Abstract
Epo-induced endocytosis of EpoR plays important roles in the down-regulation of EpoR signaling and is the primary means that regulates circulating Epo concentrations. Here we show that cell-surface EpoR is internalized via clathrin-mediated endocytosis. Both JAK2 kinase activity and EpoR cytoplasmic tyrosines are important for ligand-dependent EpoR internalization. Phosphorylated Y429, Y431, and Y479 in the EpoR cytoplasmic domain bind p85 subunit of PI3 kinase on Epo stimulation and individually are sufficient to mediate Epo-dependent EpoR internalization. Knockdown of p85α and p85β or expression of their dominant-negative forms, but not inhibition of PI3 kinase activity, dramatically impaired EpoR internalization, indicating that p85α and p85β may recruit proteins in the endocytic machinery on Epo stimulation. Furthermore, mutated EpoRs from primary familial and congenital polycythemia (PFCP) patients lacking the 3 important tyrosines do not bind p85 or internalize on stimulation. Addition of residues encompassing Y429 and Y431 to these truncated receptors restored p85β binding and Epo sensitivity. Our results identify a novel PI3 kinase activity-independent function of p85 in EpoR internalization and support a model that defects of internalization in truncated EpoRs from PFCP patients contribute to Epo hypersensitivity and prolonged signaling.
Introduction
Erythropoietin (Epo) is the primary cytokine regulating red blood cell production, and its function is mediated through the Epo receptor (EpoR). Like most cytokine receptors, EpoR lacks intrinsic enzymatic activities and relies on the cytosolic tyrosine kinase JAK2 for signal transduction. Epo, EpoR, or JAK2-deficient mice die embryonically because of severe anemia. The binding of Epo to the EpoR activates JAK2 kinase activity. Activated JAK2 then phosphorylates many of the 8 tyrosines in the EpoR cytoplasmic domain, thereby providing a platform for the recruitment and activation of signaling mediators through SH2 domain-mediated interactions. These signaling events ultimately result in the survival, proliferation, and differentiation of erythroid progenitor cells.1,2 Signaling mediators include the STAT5 transcription factor and the p85 regulatory subunit of phosphoinositide 3-kinase (PI3K). p85α binds to phosphorylated Y479 of the EpoR on stimulation.3 This recruits and activates the catalytic subunit of PI3K, which in turn activates AKT, promoting erythroid proliferation and maturation.3-5
To ensure proper amplitude and duration of Epo signaling, mechanisms are turned on activation to attenuate EpoR signal transduction. For example, phosphorylated Y429 in the EpoR recruits the tyrosine phosphatase SHP-1 (SH2 domain-containing protein-tyrosine phosphatase-1), which inactivates JAK2.6 In addition, the synthesis of Suppressor Of Cytokine Signaling (SOCS) family proteins is induced, which inactivates JAK2 and/or blocks access of STAT5 to receptor-binding sites.7 Moreover, Epo binding promotes endocytosis and degradation of the EpoR.8-11 The importance of these regulations is underscored by the association of EpoR mutations with primary familial and congenital polycythemia (PFCP), a proliferative disorder of the red cell lineage characterized by increased red blood cell mass.12 The EpoR variants associated with PFCP have deletions that remove 59 to 110 amino acids of the cytoplasmic C-terminal domain, including Y429 that recruits SHP-1, and exhibit hypersensitivity to Epo and prolonged activation of the JAK/STAT pathway.12,13 A murine model where the EpoR gene was replaced with one of the human PFCP EpoR mutants shows marked polycythemia.14
Epo-induced endocytosis is a rapid and efficient way to decrease Epo responsiveness, as the cell-surface level of EpoR controls cellular Epo sensitivity.15 It also may bring about destruction of activated protein complexes and terminate signaling. Moreover, EpoR endocytosis plays a critical role in the clearance of Epo and thus regulates circulating Epo concentrations and its bioactivity.16 On Epo stimulation, cell-surface EpoR is internalized and degraded as few, if any, EpoR molecules recycle back to the cell surface.9-11 Degradation of the EpoR is sensitive to inhibitors of both proteasomal and lysosomal function and requires ubiquitination by β-Trcp.11,17 Mechanisms underlying these processes are not well understood.
In addition to its contribution to signaling, JAK2 is required for the EpoR to exit the endoplasmic reticulum and for EpoR expression on the cell surface.18 Therefore, JAK2 is an essential subunit of the EpoR. Because the EpoR/JAK2 complex essentially functions as a receptor tyrosine kinase, we hypothesize that JAK2 might regulate EpoR endocytosis. Here we show that, after Epo stimulation, cell-surface EpoR is internalized via clathrin-mediated endocytosis, and both JAK2 kinase activity and EpoR cytoplasmic tyrosines are important for Epo-dependent EpoR internalization. We further show that phosphorylated Y429, Y431, or Y479 in the EpoR cytoplasmic domain is sufficient to mediate Epo-dependent EpoR internalization and that p85 binding to these tyrosines plays an important role in this process. In addition, we show that mutated EpoRs from PFCP patients lacking the 3 important tyrosines do not bind p85 and do not internalize on stimulation. This defect may contribute to their hypersensitivity to Epo and prolonged signaling.
Methods
Plasmid constructs, cell lines, and reagents
All EpoR mutants were generated in the pMX-IRES-GFP vector using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) and verified by sequencing. Expression vectors of p85α and p85β were from Origene (Rockville, MD). cDNAs corresponding to the SH2 domains for p85α and p85β as described19 were isolated by polymerase chain reaction and subcloned into the pcDNA3.1 vector. Ba/F3 cells and JAK2-deficient γ2A cells stably expressing wild-type or mutant HA (hemagglutinin)–tagged EpoR (HA-EpoR) and various forms of JAK2 were generated as previously described.18 Antibodies were from the following sources: HA, Covance Research Products, Princeton, NJ; JAK2, phospho-JAK2, Millipore, Billerica, MA; p85β, early endosome antigen 1 (EEA1), clathrin heavy chain, Santa Cruz Biotechnology, Santa Cruz, CA; V5, Invitrogen, Carlsbad, CA; actin, Sigma-Aldrich, St Louis, MO; SOCS3, Abcam, Cambridge, MA; and p85α, phospho-Akt, Cell Signaling Technology, Danvers, MA. HA affinity resin was from Roche Diagnostics (Indianapolis, IN). Horseradish peroxidase-coupled secondary antibodies and the enhanced chemiluminescence system were from GE Healthcare (Little Chalfont, United Kingdom). Wortmannin was from Sigma-Aldrich.
Isolation of TER119− erythroid progenitor cells from murine fetal liver
Murine fetal liver cells were isolated from E13.5 to E14.5 Balb/c or CD1 (Charles River Breeding Laboratories, Portage, MI) embryos. Cells were mechanically dissociated by pipetting in phosphate-buffered saline (PBS) containing 2% fetal bovine serum (FBS), and single-cell suspensions were prepared by passing the dissociated cells through 70-μm cell strainers. Fetal liver cells were labeled with biotin-conjugated anti-TER119 antibody (1:100; BD Biosciences PharMingen, San Diego, CA), and TER119− cells were purified through a StemSep column according to the manufacturer's instructions (StemCell Technologies, Vancouver, BC) as previously described.20 TER119− cells were transduced with retroviruses expressing wild-type or mutant HA-EpoR constructs at similar levels (judged by green fluorescent protein [GFP] fluorescence), and receptor internalization was measured by flow cytometry 40 hours after infection.
Labeling and immunoprecipitation of cell-surface EpoR (surface IP)
γ2A cells stably expressing HA-EpoR with various JAK2 constructs were incubated with blocking buffer (PBS with 5% normal mouse serum) for 30 minutes at 4°C. Subsequently, cells were incubated with 10 μg/mL anti-HA antibodies in blocking buffer for 60 minutes at 4°C. After washing with PBS, cells were lysed in 1% NP-40 lysis buffer containing 5 μg/mL HA peptide to block any residual anti-HA antibodies. After incubation with protein A/G agarose for 90 minutes at 4°C, immunoprecipitants were washed 3 times with PBS containing 1% Triton X-100 and 0.1% sodium dodecyl sulfate (SDS), run on SDS-polyacrylamide gel electrophoresis (PAGE), and immunoblotted with anti-HA antibodies. In experiments where Epo treatment was performed, cells were stimulated with Epo (30 units/mL) at 37°C for the duration indicated.
Flow cytometry and data analysis
Surface expression of the wild-type or mutant HA-EpoRs was measured in one million TER119− erythroid progenitors or in one million γ2A cells in the presence of wild-type or mutant JAK2 as described previously.21 For Epo stimulation, cells were incubated with 30 units of Epo at 37°C. The median fluorescence of allophycocyanin (APC), which is conjugated to secondary antibodies that recognize anti-HA antibodies, was used to quantify the level of receptor on the cell surface. For each sample, the EpoR surface expression was normalized to that from samples coexpressing wild-type JAK2 before Epo stimulation. Each point represents data from 3 independent experiments. To inhibit PI3K activities, cells were incubated with wortmannin at concentrations indicated for 3 hours before Epo stimulation.
Glycosidase digestion of EpoR and MTT cell proliferation assay
Glycosidase treatment of the EpoR with EndoH (Endoglycosidase H; New England Biolabs, Ipswich, MA), or with PNGaseF (Peptide N-Glycosidase F; New England Biolabs) and neuraminidase (New England Biolabs), and methyl-thiazol-tetrazolium (MTT) cell proliferation assay were performed as described.21
Immunoprecipitation and immunoblotting
γ2A cells transiently transfected with p85β in the vector pCMV6-XL6 were starved for 12 hours in Dulbecco modified Eagle medium with 1% bovine serum albumin followed by Epo induction for the appropriate time as indicated. Cells were then lysed with 1% NP-40 lysis buffer with protease and phosphatase inhibitors. Lysates were immunoprecipitated with HA affinity resin or anti-p85β antibodies. The precipitates were eluted with SDS sample buffer, separated by SDS-PAGE, transferred to nitrocellulose membranes, and probed with anti-p85β antibodies or antibodies to HA. Bound antibodies were detected by the enhanced chemiluminescence system after incubation with horseradish peroxidase-coupled secondary antibodies. The lysates were also immunoblotted with antibodies to HA, p85β, or phosphorylated JAK2.
Immunofluorescence
γ2A cells stably expressing HA-EpoR were seeded on glass coverslips. Coverslips were blocked with PBS containing 3% bovine serum albumin and 5% normal goat serum for 30 minutes at −4°C and incubated with 20 μg/mL anti-HA antibodies for 1 hour. Coverslips were then washed 3 times in cold PBS and put back in the incubator for Epo stimulation. Subsequently, cells were fixed with 3.7% formaldehyde for 10 minutes and permeabilized with methanol at 4°C for 15 minutes. Coverslips were then incubated with antibodies for EEA1 (1:100) or clathrin heavy chain (1:300) for 45 minutes in blocking buffer. After washing in PBS, coverslips were incubated with AlexaFluor 555–conjugated goat anti–mouse secondary antibodies, washed 3 times with PBS, and then incubated with AlexaFluor 647-conjugated goat anti–rabbit secondary antibodies. Coverslips were washed 3 times with PBS and mounted onto coverslides with a semipermanent Mowiol mounting medium (Calbiochem, San Diego, CA). Fluorescent images were taken on a Leica TCS SP5 confocal microscope (Leica Microsystems, Deerfield, IL) with 40× oil objective lenses with numeric aperture of 1.25N. Confocal section images were acquired by Leica acquisition software and analyzed with ImageJ (National Institutes of Health [NIH], Bethesda, MD) and Adobe Photoshop (Adobe Systems, San Jose, CA).
Short interfering RNA knockdown
Synthetic short interfering RNAs (siRNAs) to knock down the different human genes were ordered from Dharmacon RNA Technologies (Lafayette, CO) and delivered to the cells at 100 nM using Dharmafect as a transfection agent. Sequences of siRNAs used for SOCS3: CCG CUU CGA CUG CGU CGU CAA and UCG GGA GUU CCU GGA CCA GUA, and for clathrin heavy chain: UCC AA UU CG AAG ACC AAUU. On-target plus Duplex siRNA pools from Dharmacon RNA Technologies were used for p85α and p85β (J-00302017 and J-003021-12). Knockdown efficiency was determined by immunoblotting with the indicated antibodies 48 hours after treatment with siRNAs.
Results
JAK2 tyrosine kinase activity is required for ligand-induced EpoR internalization
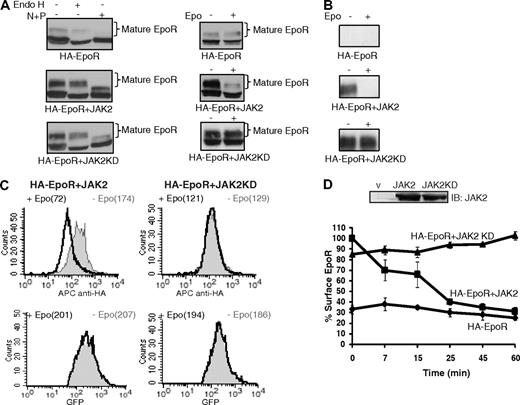
The role of JAK2 in ligand-induced EpoR down-regulation was examined in JAK2-null γ2A cells that stably coexpressed HA-tagged EpoR with JAK2, kinase-deficient JAK2 (JAK2KD), or vector. In these cells, the EpoR existed in 2 forms as assessed by SDS-PAGE: a faster migrating immature form that was Endo H sensitive and a slower migrating mature form that was Endo H resistant (Figure 1A left panels). The expression levels of JAK2 and JAK2KD in these cells were similar (Figure 1D). As previously shown,18 little mature EpoR was detected in the absence of JAK2. JAK2 coexpression markedly increased the mature Endo H-resistant form of the EpoR, and this JAK2-dependent maturation of EpoR did not depend on JAK2 kinase activity (Figure 1A).18 Stimulation with Epo down-regulated mature EpoR, and JAK2 kinase activity was essential for this process (Figure 1A right panels).11 The presence of the HA tag on the extracellular domain allowed mature cell-surface EpoR species to be specifically isolated by incubating nonpermeabilized cells with an anti-HA antibody followed by immunoprecipitation (surface IP). Consistent with previous results, cell-surface EpoRs were detected in the presence but not in the absence of JAK2 (Figure 1B − stimulation). On Epo stimulation, the majority of mature EpoRs disappeared from the cell surface in the presence of JAK2 (Figure 1B + stimulation). In contrast, in the presence of JAK2KD, mature EpoRs remained on the cell surface on stimulation (Figure 1B). These results show that JAK2 kinase activity is essential for ligand-induced EpoR internalization and degradation.
JAK2 tyrosine kinase activity is required for ligand-induced EpoR down-regulation. (A) Mature HA-EpoR species were detected in JAK2-deficient γ2A cells stably expressing HA-EpoR with wild-type or kinase-deficient JAK2 (JAK2KD) by their resistance to Endo H treatment (left). At 45 minutes after Epo induction, mature HA-EpoR was degraded, and this degradation requires JAK2 kinase activity (right). (B) HA-EpoR at the γ2A cell surface was detected by surface IP. On Epo induction, surface EpoR disappeared when coexpressed with JAK2 but not JAK2KD. (C) Cell-surface HA-EpoR was quantified by flow cytometry using APC-conjugated anti-HA antibodies in nonpermeabilized γ2A cells. Representative histograms of total receptor expression levels (GFP) and cell-surface receptor expression levels (APC) are shown. In each histogram, the uninduced sample is in gray and the induced sample is in black. Median fluorescence of each sample is in parentheses. (D) Kinetics of ligand-induced EpoR internalization. Levels of cell-surface HA-EpoR were analyzed by flow cytometry at various time points after induction as described in panel C. Immunoblotting with anti-JAK2 antibodies showed that the expression levels of JAK2 and JAK2KD are similar. All data represent results from at least 3 independent experiments. Endo H indicates endoglycosidase H; N + P, neuraminidase + PNGaseF; IB, immunoblot; and V, vector.
JAK2 tyrosine kinase activity is required for ligand-induced EpoR down-regulation. (A) Mature HA-EpoR species were detected in JAK2-deficient γ2A cells stably expressing HA-EpoR with wild-type or kinase-deficient JAK2 (JAK2KD) by their resistance to Endo H treatment (left). At 45 minutes after Epo induction, mature HA-EpoR was degraded, and this degradation requires JAK2 kinase activity (right). (B) HA-EpoR at the γ2A cell surface was detected by surface IP. On Epo induction, surface EpoR disappeared when coexpressed with JAK2 but not JAK2KD. (C) Cell-surface HA-EpoR was quantified by flow cytometry using APC-conjugated anti-HA antibodies in nonpermeabilized γ2A cells. Representative histograms of total receptor expression levels (GFP) and cell-surface receptor expression levels (APC) are shown. In each histogram, the uninduced sample is in gray and the induced sample is in black. Median fluorescence of each sample is in parentheses. (D) Kinetics of ligand-induced EpoR internalization. Levels of cell-surface HA-EpoR were analyzed by flow cytometry at various time points after induction as described in panel C. Immunoblotting with anti-JAK2 antibodies showed that the expression levels of JAK2 and JAK2KD are similar. All data represent results from at least 3 independent experiments. Endo H indicates endoglycosidase H; N + P, neuraminidase + PNGaseF; IB, immunoblot; and V, vector.
We next used flow cytometry to determine the kinetics of Epo-induced receptor internalization. In these assays, cell-surface HA-EpoRs were detected by staining nonpermeabilized cells with an anti-HA antibody followed by APC-conjugated secondary antibodies. Because HA-EpoR was expressed in a bicistronic vector that also expressed GFP, GFP fluorescence in each cell is proportional to the total amount of the receptors present, whereas APC fluorescence indicated cell-surface expression. To quantify the amounts of HA-EpoR at the cell surface, the median APC fluorescence intensity was used. γ2A cells stably expressing HA-EpoR with JAK2 or JAK2KD expressed similar total EpoR protein (as judged by median GFP fluorescence), and their cell-surface EpoR expression levels were similar (as judged by median APC fluorescence prestimulation, Figure 1C). Consistent with previous results (Figure 1A,B), Epo-induced EpoR internalization was detected as early as 7 minutes (−30%) and continued steadily to 60 minutes (−69%) in JAK2 cells (Figure 1C,D). In contrast, no receptor was lost from the cell surface in JAK2KD cells (Figure 1C,D). Similar Epo-induced internalization kinetics were observed for the EpoR in the hematopoietic cell line Ba/F3 (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
The EpoR internalizes via clathrin-coated pits
The fate of cell-surface EpoRs on Epo induction was investigated by confocal immunofluorescence microscopy. Nonpermeabilized γ2A cells were labeled with anti-HA antibodies before Epo induction. Cells were stimulated with Epo for 25 minutes and subsequently permeabilized and visualized with fluorescence-conjugated secondary antibodies. This approach thus followed only surface-tagged receptors before Epo treatment. In JAK2 cells, Epo treatment caused the EpoR staining to shift from the plasma membrane to an internal compartment that colocalized with the early endosomal marker EEA1. Importantly, no internalized receptor was observed in JAK2KD cells (Figure 2).
JAK2 kinase activity is required for ligand-induced EpoR colocalization with early endosomal marker EEA1. Cell-surface HA-EpoRs were labeled with anti-HA antibodies before Epo stimulation of 25 minutes. Cells were fixed and immunostained with anti-EEA1 antibodies followed by appropriate fluorescence-conjugated secondary antibodies. Representative confocal images (single section) for different conditions are presented. Images from negative controls with secondary antibodies alone are shown (original magnification ×40; Leica TCS SP5).
JAK2 kinase activity is required for ligand-induced EpoR colocalization with early endosomal marker EEA1. Cell-surface HA-EpoRs were labeled with anti-HA antibodies before Epo stimulation of 25 minutes. Cells were fixed and immunostained with anti-EEA1 antibodies followed by appropriate fluorescence-conjugated secondary antibodies. Representative confocal images (single section) for different conditions are presented. Images from negative controls with secondary antibodies alone are shown (original magnification ×40; Leica TCS SP5).
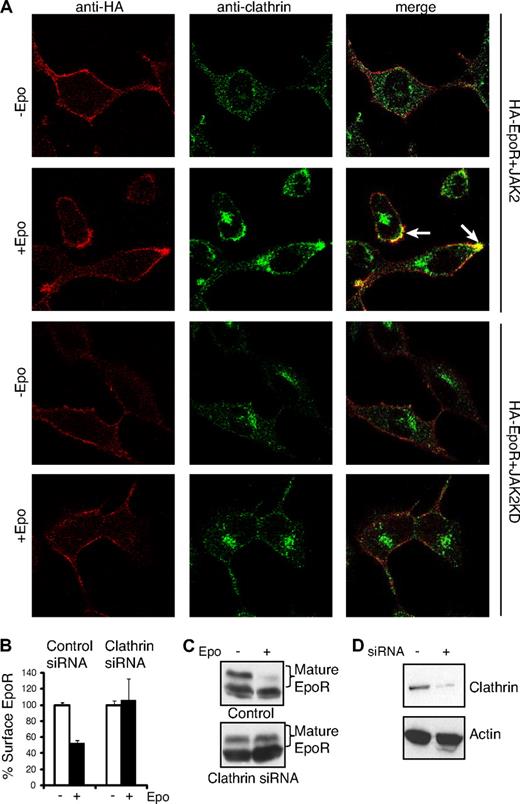
We next examined the role of clathrin in ligand-induced EpoR internalization. As shown in Figure 3A, cell-surface EpoRs colocalized with clathrin on the plasma membrane 7 minutes after Epo addition (Figure 3A), suggesting that Epo-dependent internalization of the EpoR involves clathrin-coated pits. Consistent with this possibility, siRNA-mediated knockdown of the clathrin heavy chain (to ∼ 10% of controls, Figure 3D) dramatically impaired receptor internalization and degradation, whereas control siRNA had no detectable effect on EpoR endocytosis (Figure 3B-D). In contrast, EpoR did not colocalize with clathrin in JAK2KD cells (Figure 3A). These results show that EpoR internalizes via clathrin-mediated endocytosis and that JAK2 kinase activity is required for EpoR internalization.
EpoR internalization is through a clathrin-dependent pathway. (A) γ2A cells expressing HA-EpoR with JAK2 or JAK2KD were seeded on glass coverslips. Cell-surface HA-EpoRs were labeled with anti-HA antibodies before Epo induction. Seven minutes after induction, cells were fixed and immunostained with anticlathrin antibodies followed by appropriate fluorescence-conjugated secondary antibodies. Representative confocal images (single section) are presented. Selected areas of colocalization are indicated with arrows (original magnification ×40; Leica TCS SP5). (B) Knockdown of the clathrin heavy chain abolished ligand-induced EpoR internalization. γ2A cells were transfected with siRNAs to the clathrin heavy chain, and surface EpoR was analyzed by flow cytometry. (C) Knockdown of the clathrin heavy chain abolished Epo-induced EpoR degradation. (D) Knockdown efficiency of the clathrin heavy chain shown by immunoblotting. Immunoblotting with actin was shown as a control.
EpoR internalization is through a clathrin-dependent pathway. (A) γ2A cells expressing HA-EpoR with JAK2 or JAK2KD were seeded on glass coverslips. Cell-surface HA-EpoRs were labeled with anti-HA antibodies before Epo induction. Seven minutes after induction, cells were fixed and immunostained with anticlathrin antibodies followed by appropriate fluorescence-conjugated secondary antibodies. Representative confocal images (single section) are presented. Selected areas of colocalization are indicated with arrows (original magnification ×40; Leica TCS SP5). (B) Knockdown of the clathrin heavy chain abolished ligand-induced EpoR internalization. γ2A cells were transfected with siRNAs to the clathrin heavy chain, and surface EpoR was analyzed by flow cytometry. (C) Knockdown of the clathrin heavy chain abolished Epo-induced EpoR degradation. (D) Knockdown efficiency of the clathrin heavy chain shown by immunoblotting. Immunoblotting with actin was shown as a control.
Y429, Y431, and Y479 in the EpoR cytoplasmic domain mediate EpoR internalization
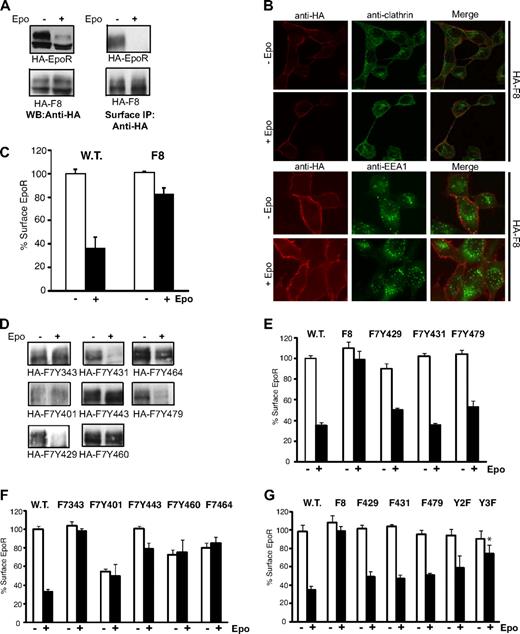
Upon Epo stimulation, JAK2 phosphorylates multiple tyrosines in the EpoR cytoplasmic domain. These phosphotyrosines serve as docking sites for proteins important for signaling. We hypothesized that these phosphorylated tyrosines also recruit proteins critical for receptor internalization. To test this hypothesis, we examined an HA-tagged mutant EpoR with all 8 tyrosines substituted by phenylalanines (HA-F8). In the presence of JAK2, little internalization of HA-F8 was detected and HA-F8 failed to degrade after Epo stimulation (Figure 4A). HA-F8 also did not colocalize with clathrin or EEA1 in the presence of Epo (Figure 4B). Defects in internalization of HA-F8 on Epo induction were also observed in the hematopoietic cell line Ba/F3 (Figure S1). To confirm these results, we examined Epo-induced internalization of HA-EpoR and HA-F8 in TER119− erythroid progenitor cells isolated from murine fetal livers. As shown in Figure 4C, internalization of HA-F8 was dramatically reduced compared with HA-EpoR after Epo stimulation, consistent with previous results. These results indicated that tyrosine residue(s) are important for EpoR internalization and subsequent degradation, possibly by the recruitment of proteins in the endocytic machinery.
Y429, Y431, and Y479 mediate ligand-induced EpoR internalization. (A) Wild-type EpoR, but not F8, is degraded (left) on Epo stimulation. Wild-type EpoR, but not F8, is internalized on Epo stimulation as detected by surface IP (right). (B) F8 does not colocalize with clathrin or EEA1 on stimulation (original magnification ×40; Leica TCS SP5). (C) Epo-induced internalization of HA-F8 is dramatically reduced in TER119− erythroid progenitor cells from murine fetal livers. Surface expression of HA-EpoR or HA-F8 was measured 60 minutes after Epo stimulation. (D) Y429, Y431, or Y479 mediates ligand-induced EpoR internalization. Surface IP was performed on γ2A cells stably expressing HA-EpoR constructs with individual cytosolic tyrosine. (E,F) Y429, Y431, or Y479, but not other tyrosines, is sufficient for ligand-induced EpoR internalization. Ligand-induced receptor internalization was measured by flow cytometry in γ2A cells. (G) Replacing Y429, Y431, or Y479 individually on the wild-type EpoR (F429, F431, and F479) or replacing both Y429 and Y431 (Y2F) did not affect receptor internalization by flow cytometry, but simultaneously mutating all 3 tyrosines (Y3F) significantly reduced Epo-induced receptor internalization. *P = .025 (unpaired t test) versus control.
Y429, Y431, and Y479 mediate ligand-induced EpoR internalization. (A) Wild-type EpoR, but not F8, is degraded (left) on Epo stimulation. Wild-type EpoR, but not F8, is internalized on Epo stimulation as detected by surface IP (right). (B) F8 does not colocalize with clathrin or EEA1 on stimulation (original magnification ×40; Leica TCS SP5). (C) Epo-induced internalization of HA-F8 is dramatically reduced in TER119− erythroid progenitor cells from murine fetal livers. Surface expression of HA-EpoR or HA-F8 was measured 60 minutes after Epo stimulation. (D) Y429, Y431, or Y479 mediates ligand-induced EpoR internalization. Surface IP was performed on γ2A cells stably expressing HA-EpoR constructs with individual cytosolic tyrosine. (E,F) Y429, Y431, or Y479, but not other tyrosines, is sufficient for ligand-induced EpoR internalization. Ligand-induced receptor internalization was measured by flow cytometry in γ2A cells. (G) Replacing Y429, Y431, or Y479 individually on the wild-type EpoR (F429, F431, and F479) or replacing both Y429 and Y431 (Y2F) did not affect receptor internalization by flow cytometry, but simultaneously mutating all 3 tyrosines (Y3F) significantly reduced Epo-induced receptor internalization. *P = .025 (unpaired t test) versus control.
To determine which of the 8 tyrosines are important for EpoR internalization, individual tyrosine residues were added back to the HA-F8 receptor. Results from surface IP (Figure 4D) and flow cytometry analyses (Figure 4E,F) demonstrated that Y429, Y431, and Y479 (as in HA-F7Y429, HA-F7Y431, and HA-F7Y479, respectively) restored EpoR internalization in γ2A cells, whereas Y343, Y401, Y443, Y460, and Y464 did not. To determine whether Y429, Y431, and Y479 are necessary for internalization in a wild-type receptor background, we mutated them individually to phenylalanine. As shown in Figure 4G, mutating any one of the 3 important tyrosine residues did not affect receptor internalization, suggesting that these tyrosines are functionally redundant. Consistent with these results, simultaneously replacing Y429 and Y431 (as in Y2F) affected EpoR internalization, although the effect was not statistically significant. Replacing all 3 tyrosines (as in Y3F) significantly impaired receptor internalization (Figure 4G, P = .025).
p85 is important for ligand-induced EpoR internalization
We investigated whether known binding proteins for Y429, Y431, or Y479 played a role in EpoR internalization. Phosphorylated Y429 binds to SHP16 ; however, SHP1 was not detected in γ2A cells, and SHP1 siRNA had no effect on EpoR internalization (data not shown). Phosphorylated Y429 and Y431 bind SOCS3,22 and phosphorylated Y479 binds to the p85α regulatory subunit of PI3K.3 Knockdown of SOCS3 or p85α by siRNA also did not affect EpoR internalization (Figure 5). Because the residues around Y429 and Y431 resemble the binding consensus sequence for the p85β C-terminal SH2 domain,23 we next tested whether p85β was required for EpoR internalization. Knockdown of p85β alone caused a small but statistically significant decrease in EpoR internalization (Figure 5, P = .001), and simultaneously knocking down p85α and p85β impaired EpoR internalization (Figure 5, P = .001). Therefore, p85α and p85β share redundant roles in EpoR internalization. The residual internalization may be the result of low knockdown efficiency.
Concurrent knockdown of p85α and p85β diminishes receptor internalization. Receptor internalization was detected by flow cytometry in γ2A cells stably expressing EpoR and JAK2 and transfected with 100 nM siRNAs to SOCS3, p85α, p85β, or p85α and p85β together. Scrambled siRNA to GFP was used as a negative control. Immunoblots of each targeted protein demonstrate knockdown efficiency. Representative immunoblotting with actin was shown as a control. *P = .001 (unpaired t test) versus control.
Concurrent knockdown of p85α and p85β diminishes receptor internalization. Receptor internalization was detected by flow cytometry in γ2A cells stably expressing EpoR and JAK2 and transfected with 100 nM siRNAs to SOCS3, p85α, p85β, or p85α and p85β together. Scrambled siRNA to GFP was used as a negative control. Immunoblots of each targeted protein demonstrate knockdown efficiency. Representative immunoblotting with actin was shown as a control. *P = .001 (unpaired t test) versus control.
Characterization of the mechanism of p85-dependent EpoR internalization
Because binding of p85α to the EpoR recruits and activates the catalytic subunit of PI3K, we tested whether PI3K kinase activity played a role in ligand-induced EpoR internalization. Treatment of JAK2 cells with the PI3K inhibitor, wortmannin, inhibited PI3K, as indicated by loss of AKT phosphorylation, but did not impair EpoR internalization (Figure 6A). These results indicate that p85 does not promote EpoR internalization through a PI3K kinase activity-dependent mechanism.
p85α and p85β are important in mediating EpoR internalization. (A) Wortmannin treatment does not affect EpoR internalization. γ2A cells stably expressing HA-EpoR and JAK2 were treated with wortmannin for 2 hours at indicated concentrations followed by Epo stimulation. At 45 minutes after Epo stimulation, surface EpoRs were quantified by flow cytometry. Wortmannin inhibited AKT activation as detected by phospho-AKT antibodies. (B) Dominant-negative forms of p85α and p85β impair EpoR internalization. Epo-induced EpoR internalization was measured by flow cytometry in γ2A cells stably expressing HA-EpoR and JAK2 transiently transfected with vectors expressing the N or C terminal-SH2 domains from p85α and p85β. (1) vector control; (2) p85α N-terminal SH2; (3) p85α C-terminal SH2; (4) p85β N-terminal SH2; (5) p85β C-terminal SH2; (6) p85α and p85β N-terminal SH2s; (7) p85α and p85β C-terminal SH2s. *P = .001 (unpaired t test) versus control. (C) p85β binds EpoR on ligand stimulation. γ2A cells stably expressing HA-EpoR and JAK2 were transiently transfected with vectors expressing full-length p85β. At 48 hours after transfection, cells were starved overnight and treated with Epo for 10 minutes. Cell lysates were subjected to immunoprecipitation by p85β antibodies and immunoblotted with anti-HA antibody for the receptor. Cell lysates were also subjected to immunoblotting with antibodies to active JAK2 (P-JAK2), p85β, and HA. IP indicates immunoprecipitation; and IB, immunoblot.
p85α and p85β are important in mediating EpoR internalization. (A) Wortmannin treatment does not affect EpoR internalization. γ2A cells stably expressing HA-EpoR and JAK2 were treated with wortmannin for 2 hours at indicated concentrations followed by Epo stimulation. At 45 minutes after Epo stimulation, surface EpoRs were quantified by flow cytometry. Wortmannin inhibited AKT activation as detected by phospho-AKT antibodies. (B) Dominant-negative forms of p85α and p85β impair EpoR internalization. Epo-induced EpoR internalization was measured by flow cytometry in γ2A cells stably expressing HA-EpoR and JAK2 transiently transfected with vectors expressing the N or C terminal-SH2 domains from p85α and p85β. (1) vector control; (2) p85α N-terminal SH2; (3) p85α C-terminal SH2; (4) p85β N-terminal SH2; (5) p85β C-terminal SH2; (6) p85α and p85β N-terminal SH2s; (7) p85α and p85β C-terminal SH2s. *P = .001 (unpaired t test) versus control. (C) p85β binds EpoR on ligand stimulation. γ2A cells stably expressing HA-EpoR and JAK2 were transiently transfected with vectors expressing full-length p85β. At 48 hours after transfection, cells were starved overnight and treated with Epo for 10 minutes. Cell lysates were subjected to immunoprecipitation by p85β antibodies and immunoblotted with anti-HA antibody for the receptor. Cell lysates were also subjected to immunoblotting with antibodies to active JAK2 (P-JAK2), p85β, and HA. IP indicates immunoprecipitation; and IB, immunoblot.
We next tested whether p85α and p85β act as adaptors for proteins other than the catalytic subunit of PI3K that are important in receptor internalization. We generated vectors expressing either the N- or the C-terminal SH2 domain from p85α and p85β, respectively. We reasoned that these SH2 domains would interact with signaling partners and phosphotyrosines in the EpoR and function as dominant-negative proteins. γ2A cells stably expressing HA-EpoR and JAK2 were transfected with individual or combinations of these SH2 domains, and Epo-induced EpoR internalization was examined. Interestingly, simultaneous expression of the N-terminal SH2 domains from p85α and p85β significantly decreased EpoR internalization (Figure 6B, P = .001). The N-SH2 domains of p85α and p85β have been shown to bind to the YXXM motif24 found in proteins, such as c-Cbl,25 which plays important roles in clathrin-mediated endocytosis of receptor tyrosine kinases.26 Together, these observations suggest that p85 proteins act as adaptors linking the EpoR to the endocytic machinery.
p85α binds to phosphorylated Y479 in the EpoR cytoplasmic domain on Epo stimulation27 ; however, whether p85β also interacts with the EpoR is not clear. On Epo induction, p85β interacted with wild-type EpoR but not F8, and this interaction is lost when JAK2KD rather than JAK2 was coexpressed (Figure 6C). Therefore, p85β interacts with the EpoR, and this binding likely requires phosphorylated tyrosine(s) in the EpoR cytoplasmic domain. We thus examined the ability for p85β to interact with Y2F or Y3F on induction. Y2F and Y3F activated JAK2 to the same degree by wild-type EpoR or F8 as shown by antibodies specifically recognizing activated JAK2 (P-JAK2) (Figure 6C). Consistent with our results showing that internalization is defective in Y3F but not Y2F (Figure 4G), p85β was able to bind Y2F but not Y3F (Figure 6C). Therefore, Y479 is probably a p85β-binding site. We also tested binding between p85β and EpoR harboring a single tyrosine Y429 or Y431. However, no interaction was detected (data not shown), possibly because both tyrosines are required for optimal p85β binding.
Truncated EpoRs from PFCP patients do not internalize on Epo stimulation
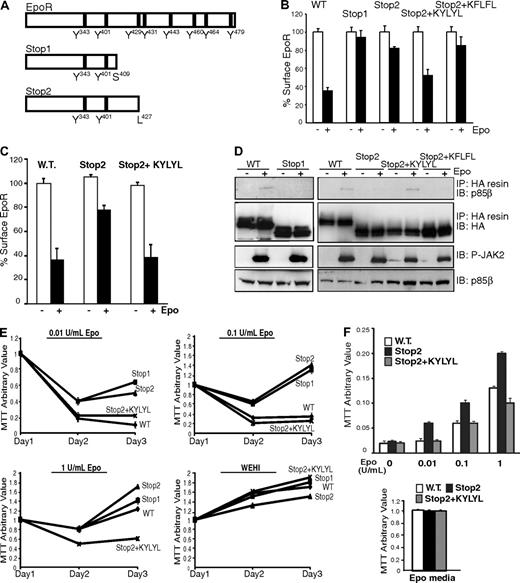
C-terminal deletions of the EpoR are associated with PFCP. These EpoR variants exhibit increased Epo sensitivity and prolonged signal transduction compared with wild-type EpoR.12,13 Interestingly, all truncated PFCP receptors lack Y429, Y431, and Y479. We hypothesized that the deletions associated with PFCP impair EpoR internalization and that this defect contributes to the prolonged signaling from these truncated receptors. To test this hypothesis, we engineered 2 EpoR truncations based on truncations identified in PFCP patients. The first construct, Stop1, lacks residues after S409 and corresponds to the most extensive EpoR truncation identified from PFCP patients.28 The second construct, Stop2, lacks residues after L427 and is analogous to the minimal EpoR truncation identified from PFCP patients (Figure 7A). The ability of γ2A cells stably expressing these receptors to internalize EpoR was tested by our flow cytometry internalization assay. As shown in Figure 7B, Epo-induced internalization was virtually absent with Stop1, whereas only 20% of the receptors internalized with Stop2 compared with wild-type EpoR. Consistent with these results, no degradation was observed for Stop1 and Stop2 on Epo stimulation (data not shown). Interestingly, when KYLYL, the residues surrounding Y429 and Y431, were appended to the C-terminus of Stop2, Epo-stimulated internalization was restored. Furthermore, Stop2+KFLFL, where the 2 Ys (corresponding to Y429 and Y431) in the KYLYL sequence were mutated to F, internalized similarly to Stop2 (Figure 7B). Importantly, similar results were observed in TER119− erythroid progenitor cells. As shown in Figure 7C, Epo-induced internalization of Stop2 was greatly reduced, whereas internalization of Stop2+KYLYL was similar to wild-type EpoR. Together, these results suggest that truncated EpoRs from PFCP patients have defective internalization and that the Y429 and Y431 in KYLYL are essential for Stop2+KYLYL internalization. These results are also consistent with our data showing that Y429 and Y431 are sufficient for EpoR internalization (Figure 4E).
Truncated EpoR mutants fail to bind p85β or internalize on Epo stimulation. (A) Schematic diagram for truncated EpoR mutants used in our studies based on those from PFCP patients. (B) Truncated EpoR mutants did not internalize on Epo treatment by flow cytometry in γ2A cells. (C) Epo-induced internalization was defective for Stop2, but not Stop2-KYLYL, in erythroid progenitor cells. (D) Stop2+KYLYL, but not Stop1, Stop2, or Stop2+KFLFL, interacted with p85β on Epo induction. (E) Truncated EpoR mutants are hypersensitive to Epo in Ba/F3 cells. Ba/F3 cells stably expressing wild-type or truncated EpoR mutants were grown under different Epo concentrations. Cell numbers were measured by MTT assays every 24 hours for 3 days. Cell numbers from cultures in WEHI media as a source of IL-3 were shown as controls. (F) Erythroid progenitor cells expressing Stop2, but not Stop2+KYLYL, are hypersensitive to Epo. Erythroid progenitor cells were transduced with retroviruses expressing wild-type or truncated EpoR mutants. At 24 hours after infection, cells were washed and cultured in media containing different Epo concentration and 2% FBS (top panel), and cell numbers were measured by MTT assays after 24 hours. Cell numbers from cultures in media containing 10% FBS and 2 U/mL Epo (Epo media) were shown as controls (bottom panel). IP indicates immunoprecipitation; IB, immunoblot; P-JAK2, phosphorylated active JAK2.
Truncated EpoR mutants fail to bind p85β or internalize on Epo stimulation. (A) Schematic diagram for truncated EpoR mutants used in our studies based on those from PFCP patients. (B) Truncated EpoR mutants did not internalize on Epo treatment by flow cytometry in γ2A cells. (C) Epo-induced internalization was defective for Stop2, but not Stop2-KYLYL, in erythroid progenitor cells. (D) Stop2+KYLYL, but not Stop1, Stop2, or Stop2+KFLFL, interacted with p85β on Epo induction. (E) Truncated EpoR mutants are hypersensitive to Epo in Ba/F3 cells. Ba/F3 cells stably expressing wild-type or truncated EpoR mutants were grown under different Epo concentrations. Cell numbers were measured by MTT assays every 24 hours for 3 days. Cell numbers from cultures in WEHI media as a source of IL-3 were shown as controls. (F) Erythroid progenitor cells expressing Stop2, but not Stop2+KYLYL, are hypersensitive to Epo. Erythroid progenitor cells were transduced with retroviruses expressing wild-type or truncated EpoR mutants. At 24 hours after infection, cells were washed and cultured in media containing different Epo concentration and 2% FBS (top panel), and cell numbers were measured by MTT assays after 24 hours. Cell numbers from cultures in media containing 10% FBS and 2 U/mL Epo (Epo media) were shown as controls (bottom panel). IP indicates immunoprecipitation; IB, immunoblot; P-JAK2, phosphorylated active JAK2.
Because our results suggest a role of p85β in EpoR internalization, we examined the interaction between p85β and truncated EpoRs based on PFCP patients. p85β did not interact with Stop1 or Stop2 (Figure 7D). Importantly, we detected Epo-inducible interaction between p85β and Stop2+KYLYL, which contains Y429 and Y431, whereas no interaction was detected between p85β and Stop2+KFLFL in which Y429 and Y431 are mutated (Figure 7D). Therefore, p85β probably binds via Y429 and/or Y431 in Stop2+KYLYL. Together with data from Figures 5 and 6, our results indicate that p85β, together with p85α, plays an important role in ligand-induced EpoR internalization via a PI3K kinase activity-independent mechanism. It does so by binding to Y429 and/or Y431, as well as Y479, in the EpoR cytoplasmic domain on stimulation, and acts as an adaptor to recruit proteins in the endocytic machinery.
Previous reports demonstrated that truncated EpoRs from PFCP patients display an increased sensitivity to Epo compared with wild-type EpoR.29 We first examined our EpoR truncations for Epo sensitivity in the interleukin-3 (IL-3)–dependent hematopoietic Ba/F3 cells. Ba/F3 cells stably expressing the different truncated EpoRs at similar levels were grown in the absence of IL-3 but with various concentrations of Epo, and their mitogenic activity was measured. As shown in Figure 7E, cells expressing Stop1 and Stop2 grew normally in Epo at 1 U/mL. However, cells expressing Stop1 and Stop2 were able to grow in 0.1 U/mL or 0.01 U/mL of Epo, whereas cells expressing wild-type receptor did not (Figure 7E). Importantly, cells expressing Stop2+KYLYL, which internalized after Epo stimulation similar to wild-type EpoR, showed similar Epo sensitivity compared with wild-type EpoR. As controls, cells expressing all constructs grew similarly in WEHI IL-3–conditioned media (Figure 7E). At 1 U/mL Epo, the mitogenic activity of Stop2+KYLYL was lower than wild-type EpoR (Figure 7E). Because Stop2+KYLYL lacks 4 of the 8 tyrosines in the EpoR cytoplasmic domain important for signaling, it may not be able to recapitulate wild-type EpoR signaling completely. We also examined Epo sensitivity of Stop2 and Stop2+KYLYL in TER119− erythroid progenitor cells. Erythroid progenitors stably expressing Stop2, Stop2+KYLYL, or wild-type EpoR at comparable levels grew similarly in media containing 10% FBS and 2 U/mL Epo (Figure 7F bottom panel). However, in media containing 2% FBS, cells expressing Stop2 had higher mitogenic activities in 3 different Epo concentrations, whereas cells expressing Stop2+KYLYL grew similarly to those expressing wild-type EpoR (Figure 7F top panel). Cells expressing Stop2, Stop2+KYLYL, or wild-type EpoR did not grow in media containing 2% FBS but without Epo (Figure 7F top panel). These results suggest that, on Epo stimulation, JAK2-dependent phosphorylation of Y429, Y431, and Y479 results in the recruitment of p85, which promotes EpoR internalization by acting as an adaptor to engage the endocytic machinery. Moreover, truncated EpoRs in PFCP patients lacking these tyrosines have impaired internalization, which may contribute to their Epo hypersensitivity.
Discussion
Receptor endocytosis is a major mechanism whereby cells control the magnitude and duration of signaling induced by extracellular ligands. For the EpoR, this process also controls cellular sensitivity to Epo15 and is the primary means that regulates circulating Epo concentrations.16 Our results demonstrate that cell-surface EpoR is internalized via clathrin-mediated endocytosis, and both JAK2 kinase activity and EpoR cytoplasmic tyrosines are important for this process. We further show that phosphorylated Y429, Y431, and Y479 in the EpoR cytoplasmic domain share redundant functions and individually are sufficient to mediate ligand-dependent EpoR internalization. These phosphotyrosines bind p85 on Epo stimulation and may recruit proteins in the endocytic machinery. In addition, mutated EpoRs from PFCP patients lacking the 3 important tyrosines do not bind p85 and do not internalize on stimulation, supporting a model that this defect may contribute to their hypersensitivity to Epo and prolonged signaling.
We show that Epo induces EpoR internalization via clathrin-mediated endocytosis, similar to other cytokine receptors, such as the prolactin receptor, thrombopoietin receptor, growth hormone receptor, and gp130.30-33 Importantly, Epo-induced EpoR internalization requires both JAK2 kinase activity and the tyrosine residues of the EpoR cytoplasmic domain. These results are consistent with studies demonstrating that ligand-stimulated internalization of the prolactin receptor34 and the thrombopoietin receptor31 also depends on associated JAK2 kinase activities. Moreover, as the EpoR/JAK2 complex functions as a unit that is equivalent to a receptor tyrosine kinase, these results are consistent with experiments showing that kinase activity is necessary for maximal rate of internalization and down-regulation of receptor tyrosine kinases.35-37
Our results differ from 2 previous reports. One study showed that a mitogenically inactive EpoR mutant EpoR(W282R) can still internalize radiolabeled Epo and that a tyrosine-null EpoR still internalizes Epo, albeit less efficiently.10 Because W282 is part of the JAK2 interaction site and changing it to Ala abolishes JAK2-dependent EpoR surface expression,18 the mutated receptors that reached the plasma membrane in that study are not likely to have formed a normal complex with JAK2. The other study showed that inhibiting JAK2 kinase activity with the AG490 inhibitor impaired internalization of radiolabeled Epo, and monensin treatment partially restored internalization.11 It was concluded that EpoR was internalized when JAK2 activity was blocked but that internalized receptors recycled to the plasma membrane. In addition, proteasomal degradation removes the portion of the EpoR cytoplasmic domain containing the tyrosine residues before the receptor is internalized.11 We did not observe any recycling of EpoR in our JAK2KD cells (data not shown). We reason that the residual JAK2 activity after AG490 treatment may be sufficient for receptor internalization. In addition, monensin is known to have pleiotropic effects on vesicular trafficking. We did observe small but consistent internalization of F8 (Figures 4C,E, S1); and at higher Epo concentrations (60 U/mL and 90 U/mL) than used herein (30 U/mL), this internalization is more pronounced (Figure S2). In contrast, no internalization was detected in the presence of JAK2KD under all Epo concentrations tested (Figure S2). Therefore, there seems to exist an internalization mechanism that depends on JAK2 but not cytoplasmic EpoR tyrosines, and this mechanism may involve proteasomal removal of part of the EpoR cytoplasmic domain. Our preliminary results suggested that this mechanism also involves clathrin. As the endocytosis of EGF receptors (EGFRs) varies with EGF concentrations,38 the contribution of different internalization mechanisms of the EpoR may differ depending on Epo concentrations.
Our characterization of the mechanism of EpoR internalization suggests a novel function for p85 that is independent of PI3K kinase activities. On receptor activation, p85α binds to Y479,3 whereas p85β binds to Y429/Y431 and Y479 in the EpoR cytoplasmic domain (Figures 6, 7). p85 may act as a scaffold that recruits proteins of the endocytic machinery. p85α and p85β interact directly,25 or indirectly through Grb2,39-41 with the ubiquitin ligase c-Cbl, which recruits endophilin and AP-2 (through CIN85) of the endocytic machinery.42 Both c-Cbl and Grb2 have previously been shown to be important for recruitment of EGFRs into clathrin-coated pits.41,43 p85 may also bind dynamin, a protein that plays important roles in clathrin-mediated endocytosis,44,45 or interact through the Grb2-SOS-Ras linkage to activate Rab5,46 a protein implicated in the formation of clathrin-coated vesicles and fusion of these vesicles to early endosomes.47,48 Alternatively, p85 may exert GAP activities toward GTPases critical for clathrin-mediated endocytosis, thus regulating their function. This hypothesis is based on the observation that p85α has GAP activity for Rab5, and for Cdc42, which was implicated in vesicle movement.49 Although no GAP activities have yet been demonstrated for p85β, p85β shares the GAP domain of p85α.
Our results add to the emerging concept that p85 has functions besides regulating PI3K kinase activity.50-54 For example, cytokinesis defects observed in p85α-deficient cells are restored by expression of a p85α mutant that does not bind the PI3K catalytic subunit,51 and that a p85α mutant with defective GAP activities caused cellular transformation via a kinase-independent mechanism.54 PI3K is activated by most cytokine receptors, and a common step in the activation involves the recruitment and interaction of p85 with components of the cytokine receptor complex. Whether p85 also contributes to ligand-dependent internalization of other cytokine receptors is currently being investigated.
Y429, Y431, and Y479 in the EpoR cytoplasmic domain are individually sufficient to support Epo-stimulated EpoR internalization. Mutations of all these tyrosines to phenylalanines or deletions that remove these tyrosines, such as those found in PFCP patients, prevent Epo-induced EpoR internalization and degradation. Consistent with a role of p85β in EpoR internalization, both Stop1 and Stop2 failed to interact with p85β. Importantly, fusion of KYLYL but not KFLFL, residues encompassing Y429 and Y431, to Stop2 restored both p85β binding and ligand-induced internalization. Moreover, the hypersensitivity to Epo of both hematopoietic Ba/F3 cells and erythroid progenitor cells expressing Stop2 was reversed when KYLYL was fused to Stop2. Therefore, the PFCP EpoR truncations not only prevent recruitment of SHP-1 that inactivates JAK2 but also impair endocytosis of EpoR. These results support a model that both defects prolong the duration of signaling. Consistent with the notion that defects in internalization prolong signaling, mutations in the granulocyte colony-stimulating factor receptor that inhibit ligand-induced internalization lead to prolonged receptor activation and acute myeloid leukemia.55,56 In addition, oncogenic forms of human EGFR are less efficiently internalized.57 Understanding the precise mechanism responsible for attenuating receptor signaling will provide insight into PCFP and other diseases that are caused by excessive receptor signaling.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Alethia Villasenor and Diana Chong for generously providing CD1 embryos and sharing advice on timed-breeding of mice, Drs Bill Snell, Joachim Seemann, and Peter Michaely for helpful discussions, and Hongyun Dong for technical assistance.
This work was supported by NIH grant R01 HL089966.
National Institutes of Health
Authorship
Contribution: R.S. and L.J.-s.H. designed and performed research, analyzed data, and wrote the paper; and O.C. provided murine embryos and helped write the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lily Jun-shen Huang, Department of Cell Biology, J2.116, University of Texas Southwestern Medical Center, 5323 Harry Hines Blvd, Dallas, TX 75390-9039; e-mail: lily.huang@utsouthwestern.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal