Abstract
Patients with myeloproliferative disorders are at a high risk of developing thrombotic events. Several investigators have hypothesized that endothelial cell (EC) abnormalities might contribute to this prothrombotic state. Budd-Chiari syndrome (BCS) and portal vein thrombosis have been reported to be associated with JAK2V617F-positive hematopoiesis. We explored whether JAK2V617F was present in ECs in the vessels of polycythemia vera (PV) patients with BCS using laser capture microdissection followed by nested polymerase chain reaction or reverse-transcribed polymerase chain reaction. The ECs of the 2 BCS patients with PV were homozygous for the JAK2V617F and were shown to express transcripts characteristic of ECs but not hematopoietic cells. ECs of the other BCS patient with PV and 2 patients with hepatoportal sclerosis without PV contained exclusively wild-type JAK2. The presence of JAK2V617F in both ECs and hematopoietic cells belonging to BCS patients with PV indicate that ECs in PV are involved by the malignant process and that in a subpopulation of the patients the disease might originate from a common cell of origin for hematopoietic and ECs.
Introduction
Polycythemia vera (PV) is a Philadelphia chromosome–negative myeloproliferative disorder (MPD) that is associated with mutations of a cytosolic tyrosine kinase, JAK2.1,2 A gain-of-function mutation (JAK2V617F) is present in more than 90% of patients with PV.1,2 PV has been associated with the intraabdominal thromboses.3 Some persons with normal blood counts who develop splanchnic vein thrombosis, including Budd-Chiari syndrome (BCS) and portal vein thrombosis, have been reported to have JAK2V617F-positive hematopoiesis, indicating that this thrombotic tendency may precede the development of an MPD.4,5
During early mammalian development, a common cell of origin for hematopoietic and endothelial cell (EC) elements has been identified.6-8 This so-called hemangioblast has been characterized in adults, but its role in normal hematopoiesis or the pathogenesis of the MPDs remains poorly defined.9,10 Since 1997, postnatal vasculogenesis has been proposed to involve a hierarchy of circulating endothelial progenitor cells.11 Some of these endothelial progenitor cells are of myeloid origin, whereas others have a more robust proliferative potential and are solely of EC origin.12,13 Involvement of endothelial progenitor cells by JAK2V617F has been studied by several groups.12,14,15 Vascular endothelium provides a nonadhesive surface to circulating neutrophils and platelets while helping to prevent blood clotting. Several groups have hypothesized that EC dysfunction might contribute to the hypercoagulable state associated with PV by orchestrating the recruitment of blood cell elements to sites of injury or by regulating vascular tone by impairing the release of nitric oxide.16-18
To further explore the origins of ECs in MPD, we examined whether ECs in nonhematopoietic organs harbored JAK2V617F. We tested this hypothesis by studying ECs in venules of liver biopsy specimens obtained from patients with BCS and PV using laser capture microdissection (LCM) followed by nested polymerase chain reaction (PCR) or reverse transcription (RT)–PCR.
Methods
Patients
Archived formalin-fixed, paraffin-embedded sections of liver biopsy specimens from 3 BCS patients with PV and 2 patients with hepatoportal sclerosis without PV were studied (Table 1). Hepatoportal sclerosis is a form of noncirrhotic portal hypertension and histologic portal venule abnormalities.19 Access to the archived specimens was approved by the Institutional Review Board of the Mount Sinai School of Medicine. The diagnosis of PV was made according to criteria outlined by the World Health Organization.20 Granulocyte JAK2V617F allele burden was studied by previously published methods in only one patient.21 The other patients were not available for such testing at the time of study.
Clinical profile of patients with intraabdominal thrombosis
| . | Patient BCS29 . | Patient BCS18 . | Patient BCS56 . | Patient PVT36 . | Patient PVT54 . |
|---|---|---|---|---|---|
| Diagnosis | PV | PV | PV | Hepatoportal sclerosis | Hepatoportal sclerosis |
| Site of thrombosis | Hepatic vein | Hepatic vein, portal vein, right jugular vein and splenic vein | Hepatic vein, portal vein and splenic vein | Portal vein | Portal vein |
| Age, y | 38 | 33 | 67 | 47 | 74 |
| Sex | Female | Female | Male | Female | Male |
| Year of diagnosis | 1996 | 1994 | 1998 | 2002 | 2000 |
| JAK2V617F status | + | + | + | − | − |
| Treatment | Coumadin/aspirin/hydroxyurea | Coumadin/hydroxyurea | Hydroxyurea | TIPS | Liver transplantation |
| . | Patient BCS29 . | Patient BCS18 . | Patient BCS56 . | Patient PVT36 . | Patient PVT54 . |
|---|---|---|---|---|---|
| Diagnosis | PV | PV | PV | Hepatoportal sclerosis | Hepatoportal sclerosis |
| Site of thrombosis | Hepatic vein | Hepatic vein, portal vein, right jugular vein and splenic vein | Hepatic vein, portal vein and splenic vein | Portal vein | Portal vein |
| Age, y | 38 | 33 | 67 | 47 | 74 |
| Sex | Female | Female | Male | Female | Male |
| Year of diagnosis | 1996 | 1994 | 1998 | 2002 | 2000 |
| JAK2V617F status | + | + | + | − | − |
| Treatment | Coumadin/aspirin/hydroxyurea | Coumadin/hydroxyurea | Hydroxyurea | TIPS | Liver transplantation |
BCS indicates Budd-Chiari syndrome; PV, polycythemia vera; TIPS, transjugular intrahepatic portosystemic shunt; +, positive; and −, negative.
EC isolation by LCM
ECs along the terminal hepatic venules were observed in archived formalin-fixed, paraffin-embedded sections of liver biopsy specimens and were captured by LCM from 5-μm-thick sections after either hematoxylin and eosin staining or immunochemical staining with an anti-CD34 monoclonal primary antibody (clone QBEnd/10; CONFIRM, Ventana Medical Systems, Tucson, AZ) followed by staining with conjugated streptavidin horseradish peroxidase secondary antibody. ECs were identified by their fusiform nuclei and their location along the lining of the terminal hepatic venules as well as their dark brown color after anti-CD34 staining. Hepatocytes were identified by their morphologic criteria, including round centrally located nuclei and abundant cytoplasm with a trabecular arrangement. Cells were captured within one hour of the staining procedure. At least 10 ECs and 10 hepatocytes from each biopsy specimen were captured using the ArcturusXT system (Arcturus Bioscience, Mountain View, CA; MDS Analytical Technologies, Mountain View, CA). The isolation of EC or hepatocytes with LCM was repeated in at least in 3 different sections from the same liver biopsy specimen and in at least in 3 different vessel walls from each of the sections and the results remained the same.
Detection of the JAK2V617F by nested allele-specific PCR
Laser-captured cells, blood cells, hepatocytes, and ECs were processed for DNA isolation, and DNA was extracted using a PicoPure DNA Extraction Kit (Arcturus Bioscience; MDS Analytical Technologies) according to the manufacturer's instructions. The JAK2V617F mutation was detected using nested allele-specific PCR as previously described.22
Real-time RT-PCR assay of laser-captured cells
Total RNA was extracted from laser-captured cells using a PicroPure RNA Isolation Kit (Arcturus Bioscience). First-strand complimentary DNA (cDNA) was synthesized from total RNA with SuperScript III Reverse Transcriptase (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. The sequences of the primers used for the amplification and the cycling conditions of VE-cadherin, VEGFR-2, VWF, CD45, CD14, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and albumin have been previously described.12,23-26 The real-time RT-PCR assays were performed with the ABI Prism 7900 Sequence Detection System (Applied Biosystems, Foster City, CA), and the PCR products were detected with the use of SYBR green I technology (QuantiTect SYBR Green PCR Kit; QIAGEN, Valencia, CA). PCR amplification was performed in a total volume of 20 μL; each reaction contained 2× SyberGreen mix, 200 nM primers, and cDNA. All assays were performed in duplicate, and for all assays a positive control, a negative control, and a no template control were included. To assess EC or hematopoietic cell mRNA expression, the fluorescence emitted by the reporter dye above baseline signal was detected using software in real-time, recorded, and represented as the cycle threshold (CT). The CT value was used to determine the presence or absence of the PCR product in the sample. The detection of a CT value for GAPDH served as means of documenting that sufficient amount of RNA was available. The absence of a CT value for the gene of interest in the presence of a GAPDH signal represented a negative signal for the gene of interest, whereas the presence of a CT value for the gene of interest in the presence of GAPDH was indicative of a positive signal. The actual CT values are provided in Figure 1C.
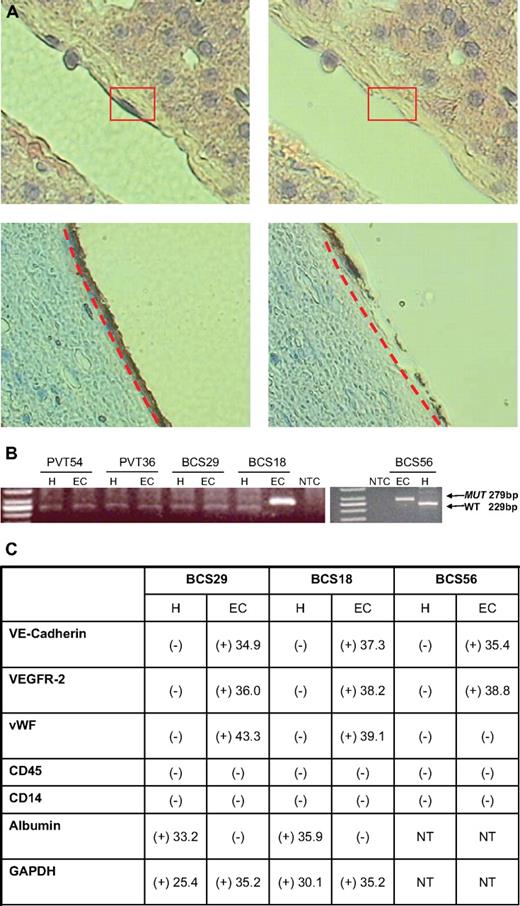
Presence of JAK2V617F in the ECs lining the terminal hepatic venules in the sections of archived liver biopsy tissue specimens from BCS patients. (A) The ECs from a terminal hepatic venule with less congestion from a liver biopsy of a patient with BCS with PV captured by LCM after hematoxylin and eosin staining (top panel) or immunohistochemical staining with an anti-CD34 antibody (bottom panel). These photomicrographs show a portion of a terminal hepatic venule lined by ECs. There is a thin rim of underlying connective tissue in the subendothelial space. The boxed or underlined area shows longitudinally sectioned endothelial cells. The rest of the photo shows plates of hepatocytes arranged in trabeculae. The right panel demonstrates the same areas after laser capture of the ECs. The remaining adjacent tissue remains intact (original magnification ×400; ArcturusXT System (MDS Analytical Technologies). (B) JAK2V617F status of LCM captured hepatocytes (H) and endothelial cells (EC) of patients by allele-specific nested PCR for JAK2V617F. (C) Table summarizing the transcripts present in hepatocytes (H) and ECs isolated by LCM from archived liver biopsy tissue specimens. The numbers represent the average CT values of the samples, which were run in duplicate and repeated on 3 separate occasions.
Presence of JAK2V617F in the ECs lining the terminal hepatic venules in the sections of archived liver biopsy tissue specimens from BCS patients. (A) The ECs from a terminal hepatic venule with less congestion from a liver biopsy of a patient with BCS with PV captured by LCM after hematoxylin and eosin staining (top panel) or immunohistochemical staining with an anti-CD34 antibody (bottom panel). These photomicrographs show a portion of a terminal hepatic venule lined by ECs. There is a thin rim of underlying connective tissue in the subendothelial space. The boxed or underlined area shows longitudinally sectioned endothelial cells. The rest of the photo shows plates of hepatocytes arranged in trabeculae. The right panel demonstrates the same areas after laser capture of the ECs. The remaining adjacent tissue remains intact (original magnification ×400; ArcturusXT System (MDS Analytical Technologies). (B) JAK2V617F status of LCM captured hepatocytes (H) and endothelial cells (EC) of patients by allele-specific nested PCR for JAK2V617F. (C) Table summarizing the transcripts present in hepatocytes (H) and ECs isolated by LCM from archived liver biopsy tissue specimens. The numbers represent the average CT values of the samples, which were run in duplicate and repeated on 3 separate occasions.
Results and discussion
The ECs lining the terminal hepatic venules present in the sections of archived liver biopsy tissue specimens from BCS and hepatoportal sclerosis patients (Table 1) were easily identified by the location and morphology after hematoxylin and eosin staining and by the characteristic brown color after immunochemical staining with an anti-CD34 antibody (Figure 1A). The hepatocytes from each of the patients contained exclusively the wild-type JAK2. The ECs from 2 BCS patients with PV were homozygous for the JAK2V617F. The ECs from a third patient with BCS and PV and 2 hepatoportal sclerosis patients without PV contained wild-type JAK2 (Figure 1B). Two PV patients were shown to be JAK2V617F-positive as assessed by the LCM of blood cells within the vessel lumens of the liver biopsy specimens (Table 1). The other BCS patient was shown to have a JAK2V617F peripheral blood granulocyte allele burden of 50%.
The EC identity of the cells that was captured by the LCM and expressed wild-type JAK2 or JAK2V617F mutation was confirmed by the presence of transcripts associated with ECs: VE-cadherin, VEGF-R2, and VWF and the absence of transcripts associated with hematopoietic cells (CD45 and CD14). The nature of the hepatocytes captured by LCM was confirmed by the presence of albumin and the absence of EC and hematopoietic cell markers (Figure 1C).
The possibility that ECs are involved by the malignant process in hematologic malignancies has been previously explored by several groups,24,27-30 but the involvement of ECs within the vessels of patients with MPD has not been previously examined. In this report, ECs from the terminal hepatic venules of 3 BCS patients with PV were isolated by LCM and analyzed for JAK2V617F. The hepatocytes of each of the patients contained exclusively wild-type JAK2, whereas the ECs for the 2 of the 3 BCS patients with PV were homozygous for the JAK2V617F. The EC identity of the cells harboring the JAK2V617F mutation was confirmed by the expression of transcripts characteristic of ECs but not hematopoietic cells.
The vascular wall is complex structure in which at least 3 different cell types are present: ECs, pericytes, and vascular wall resident progenitor cells, which are CD34+VEGFR2+TIE2+CD31−CD45+.31,32 These cells reside in a distinct zone of the vascular wall, which is localized beneath the smooth muscle and adventitial layer of human adult vascular wall, termed the vasculogenic zone. Such cells are potentially capable of differentiating into mature ECs, hematopoietic cells, and local immune cells, such as macrophages. Because the cells analyzed in the livers of patients with BCS were localized to the tunica intima of the vascular wall rather than the vasculogenic zone of the vascular wall and were negative for CD45 (Figure 1A,C), it is doubtful that these cells were contaminated with the vascular wall resident progenitor cells.
MPDs are thought to originate at the level of a primitive hematopoietic progenitor or stem cell. Although a common cell of origin for ECs and hematopoietic cells has been described during embryogenesis, its postnatal existence continues to be a subject of intense investigation. The presence of the mutant JAK2 in both ECs and hematopoietic cells in this report could be attributed to the MPD in these patients originating from a so-called hemangioblast. The data provided in this report indicate that hepatic venule ECs are involved by the malignant MPD in a subpopulation of PV patients. The lack of uniformity of EC involvement by the JAK2V617F mutation in the BCS patients with PV could be the result of at least 2 alternative explanations: (1) JAK2V617F involvement might be restricted to localized areas within the liver, which might be missed when studying a single liver biopsy specimen; and (2) not all the BCS patients with PV have JAK2V617F-positive EC, indicating heterogeneity in this patient population.
Because the number of patients studied in this report is limited by access to extrahematopoietic tissues, it is not possible to estimate the frequency or extent of such involvement by the malignant process in PV. Furthermore, the relationship between such EC involvement by the malignant process and the development of thromboses in MPD will require further careful evaluation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by grants from the Myeloproliferative Disorders Foundation (R.H.), National Cancer Institute (1P01CA108671 to R.H.), and Department of Defense.
National Institutes of Health
Authorship
Contribution: S.S designed and performed the research and analyzed data; M.I.F collected and analyzed the data and revised the manuscript; T.S. collected data, analyzed the data, and revised the manuscript; J.M. collected the data; M.X. revised the manuscript; and R.H designed the research, interpreted the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ronald Hoffman, Division of Hematology/Oncology, Tisch Cancer Institute, Department of Medicine, Mount Sinai School of Medicine, One Gustave L. Levy Pl, Box 1079, New York, NY 10029; e-mail: ronald.hoffman@mssm.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal