Because the size of the thymus in children is much larger than in adults, thymus output is widely believed to be responsible for the development of the peripheral T-cell pool during childhood. This idea is strengthened by the associations between age and the rate of T-cell reconstitution in lymphopenic patients,1 and between thymus involution and decreasing naive T-cell numbers per microliter of blood. These arguments are indirect however, and do not take into account any changes in blood volume that occur upon growth of the body during childhood.2 We have previously shown that—despite the gradual decline in naive T-cell numbers per microliter of blood—total body naive T-cell numbers in fact increase in early life.3
In this issue of Blood, Bains et al4 present a mathematical model that integrates various experimental findings on naive T cells and T-cell receptor excision circle (TREC) dynamics 5 as well as thymus output6 in young humans up to 20 years of age. They show that at all ages—even in the very young, when thymus output is still at its maximum—the contribution of peripheral T-cell proliferation to the establishment of the naive T-cell pool prevails over thymus production. These findings are in line with the observation that in young healthy people, naive T-cell numbers increase while total TREC numbers do not, implying that peripheral T-cell proliferation contributes considerably to the establishment of the naive T-cell pool in early childhood.3 Combining the decreasing output of the thymus with the increase of total body naive CD4+ T-cell numbers during childhood, one would perhaps expect the rate of peripheral T-cell proliferation to increase with age. The study by Bains et al shows, however, that the rate of peripheral T-cell proliferation decreases when thymus output declines, concomitant with increasing naive T-lymphocyte residence times.
Despite the undisputed role of thymus involution during aging, the contribution of peripheral T-cell proliferation to the establishment of the naive T-cell pool is thus much larger than previously thought, especially in the very young. Bains et al thereby demonstrate the risk of indirect intuitive reasoning and the strength of collective analysis of experimental data with the help of mathematical modeling.7 The strength of their modeling approach is that it makes no a priori assumptions on how lymphocyte proliferation and death rates are affected by age or by total lymphocyte numbers. Provided that the average TREC content of naive T cells stays constant between birth and the age of 20, no other conclusions could be drawn from the data. The amount of naive T-cell TREC content data are, however, very limited, and TREC measurements are notorious for interassay variation. Therefore, this study also calls for more extensive measurement of TREC changes in naive T cells during childhood and warrants more direct measures of T-cell death rates in children, for example, based on stable-isotope labeling.
The large contribution of peripheral T-cell proliferation to the establishment of the naive T-cell pool in children goes against current beliefs, which may in part stem from improper extrapolation of insights obtained from young thymectomized mice. Even though lymphocyte dynamics in young and old mice are widely believed to be comparable to those in young and old humans, there is little data showing that such extrapolations can indeed safely be made. Similar comparative studies in mice of different ages and in humans older than 20 years of age should point out whether the processes responsible for the establishment and maintenance of the naive T-cell pool in mice and men are indeed comparable.
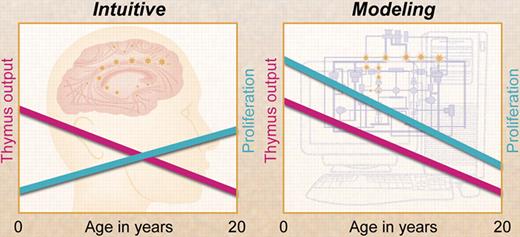
Contrary to intuition, mathematical modeling by Bains and colleagues has shown that T-cell proliferation declines with age and, at any age during childhood, contributes more to the naive T-cell pool than thymus output. Professional illustration by A. Y. Chen.
Contrary to intuition, mathematical modeling by Bains and colleagues has shown that T-cell proliferation declines with age and, at any age during childhood, contributes more to the naive T-cell pool than thymus output. Professional illustration by A. Y. Chen.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■
Acknowledgment:
J.B. was financially supported by The Netherlands Organisation for Scientific Research (NWO grant 836.07.002).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal