Abstract
Anemia as associated with numerous clinical conditions can be debilitating, but frequently can be treated via administration of epoetin-alfa, darbepoietin-alfa, or methoxy-PEG epoetin-beta. Despite the complexity of EPO-EPO receptor interactions, the development of interesting EPO mimetic peptides (EMPs) also has been possible. CNTO 530 is one such novel MIMETIBODY Fc-domain dimeric EMP fusion protein. In a mouse model, single-dose CNTO 530 (unlike epoetin-alfa or darbepoietin-alfa) bolstered red cell production for up to 1 month. In 5-fluorouracil and carboplatin-paclitaxel models, CNTO 530 also protected against anemia with unique efficiency. These actions were not fully accounted for by half-life estimates, and CNTO 530 signaling events therefore were studied. Within primary bone marrow erythroblasts, kinetics of STAT5, ERK, and AKT activation were similar for CNTO 530 and epoetin-alfa. p70S6K activation by CNTO 530, however, was selectively sustained. In vivo, CNTO 530 uniquely stimulated the enhanced formation of PODXLhighCD71high (pro)erythroblasts at frequencies multifold above epoetin-alfa or darbepoietin-alfa. CNTO 530 moreover supported the sustained expansion of a bone marrow–resident KitnegCD71highTer119neg progenitor pool. Based on these distinct erythropoietic and EPOR signaling properties, CNTO 530 holds excellent promise as a new EPO mimetic.
Introduction
As recombinant injectables, epoetin-alfa and darbepoietin-alfa are important therapeutics for anemia.1,2 Via its canonical dimeric single-transmembrane receptor (EPOR),3,4 and possibly a hypothesized accessory chain,5,6 erythropoietin (EPO) also has been shown to confer significant cytoprotection for certain injured nonhematopoietic tissues.7 This includes renal, cardiac, brain, and retinal cells as damaged by ischemia, neurotoxins, or UV irradiation.8–11 However, epoetin-alfa can require frequent injections in anemia contexts, as well as relatively high dosing to cytoprotect nonhematopoietic cells.7 To address these issues, several EPO-related erythropoiesis stimulating agents (ESAs) recently have been formulated and shown to possess one, or another, advantaged property. One orthologue is darbepoietin-alfa in which nonessential residues are mutated to provide for 2 additional N-linked glycosylation sites.12 This can enhance darbepoietin-alfa's in vivo half-life,13 and EPO receptor occupancy.14 Together, these properties can lessen dosing frequency.13 A second example is provided by “Epo-Epo” in which EPO is configured as a peptide-linked head-to-tail dimer.15 For Epo-Epo, unique properties include enhanced secretion and apparent advantaged in vivo activity after subcutaneous injection.16 In addition, a pegylated epoetin-beta has been developed (CERA) that possesses a significantly extended half-life,17 and likewise can be effectively used with less frequent dosing.18
A distinct approach to preparing ESAs involves the development of monoclonal antibodies that can bind and activate the EPOR. In particular, Liu et al19 recently described ABT007 as one such agent and via crystallographic analyses provided evidence for unique EPOR domain interactions. In an alternate approach, initial success has also been indicated for small molecule ESAs in the form of HIF prolyl hydroxylase inhibitors.20 As activated by hypoxia, hypoxia inducible factors (HIFs) act as heterodimeric HIF-ARNT PAS-domain transcription factors to promote the expression of several hypoxia response genes including Epo.21 HIF degradation is regulated, in part, via prolyl hydroxylation.20 Proline hydroxylase inhibitors act to stabilize HIFs and can substantially elevate endogenous EPO production and red cell formation.21
In a third unique approach to ESA development, Jolliffe and coworkers (Johnson et al22 and Wrighton et al23 ) previously applied phage display technologies to discover EPO mimetic peptides (EMPs) that, despite a lack of sequence homology to EPO, bind and biologically stimulate the EPOR. Crystallographic analyses have revealed structural details of EMP1-EPOR binding and provided direct evidence for EPOR's occurrence as a homodimeric receptor.3,4 In monomeric form, EMP activity in vivo, however, is limited.24
Presently, we have characterized the activities of a unique ESA, CNTO 530, which is constructed as a dimeric EMP fused to a human lgG4 Fc scaffold. Analyses in murine models first reveal uniquely high and sustained erythropoietic activity for CNTO 530 compared with epoetin-alfa and darbepoietin-alfa. CNTO 530's erythropoietic effects also were exhibited via advantaged amelioration of the anemia induced by 5-fluorouracil or carboplatin plus paclitaxel. In ex vivo analyses using primary bone marrow erythroblasts, several common phosphoprotein signal transduction factors and target response genes were defined for CNTO 530 and epoetin-alfa. However, CNTO 530 more strongly and persistently stimulated p70S6K. In vivo, CNTO 530 uniquely enforced the enhanced formation of PODXLhighCD71high erythroblasts, as well as the sustained expansion of a KitnegCD71highTer119neg erythroid progenitor pool in bone marrow at levels several-fold above epoetin-alfa or darbepoietin-alfa. CNTO 530 therefore represents a highly effective ESA whose actions interestingly appear to depend, in part, on its unique engagement of the EPOR, together with the persistent stimulation of a defined cohort of targeted proerythroblasts.
Methods
Mouse and rat models
For all in vivo and ex vivo analyses, mice used were strain C57BL/6 at age 8 to 12 weeks. Epoetin-alfa (EPO; Ortho Biotech, Raritan, NJ), darbepoietin-alfa (Amgen, Thousand Oaks, CA), and CNTO 530 injections were intraperitoneal in 0.2 mL saline. In tail vein injections, epoetin-alfa or CNTO 530 was administered in saline (100 μL injection volume). In 5-fluorouracil (5-FU) experiments, 5-FU was injected at 150 mg/kg (single intraperitoneal dose). In carboplatin and paclitaxel anemia models, female Sprague-Dawley CD rats at approximately 300 grams were used (Charles River Laboratories, Raleigh, NC). Carboplatin (50 mg/kg; Polymed, Houston, TX) and paclitaxel (10 mg/kg; Polymed) were administered intraperitoneally (∼ 60 minutes apart) on days 1, 8, and 15. Control rats received cremophore (Sigma, St Louis, MO) and mannitol (Sigma) vehicles (1.0 mL/kg each). All procedures, and the use of all mice and rat models, were reviewed and approved by the institutional animal care and use committee of the Maine Medical Center Research Institute.
Hematologic analyses
For peripheral blood samples, hematocrits, hemoglobin levels, and reticulocyte frequencies were determined using an Advia 120 hematology analyzer (Siemens Medical Solutions Diagnostics, Tarrytown, NY). Hematocrits and reticulocytes also were assayed independently via microcapillary centrifugation, and flow cytometry as described previously.25
Primary erythroblast preparations
For ex vivo expansions, marrow cells were washed in IMDM and resuspended in 1 mL phosphate-buffered saline (PBS; Invitrogen, Carlsbad, CA). Red cells were lysed,25 and progenitors were collected through 50% FBS in PBS. Cells were then plated at 1.8 × 106 cells/mL (7 mL/100 mm dish) in StemPro-34 (Invitrogen) supplemented with 2.5 U/mL epoetin-alfa, 100 ng/mL SCF, 1 μM dexamethasone, 1 μM beta-estradiol, 75 μg/mL h-transferrin, (Sigma), 0.5% BSA (StemCell Technologies, Vancouver, BC), 0.1 mM 2-mercaptoethanol, 100 U/mL penicillin-G, 100 μg/mL streptomycin, 0.25 μg/mL amphotericin-B (1XPSF; Invitrogen), and 1.5 mM l-glutamine (ie, “SP34EX” medium). At 24 hours, 4 mL fresh medium was added. At 48 hours, cells were collected, resuspended in 1 mL conditioned medium, combined with 9 mL fresh medium, and cultured for an additional 24 hours. Proerythroblasts then were purified as a KitposCD71highTer119neg cohort. Specifically, Linpos cells first were depleted from ex vivo cultures using biotinylated antibodies to CD5 (Ly-1), CD45R/B220, CDllb (Mac1), Ter119, and Ly6G (Gr1; StemCell Technologies). KitposCD71highTer119neg cells then were retrieved at purities of more than 99.9% via CD117 magnetic activated cell separation (Miltenyi Biotec, Auburn, CA). In analyses of bone marrow progenitor cell populations, femurs were isolated and thoroughly cleaned of all peripheral blood and tissue. Marrow was then extruded, gently dispersed in Iscove modified Dulbecco medium (IMDM; Invitrogen), 2% FBS, and passed through a 40-micron cell sieve. Cells were washed once in 0.1% BSA, IMDM before flow cytometric analyses.
Flow cytometry
In flow cytometry, 106 cells were washed, resuspended, and incubated at 4°C in 0.2 mL PBS, 0.1% BSA, and 1 μg rat IgG, and then with 1 μg primary antibody for 45 minutes. Primary antibodies used were as follows: biotin-anti-PODXL (R&D Systems, Minneapolis, MN), allophycocyanin (APC)–CD117, fluorescein (FITC)–CD71, and phycoerythrin (PE) Ter119 (BD Biosciences, San Jose, CA). Bound PODXL antibodies were detected using Alexa Fluor 647 streptavidin (Molecular Probes, Eugene, OR). Nucleated erythroblasts were assayed by staining with DRAQ5 (10 μM; Alexis Biochemicals, San Diego, CA). Reticulocytes were stained with Retic-COUNT (BD Biosciences). Equivalent numbers of gated events were analyzed using a BD Biosciences FACSCalibur flow cytometer.
Signal transduction assays and Western blotting
Purified KitposCD71highTer119neg erythroblasts were isolated, washed, and incubated at 8 × 105 cells/mL for 5.5 hours in 0.5% BSA, 50 μg/mL transferrin, 15 ng/mL insulin, 0.1 mM 2-mercaptoethanol in IMDM. Cells then were exposed to epoetin-alfa (2 U/mL) or CNTO 530 (0.5 μg/mL) for the time courses indicated, washed in 2°C PBS, and lysed initially in 0.2 mL 1% Igepal, 150 mM NaCl, 50 mM NaF, 2 mM Na2EDTA, 0.1 mM NaVO3, 1 mM dithiothreitol, 10 mM sodium pyruvate, 25 mM beta-glycerol phosphate, 10% glycerol, 50 mM HEPES (pH 7.5) plus 0.25 mg/mL phenylmethylsulfonylfluoride, 1× protease inhibitor, and 1 × phosphatase inhibitor cocktails (Sigma). Triton-X 100 (1%), 0.5% sodium deoxycholate, 0.1% SDS, 112.5 mM NaCl, and 37.5 mM Tris-HCL (pH 7.4) then were added (0.15 mL). Cleared extracts were assayed for protein content, denatured, electrophoresed, and blotted. Antibodies used were phospho-p70S6K and p70S6K (nos. 9204 and 9202; Cell Signaling, Danvers, MA), phospho-AKT and AKT (nos. 9271 and 9272; Cell Signaling); phospho-STAT5 and STAT5 (nos. 9351 and 9352; Cell Signaling); and phospho-ERK1,2 and ERK1,2 (nos. 9106 and 9102; Cell Signaling). In chemiluminescence, HRP-conjugated antibodies (Jackson Immunoresearch Laboratories, West Grove, PA) and Super-Signal West-Dura reagent (Pierce Biotechnology, Rockford, IL) were used. Signals were analyzed quantitatively using Image-J software (http://rsb.info.nih.gov/ij/, National Institutes of Health [NIH]).
Results
Erythropoietic properties of CNTO 530 in steady-state and anemia models
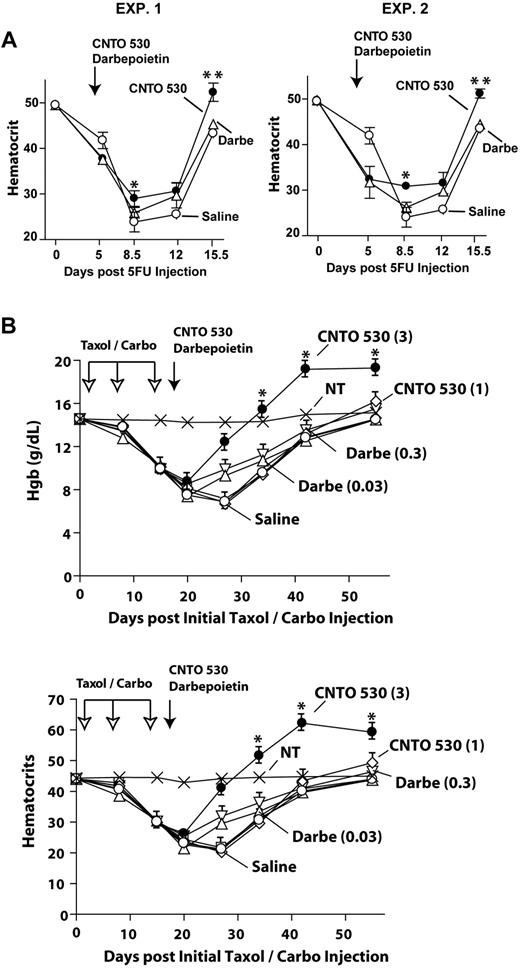
To advance a characterization of CNTO 530's in vivo biologic properties, basic dose-response studies first were performed. Primary analyses were in adult mice at steady state, and included comparisons with both epoetin-alfa (EPO) and darbepoietin-alfa. Specifically, erythropoietic activity was examined based on increases in hematocrits after dosing with CNTO 530, darbepoietin-alfa, or epoetin-alfa. CNTO 530 and darbepoietin-alfa were as single doses. Epoetin-alfa was administered at 1 and 24 hours (to compensate for epoetin-alfa's shorter half-life).13,26 Analyses revealed an ability of CNTO 530 to persistently increase hematocrits by day 8 to 15 or more points (Figure 1A). This differed from response profiles for epoetin-alfa and darbepoietin-alfa in which increases in hematocrits peaked at somewhat lower levels at day 4, and subsequently decreased by day 8. Extended time course experiments next were performed using CNTO 530, epoetin-alfa, and darbepoietin-alfa each at near-maximal doses (ie, 0.15 μg, 1.5 U, and 0.012 μg per gram-mouse, respectively). Here, single dosing of CNTO 530 was observed to provide significant increases in hematocrits for up to 25 days (Figure 1B). For epoetin-alfa and darbepoietin-alfa, increases in red cell production persisted for approximately 10 to 12 days (Figure 1B). These experiments established CNTO 530 as a potent ESA with apparently unique biologic properties that are not well accounted for by in vivo half-life estimates (∼ 40 hours for CNTO 53027 ; 8 to 48 hours for darbepoietin-alfa28,29 ; and 6 to 24 hours for epoetin-alfa26,30 ).
CNTO 530, epoetin-alfa, and darbepoietin-alfa dose-dependent effects on red cell production. (A) In dose-response studies, the capacity of CNTO 530 to stimulate red cell production in wild-type C57BL/6 mice was analyzed and was compared with dose responses for epoetin-alfa (EPO) and darbepoietin-alfa. CNTO 530 and darbepoietin-alfa were as single intraperitoneal injections, whereas epoetin-alfa (EPO) was injected at 1 and 24 hours (as indicated by ). Doses are as microgram or unit (U, EPO) per mouse gram-weight. Hematocrits are mean values ± SE (n = 5). (B) Time-course analysis of CNTO 530–induced red cell and reticulocyte production: Mice were injected with CNTO 530, epoetin-alfa (EPO), or darbepoietin-alfa at near maximal doses (0.15 μg/mouse gram-weight, 1.5 U/mouse gram-weight, and 0.012 μg/mouse gram-weight, respectively). Responses in hematocrits and reticulocytes then were determined on days 4, 8, 12, 16, and 24, and are graphed as mean values ± SE (n = 4).
CNTO 530, epoetin-alfa, and darbepoietin-alfa dose-dependent effects on red cell production. (A) In dose-response studies, the capacity of CNTO 530 to stimulate red cell production in wild-type C57BL/6 mice was analyzed and was compared with dose responses for epoetin-alfa (EPO) and darbepoietin-alfa. CNTO 530 and darbepoietin-alfa were as single intraperitoneal injections, whereas epoetin-alfa (EPO) was injected at 1 and 24 hours (as indicated by ). Doses are as microgram or unit (U, EPO) per mouse gram-weight. Hematocrits are mean values ± SE (n = 5). (B) Time-course analysis of CNTO 530–induced red cell and reticulocyte production: Mice were injected with CNTO 530, epoetin-alfa (EPO), or darbepoietin-alfa at near maximal doses (0.15 μg/mouse gram-weight, 1.5 U/mouse gram-weight, and 0.012 μg/mouse gram-weight, respectively). Responses in hematocrits and reticulocytes then were determined on days 4, 8, 12, 16, and 24, and are graphed as mean values ± SE (n = 4).
Given CNTO 530's capacity to promote erythropoiesis in a sustained fashion, its performance in 5-fluorouracil (5-FU)– and paclitaxel plus carboplatin–induced anemia models next was assessed (Figure 2). In 5-FU experiments, mice received 150 mg/kg, and were then dosed with either CNTO 530 or darbepoietin-alfa at day 4.5 at doses of 0.3 μg/rat gram-weight and 0.02 μg/rat gram-weight, respectively. 5-FU–induced anemia proved to be lessened in severity by CNTO 530 (eg, by 7.5 hematocrit points at day 15.5), but not significantly so by darbepoietin-alfa (Figure 2A).
Efficient CNTO 530 lessening of the anemia induced by 5-fluorouracil or paclitaxel plus carboplatin. (A) Anemia was induced via single 5-FU injection (150 mg/kg). On day 4.5, CNTO 530 (0.3 μg/mouse gram-weight), darbepoietin-alfa (0.02 μg/mouse gram-weight), or saline then was administered. Hematocrits were determined at the indicated time points. Results for 2 independent experiments are shown (means ± SE, n = 4 for each experiment). **P ≤ .001; *P ≤ .024. (B) In Sprague-Dawley rats, anemia was induced via dosing on days 1, 8, and 15 with 10 mg/kg paclitaxel (pac) + 50 mg/kg carboplatin (carbo). On day 16, CNTO 530 or darbepoietin-alfa then was administered and effects on hemoglobin levels and hematocrits were determined over a 50-day interval. Dosing for CNTO 530 was at 1 μg and 3 μg/rat gram-weight, and for darbepoietin-alfa at 0.03 μg and 0.3 μg/rat gram-weight. Mean values ± SD are graphed (n = 8 for controls, for pac/carbo, for pac/carbo + CNTO 530, and for pac/carbo + darbepoietin-alfa). The untreated group is indicated as NT (not treated). Top and bottom panels illustrate representative effects on hemoglobin levels and hematocrits.
Efficient CNTO 530 lessening of the anemia induced by 5-fluorouracil or paclitaxel plus carboplatin. (A) Anemia was induced via single 5-FU injection (150 mg/kg). On day 4.5, CNTO 530 (0.3 μg/mouse gram-weight), darbepoietin-alfa (0.02 μg/mouse gram-weight), or saline then was administered. Hematocrits were determined at the indicated time points. Results for 2 independent experiments are shown (means ± SE, n = 4 for each experiment). **P ≤ .001; *P ≤ .024. (B) In Sprague-Dawley rats, anemia was induced via dosing on days 1, 8, and 15 with 10 mg/kg paclitaxel (pac) + 50 mg/kg carboplatin (carbo). On day 16, CNTO 530 or darbepoietin-alfa then was administered and effects on hemoglobin levels and hematocrits were determined over a 50-day interval. Dosing for CNTO 530 was at 1 μg and 3 μg/rat gram-weight, and for darbepoietin-alfa at 0.03 μg and 0.3 μg/rat gram-weight. Mean values ± SD are graphed (n = 8 for controls, for pac/carbo, for pac/carbo + CNTO 530, and for pac/carbo + darbepoietin-alfa). The untreated group is indicated as NT (not treated). Top and bottom panels illustrate representative effects on hemoglobin levels and hematocrits.
Next, in paclitaxel plus carboplatin experiments, rats were dosed with these chemotherapeutic agents on days 1, 8, and 15. On day 16 (a point at which hematocrits fell to ∼ 25%; Figure 2B bottom panel), CNTO 530 or darbepoietin-alfa was administered, each at 2 doses as 1.0 and 3.0 μg/rat gram-weight (CNTO 530) or 0.03 and 0.3 μg/rat gram-weight (darbepoietin-alfa). By day 28, both darbepoietin-alfa and CNTO 530 provided 50 g/L (5 g/dL) or more advantage in hemoglobin levels (Figure 2B top panel). At days 35, 42, and 56, however, CNTO 530 when dosed at 3.0 μg/mouse gram-weight uniquely continued to provide marked advantages in hemoglobin levels. As analyzed in parallel, 5 to 10 or more point advantages in hematocrits also were realized at these time points due to CNTO 530 dosing (Figure 2B bottom panel). Therefore, anemia in this model was selectively lessened by high-dose CNTO 530.
CNTO 530 stimulation of signal transduction pathways in primary bone marrow erythroblasts
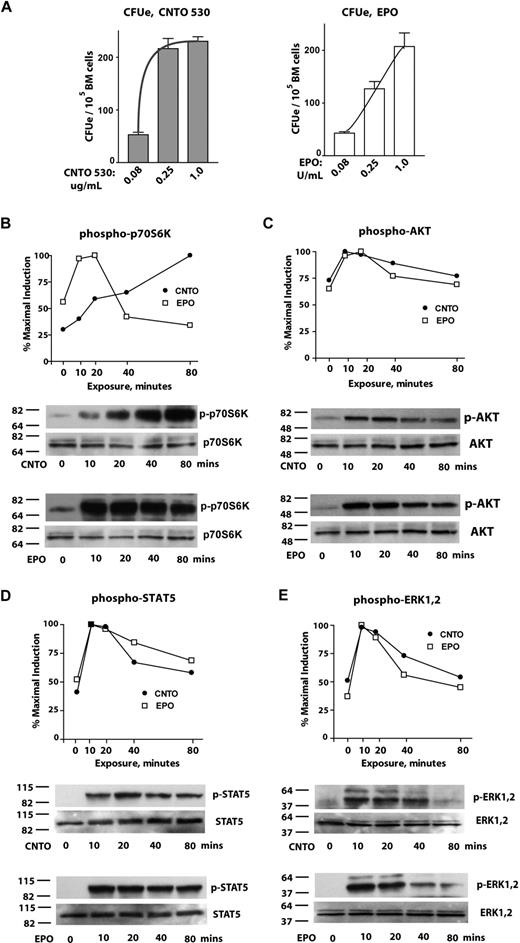
Based on CNTO 530's potent erythropoietic activities, and unique configuration as an IgG4 Fc-fused homodimeric EMP, analyses next focused on CNTO 530–stimulated signal transduction events in primary proerythroblasts. Via an initial basic analysis, the abilities of CNTO 530 versus epoetin-alfa to support bone marrow erythroid colony-forming unit (CFUe) formation first were compared. Specifically, low doses of each ESA required for essentially matched submaximal CFUe stimulation first were defined (ie, 0.08 U/mL EPO and 0.08 μg/mL CNTO 530). Activities of these and equivalent fold-increased doses of each ESA then were assessed. Interestingly, as doses increased, CNTO 530 more readily stimulated the formation of 8 to 16 cell CFUe colonies (Figure 3A).
CNTO 530 activates known EPO signal transduction pathways in primary bone marrow proerythroblasts and induces sustained p70S6K activation. (A) CNTO 530 and EPO dose-dependent support of CFUe formation: To initially examine ex vivo effects on CFUe progenitors, adult murine bone marrow cells were plated in methyl cellulose colony-forming assays in the presence of various concentrations of CNTO 530 (0.08, 0.25, 1.0 μg/mL) or EPO (epoetin-alfa; 0.08, 0.25, 1.0 U/mL) for direct comparison. At 50 hours of culture, frequencies of 8- to 16-cell CFUe were determined; values are mean ± SE and are means of 3 35-mm culture dishes for each dose. (B-E) CNTO 530 and EPO modulation of p70S6K, AKT, STAT5, and ERK1,2 in primary bone marrow proerythroblasts: Primary erythroid progenitor cells from adult bone marrow were expanded in SP34EX medium. At 72 hours, KitposCD71highTer119neg proerythroblasts were then isolated. Cytokines were withdrawn and cells (at 106 cells/mL) were incubated for 5.5 hours in IMDM, 0.5% BSA, 15 ng/mL insulin, and 0.1 mM 2-ME. Cells next were exposed in parallel to either CNTO 530 (0.5 μg/mL) or EPO (2 U/mL) for 0, 10, 20, 40, and 80 minutes. Levels of activated phospho-T421/S424-p70S6K (A); phospho-S473-AKT (B); phospho-Y694-Stat5 (C); and phospho-T202/Y204-ERK1,2 (D) then were determined. Quantitation was by Image-J analysis and was normalized based on levels of total p70S6K, AKT, STAT5, and ERK1,2. Values are percentage maximal signals for CNTO 530 and EPO.
CNTO 530 activates known EPO signal transduction pathways in primary bone marrow proerythroblasts and induces sustained p70S6K activation. (A) CNTO 530 and EPO dose-dependent support of CFUe formation: To initially examine ex vivo effects on CFUe progenitors, adult murine bone marrow cells were plated in methyl cellulose colony-forming assays in the presence of various concentrations of CNTO 530 (0.08, 0.25, 1.0 μg/mL) or EPO (epoetin-alfa; 0.08, 0.25, 1.0 U/mL) for direct comparison. At 50 hours of culture, frequencies of 8- to 16-cell CFUe were determined; values are mean ± SE and are means of 3 35-mm culture dishes for each dose. (B-E) CNTO 530 and EPO modulation of p70S6K, AKT, STAT5, and ERK1,2 in primary bone marrow proerythroblasts: Primary erythroid progenitor cells from adult bone marrow were expanded in SP34EX medium. At 72 hours, KitposCD71highTer119neg proerythroblasts were then isolated. Cytokines were withdrawn and cells (at 106 cells/mL) were incubated for 5.5 hours in IMDM, 0.5% BSA, 15 ng/mL insulin, and 0.1 mM 2-ME. Cells next were exposed in parallel to either CNTO 530 (0.5 μg/mL) or EPO (2 U/mL) for 0, 10, 20, 40, and 80 minutes. Levels of activated phospho-T421/S424-p70S6K (A); phospho-S473-AKT (B); phospho-Y694-Stat5 (C); and phospho-T202/Y204-ERK1,2 (D) then were determined. Quantitation was by Image-J analysis and was normalized based on levels of total p70S6K, AKT, STAT5, and ERK1,2. Values are percentage maximal signals for CNTO 530 and EPO.
In subsequent signal transduction experiments, primary bone marrow–derived erythroblasts were used that were isolated as a highly EPO responsive KitposCD71highTer119neg CFUe-like cohort.31,32 Here, such upstream signaling events as induced by CNTO 530 versus epoetin-alfa were analyzed in parallel. This included analyses of p70S6K, AKT, STAT5, and ERK1,2 activation. In particular, KitposCD71highTer119neg proerythroblasts were expanded and purified. Cytokines then were withdrawn, and cells were incubated for a 5.5-hour interval in 0.5% BSA, 50 μg/mL transferrin, 0.1 mM 2-ME, 10 μg/mL insulin in IMDM. Proerythroblasts then were exposed to either CNTO 530 (0.5 μg/mL) or epoetin-alfa (2 U/mL). At 0, 10, 20, 40, and 80 minutes, lysates were prepared and analyzed by Western blotting for phosphoactivation of the above signal transduction factors (Figure 3B-D). For P-S473-AKT, P-Y694-STAT5, and P-T202/Y204-ERK1,2, CNTO 530–stimulated activation profiles largely paralleled those generated by epoetin-alfa (Figure 3B-D). For p70S6K, however, its activation by CNTO 530 at late time points interestingly persisted compared directly with EPO. To confirm this interesting result, adjusted doses of CNTO 530 and EPO were defined that stimulated p70S6K T421/S424 phosphorylation at comparable levels. Blots were then probed for ERK1,2 T202/Y204 phosphorylation levels. These analyses of a coprocessed and ECL-developed blot again confirmed differentials, here, with comparably decreased ERK1,2 activation for CNTO 530 relative to p70S6K (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Within bone marrow, CNTO 530 drives the formation of PODXLhighCD71high erythroblasts and persistently expands a KitnegCD71highTer119neg erythroid progenitor pool
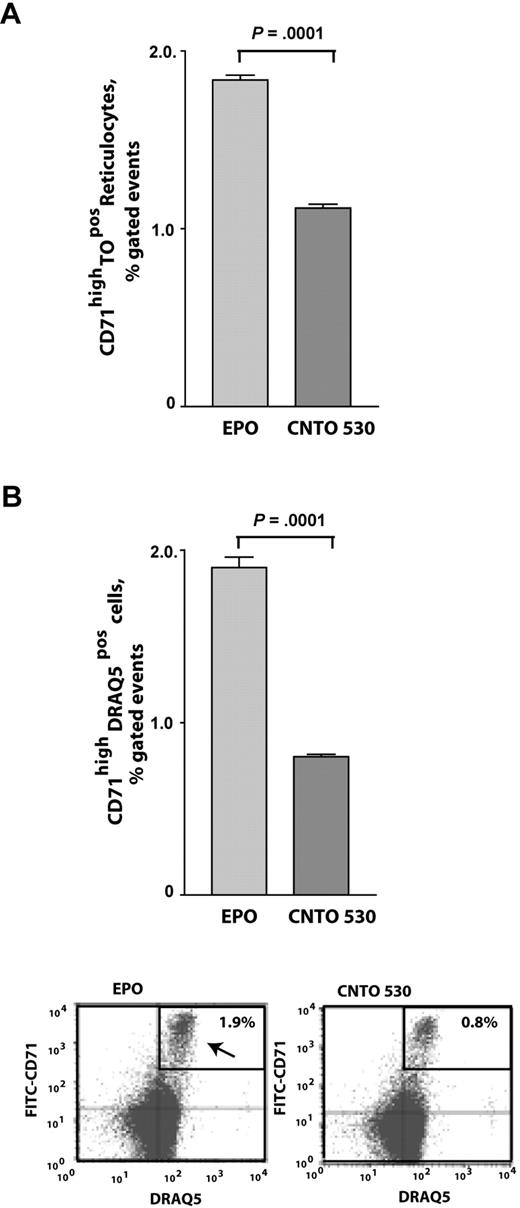
Previously, epoetin-alfa has been demonstrated to stimulate a rapid release of reticulocytes from marrow into peripheral blood.33 We therefore first examined effects of epoetin-alfa and CNTO 530 on this possible response in 2-hour time-frame experiments. Epoetin-alfa proved to stimulate a significant efflux of reticulocytes to blood at 2 hours to levels that were approximately 180% over levels elicited by CNTO 530 (Figure 4A). This provoked the notion that late-stage nucleated erythroid progenitor cells also might be affected, and this was studied by costaining for DRAQ5 positivity among CD71high peripheral blood cells (again at a 2-hour interval). Notably, a significant efflux of these progenitors also was observed in response to epoetin-alfa, but again was lesser in response to CNTO 530 (with epoetin-alfa–induced levels 238% over CNTO 530; Figure 4B). Results therefore are consistent with the notion that epoetin-alfa, more than CNTO 530, may rapidly stimulate an emptying of a late pool of red cell progenitors from marrow.
Shortly after administration, a rapid release of reticulocytes and nucleated DRAQ5pos erythroid progenitors is stimulated by EPO (epoetin-alfa), but less so by CNTO 530. Mice (n = 3 per treatment group) were administered CNTO 530 (0.15 μg/mouse gram-weight), epoetin-alfa (EPO; 2 U/mouse gram-weight), or 0.9% saline (to account for background staining levels) via tail vein injections. At 2 hours, peripheral blood samples were analyzed for levels of TOpos DRAQ5neg reticulocytes (A) and for DRAQ5posCD71high progenitors (B). (Note baseline levels of CD71+ TO− and CD71+ DRAQ5+ cells were 1.2% and 1.4%, respectively.)
Shortly after administration, a rapid release of reticulocytes and nucleated DRAQ5pos erythroid progenitors is stimulated by EPO (epoetin-alfa), but less so by CNTO 530. Mice (n = 3 per treatment group) were administered CNTO 530 (0.15 μg/mouse gram-weight), epoetin-alfa (EPO; 2 U/mouse gram-weight), or 0.9% saline (to account for background staining levels) via tail vein injections. At 2 hours, peripheral blood samples were analyzed for levels of TOpos DRAQ5neg reticulocytes (A) and for DRAQ5posCD71high progenitors (B). (Note baseline levels of CD71+ TO− and CD71+ DRAQ5+ cells were 1.2% and 1.4%, respectively.)
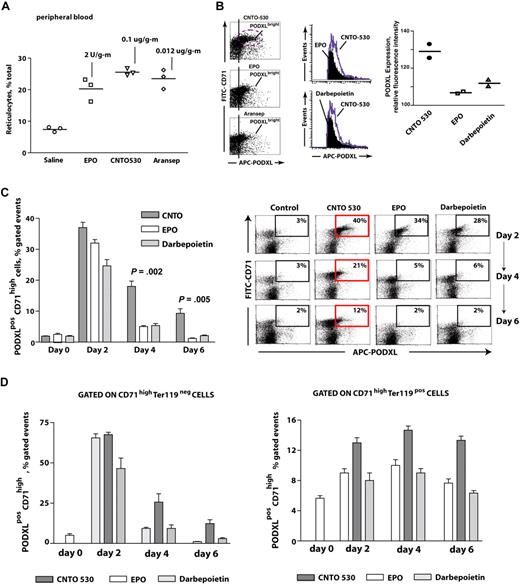
Effects of CNTO 530 on erythropoiesis within bone marrow, per se, next were analyzed, and further were compared directly with epoetin-alfa and darbepoietin-alfa. To limit effects due to differing half-lives, epoetin-alfa was administered at both 1 and 24 hours at a dose (2 U/mouse gram-weight) that by day 4 supported reticulocyte (and red blood cell) formation at levels highly similar to those afforded by CNTO 530 (at 0.1 μg/mouse gram-weight), and darbepoietin-alfa (at 0.012 μg/mouse gram-weight; Figure 5A). Under these balanced dosing conditions, marker expression among developing bone marrow erythroid cells was analyzed, including podocalyxin (PODXL) as a marker of EPO-induced erythropoiesis.34 Interestingly, CNTO 530 first was observed to support clear increase in PODXL expression intensities among CD71high cells within bone marrow (Figure 5B). CNTO 530 also was observed to promote increases in frequencies of a PODXLhighCD71high erythroblast cohort (Figure 5C). In particular, and as analyzed in time-course experiments, CNTO 530 at day 2 was modestly yet significantly more effective than epoetin-alfa and darbepoietin-alfa in expanding this progenitor pool. At day 4, this advantage increased to approximately 4-fold. At day 6, this PODXLhighCD71high progenitor pool remained elevated as selectively supported by CNTO 530 (12% positivity) and was 6-fold above epoetin-alfa– and darbepoietin-alfa–stimulated levels (which were essentially background by day 6 after dosing). PODXL expression among earlier stage CD71posTer119neg and CD71highTer119pos erythroid progenitors also was assessed (Figure 5D). Enhanced effects of CNTO 530 on PODXL expression were realized within each bone marrow progenitor pool.
CNTO 530 enhances the formation of PODXLhighCD71high bone marrow erythroid progenitors. (A) To investigate possible differential effects of CNTO 530 and epoetin-alfa or darbepoietin-alfa on bone marrow erythroid progenitor cell populations, mice were administered each at doses that at day 4 provided near maximum, and highly comparable levels of reticulocyte and red cell production. Near equivalency in reticulocyte production at day 4 was observed after dosing with CNTO 530 (0.15 μg/mouse gram-weight), epoetin-alfa (EPO; 2 U/mouse gram-weight at 1 and 24 hours), or darbepoietin-alfa (0.012 μg/mouse gram-weight). Values for each injected mouse are shown together with mean values (horizontal bars). (B) For bone marrow–resident erythroid progenitors, CNTO 530 induced significant increases in the intensity (ie, cell surface density) of PODXL expression. Here, this is shown in representative flow cytometric analyses that compare PODXL expression levels after CNTO 530 versus EPO dosing, and CNTO 530 versus darbepoietin-alfa. (C) At days 2, 4, and 6, frequencies of PODXLhighCD71high progenitors within bone marrow were determined by flow cytometry. Top panels illustrate representative flow cytometry analyses and demonstrate a clear advantage for CNTO 530 in stimulating the formation of this PODXLhighCD71high erythroid progenitor cell pool, especially at days 4 and 6. Mean frequencies (± SE) of such PODXLhighCD71high progenitors are graphed as analyzed in n = 3 mice after CNTO 530, EPO, or darbepoietin-alfa injections. (EPO was administered at 1 and 24 hours.) (D) Gating on, or off, Ter119posCD71high erythroid progenitors revealed that CNTO 530's effects on enhanced PODXL expression was exerted among not only later stage Ter119pos cells, but also their CD71highTer119neg progenitors. Values are means ± SE (n = 3).
CNTO 530 enhances the formation of PODXLhighCD71high bone marrow erythroid progenitors. (A) To investigate possible differential effects of CNTO 530 and epoetin-alfa or darbepoietin-alfa on bone marrow erythroid progenitor cell populations, mice were administered each at doses that at day 4 provided near maximum, and highly comparable levels of reticulocyte and red cell production. Near equivalency in reticulocyte production at day 4 was observed after dosing with CNTO 530 (0.15 μg/mouse gram-weight), epoetin-alfa (EPO; 2 U/mouse gram-weight at 1 and 24 hours), or darbepoietin-alfa (0.012 μg/mouse gram-weight). Values for each injected mouse are shown together with mean values (horizontal bars). (B) For bone marrow–resident erythroid progenitors, CNTO 530 induced significant increases in the intensity (ie, cell surface density) of PODXL expression. Here, this is shown in representative flow cytometric analyses that compare PODXL expression levels after CNTO 530 versus EPO dosing, and CNTO 530 versus darbepoietin-alfa. (C) At days 2, 4, and 6, frequencies of PODXLhighCD71high progenitors within bone marrow were determined by flow cytometry. Top panels illustrate representative flow cytometry analyses and demonstrate a clear advantage for CNTO 530 in stimulating the formation of this PODXLhighCD71high erythroid progenitor cell pool, especially at days 4 and 6. Mean frequencies (± SE) of such PODXLhighCD71high progenitors are graphed as analyzed in n = 3 mice after CNTO 530, EPO, or darbepoietin-alfa injections. (EPO was administered at 1 and 24 hours.) (D) Gating on, or off, Ter119posCD71high erythroid progenitors revealed that CNTO 530's effects on enhanced PODXL expression was exerted among not only later stage Ter119pos cells, but also their CD71highTer119neg progenitors. Values are means ± SE (n = 3).
Findings further prompted analyses of CNTO 530 versus darbepoietin-alfa effects on the expansion of subpopulations of erythroid progenitors within bone marrow. These included KitposCD71highTer119neg, KitnegCD71highTer119neg, and KitnegCD71highTer119pos cohorts (designated here as sequential stages E1, E2, and E3, respectively; Figure 6A). In particular, mice were dosed with CNTO 530 (single 0.2 μg/mouse gram-weight injection), darbepoietin-alfa (single 0.015 μg/mouse gram-weight injection), or saline as a baseline (or steady-state) control. E1 and E2 cells were assayed by gating off Ter119pos cells and analyzing frequencies of Kitpos versus Kitneg CD71high erythroid progenitors. E3 cells were later stage Ter119posCD71high erythroblasts (and were uniformly Kitneg). At steady state, stage-E3 cells represented approximately 20% of nucleated marrow cells, whereas E1 and E2 progenitors were relatively infrequent and occurred at approximately 1% and approximately 1.3%, respectively (Figure 6B). At day 2.5 after ESA dosing, darbepoietin-alfa and CNTO 530 each markedly modulated stage-E2 cell frequencies, with 10- and 13-fold increases realized, respectively. E1 progenitor pools remained low and were comparably unaffected (Figure 6C left panel). Perhaps most strikingly, at day 4.5 after ESA administration, the expansion of stage-E2 progenitor cells proved to be sustained uniquely in response to CNTO 530 (Figure 6C right panel). In primary flow cytometry analyses, this effect was visibly obvious for a CD71highTer119neg population of bone marrow erythroid progenitors. Although these progenitors have not yet been tagged and tracked, it is considered likely that they possess high potential for populating the adult erythron. By comparison, levels of stage-E3 cells were not so markedly affected by CNTO 530 or darbepoietin-alfa (data not shown).
CNTO 530 uniquely induces the sustained expansion of a bone marrow–resident KitnegCD71highTer119neg E2 progenitor pool. (A) Based in part on recent ex vivo analyses of murine bone marrow erythroid progenitor pools,32,34,35 a course of stepwise development can be defined as a Kitpos CD71highTer119neg → Kitneg CD71highTer119neg → Kitneg CD71highTer119pos cell progression (ie, stages E1 → E2 → E3). (B) At steady state, stage-E3 bone marrow progenitors were predominant as assayed via costaining for Kit, CD71, and Ter119 markers, and gating-off red blood cells (RBCs) and reticulocytes. E1 and E2 progenitors were assayed by gating off Ter119pos cells and on Kitpos vs Kitneg CD71high cells. Each symbol in each set represents the value for an independently treated and analyzed mouse. (C) CNTO 530 uniquely and markedly expands stage-E2 bone marrow progenitors: C57BL/6 mice were dosed with either CNTO 530 (0.2 μg/mouse gram-weight) or darbepoietin (0.015 μg/mouse gram-weight). At days 2.5 and 4.5, levels of E1, E2, and E3 progenitors were then determined via flow cytometry. Graphed values (top panel) are means ± SE (n = 3). (D) Primary flow cytometry data illustrate CNTO 530 expansion of stage-E2 erythroid progenitors within bone marrow (here at day 4.5 after dosing with either CNTO 530, darbepoietin, or saline).
CNTO 530 uniquely induces the sustained expansion of a bone marrow–resident KitnegCD71highTer119neg E2 progenitor pool. (A) Based in part on recent ex vivo analyses of murine bone marrow erythroid progenitor pools,32,34,35 a course of stepwise development can be defined as a Kitpos CD71highTer119neg → Kitneg CD71highTer119neg → Kitneg CD71highTer119pos cell progression (ie, stages E1 → E2 → E3). (B) At steady state, stage-E3 bone marrow progenitors were predominant as assayed via costaining for Kit, CD71, and Ter119 markers, and gating-off red blood cells (RBCs) and reticulocytes. E1 and E2 progenitors were assayed by gating off Ter119pos cells and on Kitpos vs Kitneg CD71high cells. Each symbol in each set represents the value for an independently treated and analyzed mouse. (C) CNTO 530 uniquely and markedly expands stage-E2 bone marrow progenitors: C57BL/6 mice were dosed with either CNTO 530 (0.2 μg/mouse gram-weight) or darbepoietin (0.015 μg/mouse gram-weight). At days 2.5 and 4.5, levels of E1, E2, and E3 progenitors were then determined via flow cytometry. Graphed values (top panel) are means ± SE (n = 3). (D) Primary flow cytometry data illustrate CNTO 530 expansion of stage-E2 erythroid progenitors within bone marrow (here at day 4.5 after dosing with either CNTO 530, darbepoietin, or saline).
Discussion
For CNTO 530, several unique properties and apparently distinct action modes presently are defined that merit discussion. These include, first, CNTO 530's notable erythropoietic activity as demonstrated at steady state and within 2 chemotherapeutic agent–induced anemia models. Second, CNTO 530's capacities to activate known EPO/EPOR signal transduction pathways are defined, together with several distinguishing signaling effects. Finally, a third property of significant interest involves CNTO 530's unique in vivo abilities to enhance the formation of podocalyxin-positive erythroid progenitors, and to also promote the sustained expansion of a defined (pro)erythroblast pool within bone marrow.
In basic concentration-dependent, and time-course experiments, CNTO 530 efficiently promoted red cell production for up to 25 days after a single injection at submaximum dosing (Figure 1). This amplification of the adult erythron was more robust than that provided by epoetin-alfa or darbepoietin-alfa. In part, this may reflect differential half-lives, which for CNTO 530 recently has been shown to approximate 40 hours.27 By day 10, however, more than 95% of CNTO 530 is predicted to become unavailable. This therefore points to additional mechanistic explanations for CNTO 530's potency (Figure 1B). Here, it also should be considered that on a molar basis, an approximate 10-fold higher mass of CNTO 530 over epoetin-alfa is required to achieve maximal bioactivity. This may reflect properties involved with marrow entry, potential EPOR on- and/or off-rates, and/or other as yet undefined features. As an IgG4 Fc fusion protein, CNTO 530 is readily prepared and purified. Requirements for higher mass dosing therefore are not disadvantageous. In its single-dose format, CNTO 530 also proved to be uniquely advantageous in limiting the anemia induced by 5-fluorouracil or paclitaxel plus carboplatin. In particular, and at essentially matched doses, CNTO 530 but not darbepoietin was observed to lessen the severity of anemia incurred after 5-FU–mediated myelodepletion (Figure 2A). In the case of an optimized paclitaxel/carboplatin model, however, anemia was not so much affected at these doses by either darbepoietin or CNTO 530. When doses were increased to approximately 10-fold over maximal steady-state doses, however, CNTO 530 (but not darbepoietin-alfa) interestingly was capable of significantly lessening this anemia in severity and duration (Figure 2B). Why this dosing difference exists between effects in a 5-FU (mouse) model versus paclitaxel/carboplatin (rat) model presently is unclear. Nonetheless, the unique ameliorating effects of CNTO 530 in each model are apparent. In future studies, it will be of significant interest to establish CNTO 530's effectiveness in additional genetically based anemia models (eg, thalassemia and/or sickle cell). Phase 1 clinical trials for CNTO 528 likewise indicate initially promising erythropoietic effects.36
In initial characterizations of CNTO 530's molecular action modes,27 we demonstrated this EMP's ability to competitively bind to the EPOR and to stimulate the growth and survival of an EPO-dependent cell line, UT7epo.27,37 Presently, we have used primary bone marrow–derived KitposCD71highTer119neg erythroblasts to analyze CNTO 530 signal transduction factor activation responses, and have compared these responses directly with those modulated by epoetin-alfa. In the activation of P-S473-AKT, P-Y694-STAT5, and P-T202/Y204-ERK1,2, signal transduction factor analyses demonstrated similar time-course responses for CNTO 530 and epoetin-alfa. For p70S6K, however, CNTO 530 interestingly proved to selectively enforce a prolonged stimulation (Figure 3B). In addition, a significant direct differential in the relative stimulation of p70S6K versus ERKs by CNTO 530 versus EPO was observed (Figure S1). p70S6K lies downstream of mTOR (especially mTOR-C1).38,39 However, p70S6K activation also can be regulated by PDK1,240 ; PKC41 ; PP2A and PTP1b phosphatases42,43 ; and ubiquitination.44 Which pathway might be engaged by CNTO 530 to sustain S6K activation is presently undefined, but is of significant interest. Functionally, S6K can exert diverse effects on cell growth, development, homeostasis, and protein translation,45 and therefore comprises an interesting effector for future investigations of CNTO 530's action mechanisms. With regard to ERK1,2, increased activation via a PY-mutated EPOR-HM allele interestingly has been observed and has been functionally linked to perturbed proerythroblast differentiation events.46 How interactions of this homodimeric EPO mimetic peptide with the EPOR might differentially sustain this response also is of interest, especially in the context of a conformation-mediated model for the activation of dimeric EPOR plus JAK2 complexes.47
In vivo, CNTO 530 was further first observed to selectively enhance the formation of podocalyxin-positive CD71high erythroid bone marrow progenitor cells. This was obvious as early as day 2 after dosing, and subsequently was reinforced at days 4 and 6 (Figure 5C). At day 6, this effect of CNTO 530 on PODXLpos progenitors expansion in marrow, in fact, was realized at levels 6-fold beyond those promoted by either epoetin-alfa or darbepoietin-alfa. Using primary bone marrow erythroblasts and a global profiling approach, we recently discovered Podxl as a major EPO response gene.34 PODXL is a transmembrane sialomucin and CD34 orthologue.48 In renal podocytes, PODXL is thought to function in maintaining diaphragm slits.49 In contrast, in hematopoietic stem cells50 and potentially in certain cancers (eg, prostate and certain leukemias),51,52 PODXL may act as an adhesion and/or cell migration factor. It therefore might be speculated through correlation that PODXL, as persistently induced by CNTO 530, may affect the residency of erythroid progenitors in marrow.
A second effect as discovered via extended analyses of KitposCD71highTer119neg, KitnegCD71highTer119neg, and KitnegCD71highTer119pos cell formation (“E1,” “E2,” and “E3” progenitors, respectively) involved CNTO 530's uniquely sustained effects on stage-E2 progenitor expansion within bone marrow. Specifically, when E1 to E3 progenitor cell pools were assayed in bone marrow after CNTO 530 or darbepoietin-alfa dosing, CNTO 530 uniquely supported the enhanced expansion of stage-E2 10-fold over those stimulated by maximal dose darbepoietin-alfa (Figure 6). Here, it is proposed that this unique activity of CNTO 530 in persistently stimulating this erythroid progenitor pool at least in part accounts for its potent and sustained activity as an EPO mimetic peptide. Precisely, how CNTO 530 exerts this effect presently is less clear. Previously, select ligand-receptor interactions within discrete niches have been described for activated B cells in a BLyS signaling context53 and for T-cell development in IL7 signaling contexts.54 Potential bone marrow niche sequestration of CNTO 530 therefore represents one possibly enhanced action mode. Potential effects on macrophage-dependent erythroblast island formation, alternatively, are possible. Erythroblast island–associated events, however, are thought to predominantly affect late-stage development, including enucleation.55 Understanding specific bases for CNTO 530's unique in vivo properties therefore will require further investigation. The present investigations nonetheless demonstrate CNTO 530 to comprise a unique and potent ESA with significant promise for prospective clinical utility in the treatment of anemia.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was supported by National Institutes of Health (NIH) R01 HL044491, and core facilities sponsored by NIH National Center for Research Resources (NCRR) COBRE grants P20-RR18789 and P20-RR15555 (Bethesda, MD).
National Institutes of Health
Authorship
Contribution: All authors contributed to bench investigations and paper construction; lead roles assumed by individual authors include the following: P.S. performed phosphoprotein analyses (with D. Marshall) and gene profiling; E.H. performed in vivo CNTO 530, EPO, and darbepoietin-alfa dosing experiments and phosphoprotein analyses (with P.S.); C.E. and A.P. performed analyses of ESA effects on erythroid progenitor pools in vivo; D. Marshall performed phosphoprotein analyses (with P.S.) and carboplatin/paclitaxel model and experiments (with A.V. and D. Makropoulos); A.V. and D. Makropoulos performed carboplatin/paclitaxel experiments (with D. Marshall); and D.M.W. and P.J.B. designed and directed all investigations and were the lead authors of this paper.
Conflict-of-interest disclosure: D. Marshall, A.V., D. Makropoulos, and P.J.B. are employees of Centocor (the developer and source of CNTO 530) and have equity stake in Johnson & Johnson, the parent company of Centocor R&D. D.M.W. also was the recipient of sponsored program support from Centocor. The remaining authors declare no competing financial interests.
Correspondence: Don M. Wojchowski, Director, Stem and Progenitor Cell Biology Program, Maine Medical Center Research Institute, 81 Research Dr, Scarborough, ME 04074; e-mail: wojchd@mmc.org.
References
Author notes
*P.S. and E.H. contributed equally to this work.