To the editor:
We read with interest the article by Wang et al1 that concluded that the presence of higher levels of anti–HIV-1 microRNAs (miRNAs) in monocytes may be responsible for their relative resistance to HIV-1 infection compared with macrophages.
They rightly point out that differential expression of the CCR5 coreceptor has been postulated as a reason for the differential susceptibility of monocytes and macrophages to HIV-1 infection. In our experience, only a small subset of monocytes (< 5%) express CCR5 and this is at low level, whereas CD4 expression is also uniformly low compared with CD4+ T cells. It is unclear from the experiments presented whether the CCR5 tropic viruses tested bind to monocytes or are internalized. A defect in adhesion or internalization would occur prior to any opportunity for miRNA to directly affect viral replication. Figure 1A from Wang et al1 clearly demonstrates the negligible reverse transcriptase (RT) activity detected in supernatants of monocyte cultures after viral inoculation. However, it is unclear from the data presented the stage of the viral life cycle at which inhibition occurs. Therefore, despite the higher levels of anti-HIV miRNAs in monocytes, it has not been demonstrated that low-level expression of both CCR5 and CD4 is not the more significant factor in explaining their relative resistance to infection.2
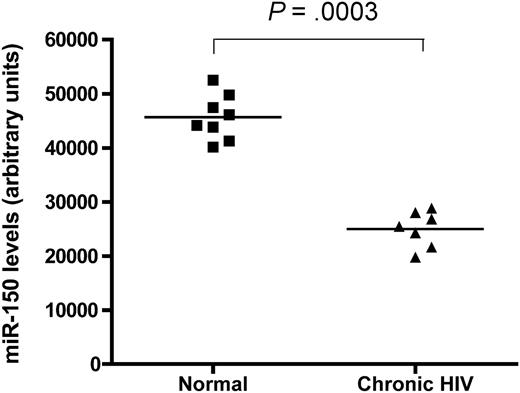
Furthermore, in preliminary work from our laboratory, we checked miRNA expression in CD4+ T cells from 8 healthy, uninfected controls and from 7 patients with chronic HIV-1 infection using a microarray system. Analysis of expression levels of the 4 miRNAs examined by Wang and colleagues showed that healthy control CD4+ T cells expressed significantly higher levels of miR-28-5p and miR-150 (Figure 1), whereas miR-223 and miR-382 levels were not different between the 2 groups. It therefore appears that there is infection-related down-regulation of miR-150 and miR-28-5p in CD4+ T cells during chronic HIV-1 infection. Using the same argument as the authors, we could conclude that because the uninfected controls had higher levels of miR-150 and miR-28-5p compared with the HIV-1–infected patients, CD4+ T cells from healthy controls are relatively protected against HIV-1 infection. This conclusion is clearly incorrect with regard to CD4+ T cells.
miR-150 levels in CD4+ T cells. Shown are levels from 8 healthy volunteers (■) and 7 patients with chronic HIV-1 infection (▴) demonstrating a highly significant difference between the 2 groups. P value shown at top is by Mann-Whitney comparison of the 2 test groups.
miR-150 levels in CD4+ T cells. Shown are levels from 8 healthy volunteers (■) and 7 patients with chronic HIV-1 infection (▴) demonstrating a highly significant difference between the 2 groups. P value shown at top is by Mann-Whitney comparison of the 2 test groups.
Our other concern is with the use of combined miRNA inhibitors to demonstrate changes in RT activity. Treatment of HIV-1–infected monocytes with miRNAs resulted in a rise in RT, but the effects were modest in comparison to RT levels achieved in HIV-1 infection of macrophages. It has been recently demonstrated that even inhibition of a single miRNA may modulate protein synthesis of thousands of genes.3 The use of multiple inhibitors combined could lead to many unanticipated off-target effects. These may have unforeseen indirect effects on viral replication through changes in cellular phenotype or function. We contend that further studies confirming a lack of alteration in phenotype of monocytes after the use of these inhibitors is required before concluding that the change is a direct result of the actions of miRNAs on HIV protein synthesis.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sanjay Swaminathan, St Vincent's Centre for Applied Medical Research, National Centre in HIV Epidemiology & Clinical Research, 405 Liverpool St, Darlinghurst NSW Australia 2010; e-mail: s.swaminathan@cfi.unsw.edu.au.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal