Abstract
Activated platelets express ligands, which are recognized by counterreceptors on neutrophils. Here, we show that the ensuing cell-to-cell interaction programs neutrophil phagocytic function, resulting in activated platelet clearance. Neutrophils that have internalized platelets circulate in the blood of patients with acute myocardial infarction, and the extent of platelet clearance correlates with expression of platelet activation, including P-selectin. Activated platelets injected intravenously in experimental animals are detectable in circulating neutrophils 60 minutes after, and within 3 hours, more than 70% circulating neutrophils have internalized platelets. Platelet clearance comprises 2 events: adhesion to neutrophils, which requires divalent cations and depends on P-selectin, on the P-selectin glycoprotein ligand-1 (PSGL-1), and on the CD11b/CD18 β2 integrin; and internalization, which is abrogated by the phosphatidylserine-binding protein annexin A5. Adhesion to platelets causes neutrophil degranulation and is blocked by antibodies specific for P-selectin and PSGL-1, either in a synthetic medium in vitro or in the whole blood, therefore in the presence of a physiologic array of plasma cofactors and opsonins. The data suggest that the interaction between circulating platelets and neutrophils influences innate immune functions, possibly contributing to regulate vascular inflammation.
Introduction
Neutrophils are recruited to inflamed sites, where they are required for microbial clearance. Neutrophil recruitment is a multistep process, which comprises initial tethering and rolling along the vessel wall, firm adhesion to endothelial cells, and eventual extravasation. It involves consecutively various adhesion molecules, including selectins and β2 integrins.1-4
Granules of endothelial cells (Weibel-Palade bodies) and of platelets (α-granules) contain P-selectin. Inflammatory stimuli cause its translocation at the endothelial cell surface. The interaction with the counterreceptor, PSGL-1, prompts leukocyte tethering and rolling and initiates the second phase of the process, firm adhesion. During these events, integrins shift to an active conformation.5-7 Integrin activation depends on the signaling cascade downstream the P-selectin/PSGL-1 interaction.8-10
Platelets adhere and are activated at sites of vascular injury: there, they produce a releasate, some contents of which penetrate and/or interact biochemically with neutrophils. This includes unprocessed free arachidonic acid which can then be transformed into leukotriene A4 and B4.11-14 Furthermore, activated platelets express P-selectin. Platelet P-selectin guarantees the access of leukocytes to perivascular tissues even when dying or severely damaged endothelial cells fail to sustain leukocyte rolling and adhesion.15,16 Indeed, endothelial cells surrounding the lesion release signals that amplify the expression of P-selectin on adhering platelets.17
The P-selectin–dependent interaction of neutrophils and platelets amplifies mutually, promoting the exchange of metabolites and protecting released molecules from plasma inhibitors.14 All together, neutrophil recruitment contributes to local inflammation, defense against invading microbes and possibly tissue repair. Neutrophils could however also play a detrimental role, exacerbating tissue damage.
The interaction between neutrophils and activated platelets also occurs in the blood, yielding cell aggregates, which are hallmarks of acute myocardial infarction, sepsis, and inflammatory and myeloproliferative disorders.18,19 Neutrophils that recognize P-selectin on endothelia and on platelets attached to the vessel wall activate intracellular signaling pathways required to cross the endothelial barrier and enter in the tissue.10 Flowing neutrophils, after recognition of P-selectin on platelets, activate identical intracellular signaling cascades5,20-22 but do not leave the vessels.18 Recognition of P-selectin initiates phagocytosis.23
Here, we describe a cell clearance program downstream platelet P-selectin recognition, which results in platelet phagocytosis and neutrophil degranulation.
Methods
Reagents
BCECF-AM (2′,7′-bis-(2-carboxyethyl)-5(6)-carboxy-fluorescein triacetoxy methyl ester), the pHrodo Phagocytosis Particle Labeling Kit for Flow Cytometry, and the Zenon IgG Labeling Kit were purchased from Invitrogen (Milan, Italy); prostaglandin E1 (PGE1) was from Cayman (Milan, Italy); thrombin receptor agonist peptide-6 (TRAP-6) was from Bachem (Milan, Italy); collagen was from Horm (Milan, Italy); Thrombofix IO test 3 fixing solution was from Immunotech (Instrumentation Laboratories, Milan, Italy); and the FIX & PERM Kit was from Caltag (Turin, Italy). Recombinant chicken annexin A524 was a generous gift of Professor Martin Herrmann (University of Erlangen, Erlangen, Germany). EDTA (ethylenediamine-tetraacetic acid), HEPES (N-2-hydroxyethyl piperazine-N′-2-ethanesulfonic acid), ADP (adenosine diphosphate), fMLP (N-Formyl-Met-Leu-Phe), cytochalasin D, and the other chemicals were from Sigma-Aldrich (St Louis, MO).
Monoclonal antibodies (mAbs) against CD14 (clone RMO52), β2 integrin (CD18, clone 7E4), CD11b/CD18 (αMβ2, also called Mac-1, clone Bear-1), CD42a (platelet glycoprotein Ibα, clone SZ2), CD45 (clone J33), CD61 (platelet glycoprotein IIIa, also called β3, clone SZ21), CD62P (P-selectin, clone CLBThromb/6), CD66b (clone 80H3), CD162 (PSGL-1, clone PL1), MPO (clone CLB-MPO-1), the isotype-matched control (clone 679.1Mc7), and FITC-labeled annexin A5 were from Immunotech (Milan, Italy). The nonblocking, P-selectin–specific AK-4 mAb, anti–mouse CD61 (clone 2C9.G2), anti-CD14 (clone mC5-3), and anti-CD45 (clone 30-F11) and its appropriate isotypic controls were from Becton Dickinson (San Jose, CA).
Patients
Venous peripheral blood was obtained upon informed consent from 15 consecutive patients with Segment T elevation acute myocardial infarction within 12 hours from onset of symptoms. Exclusion criteria were as follows: age below 18 or above 75 years, serum creatinine above 2 mg/dL, anemia, history of cancer, or immune-mediated disorders. Venous blood was obtained in parallel from 15 healthy controls. The institutional ethic committee approved the study. Blood was collected in plastic tubes containing sodium citrate, sodium EDTA, N-ethylmaleimide, and aprotinin, processed as described,18 and prepared for flow cytometry and confocal microscopy studies. The institutional ethics committee approved the study and informed consent was obtained from all patients in accordance with the Declaration of Helsinki.
Platelets and neutrophils
Venous blood was collected after informed consent from healthy volunteers, who did not receive any medication for at least 2 weeks. When indicated, blood was collected 4 hours after aspirin (500 mg) ingestion. Blood was drawn with an 18-G needle, dripping freely into open tubes, containing 3.8% sodium citrate (1:9, vol:vol). Neutrophils and platelets were isolated as described5 and resuspended in HEPES-Tyrode buffer (pH 7.4) containing CaCl2 (1 mM). Cells were routinely assessed by flow cytometry using anti-CD14, anti-CD45, and anti-CD61 mAbs to exclude potential contaminations.
Adhesion and phagocytosis
Platelets (2 × 109/mL) were treated at 37°C with TRAP-6 (25 μM, activated platelets) or with PGE1 (5 μM, resting platelets) for 2 minutes. Then, neutrophil suspensions (2 × 107 cells/mL) were added at a physiologic 20:1 platelet-to-neutrophil ratio (100 000 platelets to 5000 neutrophils). Reactions were stopped after 5 minutes and aliquots for the assessment of activation markers were retrieved and maintained at 4°C until analysis. When indicated, cells were preincubated with CLBThromb/6, PL1, SZ2, 7E4, Bear-1, or with the irrelevant isotype-matched 679.1Mc7 mAb at a final concentration of 20 μg/mL. Alternatively, the recognition of anionic phospholipids on platelets was hindered using recombinant chicken annexin A5, as described24 (15 μg/mL, final concentration), or to evaluate the role of divalent cations EDTA was added to neutrophils at a final concentration of 5 mM before cell-to-cell interaction. Cytochalasin D at a final concentration of 20 μg/mL (5 minutes at 4°C) was used to evaluate the role of neutrophil actin polimerization. Alternatively, phagocytosis experiments were carried out in parallel at 4°C and 37°C. Samples were washed to remove noninteracting platelets. Pellets were then fixed and permeabilized in HEPES-Tyrode with the FIX & PERM kit (Caltag), before labeling with PE-conjugated mAb against the CD61 platelet antigen (clone SZ21; Immunotech) and with FITC-conjugated mAb against MPO. Phagocytosis by electron and confocal microscopy was scored on coded samples and expressed as the percentage of neutrophils ingesting platelets. Platelets were considered phagocytosed only when their whole volume was contained within the border of the neutrophil, which is believed to underestimate the extent of phagocytosis. Phagocytosis was also assessed by flow cytometry and expressed as the percentage of neutrophils (ie, cells with a high side scatter within the CD45+ population, comprising > 99% CD66b+ and < 1% CD14+ events; Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article) ingesting platelets. Phagocytosis was identified by the gain of fluorescence associated to the CD61 platelet antigen after neutrophil plasma membrane permeabilization, a treatment that allows the mAb to penetrate calculated as follows: phagocytosing neutrophil percentage = percentage of permeabilized neutrophils positive for the platelet CD61 antigen-associated fluorescence (extracellular + intracellular platelets) − percentage of intact neutrophils positive for the platelet CD61 antigen-associated fluorescence (extracellular platelets only; Figure S1B). When indicated, extracellular adherent platelets were removed by incubation with 0.25% trypsin/EDTA solution for 2 minutes at 37°C.
In preliminary experiments, purified platelets were stimulated with either ADP (5 μM), collagen (1 μg/mL), or TRAP-6 (25 μM). When platelets were stimulated before the challenge with resting neutrophils, platelet phagocytosis was similar with all stimuli. However, at the concentrations we used, ADP or collagen failed to induce platelet-neutrophil adhesion in whole blood samples. Collagen and ADP directly activate neutrophils.25 We therefore verified the down-regulation of the PSGL-1 receptor (a bona fide event associated to neutrophil activation) after 60 seconds; that is, before platelet P-selectin expression (Table S1). Indeed, both ADP and collagen, but not TRAP-6, significantly down-regulated PSGL-1 expression as effectively as the positive control for neutrophil activation, fMLP (Table S1). We therefore selected TRAP-6 to study the platelet-neutrophil interaction: this peptide has the extra bonus, compared with the whole thrombin molecule, not to activate the coagulation cascade. It thus represents a suitable choice for use in whole blood samples.26 For confocal microscopy, samples were fixed, permeabilized, and labeled. Primary antibodies were labeled with Zenon IgG Labeling Kits: Alexa Fluor 488 (green), Alexa Fluor 594 (red), or Alexa Fluor 674 (pink). DAPI was used for counterstaining of nuclei. Labeled samples were washed and plated to glass coverslips before analysis with a Leica TCS SP2 Laser Scanning Confocal Microscope, objective 63× (numeric aperture [NA], 1.4). In selected experiments, the percentage of phagocytosing neutrophils was evaluated in parallel by flow cytometry and confocal microscopy; cells with discrete round fluorescent inclusions were scored as those that had ingested platelets. Sample preparation and electron microscopy were performed as described.5 When indicated, adhesion and phagocytosis were assessed in whole blood, without any previous cell isolation or sample manipulation. In these experiments, whole blood samples were treated with TRAP-6 (25 μM final concentration, activated platelets) or PGE1 (5 μM final concentration, resting platelets). After 5 minutes, samples were processed for flow cytometry or confocal microscopy; parallel aliquots were cleared by centrifugation for 1 minute at 16 000g. Released MPO concentration was assessed by enzyme-linked immunosorbent assay (ELISA) using a commercial kit (Enzyme Immunoassay; Bioxytech MPO/Oxis International, Portland, OR).
To further characterize the features of the phagocytic vesicles, platelets were labeled with the cell-permeant, dual-excitation ratiometric pH indicator BCECF-AM before interaction with neutrophils. Measurements were carried out by determining the pH-dependent emission intensity by flow cytometry (detected at 535 nm) when the dye is excited at approximately 490 nm versus the emission intensity when excited at its isobestic point of approximately 440 nm, following the manufacturer's instruction. To verify that the observed acidification of platelets was dependent on their internalization within a bona fide sealed phagocytic vacuole, the pHrodo Phagocytosis Particle Labeling Kit (Invitrogen, Milan, Italy) was used according to the manufacturer's instructions. As a positive control, Escherichia coli labeled and processed exactly as platelets were used.
Activation of platelets and neutrophils
Anionic phospholipids exposure was assessed by PE-conjugated CD61-specific mAb and FITC-conjugated annexin A5.27 Results were expressed as the percentage of annexin A5+ events in the whole CD61+ platelet population. To determine P-selectin expression, platelets were simultaneously fixed (Thrombofix18 ), labeled with FITC-conjugated mAb against CD61 and PE-conjugated against P-selectin, and analyzed by flow cytometry in purified platelet suspensions as well as in whole-blood samples. Results were expressed as the percentage of CD61+ platelets that express P-selectin. An Alexa 488–labeled irrelevant isotype control mAb was used to assess the background fluorescence. When indicated, MPO expression was assessed using specific mAbs. Neutrophil expression of Mac-1, PSGL-1, MPO, platelet/neutrophil aggregates, and intracellular platelet antigen were assessed by flow cytometry using conjugated mAbs. Results are expressed as the mean of fluorescence intensity (MFI) associated to the specific fluorochrome-labeled mAb or as the percentage of positive cells.
Clotting assay
Activated platelets at a concentration of 100 000 cells/μL were incubated for 1 minute at 37°C alone or in the presence of an equal volume of neutrophils, at a neutrophil-to-platelet ratio of 1:20, 1:10, or 1:5. Neutrophils at the 1:5 ratio were either untreated or pretreated with cytochalasin D to prevent actin-based cytoskeleton assembly. Normal human plasma (100 μL) was added for 1 minute, followed by an equal volume of a 25 mM CaCl2 solution. The coagulation time was determined.
Plate flow adhesion assay
Neutrophil interaction with adherent platelets under physiologic flow was investigated in a parallel plate flow chamber, as described.10 Platelets (3 × 107/mL) were allowed to adhere to a glass slide previously coated with aminopropyltriethoxysilane (APES; Sigma-Aldrich). Platelet-coated slides were washed and served as the chamber's bottom plate. The chamber (width, 1.4 cm; height, 0.250 mm) was placed in a thermoregulated Plexiglas box maintained at 37°C by an electric heating element. Neutrophils (5 × 106 cells/mL in medium containing 0.1% BSA) were perfused through the chamber over the platelet surface at a shear stress of 2 dyn/cm2 for 2 minutes. The chamber was then perfused with medium alone for an additional 10 minutes at 20 dyn/cm2. Adherent neutrophils were observed by contrast phase video microscopy with a 20×/0.40 NA objective (Olympus, Munich, Germany). Images were continuously recorded (Pro-Series video camera, high-performance CCD camera; Media Cybernetics, Silver Spring, MD). Firmly adhered neutrophils were quantified in the last 20 seconds of flow using ad hoc software for image analysis (Image Pro-Plus for Windows; Media Cybernetics).
Time-lapse videomicroscopy
Platelets were labeled with the BCECF-AM intracellular dye. Fluorescence was acquired by exciting at 488 nm and emission was recorded at 560 nM. Platelet-associated fluorescence abates once platelets are internalized and reach an acidic environment. BCECF-loaded platelets were activated with TRAP-6 and placed on slides (final volume 500 μL). Immediately after, autologous neutrophils were added (500 μL) and time-lapse images were acquired at 37°C for 5 minutes at 5-second intervals. Microscopy was performed with a Leica TCS SP2 laser scanning confocal. When indicated, platelets were treated with annexin A5 (15 μg/mL, final concentration) immediately before challenge with neutrophils.
In vivo injection and clearance of activated platelets
Whole blood from female C57BL/6N mice (Harlan, Correzzana, Italy) was obtained by cardiac puncture and collected in syringes containing Na citrate, EDTA, and PGE1. Mouse platelets were isolated and activated as described.26 Platelets (500 000) were resuspended in the presence of PGE1 to prevent further activation (final concentration 4 μM) or activated with thrombin (final concentration 0.5 U/mL). Thrombin was then inactivated by addition of hirudin (0.5 U/mL). Platelets were injected in the tail vein. After 1, 2, 3, and 24 hours animals were killed and blood retrieved as noted. Blood samples were fixed and lysed, washed twice, treated with the FIX & PERM Kit and incubated with anti–mouse CD61, anti-CD14, and anti-CD45 mAbs or appropriate isotypic controls. Neutrophils were identified within the CD45+ population by their forward scatter characteristics. In preliminary experiments, platelets were labeled with the intracellular dye BCECF before injection. All procedures were performed according to protocols approved by the animal care and use committee of the Fondazione San Raffaele del Monte Tabor (IACUC344) and communicated to the Ministry of Health and local authorities according to Italian law.
Statistical analysis
The data are presented as the mean plus or minus the standard error of the mean (SEM) unless otherwise stated. All statistics were calculated using PRISM version 2.0 (GraphPad Software, San Diego, CA), using analysis of variance (ANOVA) followed by multiple pairwise comparison tests, with differences being considered significant for P values less than .05.
Results
Phagocytic clearance of human platelets in vivo: clinical evidence
We first analyzed the interaction between platelets and neutrophils in patients with early acute myocardial infarction, because in these patients both circulating platelets and neutrophils are substantially, although transiently, activated.28-30
We observed that platelets from 15 consecutive patients with acute myocardial infarction had a significant increase in the expression of P-selectin and in the percentage of circulating platelet-neutrophil aggregates, as detected by flow cytometry, compared with healthy controls (Table 1). Moreover, neutrophils had a reduced intracellular expression of MPO, and up-regulated the Mac-1 β2 integrin (Table 1). In all patients, a fraction (31.2% ± 7.5%) of circulating neutrophils were completely devoid of intracellular MPO.
Neutrophils that have internalized platelets circulate in the blood of patients with acute myocardial infarction
| . | Healthy subjects (n = 15) . | AMI patients (n = 15) . | P . |
|---|---|---|---|
| P-selectin, % of positive platelets | 7 ± 1 | 29 ± 3 | < .001 |
| Neutrophils MPO content, MFI | 60 ± 7 | 158 ± 8 | < .001 |
| Neutrophils with complete MPO depletion, % | 1.0 ± 0.5 | 31.2 ± 7.5 | < .001 |
| Neutrophils Mac-1 expression, MFI | 319 ± 20 | 556 ± 26 | < .001 |
| Neutrophils with adherent platelets, % | 7 ± 1 | 15 ± 2 | < .001 |
| Neutrophils with internalized platelets, % | 3 ± 1 | 16 ± 3 | < .001 |
| . | Healthy subjects (n = 15) . | AMI patients (n = 15) . | P . |
|---|---|---|---|
| P-selectin, % of positive platelets | 7 ± 1 | 29 ± 3 | < .001 |
| Neutrophils MPO content, MFI | 60 ± 7 | 158 ± 8 | < .001 |
| Neutrophils with complete MPO depletion, % | 1.0 ± 0.5 | 31.2 ± 7.5 | < .001 |
| Neutrophils Mac-1 expression, MFI | 319 ± 20 | 556 ± 26 | < .001 |
| Neutrophils with adherent platelets, % | 7 ± 1 | 15 ± 2 | < .001 |
| Neutrophils with internalized platelets, % | 3 ± 1 | 16 ± 3 | < .001 |
Platelet P-selectin expression, neutrophil myeloperoxidase content, Mac-1 expression (CD11b/CD18) on neutrophil surface, platelet-neutrophil aggregates and neutrophils with internalized platelets were determined in whole blood samples by 4-color flow cytometry as described in “Adhesion and phagocytosis.” Statistically significant differences were calculated by ANOVA and the Tukey test.
MFI indicates mean fluorescence intensity.
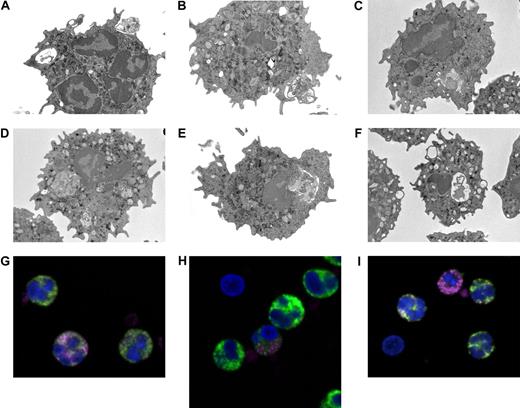
The analysis by electron microscopy of neutrophils purified from the blood of acute myocardial infarction patients indicated that phagosomes contained large particulate corpuscles, whose size and morphology was compatible with internalized platelets (Figure 1A-F). Confocal microscopy analysis confirmed that neutrophils, including those circulating as aggregates, contained intracellular platelet antigens, as assessed by staining with the antiplatelet glycoprotein Ib alpha mAb after permeabilization (Figure 1G-I, Table 1). Interestingly, neutrophils with adherent and/or intracellular platelets had an apparently lower, or absent, content of MPO (Figure 1G-I).
Neutrophils circulating in the blood of patients with acute myocardial infarction contain intracellular platelets. (A-F) Representative images by electron microscopy of neutrophils from 4 patients with acute myocardial infarction. Neutrophils were immediately purified from blood and processed as described in “Adhesion and phagocytosis.” (G-I) Representative confocal microscopy of neutrophils from 3 patients with acute myocardial infarction. Whole blood was processed and stained as described in “Adhesion and phagocytosis.” MPO, green; platelet glycoprotein Ib (CD42), pink. DAPI, blue, was used for counterstaining of nuclei (n = 15).
Neutrophils circulating in the blood of patients with acute myocardial infarction contain intracellular platelets. (A-F) Representative images by electron microscopy of neutrophils from 4 patients with acute myocardial infarction. Neutrophils were immediately purified from blood and processed as described in “Adhesion and phagocytosis.” (G-I) Representative confocal microscopy of neutrophils from 3 patients with acute myocardial infarction. Whole blood was processed and stained as described in “Adhesion and phagocytosis.” MPO, green; platelet glycoprotein Ib (CD42), pink. DAPI, blue, was used for counterstaining of nuclei (n = 15).
Phagocytic clearance of human platelets: in vitro determinations
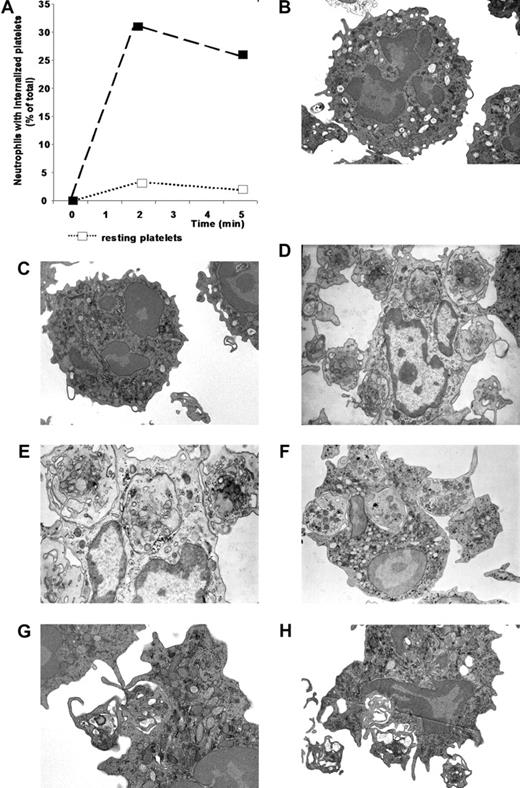
Freshly purified platelets, activated with TRAP-6, were readily ingested by resting neutrophils after a 5-minute interaction assay at a 20:1 platelet-to-neutrophil ratio, which is representative of the circulating blood, as easily evinced by electron microscopy. Several platelets closely adhering to neutrophils were evident, partially enveloped by pseudopods extending from the neutrophil plasma membrane, that ultimately encircle and engulf platelets, most of which can be traced into phagocytic vacuoles (Figure 2). Adherent or internalized platelets were not detectable when neutrophils were cultured alone (Figure 2B) or challenged with resting platelets (Figure 2C). The semiquantitative assessment indicated that a substantial fraction of neutrophils phagocytosed autologous activated platelets (Figure 2A). In contrast, less than 5% neutrophils internalized nonactivated platelets (Figure 2A).
Neutrophils effectively phagocytose activated platelets. Resting or activated platelets were incubated with neutrophils at a 20:1 ratio. At different times (x axis, minutes) cells were fixed and processed for electron microscopy. (A) For each sample, 40 to 50 neutrophils were randomly selected and analyzed for the presence of internalized platelets. Neutrophils with at least 1 platelet completely included in a phagosome were considered positive. Data are expressed as mean ± SD of the percentage of neutrophils with internalized platelets; n = 5. (B) Representative electron microscopy of neutrophils cultured alone. (C) Representative electron microscopy of neutrophils incubated with resting platelets. (D-H) Representative electron microscopy of neutrophils incubated with activated platelets.
Neutrophils effectively phagocytose activated platelets. Resting or activated platelets were incubated with neutrophils at a 20:1 ratio. At different times (x axis, minutes) cells were fixed and processed for electron microscopy. (A) For each sample, 40 to 50 neutrophils were randomly selected and analyzed for the presence of internalized platelets. Neutrophils with at least 1 platelet completely included in a phagosome were considered positive. Data are expressed as mean ± SD of the percentage of neutrophils with internalized platelets; n = 5. (B) Representative electron microscopy of neutrophils cultured alone. (C) Representative electron microscopy of neutrophils incubated with resting platelets. (D-H) Representative electron microscopy of neutrophils incubated with activated platelets.
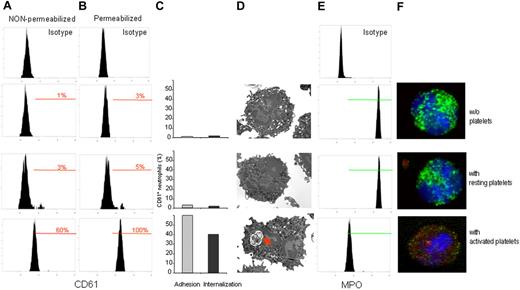
To better characterize the platelet-neutrophil interaction that resulted in platelet clearance, as assessed by electron microscopy (Figures 2, 3D) we developed a flow cytometric method (Figure S1) that was dependent on incubating isolated human neutrophils with purified autologous platelets, either resting or activated. Neutrophils were readily resolved from platelets by physical characteristics and expression of the CD66b marker. Neutrophil-associated platelet fluorescence (CD61 platelet glycoprotein IIIa antigen; Figure 3A,B,F) and MPO content (Figure 3E,F) were then assessed. To discriminate between adherence and ingestion, permeabilized and nonpermeabilized samples were processed in parallel. The fluorescence in nonpermeabilized samples depends on platelets that adhere to the surface of neutrophils. After permeabilization, the fluorescence depends on both platelets that were ingested (intracellular) or that adhered to the neutrophil membrane (extracellular; Figure 1). Results obtained by flow cytometry were in keeping with those obtained by electron microscopy and confocal microscopy (Figure 3).
Interaction with activated platelets results in adhesion, phagocytosis, and neutrophil MPO depletion. Purified neutrophils were incubated alone (without platelets) or challenged with resting or activated platelets at a platelet-to-neutrophil ratio of 20:1. (A) Platelet adhesion was evaluated by flow cytometry, assessing the CD61 platelet antigen in nonpermeabilized neutrophils. (B) Internalization was assessed by the CD61 expression after permeabilization of the neutrophil plasma membrane. (C) Neutrophils with adhering ( ) or internalized platelets (■) were calculated. (D) Representative electron micrographs show that only neutrophils challenged with activated platelets were involved in phagocytosis (red arrow). (E) MPO content was assessed in parallel by flow cytometry: neutrophils degranulate after recognition of activated platelets. (F) Confocal microscopy allows the simultaneous assessment of intracellular platelets, revealed by the platelet GPIbα antigen (red), and MPO (green). Nuclei were counterstained with DAPI (blue). Neutrophils involved in recognition and clearance of activated platelets underwent MPO depletion. Data reported are from representative experiments (flow cytometry n = 16, confocal microscopy n = 10, electron microscopy n = 7).
) or internalized platelets (■) were calculated. (D) Representative electron micrographs show that only neutrophils challenged with activated platelets were involved in phagocytosis (red arrow). (E) MPO content was assessed in parallel by flow cytometry: neutrophils degranulate after recognition of activated platelets. (F) Confocal microscopy allows the simultaneous assessment of intracellular platelets, revealed by the platelet GPIbα antigen (red), and MPO (green). Nuclei were counterstained with DAPI (blue). Neutrophils involved in recognition and clearance of activated platelets underwent MPO depletion. Data reported are from representative experiments (flow cytometry n = 16, confocal microscopy n = 10, electron microscopy n = 7).
Interaction with activated platelets results in adhesion, phagocytosis, and neutrophil MPO depletion. Purified neutrophils were incubated alone (without platelets) or challenged with resting or activated platelets at a platelet-to-neutrophil ratio of 20:1. (A) Platelet adhesion was evaluated by flow cytometry, assessing the CD61 platelet antigen in nonpermeabilized neutrophils. (B) Internalization was assessed by the CD61 expression after permeabilization of the neutrophil plasma membrane. (C) Neutrophils with adhering ( ) or internalized platelets (■) were calculated. (D) Representative electron micrographs show that only neutrophils challenged with activated platelets were involved in phagocytosis (red arrow). (E) MPO content was assessed in parallel by flow cytometry: neutrophils degranulate after recognition of activated platelets. (F) Confocal microscopy allows the simultaneous assessment of intracellular platelets, revealed by the platelet GPIbα antigen (red), and MPO (green). Nuclei were counterstained with DAPI (blue). Neutrophils involved in recognition and clearance of activated platelets underwent MPO depletion. Data reported are from representative experiments (flow cytometry n = 16, confocal microscopy n = 10, electron microscopy n = 7).
) or internalized platelets (■) were calculated. (D) Representative electron micrographs show that only neutrophils challenged with activated platelets were involved in phagocytosis (red arrow). (E) MPO content was assessed in parallel by flow cytometry: neutrophils degranulate after recognition of activated platelets. (F) Confocal microscopy allows the simultaneous assessment of intracellular platelets, revealed by the platelet GPIbα antigen (red), and MPO (green). Nuclei were counterstained with DAPI (blue). Neutrophils involved in recognition and clearance of activated platelets underwent MPO depletion. Data reported are from representative experiments (flow cytometry n = 16, confocal microscopy n = 10, electron microscopy n = 7).
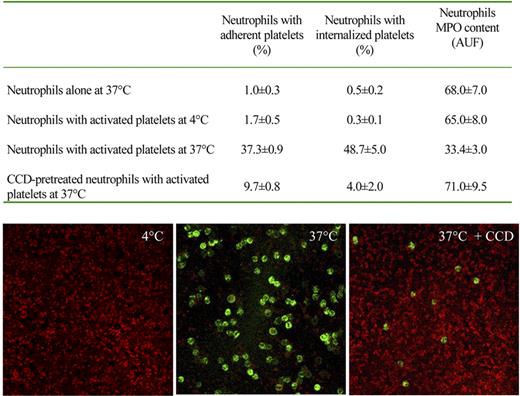
Neutrophils readily ingested platelets (Figures 2,3). To rule out possible artifacts, we removed extracellular platelets by trypsin/EDTA, after phagocytosis. As expected, adherent platelets were not detectable anymore, whereas those internalized were unaffected (Table 2). In contrast, internalization abated when the neutrophil-platelet interaction was carried out at 4°C (ie, at a temperature that does not permit actual phagocytosis), even if adhesion was partially conserved (Figure 4). Confocal microscopy clearly indicates that phagocytosis associates with loss of extracellular platelets. The phagocytosis was also greatly inhibited when the actin-based cytoskeleton was disrupted by treatment with cytochalasin D (Figure 4). Time-lapse videomicroscopy, carried out using platelets prelabeled with an intracellular red fluorescing reagent (BCECF-AM), further confirmed that neutrophils internalized whole platelets (Videos S1,S2).
Neutrophils depend upon phosphatidylserine exposure to phagocytose activated platelets
| . | Neutrophils with adherent platelets, % . | Neutrophils with internalized platelets, % . |
|---|---|---|
| Resting platelets | 1.7 ± 0.8 | 0.3 ± 0.2 |
| Activated platelets | 25.6 ± 3.0 | 37.1 ± 4.4 |
| Activated platelets + trypsin-EDTA | 9.7 ± 0.8* | 0.1 ± 15.2 |
| Annexin A5–treated activated platelets | 20.1 ± 3.3 | 5.1 ± 1.2* |
| Annexin A5–treated activated platelets + trypsin-EDTA | 7.1 ± 3.7* | 2.0 ± 1.2* |
| . | Neutrophils with adherent platelets, % . | Neutrophils with internalized platelets, % . |
|---|---|---|
| Resting platelets | 1.7 ± 0.8 | 0.3 ± 0.2 |
| Activated platelets | 25.6 ± 3.0 | 37.1 ± 4.4 |
| Activated platelets + trypsin-EDTA | 9.7 ± 0.8* | 0.1 ± 15.2 |
| Annexin A5–treated activated platelets | 20.1 ± 3.3 | 5.1 ± 1.2* |
| Annexin A5–treated activated platelets + trypsin-EDTA | 7.1 ± 3.7* | 2.0 ± 1.2* |
Neutrophils effectively adhere to and phagocytose activated but not resting platelets after a 5-minute interaction at 37°C. Treatment with trypsin-EDTA, which acts in the extracellular environment, strips adherent but not internalized platelets (*P < .05 by paired t test). Hindrance with phosphatidylserine recognition by annexin A5 blocks platelet internalization but does not influence adhesion (*P < .05 by paired t test). Both platelet adhesion and internalization were nondetectable when (1) phagocytosis was blocked by annexin A5 and (2) extracellular platelets were removed after the assay by trypsin-EDTA (*P < .05 by paired t test). Platelet adherence and internalization were determined by flow cytometry, as described in “Adhesion and phagocytosis.” Results are expressed as mean ± SEM of 3 independent experiments performed in duplicate.
Platelet phagocytosis requires integrity and function of the actin-based cytoskeleton. Neutrophils were incubated in the presence or the absence of activated platelets for 5 minutes at 37°C or 4°C. Phagocytosis of platelets, assessed by flow cytometry as described in “Adhesion and phagocytosis,” occurred at 37°C and abated in conditions in which the assembly of the actin-based cytoskeleton was prevented by pretreatment with cytochalasin D (CCD) or by nonpermissive temperatures (4°C). In these conditions, degranulation was also inhibited, as revealed by MPO content of neutrophils. Consistent results were observed by confocal microscopy: glass-adhered platelets were activated with thrombin (0.5 U/mL) for 2 minutes, washed, and incubated in the presence of purified autologous neutrophils. Platelets were revealed by staining with antiplatelet glycoprotein Ib mAbs (red) and neutrophils with anti-CD66b mAb (green). At 4°C, sample washings removed neutrophils that did not firmly adhere; at 37°C neutrophils are evident, whereas extracellular platelets were not any more detectable; in the presence of cytochalasin D, few scattered neutrophils and extracellular platelets are evident.
Platelet phagocytosis requires integrity and function of the actin-based cytoskeleton. Neutrophils were incubated in the presence or the absence of activated platelets for 5 minutes at 37°C or 4°C. Phagocytosis of platelets, assessed by flow cytometry as described in “Adhesion and phagocytosis,” occurred at 37°C and abated in conditions in which the assembly of the actin-based cytoskeleton was prevented by pretreatment with cytochalasin D (CCD) or by nonpermissive temperatures (4°C). In these conditions, degranulation was also inhibited, as revealed by MPO content of neutrophils. Consistent results were observed by confocal microscopy: glass-adhered platelets were activated with thrombin (0.5 U/mL) for 2 minutes, washed, and incubated in the presence of purified autologous neutrophils. Platelets were revealed by staining with antiplatelet glycoprotein Ib mAbs (red) and neutrophils with anti-CD66b mAb (green). At 4°C, sample washings removed neutrophils that did not firmly adhere; at 37°C neutrophils are evident, whereas extracellular platelets were not any more detectable; in the presence of cytochalasin D, few scattered neutrophils and extracellular platelets are evident.
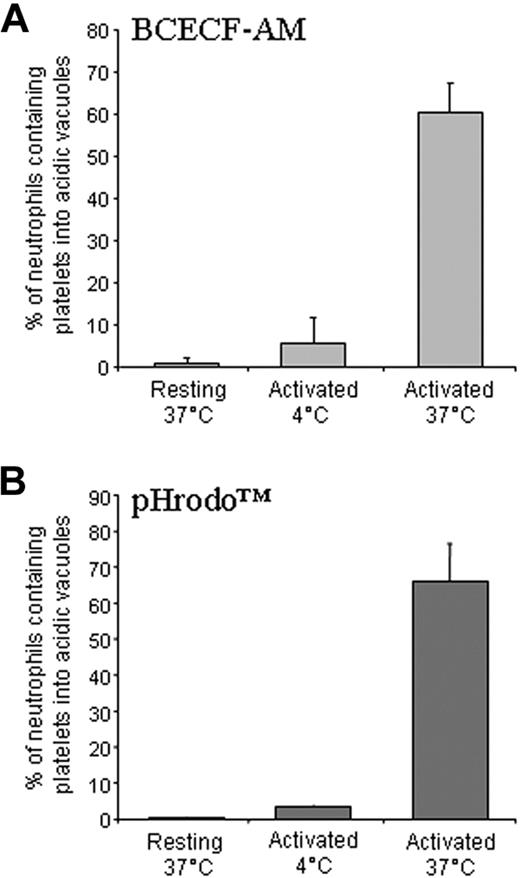
The degree of phagocytosis was dependent on platelet activation. Typically, after a 5-minute interaction, greater than 30% of human neutrophils (37.1% ± 4.4%) ingested activated autologous platelets, compared with only 5.2% ± 3.3% of neutrophils challenged with resting platelets (Figures 2A,3,4). Time-lapse videomicroscopy shows that neutrophils interacting with activated adherent platelets under arterial shear stress also extensively phagocytosed; their extreme motility in these conditions enables recognition and clearance not only of adherent, immediately interacting, platelets but also of bystander platelets (Video S3, Figure 4). Once internalized, BCECF-AM–labeled platelet-associated fluorescence abated (Videos S1, S2), suggesting that they have reached an acidic compartment. To verify this issue, BCECF-AM–labeled platelet-associated fluorescence was monitored by flow cytometry in conditions in which neutrophils adhere to and phagocytose platelets, or in which these events are abrogated (ie, at 4°C). Emission at approximately 535 nm (FL2) revealed the intracellular acidification of BCECF-AM–labeled platelets. This was prevented when neutrophils were incubated with resting platelets or with activated platelets at 4°C (Figure 5). We confirmed that platelet acidification took place in a bona fide sealed phagosome by the rhodamine-based pHrodo, which is nonfluorescent at neutral pH. Neutrophils acquired a bright-red color, indicative of acidic environment (Figure 5): the pattern was similar when platelets or killed bacteria (E coli) were used as phagocytic substrates (not shown).
Phagocytosed platelets reach intracellular acidic vacuoles. (A) Neutrophils were incubated with resting or activated platelets labeled with BCECF-AM for 5 minutes at 37°C or 4°C and analyzed by flow cytometry. A bright-red fluorescence associated with the phagocytic events indicates that intracellular platelets reached acidic vacuoles. Platelet acidification did not occur in conditions in which phagocytosis did not occur (ie, when neutrophils were challenged with resting platelets or when the assay was carried out at 4°C). (B) Neutrophils were incubated with resting or activated platelets labeled with pHrodo for 5 minutes at 37°C or 4°C and analyzed by flow cytometry. A bright-red fluorescence associated with the phagocytic events indicates that platelets reached acidic vacuoles. Acidification did not occur in conditions in which phagocytosis did not occur (ie, when neutrophils were challenged with resting platelets or when the assay was carried out at 4°C).
Phagocytosed platelets reach intracellular acidic vacuoles. (A) Neutrophils were incubated with resting or activated platelets labeled with BCECF-AM for 5 minutes at 37°C or 4°C and analyzed by flow cytometry. A bright-red fluorescence associated with the phagocytic events indicates that intracellular platelets reached acidic vacuoles. Platelet acidification did not occur in conditions in which phagocytosis did not occur (ie, when neutrophils were challenged with resting platelets or when the assay was carried out at 4°C). (B) Neutrophils were incubated with resting or activated platelets labeled with pHrodo for 5 minutes at 37°C or 4°C and analyzed by flow cytometry. A bright-red fluorescence associated with the phagocytic events indicates that platelets reached acidic vacuoles. Acidification did not occur in conditions in which phagocytosis did not occur (ie, when neutrophils were challenged with resting platelets or when the assay was carried out at 4°C).
Flow cytometry shows that neutrophils interacting with activated (but not with resting) platelets had a significantly reduced MPO content compared with neutrophils alone (Figures 3E,F and 4). The observation is in keeping with the relative low MPO content of the neutrophils from patients with acute myocardial infarction that had internalized platelets (Figure 1G-I). Interestingly, neutrophils that had degranulated upon interaction with activated platelets, but not those that interacted with resting platelets, were not any more able to further up-regulate Mac-1 expression when exposed fMLP, the best-characterized neutrophil agonist (Table 3).
Neutrophils fail to respond to fMLP after interaction with activated platelets
| . | Mac-1, % basal value . | Neutrophils with internalized platelets, % . |
|---|---|---|
| Neutrophils with resting platelets | 100 | 0.3 ± 0.2 |
| Neutrophils with activated platelets | 158.6 ± 25.8 | 43.7 ± 5.1 |
| Neutrophils alone + fMLP | 242.8 ± 31.2 | 0.3 ± 0.2 |
| Neutrophils with resting platelets + fMLP | 246.2 ± 29.3 | 0.3 ± 0.2 |
| Neutrophils that phagocytosed activated platelets + fMLP | 126.5 ± 12.1 | 43.7 ± 5.1 |
| . | Mac-1, % basal value . | Neutrophils with internalized platelets, % . |
|---|---|---|
| Neutrophils with resting platelets | 100 | 0.3 ± 0.2 |
| Neutrophils with activated platelets | 158.6 ± 25.8 | 43.7 ± 5.1 |
| Neutrophils alone + fMLP | 242.8 ± 31.2 | 0.3 ± 0.2 |
| Neutrophils with resting platelets + fMLP | 246.2 ± 29.3 | 0.3 ± 0.2 |
| Neutrophils that phagocytosed activated platelets + fMLP | 126.5 ± 12.1 | 43.7 ± 5.1 |
Neutrophils were stimulated or not with fMLP 0.5 μM after a 5-minute incubation at 37°C with resting or activated platelets. Reactions were blocked after further 5 minutes. Mac-1 expression and internalized platelets were determined by flow cytometry. Effects on Mac-1 expression are expressed as the percentage of basal value. Values are the mean ± SEM of 3 independent experiments performed in duplicate.
Phagocytic clearance of activated platelets in vivo: experimental animal model
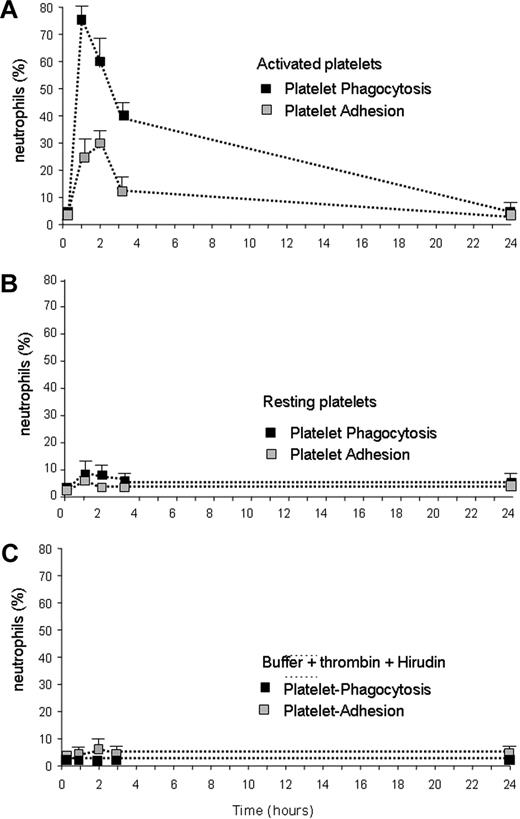
To further verify the in vivo relevance of these observations, we injected purified platelets, either activated or resting, in the tail vein of C57BL/6 mice. Blood was retrieved at different times after injection (1, 2, 3, and 24 hours). Figure 6 shows that, within the first hour, most circulating neutrophils became engaged in the clearance of activated platelets. The fraction of circulating neutrophils with internalized platelets returned to background levels at 24 hours (Figure 6A), when injected platelets had been cleared. We failed to observe significant increase over the background levels when mice were injected with nonactivated platelets (Figure 6B) or with vehicle alone (Figure 6C).
In vivo evidence of the phagocytosis of activated platelets by circulating neutrophils. Purified platelets, either activated (A) or resting (B), were injected in the tail vein of C57BL/6 mice. Platelet activation was achieved with thrombin. The molecule was then inactivated by addition of hyrudin, as described in “In vivo injection and clearance of activated platelets.” (C) The effect of the in vivo injection of the buffer used for platelet activation, containing both thrombin and hyrudin. Blood was retrieved at different times after injection (x axis, hours) and neutrophils with adherent (■) or internalized ( ) platelets were identified by flow cytometry. More than 70% of circulating neutrophils were engaged in the internalization and adhesion of activated, but not resting, platelets after 1 hour. The fraction of circulating neutrophils with internalized and adherent platelets returned to background levels 24 hours after the injection. Results are expressed as mean ± SEM; 5 to 7 animals were assessed per point.
) platelets were identified by flow cytometry. More than 70% of circulating neutrophils were engaged in the internalization and adhesion of activated, but not resting, platelets after 1 hour. The fraction of circulating neutrophils with internalized and adherent platelets returned to background levels 24 hours after the injection. Results are expressed as mean ± SEM; 5 to 7 animals were assessed per point.
In vivo evidence of the phagocytosis of activated platelets by circulating neutrophils. Purified platelets, either activated (A) or resting (B), were injected in the tail vein of C57BL/6 mice. Platelet activation was achieved with thrombin. The molecule was then inactivated by addition of hyrudin, as described in “In vivo injection and clearance of activated platelets.” (C) The effect of the in vivo injection of the buffer used for platelet activation, containing both thrombin and hyrudin. Blood was retrieved at different times after injection (x axis, hours) and neutrophils with adherent (■) or internalized ( ) platelets were identified by flow cytometry. More than 70% of circulating neutrophils were engaged in the internalization and adhesion of activated, but not resting, platelets after 1 hour. The fraction of circulating neutrophils with internalized and adherent platelets returned to background levels 24 hours after the injection. Results are expressed as mean ± SEM; 5 to 7 animals were assessed per point.
) platelets were identified by flow cytometry. More than 70% of circulating neutrophils were engaged in the internalization and adhesion of activated, but not resting, platelets after 1 hour. The fraction of circulating neutrophils with internalized and adherent platelets returned to background levels 24 hours after the injection. Results are expressed as mean ± SEM; 5 to 7 animals were assessed per point.
Role of phosphatidylserine, P-selectin/PSGL-1 interaction, and β2 integrin
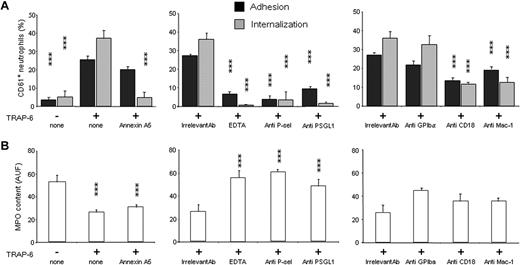
The results described in the previous paragraph indicate that moietie(s) expressed by platelets upon activation are critical to trigger their clearance. P-selectin and phosphatidylserine, which both increased on platelet outer surface after activation (Table 1, Figure S2), are promising candidates.31-33 Annexin A5 binds to exposed phosphatidylserine residues, thus inhibiting the events downstream of its recognition, including phagocytic clearance.24 When activated platelets were treated with annexin A5, the phagocytosis abated (percentage of neutrophils phagocytosing platelets from 37.1% ± 4.4% to 5.1% ± 1.2%, P < .001). Annexin A5 in contrast did not significantly modify the percentage of neutrophils with adherent platelets (from 25.6% ± 3.0% to 20.1% ± 3.3%; Figure 7). We also failed to significantly interfere with platelet adhesion to neutrophils under flow conditions (Table 2, Figure 8; Video S4). Moreover, annexin A5 did not influence the MPO depletion induced by activated autologous platelets (from 52.9 ± 5.8 to 26.3 ± 2.1 MF1 for neutrophils challenged with activated platelets, and to 30.6 ± 2.6 MF1 for neutrophils challenged with activated, annexin A5–treated platelets; Figure 7). These results indicate that by hindrance with the recognition of phosphatydylserine we dissect the events linked to platelet adhesion and phagocytosis, thus revealing that neutrophil degranulation is dependent on adhesion.
The phagocytosis of platelets depends on exposure of phosphatidylserine, on expression of P-selectin and β2 integrins. The percentage of neutrophils with adherent (■) and internalized ( ) platelets (A) and their MPO content (B) were evaluated. (Left panels) Neutrophils were challenged with resting platelets (TRAP-6−) or with activated platelets (TRAP-6+) or with activated platelets treated with recombinant annexin A5 (15 μg/mL; TRAP-6+, annexin A5). (Middle panels) Neutrophils were challenged with activated platelets (TRAP-6+) either in the presence of irrelevant control mAb, or of EDTA, or of anti–P-selectin mAb (anti P-sel) or of anti–PSGL-1 mAb (anti PSGL-1). (Right panels) Neutrophils were challenged with activated platelets (TRAP-6+) either in the presence of irrelevant control mAb, or of anti-GPIbα mAb (anti-GPIbα), or of mAb against the β2 subunit binding site (anti-CD18), or against the Mac-1 binding site (anti Mac-1). All mAbs were incubated with the relevant cell fraction at a 20 μg/mL final concentration 5 minutes before the assay. Adhesion, phagocytosis, and MPO release are expressed as mean ± SEM of 6 to 16 independent experiments. ***P < .001; *P < .005, significantly different from control.
) platelets (A) and their MPO content (B) were evaluated. (Left panels) Neutrophils were challenged with resting platelets (TRAP-6−) or with activated platelets (TRAP-6+) or with activated platelets treated with recombinant annexin A5 (15 μg/mL; TRAP-6+, annexin A5). (Middle panels) Neutrophils were challenged with activated platelets (TRAP-6+) either in the presence of irrelevant control mAb, or of EDTA, or of anti–P-selectin mAb (anti P-sel) or of anti–PSGL-1 mAb (anti PSGL-1). (Right panels) Neutrophils were challenged with activated platelets (TRAP-6+) either in the presence of irrelevant control mAb, or of anti-GPIbα mAb (anti-GPIbα), or of mAb against the β2 subunit binding site (anti-CD18), or against the Mac-1 binding site (anti Mac-1). All mAbs were incubated with the relevant cell fraction at a 20 μg/mL final concentration 5 minutes before the assay. Adhesion, phagocytosis, and MPO release are expressed as mean ± SEM of 6 to 16 independent experiments. ***P < .001; *P < .005, significantly different from control.
The phagocytosis of platelets depends on exposure of phosphatidylserine, on expression of P-selectin and β2 integrins. The percentage of neutrophils with adherent (■) and internalized ( ) platelets (A) and their MPO content (B) were evaluated. (Left panels) Neutrophils were challenged with resting platelets (TRAP-6−) or with activated platelets (TRAP-6+) or with activated platelets treated with recombinant annexin A5 (15 μg/mL; TRAP-6+, annexin A5). (Middle panels) Neutrophils were challenged with activated platelets (TRAP-6+) either in the presence of irrelevant control mAb, or of EDTA, or of anti–P-selectin mAb (anti P-sel) or of anti–PSGL-1 mAb (anti PSGL-1). (Right panels) Neutrophils were challenged with activated platelets (TRAP-6+) either in the presence of irrelevant control mAb, or of anti-GPIbα mAb (anti-GPIbα), or of mAb against the β2 subunit binding site (anti-CD18), or against the Mac-1 binding site (anti Mac-1). All mAbs were incubated with the relevant cell fraction at a 20 μg/mL final concentration 5 minutes before the assay. Adhesion, phagocytosis, and MPO release are expressed as mean ± SEM of 6 to 16 independent experiments. ***P < .001; *P < .005, significantly different from control.
) platelets (A) and their MPO content (B) were evaluated. (Left panels) Neutrophils were challenged with resting platelets (TRAP-6−) or with activated platelets (TRAP-6+) or with activated platelets treated with recombinant annexin A5 (15 μg/mL; TRAP-6+, annexin A5). (Middle panels) Neutrophils were challenged with activated platelets (TRAP-6+) either in the presence of irrelevant control mAb, or of EDTA, or of anti–P-selectin mAb (anti P-sel) or of anti–PSGL-1 mAb (anti PSGL-1). (Right panels) Neutrophils were challenged with activated platelets (TRAP-6+) either in the presence of irrelevant control mAb, or of anti-GPIbα mAb (anti-GPIbα), or of mAb against the β2 subunit binding site (anti-CD18), or against the Mac-1 binding site (anti Mac-1). All mAbs were incubated with the relevant cell fraction at a 20 μg/mL final concentration 5 minutes before the assay. Adhesion, phagocytosis, and MPO release are expressed as mean ± SEM of 6 to 16 independent experiments. ***P < .001; *P < .005, significantly different from control.
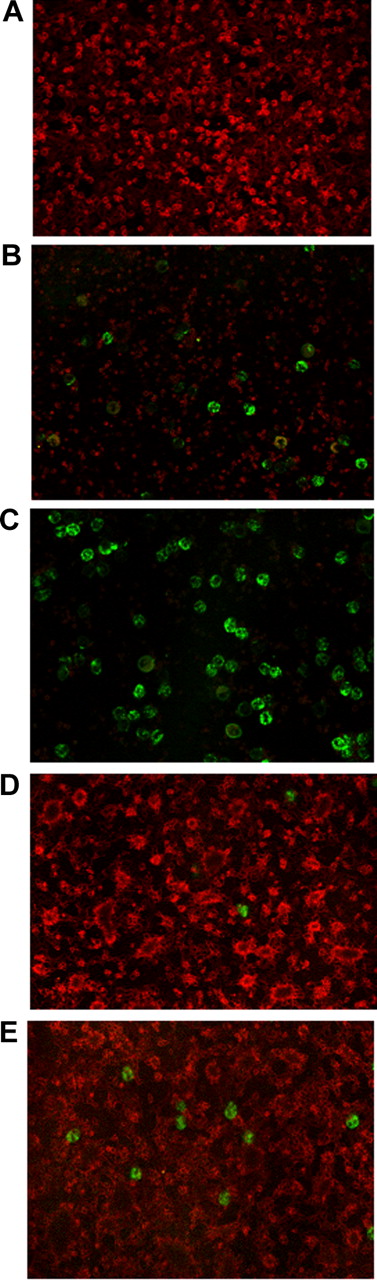
Requirements of P-selectin and phosphatidylserine for the clearance of activated platelets. Glass-adhered platelets were activated with thrombin (0.5 U/mL) for 2 minutes, washed, and incubated either alone (A) or in the presence of purified autologous neutrophils for 2 minutes (B) or 5 minutes (C-E). (D) Platelets were treated with mAb anti–P-selectin before addition of neutrophils. (E) Platelets were treated with recombinant annexin A5 before addition of neutrophils. Glass-adhered, activated platelets were revealed by confocal microscopy after staining with antiplatelet glycoprotein Ib mAbs (red) and neutrophils after staining with the anti-CD66b mAb (green).
Requirements of P-selectin and phosphatidylserine for the clearance of activated platelets. Glass-adhered platelets were activated with thrombin (0.5 U/mL) for 2 minutes, washed, and incubated either alone (A) or in the presence of purified autologous neutrophils for 2 minutes (B) or 5 minutes (C-E). (D) Platelets were treated with mAb anti–P-selectin before addition of neutrophils. (E) Platelets were treated with recombinant annexin A5 before addition of neutrophils. Glass-adhered, activated platelets were revealed by confocal microscopy after staining with antiplatelet glycoprotein Ib mAbs (red) and neutrophils after staining with the anti-CD66b mAb (green).
Divalent cations, which are necessary for selectin- and integrin-mediated adhesion,34 were also required. Platelet adhesion, platelet phagocytosis, and MPO release were virtually abrogated when EDTA was added to activated platelets before interaction with neutrophils (the percentage of neutrophils with adherent platelets decreased from 25.6% ± 2.0% to 6.8% ± 1.2%; the percentage of neutrophils that phagocytosed platelets from 37.1% ± 4.4% to 0.7% ± 0.4% and MPO content from 26.3 ± 1.1 to 55.8 ± 6.2 MPO-MF1; P < .001 for both adhesion and phagocytosis, P < .001 for MPO release; n = 6; Figure 7).
To determine whether P-selectin was involved, we used mAbs blocking either P-selectin or its counterreceptor on neutrophils, PSGL-1. Both adhesion and internalization abated in treated samples, and MPO depletion was prevented (the percentage of neutrophils with adherent platelets decreased from 27.1% ± 1.5% to 3.7% ± 0.4%, the percentage of neutrophils that phagocytosed platelets from 36.1% ± 2.7% to 3.5% ± 1.2%; MPO content from 25.7 ± 3.4 to 60.5 ± 4.5 MF1, respectively, upon P-selectin blockade; the percentage of neutrophils with adherent platelets decreased to 9.5% ± 2.2%, the percentage of neutrophils that phagocytosed platelets to 1.6 ± 1.1 and MPO content to 47.9 MF1 upon PSGL1 blockade). The difference with results obtained in the presence of irrelevant isotype-matched mAb was statistically significant (P < .001, n = 6; Figure 7). These results further support the role of the P-selectin/PSGL-1 interaction in the cell-to-cell interaction and unequivocally implicate this pathway as a master regulator of platelet phagocytosis.
P-selectin/PSGL-1 interaction promotes Mac-1–dependent homotypic neutrophil aggregation and neutrophil/platelet aggregation,5,35 a mechanism dependent on cytoskeletal rearrangement and Mac-1 clusterization. Neutrophils expressed the Mac-1 molecule and up-regulated the surface expression of the molecule when challenged with activated platelets (Figure S2). Blocking mAbs against the common β2 chain (CD18) or against the αM chain of the integrin significantly inhibited both platelet adhesion to neutrophils (from 27.1% ± 1.5% to 13.4% ± 3.2% with anti-CD18 mAb, n = 11, and to 19.0% ± 1.2% with the anti–Mac-1 mAb, n = 6; differences with the control isotype-matched mAb were significant, P < .001) and phagocytosis (n = 6, from 36.1% ± 2.7% to 11.7% ± 4.7% in the presence of anti-CD18 mAb and to 12.4% ± 3.7% in the presence of anti–Mac-1 mAb, P < .001 versus cells treated with the irrelevant mAb). The MPO release, although reduced, was not significantly affected by Mac-1 blockade (Figure 8). The glycoprotein Ibα interacts with Mac-1 expressed on activated leukocytes:36 its blockade did not exert a significant effect in this system (Figure 8). The results indicate that the Mac-1 integrin is required for the firm adhesion of neutrophils to platelets, and suggest that this event is required for eventual platelet internalization. In agreement, prevention of the rearrangement of the actin-based filament network at 4°C or its disruption by cytochalasin D abrogated platelet clearance by neutrophils (Figure 4).
We studied in a parallel set of experiments the interaction between neutrophils and platelets in whole-blood samples.37 This approach avoids potential artifacts due to cell isolation procedures, and maintains during the assay the physiologic array of plasma cofactors and opsonins. At 5 minutes after platelet activation, the fraction of neutrophils with adherent platelets shifted from 2.13% plus or minus 0.6% to 32.0% plus or minus 5.7% (n = 5; P < .001), and the fraction of neutrophils that phagocytosed platelets from 3.4% plus or minus 1.4% to 42.7% plus or minus 5.7% (Figure S3A). Intracellular MPO content significantly decreased (from 101.3 ± 37.1 to 60.0 ± 23.1 MF1, n = 5; P < .001; Figure S3B), with a consensual increase in the plasma concentration of the soluble protein (from 3.8 ± 1.0 ng/mL to 14.6 ± 2.0 ng/mL, n = 5; P < .001; Figure S3B). These results indicate that events associated to platelet adhesion and phagocytosis by neutrophils occurs in the blood (ie, in the physiologic environment where circulating cells interact).
Blocking mAbs against P-selectin and Mac-1, reduced adherence, phagocytosis, and MPO release (Figure S3). Results with recombinant annexin A5 were in keeping with those obtained using isolated cells, because phosphatidylserine exposure was required for internalization of platelets; its hindrance did not alter the release of MPO, further supporting the contention that phagocytosis and degranulation are not causally linked.
To address the role of cyclooxygenase pathway, experiments were performed using samples from donors before and 4 hours after ingestion of acetylsalicylic acid. The treatment did not influence either activated platelet adhesion to neutrophils or their phagocytic clearance (Figure S4). Finally, we evaluated whether phagocytosis by neutrophils influenced the acceleration of the coagulation time due to activated platelets, which physiologically represent a source of anionic phospholipids. Table 4 shows that the interaction with neutrophils significantly reduces the procoagulant activity of activated platelets. The action of neutrophils was dose-dependent, because it increased in direct proportion to the phagocyte-to-platelet ratio and was due to active phagocytosis, because it was abrogated by treatment with cytochalasin D (Table 4).
Phagocytosis by neutrophils quenches the procoagulant activity of activated platelets
| . | Time, seconds . |
|---|---|
| No platelets | 159.3 ± 17.3 |
| Activated platelets (105/μL) | 87.3 ± 4.6* |
| Activated platelets (105/μL) + neutrophils (5 × 103/μL) | 113.5 ± 3.1* |
| + neutrophils (10 × 103/μL) | 130.0 ± 4.4* |
| + neutrophils (20 × 103/μL) | 153.6 ± 5.9* |
| + neutrophils (20 × 103/μL) treated with cytochalasin D | 98.0 ± 5.5 |
| . | Time, seconds . |
|---|---|
| No platelets | 159.3 ± 17.3 |
| Activated platelets (105/μL) | 87.3 ± 4.6* |
| Activated platelets (105/μL) + neutrophils (5 × 103/μL) | 113.5 ± 3.1* |
| + neutrophils (10 × 103/μL) | 130.0 ± 4.4* |
| + neutrophils (20 × 103/μL) | 153.6 ± 5.9* |
| + neutrophils (20 × 103/μL) treated with cytochalasin D | 98.0 ± 5.5 |
Platelests (100 × 103/μL) were activated with TRAP-6 for 2 minutes and then incubated for 1 minute at 37°C alone or in the presence of an equal volume of neutrophils, at a neutrophil:platelet ratio of 1:20, 1:10, or 1:5. Neutrophils at the 1:5 ratio were either untreated or pretreated with cytochalasin D to prevent actin-based cytoskeleton assembly. After addition of normal human plasma and of a 25 mM CaCl2 solution, the coagulation (recalcification) time was recorded and expressed as seconds (mean ± SEM of 5 different experiment performed in duplicate). The platelet phagocytosis by neutrophils was confirmed in parallel by flow cytometry. *P < .05 with respect to samples with activated platelets alone (by paired t test).
Discussion
These experiments document an active pathway culminating in the clearance of activated platelets by neutrophils in vitro and in vivo, involving release of the content of the azurophilic cytoplasmic granules and active phagocytosis. The clearance comprises an adhesion step, which depends on the interaction between P-selectin and its counterreceptor on neutrophils, PSGL-1, and on the activation of the β2-integrin Mac-1. Active internalization is a second step, which is selectively abrogated by interference with the recognition of phosphatidylserine. Neutrophils that have phagocytosed platelets can be traced with relative ease in the circulating blood of patients with acute myocardial infarction who have high platelet P-selectin expression (Table 1). Depletion of neutrophil MPO across the coronary inflamed vascular bed is a well-characterized event in patients with severe unstable angina38,39 where it is unrelated to myocardial ischemia, chronic atherosclerotic burden, or to passage through the ischemic myocardium or culprit unstable lesions.39 The findings of the present study raise the possibility that the interaction between circulating activated platelets and leukocytes is mechanistically involved in such depletion.
The mechanisms controlling the removal of platelets from the circulation are poorly identified, even if the phagocytosis of effete, activated platelets possibly contributes. Extensive thrombosis, such as that which occurs during disseminated intravascular coagulation, causes thrombocytopenia, most likely because of incorporation of platelets into the small thrombi that are eventually cleared by local phagocytes.40 Even in more physiologic conditions, platelet activation would occur on injured vessel walls, and the activated platelets could be rapidly sequestered at these sites.
Autoantibodies accelerate the removal of platelets by Fc receptor–bearing phagocytes in patients with autoimmune thrombocytopenia,41 and microbial products, including bacterial endotoxin, enhance phagocytosis.42,43 Cooling primes reinfused human platelets for activation, and Mac-1 is responsible for rapidly decreasing platelet counts, preventing the induction of thrombosis by primed platelets.44 Interestingly, and in good agreement with our data on a key role of β2 integrin in the phagocytic clearance of platelets, Mac1−/− mice have an increased concentration of circulating platelets compared with wild-type littermates.44 Platelet activation in the circulating blood is a relatively common event, which results in a swift up-regulation of their adhesive45 and prothrombotic properties.46,47 The activation of circulating platelets is indeed directly associated with the risk of thrombosis; it is quite clear that several homeostatic mechanisms physiologically quench their capacity to activate the coagulation system.
The activation of circulating platelets, besides triggering their accelerated turnover, results in the cross-talk with monocytes and neutrophils.48 Our finding that the interaction with activated platelets prompts a phagocytic program is in keeping with previous observations, for which neutrophils that interact with platelets phagocytose bacteria more readily than unbound neutrophils.49
We did not observe any effect of aspirin, which eliminates thromboxane from the platelet releasate and substitute more free, unprocessed arachidonic acid as well as the hydroxy acid12-hydroxyeicosatetraenic acid. The lack of detectable differences in the presence or the absence of thromboxane apparently rules out the participation of the cyclooxygenase pathway in the results.
It is tempting to speculate that the ability of neutrophils to adhere and to selectively internalize activated platelets, expressing phosphatidylserine and P-selectin, is a mechanism to maintain the normal blood fluidity by sequestering a circulating potentially dangerous substrate, which would induce thrombi formation.50 Indeed, it has been previously clearly shown that neutrophils down-regulate platelet function.50
Neutrophils are the more abundant circulating phagocytes, and could represent an inborn circulating clearance system. A “tether and tickle” mechanism controls the clearance of other particulate substrates, like aged red blood cells, phosphatidylserine-containing liposomes, and apoptotic cells. In these models, bridging receptors function by tethering the apoptotic cell to the phagocyte, whereas the recognition of phosphatidylserine is required to transduce signals that initiate the uptake. Our data are at least partially consistent with this model. Indeed, annexin A5, forms a 2-dimensional crystalline lattice on membrane anionic phospholipids, thus hindering their recognition by leukocytes.24 The ensuing selective blockade of phosphatidylserine abolishes internalization, but does not apparently interfere with adhesive interactions.
The P-selectin/PSGL-1 engagement is required for platelet adhesion to neutrophils. Moreover, it is necessary for integrin transactivation, assembly of the phagosome, and neutrophil degranulation.5,10,21,22,51,52 Therefore, platelet adhesion and neutrophil degranulation are simultaneously blocked in our system by the antibodies for P-selectin and PSGL-1.
All together, we propose that tethering is achieved via P-selectin recognition, leading to MPO release, and activating β2 integrins for internalization; if the tethered substrate was a phosphatidylserine-expressing platelet, phagocytosis swiftly removes the activated platelets, which represent a template for thrombin formation, from the circulating blood.31,46,47
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Martin Herrmann (University of Erlangen, Erlangen, Germany) for generously providing unlabeled recombinant annexin A5, Roman Polischuk (Laboratory of Membrane Traffic, Consorzio Mario Negri Sud, Santa Maria Imbaro, Chieti, Italy) for excellent electron microscopy, Alessio di Pentima (Laboratory of Membrane Traffic, Consorzio Mario Negri Sud, Santa Maria Imbaro, Chieti, Italy) for skillful technical assistance, and Anca Dragomir (Department of Medical Cell Biology, Uppsala University, Uppsala, Sweden) for kind advice on electron microscopy.
This work was supported by the MIUR (Ministero dell'Istruzione dell'Università e della Ricerca; PRIN 2005; FIRB 2003), the EC (EVGN network), the Fondazione per il Cuore, the Associazione Italiana Per La Ricerca Sul Cancro (AIRC), and the Ministero della Salute.
Authorship
Contribution: N.M. designed and performed experiments, discussed the experimental strategy, and wrote the manuscript; P.R.-Q. discussed the experimental strategy and wrote the manuscript; V.E. and L.T. provided vital reagents and supervised the experiments of electron microscopy and of phagocytosis under flow conditions; A.P. performed experiments under flow conditions; C.C. carried out image analysis; A.C. and M.T.S.B. contributed to the establishment of the mouse model of platelet clearance; D.C. selected patients and organized clinical data; A.M. discussed the experimental strategy; and A.A.M. defined the experimental strategy, supervised experiments, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Norma Maugeri, Clinical Cardiovascular Research Laboratories, University Vita-Salute San Raffaele, via Olgettina 58, 20132 Milan, Italy; e-mail: maugeri.norma@hsr.it or normamaugeri2003@yahoo.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal