To the editor:
Graft-versus-host disease (GVHD) after transfusion of nonirradiated blood products or solid tumor transplantation is a rare but lethal complication associated with mortality of 80% to 90%1-3 and occurs when immunocompetent donor T cells from the graft recognize disparate alloantigens of host cells. This process occurs primarily in immunocompromised hosts. Symptoms are typical of GVHD affecting skin, gut, and liver. Profound pancytopenia due to graft-versus-hematopoiesis effect may occur, described in animal models of transfusion-associated GVHD.4 Therapies to reverse this process are almost uniformly unsuccessful.3 Case reports and small series have documented response with agents such as basiliximab, daclizumab, antilymphocyte antibodies, and granulocyte colony-stimulating factor (G-CSF), though response to these agents is inconsistent.3,5,6
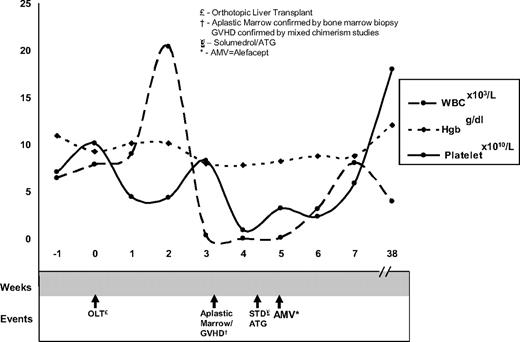
Our patient was a 60-year-old man with cirrhosis status postorthotopic liver transplantation (OLT). At day 20 after OLT the patient reported abdominal pain and emesis and had a fever. His white blood cell count (WBC) was 11.3 k/μL (ANC of 8.89 k/μL), hematocrit was 31%, and platelet count was 337 k/μL. Infectious workup and empiric antibiotics were started. At day 27 the patient developed a generalized, dusky morbilliform rash with reticulated morphology (biopsies showed cytotoxic dermatitis compatible with GVHD grade 2-3) and odynophagia (laryngoscopy revealed mucositis). His WBC nadired to 0.02 k/μL on day 39 (Figure 1). A bone marrow biopsy revealed marked hypocellularity (< 10%) with residual hematopoiesis showing apoptotic bodies, consistent with GVHD. To secure the diagnosis of alloantigen-induced marrow aplasia, 4 specimens were submitted for single tandem repeat (STR) analysis7 : (1) a buccal swab (recipient DNA); (2) donor DNA–Allogen labs; (3) peripheral blood; and (4) T-cell enriched fraction from peripheral blood. Peripheral blood DNA showed mixed chimerism (11% donor DNA), and the T cell–enriched fraction (81% donor DNA) confirmed the diagnosis of GVHD. The patient remained profoundly pancytopenic despite high-dose steroids, filgrastim support, and rabbit antithymocyte globulin.3,8 Because of the patient's deteriorating condition and lack of response to other therapies, his case was discussed in the transplantation peer review group. Off-label use of the immunosuppressant alefacept was recommended. Rationale and potential side effects were discussed with the patient, who gave consent and subsequently received 30 mg alefacept, followed by 3 additional doses of 30 mg every 3 days. After the initial alefacept dose, his blood counts improved over 9 days to a WBC of 7.49 k/μL and platelet count of 31 k/μL. His counts remain stable 13 months later. It is provocative to note the rapidity and durability of his response after alefacept dosing.
Timeline of count nadir and count recovery with respect to administration of immunosuppressive agents.
Timeline of count nadir and count recovery with respect to administration of immunosuppressive agents.
Alefacept is a novel dimeric fusion protein produced by recombinant DNA technology in a Chinese hamster ovary. It comprises the extracellular CD2-binding portion of the human leukocyte function antigen-3 (LFA-3) linked to the Fc portion of human-IgG1, and selectively targets memory T cells. It is approved for the treatment of psoriasis,9 and has been studied in patients with steroid resistant/dependent GVHD after HSCT (hematopoietic stem cell transplantation) with some success.10 Given the dismal responses seen with conventional immunosuppressive treatments for GVHD after transfusion or solid organ transplantation,1,3 alefacept may offer a reasonable treatment alternative in a setting cwhere outcomes have generally been fatal. Its use may also provide a clinical model for bone marrow failure states.
Authorship
Acknowledgment: The authors thank Dr Ramon Tiu, who helped with the graphic illustration.
Contribution: All authors contributed to the work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Christy J. Stotler, Cleveland Clinic Foundation, 9500 Euclid Ave, Desk R35, Cleveland, OH 44195; e-mail: stotlec@ccf.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal