to regulate neutrophil release, mixed bone marrow chimeras were generated in which hematopoiesis was reconstituted with a 1:1 ratio of wild-type to MKO cells. The mixed chimeras displayed chronic neutrophilia (absolute neutrophil count of 5.8 ± 0.4 × 109/L). Whereas the percentage of wild-type and MKO neutrophils in the bone marrow was similar, the great majority of neutrophils in the blood were derived from MKO cells (Figure 5A). Accordingly, the calculated neutrophil distribution index for MKO cells (23.9% ± 2.5%) was much higher than that for wild-type neutrophils (4.3% ± 0.6%; P < .001; Figure 5B). As a control, we also assessed the distribution of B lymphocytes in the mixed chimeras. Although a modest increase in MKO-derived B lymphocytes was observed, the ratio of MKO to wild-type cells in the bone marrow and blood was similar (Figure 5C). These data show that CXCR4 negatively regulates neutrophil release in a cell-autonomous fashion.
Trafficking of MKO neutrophils is altered in mixed chimeras. Whole bone marrow from wild-type (WT, Ly5.1) and MKO (Ly5.2) mice was mixed in a 1:1 ratio and transplanted into lethally irradiated wild-type (Ly5.1) recipients. After hematopoietic reconstitution (8-10 weeks), the bone marrow and blood were analyzed by flow cytometry. The numbers at the top of the columns indicate the fold increase over the wild-type cells. (A) Mature neutrophils (Gr-1bright CD115−). (B) Neutrophil distribution index. (C) B lymphocytes (B220+). The data represent the mean ± SEM of n = 18 recipients from 2 separate transplants. *P < .05 compared with wild-type cells.
Trafficking of MKO neutrophils is altered in mixed chimeras. Whole bone marrow from wild-type (WT, Ly5.1) and MKO (Ly5.2) mice was mixed in a 1:1 ratio and transplanted into lethally irradiated wild-type (Ly5.1) recipients. After hematopoietic reconstitution (8-10 weeks), the bone marrow and blood were analyzed by flow cytometry. The numbers at the top of the columns indicate the fold increase over the wild-type cells. (A) Mature neutrophils (Gr-1bright CD115−). (B) Neutrophil distribution index. (C) B lymphocytes (B220+). The data represent the mean ± SEM of n = 18 recipients from 2 separate transplants. *P < .05 compared with wild-type cells.
Neutrophil mobilization by G-CSF or GROβ is impaired in the absence of CXCR4
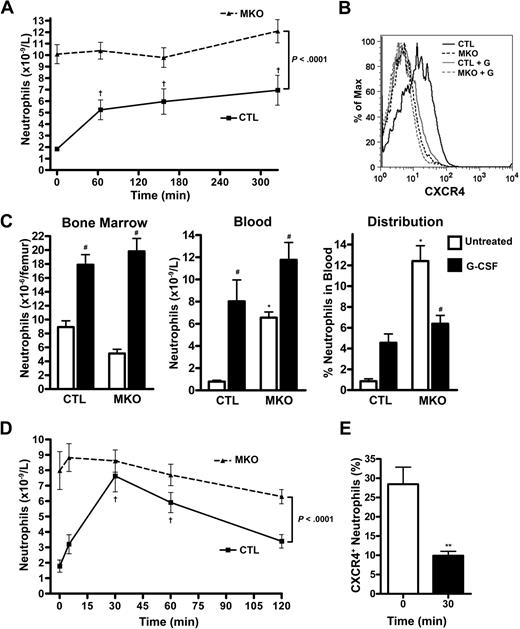
A key feature of the innate immune response is the capacity to rapidly increase neutrophil number in the blood in response to infection or other stresses. Certain cytokines, chemokines, and bacterial products are thought to mediate the stress granulopoiesis response. To examine the contribution of CXCR4 signaling in stress granulopoiesis, we first characterized the neutrophil response to G-CSF, the prototypical mobilizing cytokine. After a single injection of G-CSF, neutrophil number in the blood of control mice increased 3.9- plus or minus 0.7-fold (Figure 6A), with peak levels occurring after 6 hours before returning to near-baseline levels at 24 hours. As reported previously,25 this was associated with a marked decrease in surface CXCR4 expression on blood neutrophils (Figure 6B). In contrast, no change in neutrophil counts in the blood was observed after G-CSF treatment of MKO mice (Figure 6A). Next, we studied granulopoiesis after 5 days of G-CSF administration. In control mice, an 11.8- plus or minus 1.6-fold increase in circulating neutrophils was observed (Figure 6C). This increase was secondary to both increased production (as evidenced by a modest increase in bone marrow neutrophils) and increased neutrophil release (as evidenced by an increase in the NDI). Of note, previous studies showed that G-CSF does not alter the kinetics of neutrophil clearance from the blood.2,3,26 In MKO mice, a similar increase in bone marrow neutrophils after G-CSF treatment was observed, suggesting that G-CSF–induced increases in neutrophil production were intact. In contrast, though a modest increase in circulating neutrophils was observed in MKO mice after G-CSF treatment, this appears to be secondary mainly to increased production, rather than release, as no increase in the NDI was seen.
Neutrophil mobilization by G-CSF or GROβ is abrogated in MKO mice. (A) Mice (n = 5 per group) were given a single subcutaneous injection of G-CSF (125 μg/kg) and the absolute neutrophil count measured at the indicated times. †P < .05 compared with time 0. (B) Representative histograms showing cell surface CXCR4 expression on blood neutrophils from control (CTL) or MKO mice at baseline and 65 minutes after a single dose of G-CSF (+G). (C) Mice (n = 8-11 per group) were treated with G-CSF (125 μg/kg per day, twice daily injections) for 5 days, and neutrophils in the bone marrow and blood were quantified. The calculated neutrophil distribution index is shown in the far right panel. *P < .05 compared with control mice at the same time point; #P < .05 compared with untreated mice of the same genotype. (D) Mice (n = 9-12 per group) were given a single subcutaneous injection of GROβ (100 μg/kg), and the absolute neutrophil count was determined at the indicated times. †P < .05 compared with time 0. (E) CXCR4 cell-surface expression on peripheral blood neutrophils from control mice was determined by flow cytometry at baseline and at the time of peak mobilization, 30 minutes after GROβ administration. **P < .001. Data represent the mean ± SEM.
Neutrophil mobilization by G-CSF or GROβ is abrogated in MKO mice. (A) Mice (n = 5 per group) were given a single subcutaneous injection of G-CSF (125 μg/kg) and the absolute neutrophil count measured at the indicated times. †P < .05 compared with time 0. (B) Representative histograms showing cell surface CXCR4 expression on blood neutrophils from control (CTL) or MKO mice at baseline and 65 minutes after a single dose of G-CSF (+G). (C) Mice (n = 8-11 per group) were treated with G-CSF (125 μg/kg per day, twice daily injections) for 5 days, and neutrophils in the bone marrow and blood were quantified. The calculated neutrophil distribution index is shown in the far right panel. *P < .05 compared with control mice at the same time point; #P < .05 compared with untreated mice of the same genotype. (D) Mice (n = 9-12 per group) were given a single subcutaneous injection of GROβ (100 μg/kg), and the absolute neutrophil count was determined at the indicated times. †P < .05 compared with time 0. (E) CXCR4 cell-surface expression on peripheral blood neutrophils from control mice was determined by flow cytometry at baseline and at the time of peak mobilization, 30 minutes after GROβ administration. **P < .001. Data represent the mean ± SEM.
We also examined neutrophil release after treatment with GROβ, a well-characterized mobilizing chemokine. In control mice, GROβ treatment induced a 7.4- plus or minus 2.1-fold increase in circulating neutrophils that peaked 30 minutes after injection and nearly returned to baseline after 2 hours (Figure 6D). In contrast, no significant increase in circulating neutrophils was observed in MKO mice. Surprisingly, CXCR4 expression on neutrophils isolated from control mice treated with GROβ was significantly reduced at the time of peak mobilization compared with pretreatment levels (Figure 6E). These data suggest that G-CSF– and GROβ-induced neutrophil release from the bone marrow are dependent on CXCR4 signals.
Neutrophil mobilization in response to L monocytogenes infection is impaired in the absence of CXCR4 but homing of neutrophils to the peritoneum and bacterial clearance is normal
To further define the requirement for CXCR4 in emergency granulopoiesis, control and MKO mice were infected intraperitoneally with L monocytogenes. Infection with L monocytogenes is an established model of emergency granulopoiesis, and neutrophils are known to play a key role in the clearance of this infection.39 Clearance of bacteria was similar in MKO and wild-type mice; no difference in survival or spleen and liver bacterial load was observed after L monocytogenes challenge (Figure 7A,B). In control mice, a 3.4- plus or minus 0.6-fold increase in circulating neutrophils was observed 24 hours after bacterial challenge that remained elevated for the duration of the assay. In contrast, MKO mice failed to increase their circulating neutrophils in response to infection with this pathogen (Figure 7C). However, neutrophils in MKO mice were able to emigrate into the peritoneal space in similar numbers compared with control mice (Figure 7D). These data show that CXCR4, although required for maximal neutrophil mobilization into the blood after L monocytogenes infection, is dispensable for neutrophil emigration to the peritoneum.
MKO mice have impaired blood neutrophil mobilization but normal neutrophil recruitment to the peritoneum in response to Listeria infection. Control (CTL) or MKO mice were infected intraperitoneally with L monocytogenes. (A) Survival was assessed in mice (n = 12 per group) from 2 separate infections with 9.8 to 11.2 × 105 CFU of bacteria. (B) The bacterial titer in the spleen and liver of control and MKO mice (n = 8 per group) was determined 72 hours after infection with 2.1 to 7.2 × 105 CFU of bacteria. (C) Blood neutrophil counts were assessed by flow cytometry at the indicated times after infection with 2.1 to 9.8 × 105 CFU of bacteria (n = 8-19 mice per group depending on the time). (D) Shown is the number of neutrophils in the peritoneum at the indicated times after infection with 2.1 to 7.2 × 105 CFU of bacteria (n = 5-8 mice per group depending on the time). Data represent the mean ± SEM. †P < .05 compared with time 0.
MKO mice have impaired blood neutrophil mobilization but normal neutrophil recruitment to the peritoneum in response to Listeria infection. Control (CTL) or MKO mice were infected intraperitoneally with L monocytogenes. (A) Survival was assessed in mice (n = 12 per group) from 2 separate infections with 9.8 to 11.2 × 105 CFU of bacteria. (B) The bacterial titer in the spleen and liver of control and MKO mice (n = 8 per group) was determined 72 hours after infection with 2.1 to 7.2 × 105 CFU of bacteria. (C) Blood neutrophil counts were assessed by flow cytometry at the indicated times after infection with 2.1 to 9.8 × 105 CFU of bacteria (n = 8-19 mice per group depending on the time). (D) Shown is the number of neutrophils in the peritoneum at the indicated times after infection with 2.1 to 7.2 × 105 CFU of bacteria (n = 5-8 mice per group depending on the time). Data represent the mean ± SEM. †P < .05 compared with time 0.
Discussion
There is accumulating evidence suggesting that CXCR4 is a key regulator of neutrophil homeostasis. Perhaps most convincing is the identification of truncation mutations of CXCR4 in most cases of WHIM syndrome. Patients with WHIM syndrome display neutropenia despite normal to increased numbers of neutrophils in the bone marrow. The CXCR4 truncation mutations confer enhanced responsiveness to CXCL12, suggesting a model in which increased CXCR4 signaling in WHIM neutrophils results in their abnormal retention in the bone marrow.18,19,40,41 Consistent with this model, treatment of humans or mice with AMD3100, a specific CXCR4 antagonist, results in the rapid mobilization of neutrophils into the blood.15,16 However, direct testing of the contribution of CXCR4 to neutrophil homeostasis has been limited by the embryonic lethality of CXCR4-deficient mice and the severe engraftment defect of CXCR4−/− hematopoietic stem cells.10,11,13,32 In the present study, we characterized granulopoiesis and neutrophil trafficking in transgenic mice carrying a myeloid-specific deletion of CXCR4. The marked neutrophilia present in these mice confirms a key role for CXCR4 signaling in the regulation of neutrophil homeostasis.
Neutrophil homeostasis in the blood is maintained by balancing neutrophil production, release from the bone marrow, and clearance. There is evidence implicating CXCR4 in the regulation of all 3 of these processes. In WHIM syndrome, there are reports of dysmorphic neutrophils and increased neutrophil apoptosis, suggesting defective granulopoiesis.42,43 Moreover, in CXCR4−/− fetal liver chimeras, there is a decrease in myeloid progenitors and precursors.13 Our study was not designed to assess the contribution of CXCR4 to granulopoiesis, because CXCR4 expression in MKO mice was maintained until the final stages of myeloid development. Not surprisingly, granulopoiesis, as measured by leukocyte differentials, BrdU incorporation in myeloid precursors, and number and cytokine responsiveness of myeloid progenitors was normal in MKO mice. Despite normal granulopoiesis, marked neutrophilia and increased splenic neutrophils were observed in MKO mice, suggesting an alteration in neutrophil release or clearance.
As noted previously, the rapid mobilization of neutrophils into the blood by AMD3100 suggests a role for CXCR4 in regulating neutrophil release. Indeed, the striking redistribution of neutrophils from the bone marrow to blood in MKO mice suggests enhanced neutrophil release. Consistent with this conclusion, BrdU-labeled neutrophils appeared in the blood more rapidly in MKO versus control mice. A recent report suggested that CXCR4 may contribute to neutrophil clearance from the blood by directing senescent neutrophils to the bone marrow.31 Consistent with this finding, we observed decreased homing of CXCR4-deficient neutrophils to the bone marrow after adoptive transfer. On the other hand, the half-life of CXCR4-deficient neutrophils in the blood was comparable with wild-type neutrophils, suggesting that CXCR4 is not a major regulator of neutrophil clearance from the blood and that the bone marrow represents a nonessential site of neutrophil removal. Collectively, these data suggest that CXCR4 maintains neutrophil homeostasis primarily by regulating neutrophil release from the bone marrow.
Neutrophil homeostasis can be regulated in both a cell-autonomous and noncell-autonomous fashion. In a series of elegant studies, Forlow et al37 and Stark et al38 showed that β2-integrin–deficient neutrophils induced neutrophilia in a noncell-autonomous fashion through the suppression of a negative feedback loop that senses the number of neutrophils that have emigrated into the tissue. They showed that the presence of even a small number of wild-type neutrophils activated the feedback loop and restored normal neutrophil number in the blood. In contrast, we observed persistent neutrophilia and preferential release of CXCR4-deficient neutrophils in mixed bone marrow chimeras reconstituted with both wild-type and MKO bone marrow cells. These data show that CXCR4 acts in a cell-autonomous fashion to regulate neutrophil release from the bone marrow.
G-CSF, the prototypical neutrophil-mobilizing cytokine, is a key regulator of both basal and stress granulopoiesis.44 Previous studies have shown that treatment with G-CSF results in a decrease in CXCL12 expression in the bone marrow.26-28 Moreover, G-CSF treatment leads to decreased surface expression of CXCR4 on neutrophils, a finding confirmed in the present study.25 These data suggest the hypothesis that disruption of CXCR4 signaling may contribute to G-CSF–induced neutrophil mobilization. However, G-CSF treatment also induces other changes in the bone marrow microenvironment, such as the release of proteases,45 that might contribute to neutrophil mobilization. Thus, the relative importance of CXCR4 signaling in mediating neutrophil mobilization by G-CSF is unclear. In the present study, we show that G-CSF, though stimulating neutrophil production, did not stimulate neutrophil release from the bone marrow in the absence of CXCR4 signals.
Of note, despite the near normal number of morphologically mature neutrophils in the bone marrow, it is possible that the mobilizable pool of neutrophils in MKO mice is exhausted. Arguing against this possibility, blood neutrophil counts in MKO mice doubled after the administration of a solution of propylene glycol (data not shown). Collectively, these data suggest that disruption of CXCL12/CXCR4 signaling is the dominant pathway by which G-CSF induces neutrophil mobilization.
Besides G-CSF, there are several neutrophil mobilizing agents that are thought to contribute to the stress granulopoiesis response. Most notable among these agents are chemokines. The rapidity of neutrophil mobilization by chemokines (minutes to hours) compared with G-CSF (hours to days) suggests distinct mechanisms of mobilization. Consistent with this idea, the CXCR2-chemokine KC (CXCL1) can synergize with G-CSF or AMD3100 to induce neutrophil mobilization.17,31,46 Surprisingly, herein we show that GROβ-induced neutrophil mobilization is abrogated in MKO mice. This result suggests 2 possibilities: (1) the GROβ-mobilizable pool of neutrophils is depleted in MKO mice. (2) GROβ-induced mobilization is dependent on CXCR4 signaling in neutrophils. In support of the latter possibility, several recent reports showed that treatment of wild-type neutrophils or monocytes with CXCR2 ligands resulted in impaired CXCR4 signaling, presumably through heterologous desensitization.17,47 In addition, we observed a significant decrease in cell-surface CXCR4 expression on neutrophils after GROβ treatment. Together, these data suggest that disruption of CXCL12/CXCR4 signaling is a common mechanism by which chemokines and cytokines induce neutrophil release.
The stress granulopoiesis response to infection requires the coordinated expression of many different cytokines and chemokines. In this study, we used a model of L monocytogenes infection to further explore the contribution of CXCR4 signals in the stress granulopoiesis response. Interestingly, the neutrophil mobilization response in MKO mice after L monocytogenes infection was abrogated, suggesting that modulation of CXCR4 signaling may be a common mechanism of neutrophil release.
In summary, our data provide new evidence that CXCR4 is a key regulator of neutrophil homeostasis under basal and stress conditions. CXCR4 signals act primarily to regulate neutrophil trafficking from the bone marrow, and disruption of CXCR4 signals may represent a common mechanism by which cytokines and chemokines induce neutrophil release from the bone marrow. These data suggest that pharmacologic agents that modulate CXCR4 signaling may be effective for controlling neutrophil responses in infectious and inflammatory diseases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dan R. Littman (New York University School of Medicine) for the CXCR4flox/flox mice.
This work was supported by the Abbott Scholar Award (D.W.W.)and the National Institutes of Health (Bethesda, MD) K08 AI079011-01 (D.W.W.), RO1 HL60772 (D.C.L.).
National Institutes of Health
Authorship
Contribution: K.J.E. designed and performed research, analyzed data, and wrote the paper; J.M.M. performed research and collected data; D.W.W. designed and performed experiments and contributed reagents; and D.C.L. supervised all of the research and edited the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Daniel C. Link, Division of Oncology, Department of Medicine, 660 S Euclid Ave, Campus Box 8007, St Louis, MO 63110; e-mail: dlink@dom.wustl.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal