Abstract
The farnesyltransferase inhibitor tipifarnib exhibits modest activity against acute myelogenous leukemia. To build on these results, we examined the effect of combining tipifarnib with other agents. Tipifarnib inhibited signaling downstream of the farnesylated small G protein Rheb and synergistically enhanced etoposide-induced antiproliferative effects in lymphohematopoietic cell lines and acute myelogenous leukemia isolates. We subsequently conducted a phase 1 trial of tipifarnib plus etoposide in adults over 70 years of age who were not candidates for conventional therapy. A total of 84 patients (median age, 77 years) received 224 cycles of oral tipifarnib (300-600 mg twice daily for 14 or 21 days) plus oral etoposide (100-200 mg daily on days 1-3 and 8-10). Dose-limiting toxicities occurred with 21-day tipifarnib. Complete remissions were achieved in 16 of 54 (30%) receiving 14-day tipifarnib versus 5 of 30 (17%) receiving 21-day tipifarnib. Complete remissions occurred in 50% of two 14-day tipifarnib cohorts: 3A (tipifarnib 600, etoposide 100) and 8A (tipifarnib 400, etoposide 200). In vivo, tipifarnib plus etoposide decreased ribosomal S6 protein phosphorylation and increased histone H2AX phosphorylation and apoptosis. Tipifarnib plus etoposide is a promising orally bioavailable regimen that warrants further evaluation in elderly adults who are not candidates for conventional induction chemotherapy. These clinical studies are registered at www.clinicaltrials.gov as #NCT00112853.
Introduction
Response rates and survival in elderly (age > 70 years) acute myelogenous leukemia (AML) patients remain limited. Part of this poor response reflects the inability of very elderly patients to tolerate intensive chemotherapy.1–3 Equally important, however, are the genetic complexity and inherent resistance of these AMLs to the cytotoxic effects of traditional cytotoxic agents because of their frequent evolution from antecedent hematologic disorders, such as myelodysplasia (MDS), which itself has evolved in the setting of toxin exposures.1–5 These AMLs exhibit complex genetic profiles that probably reflect cumulative genomic damage, a feature associated with drug resistance and poor clinical outcome.1–5
Farnesyltransferase inhibitors (FTIs) are a new class of therapeutic agents that are undergoing extensive clinical testing in various hematologic malignancies.6–11 These agents inhibit farnesyltransferase, an enzyme that transfers the 15-carbon farnesyl group to various polypeptide acceptors, including the chaperone HDJ-2, nuclear lamins, and small guanosine triphosphate-binding polypeptides of the Ras, Rho, and Rheb families.12–14 Inhibiting farnesylation of these polypeptides leads to diminished cell proliferation and, in some model systems, cell death. These cytotoxic effects have been attributed to FTI-induced inhibition of prosurvival signaling by Akt,15,16 the Rheb target mammalian target of rapamycin (mTOR),17,18 or mitogen-activated protein kinases.19–21 Alternatively, it has been suggested that FTIs induce apoptosis by causing up-regulation of the proapoptotic Bcl-2 family members Bax,22 Bak,23 or Puma.24
Tipifarnib (R115777, Zarnestra), an orally bioavailable nonpeptidomimetic methylquinolinone FTI, exhibits clinical activity in patients with myeloid malignancies, including elderly adults with AML who are not candidates for traditional cytotoxic chemotherapy,25,26 high-risk MDS,27–29 myeloproliferative disorders (MPDs),30 and imatinib-resistant chronic myelogenous leukemia.31 A phase 2 study of tipifarnib 600 mg twice daily for 21 of 28 to 63 days in 158 older adults (median age, 74 years) with previously untreated, poor-risk AML yielded a complete remission (CR) rate of 14%, with an additional 10% partial remission (PR) or hematologic improvement (HI).26 Among CR patients, 82% had prior MDS and 40% had adverse cytogenetics.
Although it is possible that the antileukemic effects of tipifarnib might be enhanced by adjusting the dose or schedule, an alternative approach would be to combine this agent with existing antileukemic drugs. Toward this end, previous in vitro studies revealed that the antiproliferative effects in human AML cells are additive when tipifarnib is combined with cladrabine or fludarabine32 and synergistic when tipifarnib is combined with bortezomib33 or daunorubicin,34 possibly reflecting competitive inhibition of P-glycoprotein (Pgp) in the latter case.
In an attempt to build on earlier results, the present study examined the effects of combining tipifarnib with other oral antileukemic agents. Preclinical results demonstrated synergy when myeloid and lymphoid cell lines as well as AML clinical specimens were treated with tipifarnib and the topoisomerase II poison etoposide,35,36 which has activity in AML as a single agent37–39 and when combined with drugs, such as idarubicin.40–42 Accordingly, we conducted a phase 1 trial of oral tipifarnib plus oral etoposide (T + E), with escalating doses of both drugs and exploration of 2 different durations of tipifarnib administration (14 vs 21 days), to examine the safety and tolerability of T + E in patients who were not candidates for conventional induction chemotherapy, determine the effects of the combination on leukemic cells harvested on day 8 of therapy, and preliminarily assess the antileukemic activity of the combination.
Methods
Materials
Tipifarnib was provided by David End (Johnson & Johnson, Springhouse, PA); and etoposide was purchased from BIOMOL Research Laboratories (Plymouth Meeting, PA). Antibodies were obtained as follows: anti–phospho-Ser139-histone H2AX from Upstate Biotechnology (Charlottesville, VA), mouse IgG1 isotype control from Southern Biotechnology (Birmingham, AL), Alexa Fluor 488 goat anti–mouse IgG from Invitrogen (Carlsbad, CA), and antibodies for immunoblotting from Cell Signaling Technology (Danvers, MA) or as previously described.43
Preclinical studies
HL-60,44,45 Jurkat,46 and DoHH2 cells (from Thomas Witzig, Mayo Clinic) were cultured in RPMI 1640 with 10% heat-inactivated fetal calf serum, 100 units/mL penicillin G, 100 μg/mL streptomycin, and 2 mM glutamine. Jurkat-derived I2.1 cells, which lack Fas-associated death domain and are resistant to death ligand–induced apoptosis,47 were grown as previously described.44 U937 AML cells (ATCC, Manassas, VA) were cultured as specified by the supplier.
To assess effects on colony formation, HL-60 cells plated in 0.3% agar45,48 were exposed to increasing concentrations of one or both drugs for 10 to 14 days; and colonies containing more than 50 cells were counted. Likewise, aliquots containing 6 × 105 freshly isolated marrow mononuclear cells plated in Methocult medium (StemCell Technologies, Vancouver, BC) containing one or both drugs (or diluent) were examined on day 14 for leukemic colonies according to established morphologic criteria.49
Patient eligibility and selection
Between April 2005 and November 2006, 101 adults more than 70 years of age with Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0 to 2, pathologically confirmed (using World Health Organization criteria),50 newly diagnosed de novo or secondary (MDS, MPD, treatment-related) AML excluding acute progranulocytic leukemia, were evaluated for eligibility using previously described criteria.26 Patients were ineligible if they had peripheral blast count more than 30 000/μL or a projected doubling time of less than 2 days, but cytoreduction with hydroxyurea was permitted until 24 hours before T + E. Prior therapy for MDS (cytokines, thalidomide/lenalidomide, interferon, 5-azacytidine/decitabine) was not exclusionary. All patients provided written informed consent with the approval of the Institutional Review Boards and according to the guidelines of each participating institution, in accordance with the Declaration of Helsinki.
Treatment schema
To determine whether T + E would interact positively with respect to clinical outcome and/or negatively with respect to toxicity, T + E were varied over multiple dose levels. The planned dose-escalation schema is depicted in Table 1. Initially, we fixed the etoposide dose (100 mg orally daily on days 1-3 and 8-10) while escalating tipifarnib over 3 dose levels (300 mg, 400 mg, 600 mg orally twice a day). If no more than 1 of 6 patients experienced dose-limiting toxicity (DLT) at the highest tipifarnib dose (600 mg twice a day), then a higher etoposide dose (150 mg daily on days 1-3 and 8-10) was instituted while escalating tipifarnib across the same 3 dose levels. Tipifarnib was administered for 14 (“A” cohorts) or 21 days (“B” cohorts).
Dose escalation schema for oral tipifarnib plus oral etoposide
| Dose level (no. patients treated) . | Daily tipifarnib dose, mg BID . | Etoposide dose, days 1-3 and 8-10, mg QD . |
|---|---|---|
| Tipifarnib 14-day schedule | ||
| 1A (6) | 300 | 100 |
| 2A (6) | 400 | 100 |
| 3A (6) | 600 | 100 |
| 4A (6) | 300 | 150 |
| 5A (6) | 400 | 150 |
| 6A (6) | 600 | 150 |
| 7A (6) | 300 | 200 |
| 8A (6) | 400 | 200 |
| 9A (6) | 600 | 200 |
| Tipifarnib 21-day schedule | ||
| 1B (6) | 300 | 100 |
| 2B (6)* | 400 | 100 |
| 3B (0) | 600 | 100 |
| 4B (6) | 300 | 150 |
| 5B (6) | 400 | 150 |
| 6B (0) | 600 | 150 |
| 7B (6)* | 300 | 200 |
| 8B (0) | 400 | 200 |
| 9B (0) | 600 | 200 |
| Dose level (no. patients treated) . | Daily tipifarnib dose, mg BID . | Etoposide dose, days 1-3 and 8-10, mg QD . |
|---|---|---|
| Tipifarnib 14-day schedule | ||
| 1A (6) | 300 | 100 |
| 2A (6) | 400 | 100 |
| 3A (6) | 600 | 100 |
| 4A (6) | 300 | 150 |
| 5A (6) | 400 | 150 |
| 6A (6) | 600 | 150 |
| 7A (6) | 300 | 200 |
| 8A (6) | 400 | 200 |
| 9A (6) | 600 | 200 |
| Tipifarnib 21-day schedule | ||
| 1B (6) | 300 | 100 |
| 2B (6)* | 400 | 100 |
| 3B (0) | 600 | 100 |
| 4B (6) | 300 | 150 |
| 5B (6) | 400 | 150 |
| 6B (0) | 600 | 150 |
| 7B (6)* | 300 | 200 |
| 8B (0) | 400 | 200 |
| 9B (0) | 600 | 200 |
BID indicates twice a day and QD, once a day.
Dose-limiting toxicity reached.
Each treatment cycle was 28 days, followed by a rest period of up to 35 days to allow count recovery. Subsequent cycles began between day 29 and day 64 of the previous cycle. Patients were eligible to receive a second cycle if stable disease or any clinical improvement (CR, PR, HI) was achieved. Patients achieving CR were permitted up to 6 cycles of T + E. Patients achieving PR or HI could receive T + E until disease progression or unacceptable toxicity ensued. All patients received supportive care as previously described.26 Growth factors were not permitted.
Response and toxicity evaluation
Bone marrow aspiration and biopsy were performed before treatment and at hematologic recovery or when leukemia regrowth was suspected clinically, typically 14 to 21 days after the last dose of tipifarnib. Hematologic recovery and response criteria, as previously described,26 are consistent with Cheson et al.51
National Cancer Institute (NCI) Common Toxicity Criteria, version 3.0, were used to describe and grade all adverse events. DLT was based on toxicities incurred during the first treatment cycle. If 2 of 3 or 2 of 6 patients developed DLT, the maximum tolerated dose was defined as one dose level below which DLTs were observed. Myelosuppression was not considered dose limiting except where marrow hypoplasia persisted for more than or equal to 42 days with marrow cellularity less than or equal to 5% and no evidence of leukemia. T + E was discontinued for progressive disease or grade 4 nonhematologic toxicity. T + E was withheld temporarily for more than or equal to grade 2 neurotoxicity or nephrotoxicity, grade 3 other nonhematologic toxicity (excluding alopecia or controlled nausea and vomiting), or grade 4 granulocytopenia or platelets less than 20 000/μL lasting more than 3 weeks after completion of each 28-day cycle. T + E could be resumed at a lower tipifarnib dose after resolution to less than grade 1 nonhematologic toxicity within 28 days of first occurrence.
Statistical methods
Although this is a phase 1 study, both drugs are known to have activity in this patient population. Thus, both clinical response and toxicity criteria were used to select the recommended phase 2 dose. The ideal combination for chronic administration would evince maximal response and have no additive or synergistic nonhematologic toxicities grade 2 or greater. For each criterion (efficacy and toxicity), a response surface was constructed using a flexible 2-dimensional polynomial.52 Model building was performed to ensure that the model was not overspecified, which could lead to erroneous dose selection; and the fitted model was compared with the empirical results at each dose combination. The maximum point (clinical response) or minimum point (maximum toxicity grade) in the surface was determined using the fitted model. Multiple dose schedules were then selected for further examination based on the results of both the toxicity model and efficacy model, with the aim of designing and conducting a randomized phase 2 trial. We intended to look for dosing regions with high efficacy and acceptable toxicity. However, because of a flat surface describing the relationship between dose and toxicity, doses taken forward were determined primarily based on the efficacy response surface. Logistic regression was used to assess the association between grade 3 and higher toxicities and patient characteristics, doses of both agents, and schedule. Ordinal logistic regression was also performed to explore the same associations, treating toxicity grade as ordinal as a comparison. A multiple regression was also performed on response to determine whether patient characteristics were associated with response and how that may affect inferences comparing the single-agent tipifarnib study to this combination study of T + E. Simple regressions were first fit to models with toxicity as the outcome and patient characteristics as predictors (age, number of comorbidities, secondary AML, advanced cytogenetics). Patient characteristics that had a P value less than .10 were then included in a multiple regression with dose and schedule variables. The final model was then used to demonstrate the association of dose and schedule with toxicity, adjusting for patient characteristics. Proportions were compared using Fisher exact test. Kaplan-Meier methods were used for describing overall survival (OS) and disease-free survival (DFS). Results were analyzed as of May 1, 2008.
Laboratory correlates
AML marrow blasts obtained before therapy (day 0) and on day 8 before drug dosing were enriched to more than 70% blasts by Ficoll-Hypaque density gradient separation. The percentages of cells with extractable chromatin were estimated by calculating sub-2N DNA in ungated specimens after cell-cycle analysis using propidium iodide as described.53 Samples were stained with antibody to phosphorylated histone H2AX,54,55 which is present in cells containing unrepaired DNA damage54 or undergoing apoptosis,56 and examined by flow cytometry as described.53
Results
Preclinical study
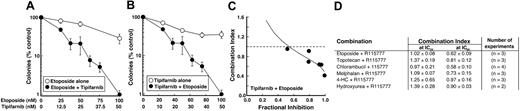
To identify orally bioavailable antileukemic agents that might enhance the antiproliferative effects of tipifarnib, the effects of tipifarnib and other agents on colony-forming ability of HL-60 cells were examined. Clinically achievable concentrations25,57,58 of T + E applied simultaneously inhibited colony formation more than either drug alone (Figure 1A,B). Formal analysis by the median effect method59 demonstrated synergy at concentrations that exceeded the IC50 of the combination, as indicated by a combination index less than 1 (Figure 1C,D). Tipifarnib also synergized with chlorambucil, melphalan, and, to a lesser extent, topotecan, but not hydroxyurea or the cyclophosphamide prodrug 4-hydroperoxycyclophosphamide (Figure 1D).
Antiproliferative effects of tipifarnib-containing combinations. (A,B) Log-phase HL-60 cells were treated with the indicated concentration of etoposide (○, A), tipifarnib (○, B), or the combination at a 2:1 ratio (●, A,B). (C) Combination index values calculated from the data shown in panels A and B when data were analyzed by the median effect method59 as previously described in detail.60 (D) Summary of results obtained when tipifarnib was combined with multiple orally bioavailable antileukemic agents. Results are expressed as the mean ± SD of combination index values at the IC50 and IC90 in the indicated number of independent experiments.
Antiproliferative effects of tipifarnib-containing combinations. (A,B) Log-phase HL-60 cells were treated with the indicated concentration of etoposide (○, A), tipifarnib (○, B), or the combination at a 2:1 ratio (●, A,B). (C) Combination index values calculated from the data shown in panels A and B when data were analyzed by the median effect method59 as previously described in detail.60 (D) Summary of results obtained when tipifarnib was combined with multiple orally bioavailable antileukemic agents. Results are expressed as the mean ± SD of combination index values at the IC50 and IC90 in the indicated number of independent experiments.
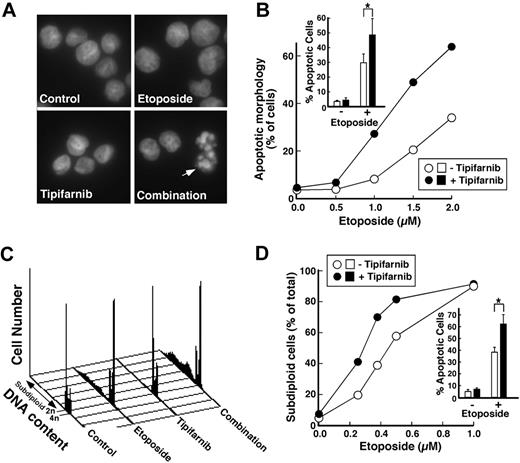
In further experiments, tipifarnib increased etoposide-induced apoptosis in diverse myeloid and lymphoid cell lines (Figure 2; Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article) as manifested by increases in apoptotic morphology (Figure 2A,B), DNA fragmentation (Figure 2C,D), phosphatidylserine exposure (Figure S1A,B), and cleavage of caspase substrates (Figure S1D). The increased histone H2AX phosphorylation observed with the combination also reflected increased apoptosis56 as evidenced by the ability of caspase inhibition to suppress this alteration (Figure S1D). In all cases, tipifarnib either increased etoposide-induced apoptosis without any effect itself, meeting one definition of synergy,61 or synergistically enhanced apoptosis by median effect analysis (Figure S1C,F,I).
Tipifarnib enhances induction of apoptosis by etoposide in AML cell lines. (A) Log-phase HL-60 cells were treated for 24 hours with diluent, 1.5 μM etoposide, 1 μM tipifarnib, or the combination of 1.5 μM etoposide plus 1 μM tipifarnib. At the completion of the incubation, cells were fixed, stained with Hoechst 33258, and examined by fluorescence microscopy. Arrow represents apoptotic cells. (B) Samples shown in panel A and additional samples treated with differing concentrations of etoposide in the absence or presence of 1 μM tipifarnib were examined by fluorescence microscopy (> 500 cells/sample) by an investigator blinded to the treatment, and the percentage of cells displaying apoptotic morphologic changes was recorded. Inset in panel B: summary of 4 independent experiments in which HL-60 cells were treated with diluent, 2 μM etoposide, 1 μM tipifarnib, or 2 μM etoposide plus 1 μM tipifarnib. Error bars represent ± 1 SD. *P = .015 by paired t test. (C) Log-phase U937 cells were treated for 24 hours with diluent, 0.375 μM etoposide, 1 μM tipifarnib, or the combination of 0.375 μM etoposide plus 1 μM tipifarnib. At the completion of the incubation, cells were permeabilized, stained with propidium iodide, and examined by flow microfluorimetry. (D) Samples shown in panel C and additional samples treated with differing concentrations of etoposide in the absence or presence of 1 μM tipifarnib were examined by flow cytometry, and the percentage of cells with less than 2n DNA content was recorded. (D inset) Summary of 4 independent experiments in which U937 cells were treated with diluent, 0.375 μM etoposide, 1 μM tipifarnib, or 0.375 μM etoposide plus 1 μM tipifarnib. Error bars represent ± 1 SD. *P = .008 by paired t test.
Tipifarnib enhances induction of apoptosis by etoposide in AML cell lines. (A) Log-phase HL-60 cells were treated for 24 hours with diluent, 1.5 μM etoposide, 1 μM tipifarnib, or the combination of 1.5 μM etoposide plus 1 μM tipifarnib. At the completion of the incubation, cells were fixed, stained with Hoechst 33258, and examined by fluorescence microscopy. Arrow represents apoptotic cells. (B) Samples shown in panel A and additional samples treated with differing concentrations of etoposide in the absence or presence of 1 μM tipifarnib were examined by fluorescence microscopy (> 500 cells/sample) by an investigator blinded to the treatment, and the percentage of cells displaying apoptotic morphologic changes was recorded. Inset in panel B: summary of 4 independent experiments in which HL-60 cells were treated with diluent, 2 μM etoposide, 1 μM tipifarnib, or 2 μM etoposide plus 1 μM tipifarnib. Error bars represent ± 1 SD. *P = .015 by paired t test. (C) Log-phase U937 cells were treated for 24 hours with diluent, 0.375 μM etoposide, 1 μM tipifarnib, or the combination of 0.375 μM etoposide plus 1 μM tipifarnib. At the completion of the incubation, cells were permeabilized, stained with propidium iodide, and examined by flow microfluorimetry. (D) Samples shown in panel C and additional samples treated with differing concentrations of etoposide in the absence or presence of 1 μM tipifarnib were examined by flow cytometry, and the percentage of cells with less than 2n DNA content was recorded. (D inset) Summary of 4 independent experiments in which U937 cells were treated with diluent, 0.375 μM etoposide, 1 μM tipifarnib, or 0.375 μM etoposide plus 1 μM tipifarnib. Error bars represent ± 1 SD. *P = .008 by paired t test.
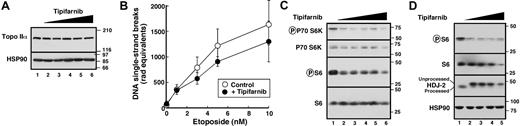
Several experiments examined the mechanistic basis for this synergy. The observation that T + E synergized in I2.1 cells (Figure S1H,I), which are resistant to death receptor-mediated apoptosis (Figure S1G),44,47 ruled out involvement of death receptors in the synergy. Immunoblotting (Figure 3A) and alkaline elution (Figure 3B) failed to show any effect of tipifarnib on levels of the etoposide target enzyme topoisomerase IIα or etoposide-induced stabilization of topoisomerase II-DNA cleavage complexes, ruling out the possibility that the FTI was enhancing etoposide uptake or interaction with its target. Additional immunoblotting failed to demonstrate increases in Bax, Bak, or Puma, (Figure S2A); suppression of cyclin B1 (Figure S2B), a change implicated in RhoB-mediated apoptosis63 ; or significant alterations in signaling through the kinases Akt and ERK (Figure S2B). Among the diverse changes previously implicated in FTI action,15–24 only diminished phosphorylations of ribosomal S6 protein and its upstream kinase p70 S6 kinase 1 was observed in multiple AML cell lines (Figure 3C,D). The occurrence of these alterations in the absence of Akt inhibition, as evidenced by unaltered GSK3β phosphorylation (Figure S2B), has in other cells been attributed to FTI-induced decreases in Rheb prenylation and consequent mTOR inhibition.17,64 Collectively, these results not only provided a potential new marker of FTI action in AML cells but also indicated a parallel between the actions of tipifarnib and rapamycin, which similarly synergizes with etoposide in myeloid cells.65
Tipifarnib preferentially inhibits signaling downstream of mTOR. (A) HL-60 cells treated with 0, 62.5, 125, 250, 500, or 1000 nM tipifarnib (lanes 1-6, respectively) were washed and examined for topoisomerase IIα content by immunoblotting. Numbers at right represent migration of molecular markers in kilodaltons. The same blot was probed with anti-Hsp90 as a loading control. (B) Alkaline elution to evaluate the possibility that tipifarnib enhances etoposide uptake and/or trapping of covalent topoisomerase II-DNA complexes. After log-phase HL-60 cells were treated for 24 hours with 1 μM tipifarnib or diluent, etoposide was added for 30 minutes. The ability of etoposide to stabilize covalent protein-DNA covalent complexes was quantitated as indicated.62 (C) HL-60 cells were treated with diluent (lane 1), 62.5 (lane 2), 125 (lane 3), 250 (lane 4), 500 (lane 5), or 1000 nM (lane 6) tipifarnib for 24 hours. Whole-cell lysates were blotted with antibodies to phospho-Thr389-p70S6 kinase, phospho-Ser235/236-S6, p70S6 kinase, and total S6 protein. (D) U937 cells were treated with diluent (lane 1), 125 (lane 2), 250 (lane 3), 500 (lane 4), or 1000 nM (lane 5) tipifarnib for 24 hours. Whole-cell lysates were blotted with antibodies to phospho-Ser235/236-S6, total S6, the farnesylated protein HDJ-2 and, as a loading control, heat shock protein 90.
Tipifarnib preferentially inhibits signaling downstream of mTOR. (A) HL-60 cells treated with 0, 62.5, 125, 250, 500, or 1000 nM tipifarnib (lanes 1-6, respectively) were washed and examined for topoisomerase IIα content by immunoblotting. Numbers at right represent migration of molecular markers in kilodaltons. The same blot was probed with anti-Hsp90 as a loading control. (B) Alkaline elution to evaluate the possibility that tipifarnib enhances etoposide uptake and/or trapping of covalent topoisomerase II-DNA complexes. After log-phase HL-60 cells were treated for 24 hours with 1 μM tipifarnib or diluent, etoposide was added for 30 minutes. The ability of etoposide to stabilize covalent protein-DNA covalent complexes was quantitated as indicated.62 (C) HL-60 cells were treated with diluent (lane 1), 62.5 (lane 2), 125 (lane 3), 250 (lane 4), 500 (lane 5), or 1000 nM (lane 6) tipifarnib for 24 hours. Whole-cell lysates were blotted with antibodies to phospho-Thr389-p70S6 kinase, phospho-Ser235/236-S6, p70S6 kinase, and total S6 protein. (D) U937 cells were treated with diluent (lane 1), 125 (lane 2), 250 (lane 3), 500 (lane 4), or 1000 nM (lane 5) tipifarnib for 24 hours. Whole-cell lysates were blotted with antibodies to phospho-Ser235/236-S6, total S6, the farnesylated protein HDJ-2 and, as a loading control, heat shock protein 90.
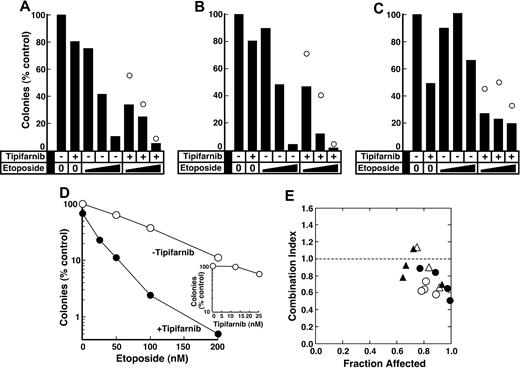
In a final series of in vitro experiments, effects of the combination on primary AML isolates were examined. As illustrated in Figure 4A-D, tipifarnib enhanced etoposide-induced decreases in colony formation even though the FTI had only a modest effect by itself. Analysis by the fractional product approach61 in 11 samples where tipifarnib inhibited colony formation only at the highest concentration tested revealed greater than additive effects in 8 (eg, Figure 4A-C) and additive effects in 3. In an additional 4 AML samples where tipifarnib was active at multiple concentrations, analysis by the median effect method59 likewise demonstrated synergy, as indicated by combination indices less than 1 at the majority of data points (Figure 4D,E).
Effect of tipifarnib on antiproliferative effects of etoposide in clinical AML isolates. (A-C) Freshly isolated mononuclear cells from 3 newly diagnosed AML patients were plated in methylcellulose in diluent, 25 nM tipifarnib plus 50 to 200 nM etoposide, or 25 nM tipifarnib plus 50 to 200 nM etoposide as indicated. Bars represent relative colony counts observed after the indicated treatment. ○ represents relative colony counts predicted from the effects of the individual agents using the fractional product method.61 (D) Freshly isolated mononuclear cells from a newly diagnosed AML patient were plated in methylcellulose in the presence of etoposide alone (○), tipifarnib alone (inset), or 25 nM tipifarnib plus the indicated concentration of etoposide (●). (E) Combination index values59 calculated from the data in panel D (●) and assays in samples from 3 other AML patients (○ and △) under conditions that are render the analysis equivalent to isobologram analysis.60 Note that a combination index less than 1.0 indicates synergy.
Effect of tipifarnib on antiproliferative effects of etoposide in clinical AML isolates. (A-C) Freshly isolated mononuclear cells from 3 newly diagnosed AML patients were plated in methylcellulose in diluent, 25 nM tipifarnib plus 50 to 200 nM etoposide, or 25 nM tipifarnib plus 50 to 200 nM etoposide as indicated. Bars represent relative colony counts observed after the indicated treatment. ○ represents relative colony counts predicted from the effects of the individual agents using the fractional product method.61 (D) Freshly isolated mononuclear cells from a newly diagnosed AML patient were plated in methylcellulose in the presence of etoposide alone (○), tipifarnib alone (inset), or 25 nM tipifarnib plus the indicated concentration of etoposide (●). (E) Combination index values59 calculated from the data in panel D (●) and assays in samples from 3 other AML patients (○ and △) under conditions that are render the analysis equivalent to isobologram analysis.60 Note that a combination index less than 1.0 indicates synergy.
Phase 1 clinical study
Patient demographics.
To assess the tolerability of the T + E combination in leukemia patients, we performed a phase 1 clinical trial. Of the 101 patients evaluated for eligibility, 84 were enrolled. Reasons for ineligibility included screen failure resulting from diagnosis being MDS or MPD rather than AML (10), patients declining therapy (4), creatinine more than 2.0 mg/dL (1), and alternate therapy (2). As detailed in Table 2, the 84 patients enrolled had a median age of 77 years (range, 70-91 years), with 58 (69%) being more than 75 years old, and median ECOG PS of 1 (range, 0-2), with only 12 of 84 (14%) having a performance status of 0. Sixty-six (79%) had at least one poor risk disease feature, including secondary AML (55%) and adverse cytogenetics (54%). All 26 patients 70 to 74 years of age had at least one poor risk disease feature: 3 presented with marked hyperleukocytosis and FLT3-ITD positivity, and 23 (88%) had secondary AML with or without adverse cytogenetics. For patients with secondary AML, the duration of antecedent hematologic disorder ranged from 1.5 months to 12+ years; and 16 (35%) had received some treatment for their antecedent hematologic disorder. Almost all patients also had at least one nonhematologic comorbidity, and 45% had 3 or more. Table 3 demonstrates the comparability of patients receiving tipifarnib for 14 days (A) versus 21 (B).
Demographic and biologic characteristics of 84 elderly adults with newly diagnosed AML treated with tipifarnib plus etoposide
| Characteristic . | Value . |
|---|---|
| Sex, n (%) | |
| Male | 40 (48) |
| Female | 44 (52) |
| Median age, y (range) | 77 (70-91) |
| Biologic disease features | |
| Secondary AML | 46 (55%) |
| MDS/AML | 36 |
| Treatment-related AML | 10 |
| Prior MDS therapy | 16/46 (35%)* |
| Adverse cytogenetics | 43 (of 79 performed, 54%) |
| Single −5 or −7 | 7 |
| Complex (> 3 lesions) | 27 |
| FLT-3 ITD (normal cytogenetics) | 6 |
| Median pretreatment peripheral blood WBC (range) | 5200/μL (300-226 000) |
| Blasts > 10 000/μL | 26 (31%) |
| Pretreatment hydroxyurea (blasts > 30 000/μL) | 15 (18%) |
| Median pretreatment marrow blast percentage (range) | 55% (18%-96%) |
| Patients with > 1 poor-risk disease feature, n (%) | 66 (79%) |
| Host comorbidities, no. of patients | |
| Cardiovascular | 30 |
| Pulmonary | 17 |
| Gastrointestinal | 13 |
| Renal | 13 |
| Diabetes | 12 |
| Neurologic (central) | 11 |
| More than 2 prior malignancies | 22 (6) |
| More than 1 comorbidity | 76 (90%) |
| More than 3 comorbidities | 35 (42%) |
| Characteristic . | Value . |
|---|---|
| Sex, n (%) | |
| Male | 40 (48) |
| Female | 44 (52) |
| Median age, y (range) | 77 (70-91) |
| Biologic disease features | |
| Secondary AML | 46 (55%) |
| MDS/AML | 36 |
| Treatment-related AML | 10 |
| Prior MDS therapy | 16/46 (35%)* |
| Adverse cytogenetics | 43 (of 79 performed, 54%) |
| Single −5 or −7 | 7 |
| Complex (> 3 lesions) | 27 |
| FLT-3 ITD (normal cytogenetics) | 6 |
| Median pretreatment peripheral blood WBC (range) | 5200/μL (300-226 000) |
| Blasts > 10 000/μL | 26 (31%) |
| Pretreatment hydroxyurea (blasts > 30 000/μL) | 15 (18%) |
| Median pretreatment marrow blast percentage (range) | 55% (18%-96%) |
| Patients with > 1 poor-risk disease feature, n (%) | 66 (79%) |
| Host comorbidities, no. of patients | |
| Cardiovascular | 30 |
| Pulmonary | 17 |
| Gastrointestinal | 13 |
| Renal | 13 |
| Diabetes | 12 |
| Neurologic (central) | 11 |
| More than 2 prior malignancies | 22 (6) |
| More than 1 comorbidity | 76 (90%) |
| More than 3 comorbidities | 35 (42%) |
Prior MDS therapy: 7, growth factors alone; 9, 5-azacytidine/decitabine or thalidomide/lenalidamide.
Comparative demographics of patients receiving 14 days versus 21 days of tipifarnib
| Characteristic . | 14-day schedule, % (n = 54) . | 21-day schedule, % (n = 30) . | P . |
|---|---|---|---|
| Age > 75 y | 69 | 68 | > .999 |
| No. of comorbidities | .85 | ||
| 0 | 9 | 13 | |
| 1 | 19 | 20 | |
| 2 | 28 | 20 | |
| 3 | 44 | 47 | |
| Adverse cytogenetics | 56 | 47 | .50 |
| Secondary AML | 50 | 60 | .49 |
| Characteristic . | 14-day schedule, % (n = 54) . | 21-day schedule, % (n = 30) . | P . |
|---|---|---|---|
| Age > 75 y | 69 | 68 | > .999 |
| No. of comorbidities | .85 | ||
| 0 | 9 | 13 | |
| 1 | 19 | 20 | |
| 2 | 28 | 20 | |
| 3 | 44 | 47 | |
| Adverse cytogenetics | 56 | 47 | .50 |
| Secondary AML | 50 | 60 | .49 |
Toxicities.
Fourteen of 18 planned dose levels were completed, with 224 cycles administered (median, 2 per patient; range, 1-7). Fifty-five patients (67%) received at least 2 cycles of T + E, with the second cycle beginning on median day 31 (range, 29-46); 31 (37%) received 3 or more cycles. Table 4 details the spectrum of nonhematologic toxicities. All 9 “A” cohorts (14 days of tipifarnib) were completed with no more than one DLT per 6 patients at a given dose level. For “B” cohorts (21 days of tipifarnib), DLTs occurred in 2 patients each in cohort 2B (mucositis, death from fungal pneumonia) and cohort 7B (hyperbilirubinemia, multiorgan failure), precluding initiation of cohorts 3B, 6B, 8B, and 9B (Tables 1, S1). Notably, more than grade 3 oropharyngeal mucositis was detected in 6 patients (7%) across all etoposide doses and was seen more frequently with the 21-day tipifarnib schedule (4 of 30) than the 14-day schedule (2 of 54), although this difference did not reach statistical significance (P = .18, Table 5).
Toxicities encountered during tipifarnib plus etoposide therapy
| Toxicity . | Cycle 1 (% of 84 patients) . | Total (% of 224 cycles) . |
|---|---|---|
| Hospitalizations | 42 (50%) | 59 (26%) |
| Documented infections | 25 (30%) | 36 (16%) |
| Skin/cellulitis | 7 | 9 |
| Pneumonia | 6 | 10 |
| Sinusitis | 2 | 3 |
| Pharyngitis/esophagitis | 3 | 3 |
| Gastrointestinal | 1 | 4 |
| Genitourinary | 3 | 3 |
| Bacteremia (no site) | 3 | 4 |
| Neutropenic fever | 20 (24%) | 30 (13%) |
| Drug-related toxicities | ||
| Neurotoxicity (grades 1-3) | 12 (14%)* | 20 (9%)† |
| Mucositis (grade > 2) | 10 (12%) | 12 (5%) |
| Gastrointestinal (grade > 2) | 9 (11%) | 12 (5%) |
| Hyperbilirubinemia (grade > 2) | 4 (5%) | 6 (3%) |
| Renal (grade > 2) | 3 (4%) | 4 (2%) |
| Rash (grade > 2) | 4 (5%) | 6 (3%) |
| Fatigue (grade > 2) | 6 (7%) | 12 (5%) |
| Cardiac (grades 1-5) | 3 (4%)‡ | 8 (4%)§ |
| Death | 9 (11%) | 13 (5.8%) |
| Infection | 5 | 5 |
| Cardiac | 1 | 4 |
| Cerebrovascular | 1 | 2 |
| Pulmonary | 2 | 2 |
| Toxicity . | Cycle 1 (% of 84 patients) . | Total (% of 224 cycles) . |
|---|---|---|
| Hospitalizations | 42 (50%) | 59 (26%) |
| Documented infections | 25 (30%) | 36 (16%) |
| Skin/cellulitis | 7 | 9 |
| Pneumonia | 6 | 10 |
| Sinusitis | 2 | 3 |
| Pharyngitis/esophagitis | 3 | 3 |
| Gastrointestinal | 1 | 4 |
| Genitourinary | 3 | 3 |
| Bacteremia (no site) | 3 | 4 |
| Neutropenic fever | 20 (24%) | 30 (13%) |
| Drug-related toxicities | ||
| Neurotoxicity (grades 1-3) | 12 (14%)* | 20 (9%)† |
| Mucositis (grade > 2) | 10 (12%) | 12 (5%) |
| Gastrointestinal (grade > 2) | 9 (11%) | 12 (5%) |
| Hyperbilirubinemia (grade > 2) | 4 (5%) | 6 (3%) |
| Renal (grade > 2) | 3 (4%) | 4 (2%) |
| Rash (grade > 2) | 4 (5%) | 6 (3%) |
| Fatigue (grade > 2) | 6 (7%) | 12 (5%) |
| Cardiac (grades 1-5) | 3 (4%)‡ | 8 (4%)§ |
| Death | 9 (11%) | 13 (5.8%) |
| Infection | 5 | 5 |
| Cardiac | 1 | 4 |
| Cerebrovascular | 1 | 2 |
| Pulmonary | 2 | 2 |
Four grade 1, five grade 2, and three grade 3.
Six grade 1, eight grade 2, and six grade 3.
One grade 5.
Four grade 5.
Comparison of 14-day versus 21-day tipifarnib administration
| . | 14 days (A) . | 21 days (B) . | P . |
|---|---|---|---|
| Toxicity, no. (%) of patients | |||
| Grade 3 mucositis | 2/54 (4) | 4/30 (13) | .18 |
| Induction death | 3/54 (6) | 6/30 (20) | .06 |
| Efficacy, day to begin cycle 2, median (range) | 30 (29-43) | 35 (29-46) | |
| Complete remission, no. (%) of patients | 16/54 (30) | 5/30 (17) | .29 |
| . | 14 days (A) . | 21 days (B) . | P . |
|---|---|---|---|
| Toxicity, no. (%) of patients | |||
| Grade 3 mucositis | 2/54 (4) | 4/30 (13) | .18 |
| Induction death | 3/54 (6) | 6/30 (20) | .06 |
| Efficacy, day to begin cycle 2, median (range) | 30 (29-43) | 35 (29-46) | |
| Complete remission, no. (%) of patients | 16/54 (30) | 5/30 (17) | .29 |
A polynomial model was fit to the data for estimating the joint association between doses of each drug and observed DLTs for each of the 14- and 21-day schedules separately, and also for both schedules together. There appeared to be no association between dose and DLTs. Based on this finding, we estimated DLT rates for each of the 14- and 21-day schedules. For the 14-day schedule, the estimated DLT rate was estimated to be 0.06 (95% confidence interval [CI], 0.02-0.16); and for the 21-day schedule, it was estimated to be 0.13 (95% CI, 0.05-0.31).
To address the concern that the higher toxicity seen in the 21-day versus 14-day schedule may have been a result of patient characteristics (Table 3), we considered age, adverse cytogenetics, number of comorbidities, and secondary AML in a multiple regression model, along with doses of each drug and schedule. Patient characteristics that exhibited a P value of less than .10 in a simple logistic regression model with toxicity as outcome were then included in a multiple logistic regression model with doses and schedule. The outcome was grade 3 or higher toxicity rather than DLTs, resulting from sparseness of DLT occurrences. This analysis (Table S2) showed that, after adjustment for patient characteristics (age and number of comorbidities), there was still a trend toward higher toxicity with the 21-day tipifarnib schedule.
A positive feature of T + E is the possibility of administering this regimen in the outpatient setting. Nonetheless, hospitalizations were required during 59 of 224 (26%) cycles (Table 4). The majority of hospitalizations occurred during the first cycle (40 of 59, 68%) and lasted a median 8 days (range, 2-45 days). Although documented infections and/or neutropenic fevers were the most common causes for hospitalization in any cycle, there were 7 hospitalizations (3 in cycle 1) for cardiac events (4), pulmonary decompensation (1), and cerebrovascular events (2).
During the first cycle of T + E, 9 (11%) patients had grade 5 events: infection (5), extension of previous cerebrovascular accidents (1), pulmonary decompensation (2), and acute myocardial infarction (1). Death in cycle 1 occurred in 3 of 54 (6%) “A” cohort patients and 6 of 30 (20%) “B” cohort patients (P = .06, Table 5). Two patients who died of infection (1 cohort 2B, 1 cohort 4A) did so with normal marrow recovery without evidence for residual AML. Four (7%) of 55 patients receiving 2 or more cycles of T + E died of cardiac (3) or cerebrovascular (1) causes during cycles 2 to 4, none in CR or PR, and all with underlying vascular disease.
Clinical outcome.
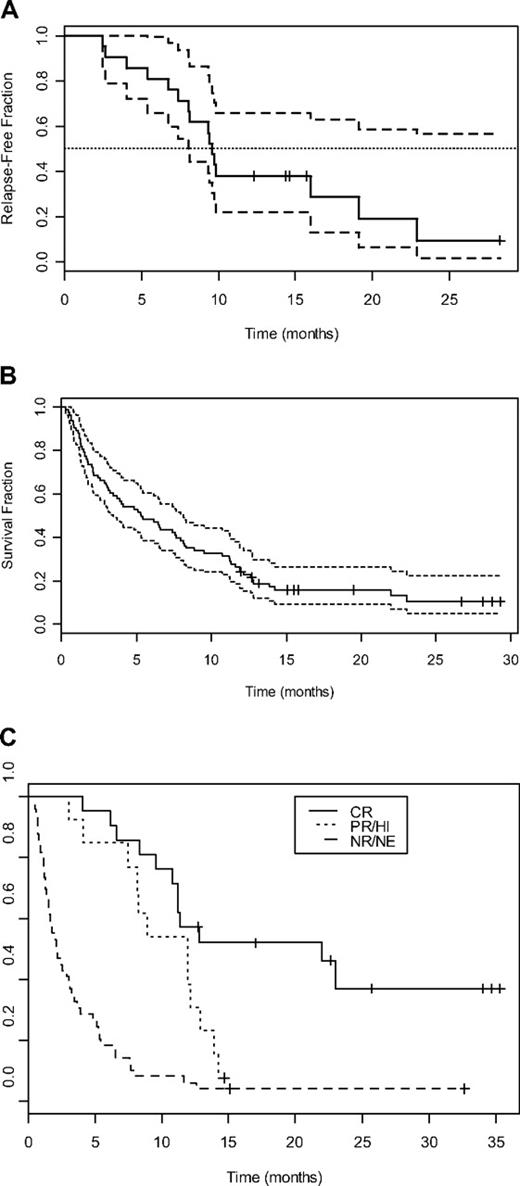
Of the 84 patients receiving T + E, 21 (25%) achieved CR, with median CR duration being 9.8 months (95% CI, 8.1-∞; Figure 5A). A summary of the 21 CR patients is presented in Table 6. Those achieving CR had a median age of 77 years (range, 71-85 years); and 16 (76%) had at least one poor-risk disease feature, including secondary AML with adverse cytogenetics in 6 (29%), secondary AML without adverse cytogenetics in 7 (33%), and adverse cytogenetics without a history of secondary AML in 3 (14%). CR was achieved after cycle 1 in 14 (67%) and by the end of cycle 2 in all but 1 patient. An additional 3 (4%) patients achieved PR and 9 (11%) achieved HI. Of 26 patients presenting with peripheral blood blast counts greater than 10 000/μL, 7 (27%) had some type of improvement (2 CR, 5 PR/HI). None of the 6 patients whose blasts expressed FLT-3 ITD achieved CR, and only 1 achieved HI.
Survival curves of patients treated with T + E. (A) DFS (median, —; 95% CI, – –) for 21 patients who achieved complete remission in response to T + E. (B) OS (median, —; 95% CI,  ) for 84 adults treated with T + E. (C) OS for 21 patients achieving complete remission (CR, —), 13 patients achieving partial remission or hematologic improvement (PR/HI,
) for 84 adults treated with T + E. (C) OS for 21 patients achieving complete remission (CR, —), 13 patients achieving partial remission or hematologic improvement (PR/HI,  ), and 49 patients who did not achieve response (NR/NE, – –).
), and 49 patients who did not achieve response (NR/NE, – –).
Survival curves of patients treated with T + E. (A) DFS (median, —; 95% CI, – –) for 21 patients who achieved complete remission in response to T + E. (B) OS (median, —; 95% CI,  ) for 84 adults treated with T + E. (C) OS for 21 patients achieving complete remission (CR, —), 13 patients achieving partial remission or hematologic improvement (PR/HI,
) for 84 adults treated with T + E. (C) OS for 21 patients achieving complete remission (CR, —), 13 patients achieving partial remission or hematologic improvement (PR/HI,  ), and 49 patients who did not achieve response (NR/NE, – –).
), and 49 patients who did not achieve response (NR/NE, – –).
Clinical and cytogenetic characteristics of patients achieving CR with tipifarnib plus etoposide
| Age, y/sex . | Prior SAML . | Prior treatment* . | PS† . | Comorbidities . | Cytogenetics . | Dose level . | DFS/OS, mo . |
|---|---|---|---|---|---|---|---|
| 85/F | Yes | 0 | 1 | Breast carcinoma, sinusitis, HBP | 46XX, 13q− | 2A | 32/37.5+ |
| 72/F | No | 0 | 1 | HBP, spinal stenosis | 46XX | 2A | 16/35+ |
| 83/F | Yes | 2 | 2 | NHL, diverticulitis, UTI | 45X,−X, t(1;11) iso8q, 20q− | 2B | 2.5/6 |
| 71/F | Yes | 2 | 1 | Breast carcinoma, osteoporosis | 46XX | 3A | 23/34+ |
| 71/M | Yes | 0 | 1 | HBP, osteoarthritis | 46XY | 3A | 9.6/23 |
| 85/F | No | 0 | 1 | None | 46XX | 3A | 9.9/12.8 |
| 83/F | Yes | 0 | 0 | HBP, hypothyroidism, arthritis | 46XX, 5q−,7q− 20q− | 4A | 6.7/9.5 |
| 76/F | No | 0 | 1 | Atrial fibrillation | 46XX | 4A | 19.1/22 |
| 81/M | Yes | 1 | 2 | Pneumonia, arthritis, HBP femoral DVT | 46XY | 4B | 8.1/11.2 |
| 76/F | No | 0 | 1 | Gastritis with ulcer, hypothyroidism | 46XX | 4B | 5.4/11 |
| 71/F | Yes | 1,2 | 1 | Breast carcinoma, gastric ulcer | 46XX | 5A | 2.8/4 |
| 77/M | Yes | 1 | 2 | Chronic renal failure, CAD pneumonia, asthmatic bronchitis | 46XY, 20q− | 5B | 4/6.6 |
| 77/F | Yes | 0 | 1 | HBP, hypothyroidism, rectal fissure | 46XX | 6A | 22.1+/24 |
| 77/F | Yes | 2 | 2 | Lung carcinoma, CAD, HBP, DM | 46XX, t(9;11;19) | 7A | 9.3/11.2 |
| 81/M | No | 0 | 2 | COPD, HBP, urosepsis | 47XY,+8 | 7B | 7.3/8.3 |
| 79/F | No | 0 | 1 | Colon carcinoma, HBP | 46XX | 8A | 20.7+/22+ |
| 76/M | No | 0 | 1 | Colon carcinoma, seizure disorder, CAD, DOE | 46XY, t(9;11) | 8A | 9.4/10.7 |
| 80/F | No | 0 | 0 | BP | 45XX, −7 | 8A | 20.5+/22+ |
| 72/F | Yes | 0 | 2 | DM, pneumonia, HBP, arthritis, essential tremor | 47XX,+1,+3q,−5Q,−7,−10,+12p, +17p,+21q | 9A | 8.8/17.2 |
| 76/F | Yes | 1,2 | 2 | Breast carcinoma, GERD, COPD, recurrent UTI | 46XX | 9A | 15.2/18.5+ |
| 79/M | Yes | 1 | 1 | HBP, gout, GERD, COPD | Not done | 9A | 9.7/19+ |
| Age, y/sex . | Prior SAML . | Prior treatment* . | PS† . | Comorbidities . | Cytogenetics . | Dose level . | DFS/OS, mo . |
|---|---|---|---|---|---|---|---|
| 85/F | Yes | 0 | 1 | Breast carcinoma, sinusitis, HBP | 46XX, 13q− | 2A | 32/37.5+ |
| 72/F | No | 0 | 1 | HBP, spinal stenosis | 46XX | 2A | 16/35+ |
| 83/F | Yes | 2 | 2 | NHL, diverticulitis, UTI | 45X,−X, t(1;11) iso8q, 20q− | 2B | 2.5/6 |
| 71/F | Yes | 2 | 1 | Breast carcinoma, osteoporosis | 46XX | 3A | 23/34+ |
| 71/M | Yes | 0 | 1 | HBP, osteoarthritis | 46XY | 3A | 9.6/23 |
| 85/F | No | 0 | 1 | None | 46XX | 3A | 9.9/12.8 |
| 83/F | Yes | 0 | 0 | HBP, hypothyroidism, arthritis | 46XX, 5q−,7q− 20q− | 4A | 6.7/9.5 |
| 76/F | No | 0 | 1 | Atrial fibrillation | 46XX | 4A | 19.1/22 |
| 81/M | Yes | 1 | 2 | Pneumonia, arthritis, HBP femoral DVT | 46XY | 4B | 8.1/11.2 |
| 76/F | No | 0 | 1 | Gastritis with ulcer, hypothyroidism | 46XX | 4B | 5.4/11 |
| 71/F | Yes | 1,2 | 1 | Breast carcinoma, gastric ulcer | 46XX | 5A | 2.8/4 |
| 77/M | Yes | 1 | 2 | Chronic renal failure, CAD pneumonia, asthmatic bronchitis | 46XY, 20q− | 5B | 4/6.6 |
| 77/F | Yes | 0 | 1 | HBP, hypothyroidism, rectal fissure | 46XX | 6A | 22.1+/24 |
| 77/F | Yes | 2 | 2 | Lung carcinoma, CAD, HBP, DM | 46XX, t(9;11;19) | 7A | 9.3/11.2 |
| 81/M | No | 0 | 2 | COPD, HBP, urosepsis | 47XY,+8 | 7B | 7.3/8.3 |
| 79/F | No | 0 | 1 | Colon carcinoma, HBP | 46XX | 8A | 20.7+/22+ |
| 76/M | No | 0 | 1 | Colon carcinoma, seizure disorder, CAD, DOE | 46XY, t(9;11) | 8A | 9.4/10.7 |
| 80/F | No | 0 | 0 | BP | 45XX, −7 | 8A | 20.5+/22+ |
| 72/F | Yes | 0 | 2 | DM, pneumonia, HBP, arthritis, essential tremor | 47XX,+1,+3q,−5Q,−7,−10,+12p, +17p,+21q | 9A | 8.8/17.2 |
| 76/F | Yes | 1,2 | 2 | Breast carcinoma, GERD, COPD, recurrent UTI | 46XX | 9A | 15.2/18.5+ |
| 79/M | Yes | 1 | 1 | HBP, gout, GERD, COPD | Not done | 9A | 9.7/19+ |
SAML indicates secondary AML; HBP, high blood pressure; NHL, non-Hodgkin lymphoma; UTI, urinary tract infection; DVT, deep vein thrombosis; CAD, coronary artery disease; DM, diabetes mellitus; COPD, chronic obstructive pulmonary disease; DOE, dyspnea on exertion; and GERD, gastroesophageal reflux disease.
Prior treatment: 0 indicates none; 1, antecedent hematologic disorder and 2, prior malignancy.
Performance status based on Eastern Cooperative Oncology Group criteria.
As depicted in Figure 5B, median OS for the entire group of 84 patients is 5.3 months (95% CI, 3.4-8.1). Figure 5C depicts OS based on clinical response. For patients with stable disease or progressive disease, median OS was 2.1 months (range, 0.25-26+ months; 95% CI, 1.6-3.4), with 1 (2%) of 51 nonresponding patients currently alive at 26.2 months. Patients achieving PR or HI had a median OS of 11.9 months (range, 3-14.2 months; 95% CI, 8.1-∞). For the 21 CR patients, median OS is 22 months (range, 3.5-36+ months; 95% CI, 11.2-∞), with 14 (67%) surviving more than 1 year and 9 (43%) still living at 15.5+ to 36+ months.
The pretreatment considerations that precluded intensive induction therapy at the time of diagnosis were also present at relapse. Accordingly, none of these patients received intensive induction therapy even at relapse. Any patient who achieved a CR of at least 4 weeks after completion of all cycles of therapy was eligible to be re-treated at the original dose level of T + E, provided that eligibility criteria were still met (eg, ECOG PS 0-2). Relapses occurred 5 to 32 months after achieving first CR (median, 10 months). Of 11 CR patients who relapsed and were retreated, 5 achieved a second CR lasting 3.5 to 17+ months. The median CR1 duration was 16 months for the 5 patients achieving a second CR and 9.3 months for the 6 patients who did not achieve a second CR.
When the relationship between dose or schedule and response was examined, CR was observed in 15 (31%) of 48 patients who received more than 400 mg tipifarnib twice a day, including 13 of 36 (36%) treated with tipifarnib on 14-day schedules. Nine of 24 (37.5%) who received etoposide 200 mg daily on days 1 to 3 and 8 to 10 achieved CR, in contrast to 12 of 60 (20%) who received lower etoposide doses. CR rate was 16 of 54 (30%) for patients receiving tipifarnib for 14 days and 5 of 30 (17%) for patients receiving tipifarnib for 21 days (P = .29, Table 5). CR rates of 50% (3 of 6 patients per cohort) were achieved in cohorts 3A (tipifarnib 600 mg twice a day, etoposide 100 mg), 8A (tipifarnib 400 mg twice a day, etoposide 200 mg), and 9A (tipifarnib 600 mg twice a day, etoposide 200 mg). There were no DLTs in cohorts 3A and 8A and only 2 of 6 in each cohort had grade 3 nonhematologic toxicities. In contrast, 4 of 6 in cohort 9A had grade 3 or 4 toxicities and one died from disseminated fungal infection.
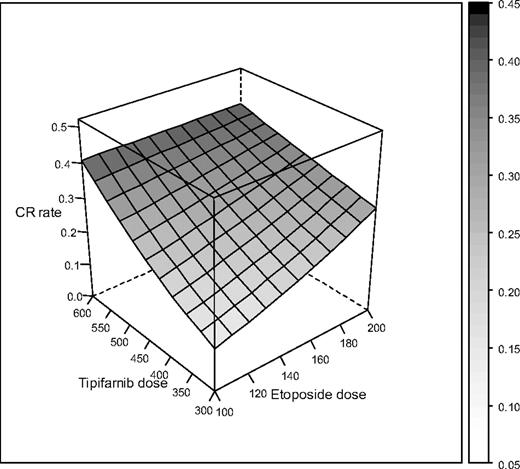
Using a polynomial model, we estimated the relationship between drug doses and CR. Compared with a nonparametric model, where empirical response rates are estimated at each dose combination, the polynomial model borrows strength across dose combinations and assumes a simpler structure with relatively few degrees of freedom. This approach provides more power and precision for estimating response rates using the fitted model. As shown in Figure 6, there appears to be an improvement in response with higher dose levels of each drug, although the dose-response relationship for tipifarnib appears to be steeper than for etoposide. Based on the toxicity and response results described herein, we recommend dose cohorts 3A and 8A for further study.
Estimated dose-response relationship between combinations of etoposide and tipifarnib. The height of the surface and its shading indicate the response rate for each dose combination.
Estimated dose-response relationship between combinations of etoposide and tipifarnib. The height of the surface and its shading indicate the response rate for each dose combination.
Laboratory correlates.
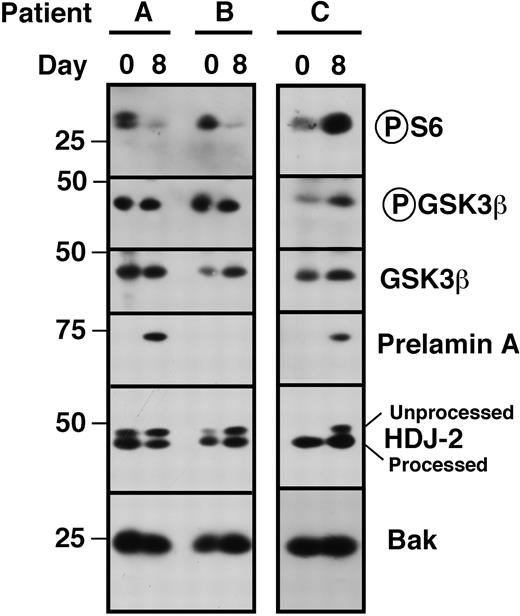
Paired bone marrow samples from a subset of patients were analyzed to determine whether the changes observed in vitro (Figures 2,Figure 3–4) could also be observed in vivo. To determine whether tipifarnib could diminish S6 phosphorylation in vivo, samples from 42 patients were probed as illustrated in Figure 7. S6 phosphorylation was detectable before therapy in 38 (90%). Of the 38, 8 were uninformative on day 8 because of a decrease in the loading controls, 10 of the remaining 30 (33%) showed a decrease in S6 phosphorylation on day 8 (eg, samples A, B), and 20 (67%) showed no decrease (eg, sample C). Importantly, the decreases occurred without decreased Akt-mediated phosphorylation of GSK3β, suggesting that tipifarnib inhibits the Rheb/mTOR pathways in some, but not all, patients without affecting upstream signaling.
Assessment of pathway inhibition in situ. Bone marrow mononuclear cells harvested before institution of therapy (day 0) and on day 8 before drug administration were subjected to immunoblotting with antibodies that recognize phosphor-Ser235/236 ribosomal protein S6, GSK3β phosphorylated by Akt on Ser9 and total GSK3β. The shift in HDJ-2 and appearance of prelamin A on day 8 served to confirm FTase inhibition,9,10 whereas Bak served as a loading control. Note that S6 phosphorylation was inhibited in 2 patients (patients A and B) but not the third (patient C).
Assessment of pathway inhibition in situ. Bone marrow mononuclear cells harvested before institution of therapy (day 0) and on day 8 before drug administration were subjected to immunoblotting with antibodies that recognize phosphor-Ser235/236 ribosomal protein S6, GSK3β phosphorylated by Akt on Ser9 and total GSK3β. The shift in HDJ-2 and appearance of prelamin A on day 8 served to confirm FTase inhibition,9,10 whereas Bak served as a loading control. Note that S6 phosphorylation was inhibited in 2 patients (patients A and B) but not the third (patient C).
H2AX phosphorylation was examined in paired samples from 33 patients. As depicted in Table 7, the 8 patients who achieved CR had a median 1.9-fold increase in the percentage of H2AX-positive marrow blasts from day 0 to day 8, whereas there was no measurable change in the remaining 25 patients (6 PR/HI, 19 NR). When examined for staining intensity, the differences were less pronounced, with median increase for CR patients' blast cells being 1.13, and no change for the remaining 25 patients.
In vivo effects of tipifarnib plus etoposide on DNA damage and apoptosis in AML marrow blasts
| . | Clinical outcome . | |
|---|---|---|
| CR (n = 8) . | Non-CR (n = 25) . | |
| Median fold increase in total population γH2AX staining (day 8/day 0), (range) | 1.9 (0.6-3.1) | 0.98 (0.01-2.6) |
| ≥ 1.2-fold | 5/8 (62.5%) | 9/25 (36%) |
| ≥ 1.5-fold | 4/8 (50%) | 4/25 (16%) |
| Median fold increase in γH2AX staining intensity (“per cell”) (day 8/day 0), (range) | 1.13 (0.75-1.6) | 0.9 (0.5-1.2) |
| ≥ 1.2-fold | 3/8 (37.5%) | 1/25 (4%) |
| Median fold increase in sub2N DNA content (day 8/day 0), (range) | 2.0 (0.6-5.2) | 0.8 (0.2-19.8) |
| ≥ 1.2-fold | 5/6 (83%) | 10/21 (48%) |
| ≥ 1.5-fold | 4/6 (67%) | 8/21 (38%) |
| . | Clinical outcome . | |
|---|---|---|
| CR (n = 8) . | Non-CR (n = 25) . | |
| Median fold increase in total population γH2AX staining (day 8/day 0), (range) | 1.9 (0.6-3.1) | 0.98 (0.01-2.6) |
| ≥ 1.2-fold | 5/8 (62.5%) | 9/25 (36%) |
| ≥ 1.5-fold | 4/8 (50%) | 4/25 (16%) |
| Median fold increase in γH2AX staining intensity (“per cell”) (day 8/day 0), (range) | 1.13 (0.75-1.6) | 0.9 (0.5-1.2) |
| ≥ 1.2-fold | 3/8 (37.5%) | 1/25 (4%) |
| Median fold increase in sub2N DNA content (day 8/day 0), (range) | 2.0 (0.6-5.2) | 0.8 (0.2-19.8) |
| ≥ 1.2-fold | 5/6 (83%) | 10/21 (48%) |
| ≥ 1.5-fold | 4/6 (67%) | 8/21 (38%) |
Non-CR includes partial remission/hematologic improvement (PR/HI) and no remission (NR).
Because increased H2AX phosphorylation might reflect the ability of tipifarnib to increase etoposide-induced apoptosis (Figure S1D), we also stained blasts from 27 patients with PI and searched for “subdiploid” apoptotic cells (Table 7). Among the 6 CR patients, there was a 2-fold increase (range, 0.6-5.2) in the percentage of cells with subdiploid DNA content on day 8 relative to day 0. For non-CR patients, treatment-related changes in the apoptotic fraction varied over a very wide range from 0.2- to 19.8-fold (median, 0.8-fold).
Paired samples from 18 patients were studied for both H2AX staining and apoptosis. Serially obtained-marrow populations from 3 of 4 CR patients (75%) demonstrated concomitant increases in both H2AX phosphorylation and sub2N DNA on day 8, whereas only 2 of 14 non-CR patients (14%) demonstrated increases in both parameters (P = .04), despite the finding that 5 of 14 exhibited increases in γH2AX staining and 6 of 14 exhibited increases in sub2N DNA. These observations suggest that linked changes in both phospho-H2AX staining and subdiploid DNA content might correlate with response.
Discussion
Our preclinical studies presented herein demonstrate that tipifarnib inhibits signaling downstream of mTOR and, like rapamycin,65 enhances the antiproliferative effects of etoposide in AML cell lines and clinical specimens. Based on these results, we performed a multicenter phase 1 trial that demonstrated the ability to safely administer T + E to elderly AML patients who are not candidates for conventional induction therapy on the basis of both host and disease biology, based on criteria set forth by multiple investigators.1,2,66,67
In our previous phase 2 study of single-agent tipifarnib administered for 21 consecutive days, grade 3 or 4 adverse events occurred in 47% of elderly AML patients.26 When the same 21-day schedule of tipifarnib was combined with etoposide in the present study, DLTs of grade 4 mucositis, grade 3 hyperbilirubinemia, and multiorgan failure precluded dose escalation beyond cohorts 2B, 5B, and 7B (Table S1). In contrast, the same etoposide schedule was better tolerated when tipifarnib was administered for 14 days, with successful accrual of patients to all “A” cohorts. Oral mucositis, a well-known toxicity of etoposide, has not accompanied previous studies of single-agent tipifarnib.25–29 The apparent relationship between tipifarnib schedule and mucositis induction may represent synergy between T + E against normal oral mucosa. In contrast, tipifarnib-related neurotoxicity did not appear to be enhanced by combination with etoposide in “A” or “B” cohorts. Taken together, the 14-day T + E regimens (“A” cohorts) were tolerable and can be recommended for further study.
The CR rate with T + E appears to be higher than that of tipifarnib alone. The CR rate in our previous phase 2 trial of single-agent tipifarnib (administered for 21 days) was 14%.26 A phase 3 study of single-agent tipifarnib versus best supportive care (including hydroxyurea) in 457 adults more than 70 years of age with newly diagnosed AML who were deemed unfit for conventional chemotherapy likewise demonstrated CRs with DFS of 8 months and OS of 22 months in only 8% of those randomized to tipifarnib (compared with no CRs in the supportive care/hydroxyurea arm).68 As indicated in Table S3, the patient populations enrolled in our previous phase 2 trial of single-agent tipifarnib26 and the present phase 1 trial are comparable with respect to most patient characteristics. In the current study, the overall CR rate with T + E was 25% (compared with 14% CR rate in our single-agent trial,26 P = .036), with a CR rate of 36% for tipifarnib doses of at least 400 mg twice a day for 14 days. Furthermore, a CR rate of 50% occurred in 3 14-day cohorts (3A, 8A, and 9A) without death or DLT in 3A and 8A. It is noteworthy that the proportion of patients with age more than 75 years in our single-agent tipifarnib study was 47%, whereas in the T + E study it was 65% (Table S3, P = .01); and the combination of T + E appears more efficacious in patients more than 75 years of age compared with younger patients (Table S2). Based on a multiple regression model adjusting for number of comorbidities, we found that for patients with 2 comorbidities (the median number of comorbidities), the estimated response rate in patients younger than 75 years was 14% and in those older than 75 years was 32%. This still suggests an improvement over our previous single-agent tipifarnib study, which had an overall response rate of 14% across both age groups.26
The mechanism for the synergy between tipifarnib and etoposide is not entirely clear. Tipifarnib inhibits the drug efflux activity of Pgp in human acute T-lymphoblastic leukemia and AML cell lines.34 Because etoposide is a Pgp substrate69 and high levels of Pgp expression correlate with clinical drug resistance in elderly AML patients,1,70 we cannot rule out the possibility that tipifarnib-induced Pgp inhibition contributes to the results observed in the present clinical trial. On the other hand, results in Figures 2 and 3 demonstrate that tipifarnib also enhances etoposide-induced apoptosis under conditions where drug uptake and action at the level of topoisomerase II are unchanged. Additional data (Figure 1D) demonstrate that tipifarnib also sensitizes cells to chlorambucil and melphalan, which are not Pgp substrates. Thus, it appears that tipifarnib can enhance the effects of certain DNA-damaging agents independent of its effect on Pgp.
Results of the present study demonstrate, for the first time, that tipifarnib can inhibit signaling downstream of mTOR in the absence of Akt inhibition. These findings are hallmarks of Rheb inhibition and, in conjunction with recently published animal studies,71 suggest that further study of this small G protein in AML is warranted. Because S6 phosphorylation was only inhibited in a subset of AML patients treated with T + E (Figure 7), further study is also needed to understand why Rheb is inhibited in some AMLs and not others.
The correlative studies also demonstrated that treatment with T + E in vivo is accompanied by drug-induced increases in histone H2AX phosphorylation and, to a lesser extent, DNA fragmentation in AML marrow blasts, with the suggestion that achievement of CR may be associated with modest but measurable increases in both parameters (Table 7). Importantly, the increased H2AX phosphorylation was observed before administration of etoposide on day 8 and, therefore, probably doesn't reflect direct topoisomerase II-mediated strand breaks. Instead, it might reflect DNA damage associated with ongoing cell death (Figure S1D).56 Consistent with this possibility, in the small subgroup of CR patients in whom both parameters were assessed concomitantly, increased phospho-H2AX staining and apoptosis were linked, although this was not necessarily the case in the non-CR patients (Table 7). On the other hand, it is also possible that the observed link between DNA damage and apoptosis in our responsive patients relates to the recent finding that response to single-agent tipifarnib is associated with lower expression of APTX,72 the gene encoding the DNA repair protein aprataxin.73 In particular, active aprataxin may be able to repair damaged DNA in a way that uncouples the detection of that damage and the eventual completion of programmed cell death. Further studies are required to examine the relationship between aprataxin expression, phospho-H2AX staining, apoptosis induction, and clinical response to T + E.
In conclusion, based in part on preclinical studies presented herein, we have performed a phase 1 trial of T + E in elderly AML patients. The combination has yielded encouraging clinical results in a group of patients for whom traditional antileukemia chemotherapy may not be appropriate because of advanced age, poor risk biologic disease features, and/or the presence of significant nonhematologic comorbidities.1,2,66,67 After this study was completed, Burnett et al reported that administration of low-dose cytarabine to adults with AML or high-risk MDS deemed unfit for intensive chemotherapy resulted in OS of 3.5 to 4 months, with a CR rate of 18%, median DFS of 8 months and OS of 19 months for CR patients.67 In addition, Burnett et al74 and Erba et al73 reported CR rates 35% to 40% and 30-day mortalities of 15% to 20% with single-agent clofarabine in adults 60 to 65 years of age and older. Although these results appear somewhat similar to ours, there are some notable differences. First, our results reflect response rates over multiple dose cohorts, and the response rate in cohorts 3A and 8A recommended for further study might actually be higher (Figure 6). To assess this possibility, a randomized phase 2 trial of these cohorts is planned. Second, no patient with adverse cytogenetics achieved a CR in Burnett's low-dose cytarabine series,67 whereas T + E induced CR in 9 of 43 (21%) with adverse cytogenetics. Although clofarabine induced CRs in patients with adverse cyogenetics and secondary AMLs, the CR durations (median DFS, 6 months; 26% survival at 1 year) may not be as long as those achieved with T + E. Nonetheless, cytogenetics remain a powerful determinant of response for all therapeutic strategies tested to date, and it remains to be seen how effective optimal doses of T + E will be in overcoming the negative impact of adverse cytogenetics. On the other hand, because low-dose cytarabine67 and oral clofarabine74,75 have activity in older adults with AML, it will also be important to compare T + E to these treatments as well as single-agent oral etoposide in the future.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Johns Hopkins Sidney Kimmel Cancer Center nursing staff for superb medical care, and the patients and families, without whose partnership we could never have conducted the trial and from whom we have learned critical information that will help us to improve the treatment of these diseases.
This work was supported in part by NCI (Cooperative Agreement U01 CA70095; J.E.K.), NCI Translational Research Initiative (contract 24XS126; J.E.K.), NCI Cancer Center (support grant 2P30 CA06973-44), National Center for Research Resources (grants M01-RR0052, R01 CA73709, and R01 CA127433), the Leukemia & Lymphoma Society Translational Research Project (White Plains, NY; grant 6047-08; S.H.K., J.E.K.), and philanthropic funds from Dr Robert Fischell in memory of his late wife Marian (J.E.K.).
National Institutes of Health
Authorship
Contribution: J.E.K., E.G.-M., and S.H.K. designed and performed research, analyzed data, and wrote the paper; E.J.F. participated in protocol design, performed research, helped to analyze data, and helped to write the paper; J.M.G. served as multicenter coordinator, coordinated sample acquisition for laboratory studies, performed research, collected data, and assisted with data analysis; K.F., R.M.R., D.A.L., S.B.L., X.W.M., P.A.S., and N.T.D. performed laboratory experiments and/or correlative assays and helped to analyze data; A.A.A. helped design preclinical studies and write the paper; L.E.M., E.R., B.D.S., V.I., G.R., S.D.G., and M.J.L. performed research and edited the paper; T.T. collected data and assisted with data analysis; and J.J.W. participated in protocol design, helped to analyze data, and performed critical review of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Judith E. Karp, Johns Hopkins Sidney Kimmel Comprehensive Cancer Center, 1650 Orleans St, CRB 1 Rm 289, Baltimore, MD 21231-1000; e-mail: jkarp2@jhmi.edu.
References
Author notes
*E.G.-M. and S.H.K. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal