Multiple innate signals regulate the genesis of effector and memory CD8+ T cells. In this study, we demonstrate that the innate cytokines interleukin (IL)–12 and interferon (IFN)–α/β regulate distinct aspects of effector and memory human CD8+ T-cell differentiation. IL-12 exclusively promoted the development of IFN-γ– and tumor necrosis factor (TNF)–α–secreting T effector memory (TEM) cells, whereas IFN-α drove the development of T central memory (TCM) cells. The development of TEM and TCM was linked to cell division. In rapidly dividing cells, IL-12 programmed TEM through induction of the IL-12 receptor β2. In contrast, IFN-α regulated TCM development by slowing the progression of cell division in a subpopulation of cells that selectively expressed elevated IFN-α/β receptor-2. The strength of signal delivered through T-cell receptor (TCR) engagement regulated the responsiveness of cells to IL-12 and IFN-α. In the presence of both IL-12 and IFN-α, these cytokine signals were amplified as the strength of the TCR signal was increased, promoting the simultaneous development of both TCM and TEM. Together, our results support a novel model in which IL-12 and IFN-α act in a nonredundant manner to regulate the colinear generation of both effector and memory cells.
Introduction
CD8+ T cells are critical mediators of adaptive inflammatory responses to intracellular pathogens. They require a series of signals for efficient expansion and acquisition of effector functions, such as cytokine secretion and lytic activity. These signals are delivered by professional antigen-presenting cells (APC) and include antigen recognition (signal 1), costimulatory activation (signal 2), and signaling provided by innate inflammatory cytokines (signal 3).1 Whereas signals 1 and 2 prime naive CD8+ T cells and initiate cell division, signal 3 cytokines program effector functions and ensure clonal survival. A variety of cytokines have the potential to act as signal 3 in CD4+ and CD8+ T cells,2,–4 and interleukin (IL)–12 and interferon (IFN)–α/β, in particular, promote efficient induction of innate immunity as well as the development of adaptive type 1 responses to intracellular infection.5,6 Thus, IL-12 and IFN-α/β may represent the predominant signal 3 during intracellular infection.
Whereas IL-12 regulates T helper (Th) 1 development in CD4+ T cells, early reports suggested that the induction of IFN-γ secretion and lytic activity in CD8+ T cells was independent of IL-12, signal transducer and activator of transcription (STAT) 4, and T-box expressed in T cells (T-bet).7,–9 However, recent studies by Schmidt and Mescher found that in vitro priming with IL-12 induced high and sustained secretion of IFN-γ and markedly enhanced lytic activity in murine CD8+ T cells.10 Furthermore, these effects were dependent on STAT4, indicating that IL-12 signaling provides a necessary third signal for the regulation of CD8+ T-cell development.3,11,12 More recent studies have indicated that IFN-α/β can act in a manner similar to IL-12 to provide signal 3 and promote the induction of cytokine secretion, cytolytic activity, and clonal expansion in murine CD8+ T cells.3,13 Collectively, these studies suggested that IL-12 and IFN-α can act as redundant signals to promote the development of effector responses in murine CD8+ T cells.
In addition to enhancing effector cell development, IFN-α/β was implicated in the generation of memory CD8+ T cells in vivo. In these studies, IFN-α/β receptor (IFNAR)–deficient, T-cell receptor (TCR)–transgenic (P14) CD8+ T cells failed to expand and generate memory populations in response to in vivo lymphocytic choriomeningitis virus infection despite their ability to proliferate efficiently in vitro.13 Alternatively, IL-12−/− mice displayed defective primary effector responses, whereas development of T central memory (TCM) cells was markedly enhanced compared with wild type, indicating that IL-12 signaling suppresses TCM development.14,–16 Considering that IFN-α/β has been implicated in effector and memory cell development, it is unclear how this signal regulates both events and whether any of these activities operate in human CD8+ T cells. Furthermore, it is not clear how IL-12 and IFN-α/β signals are integrated to balance effector and memory cell development, as many intracellular pathogens elicit the secretion of both innate cytokines from professional APCs. In this study, we demonstrate a novel pathway for the variegated programming of human CD8+ T-cell effector and memory development by IL-12 and IFN-α. In this study, we show that IL-12 and IFN-α are not redundant signals in the development of human CD8+ T-cell responses, and instead act in concert in the context of signals 1 and 2 to balance the development of effector and memory cell populations.
Methods
Human subjects
Peripheral blood was collected by venipuncture from healthy adult donors with approval of the Internal Review Board of the University of Texas Southwestern Medical Center. Informed consent was obtained from each donor in accordance with guidelines established by the Internal Review Board and the Declaration of Helsinki.
Purification and in vitro cultures of human CD8+ T cells
CD8+CD45RA+ cells were purified by flow cytometric sorting or isolated by magnetic bead enrichment. Purified CD8+CD45RA+ cells were cultured at 0.5-1 × 106 cells/mL on anti-CD3/anti-CD28–coated plates in complete Iscove modified Dulbecco medium (cIMDM) with IL-2 (50 U/mL) with cytokines as follows: neutralized (anti–IL-4, anti–IL-12, anti-IFNAR2, and anti–IFN-γ), IL-12 (recombinant human [rh]IL-12 [10 ng/mL], anti–IL-4, anti-IFNAR2, and anti–IFN-γ), IFN-α (rhIFN-α(A) [1000 U/mL], anti–IL-4, anti–IL-12, and anti–IFN-γ), or IL-12 plus IFN-α (rhIL-12, rhIFN-α(A), anti–IL-4, and anti–IFN-γ). On day 3, cells were either harvested for analysis or split 1:10 in fresh media supplemented with 50 U/mL rhIL-2 and cultured to day 7.
Flow cytometric analysis
For intracellular staining, cells were activated for 4 hours with phorbol 12-myristate 13-acetate (PMA; 80 ng/mL) and ionomycin (1 μM) in the presence of brefeldin A (1 μM) or left unstimulated before fixation, permeabilization, and staining. For T-bet detection, cells were stained with unconjugated rabbit anti–human T-bet (Santa Cruz Biotechnology, Santa Cruz, CA), followed by an anti–rabbit Ig-biotin and either a streptavidin conjugate to peridinin chlorophyll protein (PerCP) or Q-dot655 (Invitrogen, Carlsbad, CA). For analysis of cell division, cells were resuspended at 107 cells/mL in phosphate-buffered saline and treated with 1.25 μM carboxyfluorescein diacetate succinimidyl ester (CFSE; Sigma-Aldrich, St Louis, MO) or 12 μg/mL Pacific Blue succinimidyl ester (PBSE; Invitrogen Life Technologies) for 10 minutes at room temperature. Cells were then activated by culturing on anti-CD3/anti-CD28–coated plates, and cell division was assessed by CFSE/PBSE dilution at either day 3 or day 5 postactivation. All data were collected on an LSRII flow cytometer (BD Biosciences, San Jose, CA) and analyzed using FlowJo software (TreeStar, Ashland, OR).
Intracellular STAT staining
Purified CD8+CD45RA+ T cells were polarized under IL-12 plus IFN-α conditions for 6 days to generate polarized TCM and T effector memory (TEM) subpopulations. Cells were either left untreated or treated with IL-12 (10 ng/mL) or IFN-α (1000 U/mL) for 30 minutes. Detection of tyrosine-phosphorylated STAT1 and STAT4 was performed by intracellular staining, as described.17 Cells were stained with unconjugated rabbit polyclonal antibodies to STAT1 (clone SC-346), STAT-4 (clone SC-486; Santa Cruz Biotechnology), or antiphosphotyrosine STAT1 (Upstate Biotechnology, Lake Placid, NY) or antiphosphotyrosine STAT4 (Zymed Laboratories, San Francisco, CA). A goat anti–rabbit Ig-biotin (Jackson ImmunoResearch Laboratories, West Grove, PA) was used for secondary detection, followed by streptavidin-PerCP (BD Biosciences).
Cytolytic assays
Day 7 polarized cells were rested overnight at 106 cells/mL in the absence of IL-2. THP-1 target cells were labeled by culturing in the presence of 150 μCi of 51Cr Na2[51Cr]O4 for 90 minutes. THP-1 cells were left untreated or coated with anti–human CD3 (OKT3) at 1.5 μg/mL to provide antigen receptor–dependent lysis. THP-1 targets were incubated with polarized CD8+ CD45RA+ T cells at various effector:target (E:T) ratios for 4 hours at 37°C, and cytotoxic T lymphocyte (CTL) activity was assessed by measuring release of 51Cr by scintillation counting.
Live cell chemokine receptor sorting
CD8+ CD45RA+ sorted cells were polarized for 7 days and rested overnight in the absence of IL-2. Cells were labeled with either CFSE or PBSE and stained with antibodies to human C-C chemokine receptor (CCR) 7 and CXC chemokine receptor (CXCR) 3. Stained cells were sorted using a FACSAria (BD Biosciences) for either CCR7high or CXCR3high to less than 90% purity. Sorted cells were rested overnight and subjected to redirected lysis or were reactivated on anti-CD3–coated plates. Reactivated cells were analyzed for chemokine receptor expression, or lytic activity was assessed by redirected lysis assays.
Results
IL-12, but not IFN-α, regulates effector CD8+ T-cell development
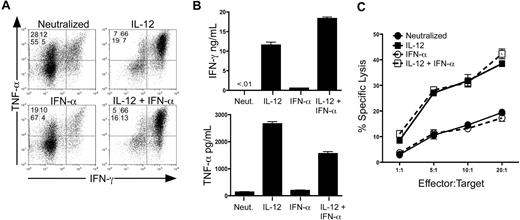
We assessed the development of effector cytokine secretion and lytic activity in purified human CD8+/CD45RA+ T cells in response to IL-12 and IFN-α. To strictly control specific cytokine signals, cells were activated with anti-CD3/anti-CD28 in the absence or presence of cytokines or anti-cytokine antibodies. As previously reported, IL-12 markedly induced the secretion of IFN-γ and TNF-α compared with neutralizing conditions (Figure 1A,B). In contrast, treatment with IFN-α alone was insufficient to induce either expression or secretion of IFN-γ or TNF-α to levels above the neutralized control. Importantly, IFN-α did not inhibit the ability of IL-12 to induce cytokine expression and secretion (Figure 1A,B), indicating that IL-12 drove effector cytokine expression independently of IFN-α signaling.
IL-12, but not IFN-α, is sufficient to program human CD8+ T-cell effector functions. (A) Intracellular expression of human IFN-γ and TNF-α from day 7 in vitro polarized human CD8+ T cells. Rested cells were reactivated for 4 hours with PMA and ionomycin in the presence of brefeldin A, and IFN-γ and TNF-α were assessed by intracellular stain and flow cytometric analysis. Data are gated on live, CD8+ cells. (B) Day 7 polarized cells were left unstimulated or stimulated with anti-CD3 for 24 hours, and supernatants were harvested for enzyme-linked immunosorbent assay. Error bars represent the SD of triplicate determinations of each condition. (C) Characterization of CTL activity by 51Cr release assay. Day 7 polarized CD8+ T cells were incubated for 4 hours with 51Cr-labeled THP-1 cells (target) at the E:T ratios shown. CTL activity was assessed by quantification of 51Cr released into the supernatant by β emission. These experiments were performed with 5 different healthy donors with similar results.
IL-12, but not IFN-α, is sufficient to program human CD8+ T-cell effector functions. (A) Intracellular expression of human IFN-γ and TNF-α from day 7 in vitro polarized human CD8+ T cells. Rested cells were reactivated for 4 hours with PMA and ionomycin in the presence of brefeldin A, and IFN-γ and TNF-α were assessed by intracellular stain and flow cytometric analysis. Data are gated on live, CD8+ cells. (B) Day 7 polarized cells were left unstimulated or stimulated with anti-CD3 for 24 hours, and supernatants were harvested for enzyme-linked immunosorbent assay. Error bars represent the SD of triplicate determinations of each condition. (C) Characterization of CTL activity by 51Cr release assay. Day 7 polarized CD8+ T cells were incubated for 4 hours with 51Cr-labeled THP-1 cells (target) at the E:T ratios shown. CTL activity was assessed by quantification of 51Cr released into the supernatant by β emission. These experiments were performed with 5 different healthy donors with similar results.
We next measured expression of the cytolytic effector molecules perforin and granzyme B. We observed induction of these molecules by both IL-12 and IFN-α; however, the magnitude and pattern by which these cytokines affected this induction were variable among the donors tested (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Therefore, to address the precise role of IL-12 and IFN-α on functional cytotoxicity, we assessed the ability of cytokine-polarized cells to directly kill target cells in a redirected lysis assay. Unlike expression of perforin and granzyme B, we observed that IL-12, but not IFN-α, promoted strong lytic activity compared with cells activated under neutralizing conditions consistently in all donors (Figure 1C). Although not enhanced by IFN-α alone, IL-12–mediated lytic activity was not inhibited by the presence of IFN-α during priming. The lytic activity observed by these cells was completely inhibited by concanamycin A, demonstrating an exclusive role for perforin and granzyme as opposed to Fas/Fas ligand-mediated killing in these assays18,19 (Figure S2). These data demonstrate that IL-12, but not IFN-α, is sufficient to program both effector cytokine secretion and perforin-mediated CTL activity in human CD8+ T cells.
IFN-α drives the development of human CD8+ TCM cells
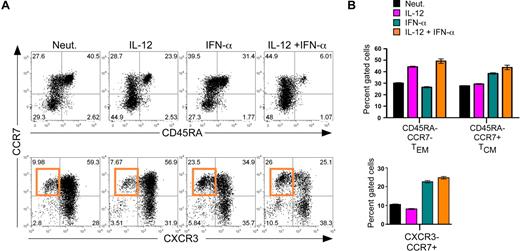
Considering recent studies in mice suggesting that IFN-α could modulate primary expansion and subsequent TCM development,20 we next assessed the ability of IL-12 and IFN-α to influence the expansion of human CD8+ TEM and TCM cells. TEM and TCM are characterized by low expression of CD45RA and differential expression of CCR7 such that TEM are CCR7low and TCM are CCR7high, whereas naive cells are CD45RAhigh/CCR7high.21,22 As expected, IL-12 enhanced a subpopulation of CCR7low/CD45RAlow cells that match the proposed TEM phenotype (Figure 2A,B top panels). Interestingly, IFN-α markedly enhanced the percentage of CCR7high/CD45RAlow TCM cells, and the induction of TCM development by IFN-α was not inhibited in the presence of IL-12, suggesting a dominant role for IFN-α in regulating TCM differentiation. We next assessed 2 differentially expressed chemokine receptors, as follows: CXCR3, which allows for traffic to the periphery and is associated with effector phenotypes,23,–25 and CCR7, which allows for efficient trafficking to lymph nodes and is associated with the TCM phenotype.26,27 Consistent with the induction of CCR7high/CD45RAlow TCM cells, we found that IFN-α promoted the development of CXCR3low/CCR7high populations either in the presence or absence of IL-12 (Figure 2A,B bottom panels). These cells are composed of a subset of the CCR7high/CD45RAlow cells, and in agreement with previous studies,24 we propose that the CCR7high/CXCR3low subset represents a more precisely defined TCM population. Together, these analyses reveal that IL-12 acts independently of IFN-α to program the development of TEM, whereas signaling via IFN-α promotes the development of TCM phenotypes.
Regulation of human CD8+ TCM development by IFN-α. Day 7 cytokine-polarized cells were stained with a panel of anti–human monoclonal antibodies, including CCR7, CD45RA, and CXCR3, to assess memory and effector phenotypes. (A) Analysis of surface markers CCR7 and CD45RA (top panel) and CCR7 and CXCR3 (bottom panel). The induction of CCR7high/CXCR3low cells by IFN-α is indicated by the orange gate. (B) Quantification of human effector and memory profile (top) and chemokine receptor profile (bottom) regulated by IL-12 and IFN-α. Black indicates neutralized; magenta, IL-12; teal, IFN-α; and orange, IL-12 + IFN-α. Error bars represent the SD of triplicate determinations of each condition. These experiments were performed with 7 different healthy donors with similar results.
Regulation of human CD8+ TCM development by IFN-α. Day 7 cytokine-polarized cells were stained with a panel of anti–human monoclonal antibodies, including CCR7, CD45RA, and CXCR3, to assess memory and effector phenotypes. (A) Analysis of surface markers CCR7 and CD45RA (top panel) and CCR7 and CXCR3 (bottom panel). The induction of CCR7high/CXCR3low cells by IFN-α is indicated by the orange gate. (B) Quantification of human effector and memory profile (top) and chemokine receptor profile (bottom) regulated by IL-12 and IFN-α. Black indicates neutralized; magenta, IL-12; teal, IFN-α; and orange, IL-12 + IFN-α. Error bars represent the SD of triplicate determinations of each condition. These experiments were performed with 7 different healthy donors with similar results.
IFN-α–driven TCM cells display functional memory activities
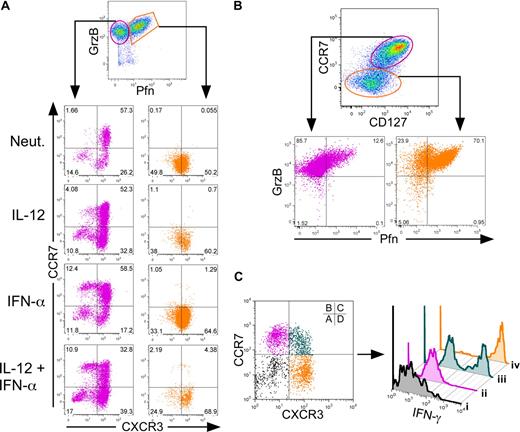
To determine whether the in vitro development of TCM and TEM paralleled their known functional roles, we examined effector molecule expression as a function of TCM and TEM cell surface phenotypes. Activation of naive CD8+ T cells led to the development of 2 distinct populations of cells, as follows: those that expressed either granzyme B alone, or both granzyme B and perforin (Figure 3A). We assessed expression of CXCR3 and CCR7 within these 2 populations. The majority of cells expressing both perforin and granzyme B (Figure 3A right panels, orange gate) uniformly expressed high levels of CXCR3 and low CCR7 regardless of cytokine treatment. In contrast, cells expressing granzyme B alone (Figure 3A left panels, magenta gate) heterogeneously expressed CCR7 and CXCR3. Within this gate, IFN-α enhanced a population of CCR7high/CXCR3low TCM cells, demonstrating that the genesis of the TCM cells in these cultures was derived from within the granzyme B single-positive population. We also assessed expression of CD127 (IL-7Rα) as TCM express relatively high levels of this receptor compared with TEM. In this study, cells within the CCR7low/CD127low gate displayed high frequencies of perforin and granzyme B double-positive cells (Figure 3B). Alternatively, cells within the CCR7high/CD127high gate expressed granzyme B with very low levels of perforin, demonstrating a direct link between effector potential and decreased expression of the IL-7R. Finally, cells expressing CXCR3 in the absence of CCR7 overwhelmingly secreted high levels of IFN-γ in response to secondary activation (Figure 3C population iv). In contrast, only 50% of cells that coexpressed CXCR3 and CCR7 (Figure 3C population iii) and less than 1% of CCR7high/CXCR3low cells (Figure 3C population ii) were capable of secreting IFN-γ upon reactivation. These data directly link TEM phenotypes with secretion of effector molecules and demonstrate that IL-12 and IFN-α differentially regulate both the phenotype and function of TEM and TCM, respectively. Expression of T-bet has been implicated in the development of effector responses in CD8+ T cells. We examined expression of T-bet within TCM and TEM subpopulations. Similar to previous reports,15,28 T-bet expression was enhanced within cells that acquired a TEM, but not a TCM phenotype (Figure S3).
Human CD8+ TEM and TCM cells display distinct effector properties. Day 7 cytokine-polarized cells were assessed for surface marker and cytokine expression. (A) Cells were gated on live CD8+ cells, and perforin and granzyme B levels were assessed. Cells were gated on either granzyme B single-positive cells (magenta) or perforin–granzyme B double-positive cells (orange) and examined for CCR7 and CXCR3 expression (bottom panels). (B) Live CD8+ cells were gated through either CCR7high, CD127high cells (magenta) or CCR7 and CD127low cells (orange) and assessed for perforin and granzyme B expression (bottom panels). (C) PMA- and ionomycin-activated cells were gated on either CCR7low/CXCR3low (i, black), CCR7high/CXCR3low (ii, magenta), CCR7high/CXCR3high (iii, teal), or CCR7low/CXCR3high (iv, orange), and examined for IFN-γ expression by intracellular staining (right panel).
Human CD8+ TEM and TCM cells display distinct effector properties. Day 7 cytokine-polarized cells were assessed for surface marker and cytokine expression. (A) Cells were gated on live CD8+ cells, and perforin and granzyme B levels were assessed. Cells were gated on either granzyme B single-positive cells (magenta) or perforin–granzyme B double-positive cells (orange) and examined for CCR7 and CXCR3 expression (bottom panels). (B) Live CD8+ cells were gated through either CCR7high, CD127high cells (magenta) or CCR7 and CD127low cells (orange) and assessed for perforin and granzyme B expression (bottom panels). (C) PMA- and ionomycin-activated cells were gated on either CCR7low/CXCR3low (i, black), CCR7high/CXCR3low (ii, magenta), CCR7high/CXCR3high (iii, teal), or CCR7low/CXCR3high (iv, orange), and examined for IFN-γ expression by intracellular staining (right panel).
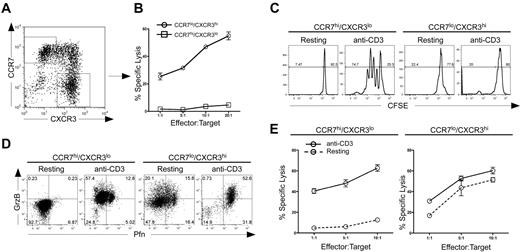
TEM cells exert immediate effector functions, are considered to be more terminally differentiated, and generally do not expand efficiently upon reactivation. In contrast, TCM cells divide rapidly to secondary challenge, giving rise to additional effector cells.29,30 Therefore, we sought to determine whether IFN-α–driven TCM cells were capable of rapid proliferation and generation of secondary effector cells. To address this, cells were polarized in the presence of both IL-12 and IFN-α for 7 days and sorted based on the following gates: TEM, CCR7low/CXCR3high (TEMXR3), and TCM, CCR7high/CXCR3low (TCMR7; Figure 4A). These purified cells were then examined for direct lytic activity. TEMXR3 cells displayed strong CTL activity, whereas the TCMR7 cells were incapable of immediate lytic activity (Figure 4B). These data are in agreement with our previous observations that TEMXR3 cells expressed higher levels of perforin compared with TCMR7 cells, and demonstrate functional differences in effector capabilities between CXCR3high and CCR7high CD8+ T cells.
CCR7 and CXCR3 expression demarcates distinct subpopulations of human CD8+ T cells with functional effector and memory properties. (A) Sorted CD8+ CD45RA+ cells were activated with IL-12 plus IFN-α to day 7. Cells were then sorted into separate CCR7high/CXCR3low or CXCR3high/CCR7low populations. (B) Sorted cells were rested overnight in the absence of IL-2 and subjected to a 51Cr-redirected lysis assay with THP-1 target cells at the indicated E:T ratios. (C) Sorted cells were labeled with CFSE and left untreated (resting) or activated with 1.5 μg/mL plate-bound anti–human CD3 for 3 days (anti-CD3). On day 3, cells were assessed for proliferation by CFSE dilution. (D) Sorted cells were activated, as described in panel C, and examined at day 3 for expression of perforin and granzyme B by bivariant dot plot analysis. (E) Sorted cells were either left untreated (resting) or activated with 1.5 μg/mL anti-CD3 for 3 days (anti-CD3). On day 3, CCR7high/CXCR3low cells (left panel) and CXCR3high/CCR7low cells (right panel) were subjected to a redirected lysis assay, as described above. Each of these experiments was performed twice with 2 separate healthy donors with similar results.
CCR7 and CXCR3 expression demarcates distinct subpopulations of human CD8+ T cells with functional effector and memory properties. (A) Sorted CD8+ CD45RA+ cells were activated with IL-12 plus IFN-α to day 7. Cells were then sorted into separate CCR7high/CXCR3low or CXCR3high/CCR7low populations. (B) Sorted cells were rested overnight in the absence of IL-2 and subjected to a 51Cr-redirected lysis assay with THP-1 target cells at the indicated E:T ratios. (C) Sorted cells were labeled with CFSE and left untreated (resting) or activated with 1.5 μg/mL plate-bound anti–human CD3 for 3 days (anti-CD3). On day 3, cells were assessed for proliferation by CFSE dilution. (D) Sorted cells were activated, as described in panel C, and examined at day 3 for expression of perforin and granzyme B by bivariant dot plot analysis. (E) Sorted cells were either left untreated (resting) or activated with 1.5 μg/mL anti-CD3 for 3 days (anti-CD3). On day 3, CCR7high/CXCR3low cells (left panel) and CXCR3high/CCR7low cells (right panel) were subjected to a redirected lysis assay, as described above. Each of these experiments was performed twice with 2 separate healthy donors with similar results.
We next assessed the ability of sorted cells to proliferate and expand in response to secondary activation. As expected, activated TEMXR3 cells did not divide, whereas TCMR7 cells displayed robust proliferation in response to anti-CD3 stimulation (Figure 4C). The lack of proliferation in the TEMXR3 cells correlated well with a decrease in the total live population of cells as assessed by forward/side scatter analysis and by 7-amino-actinomycin D staining, whereas the TCMR7 cells maintained a live lymphocyte profile (Figure S4). We examined the ability of sorted cells to expand and give rise to new effector and memory subpopulations in response to secondary activation. The TEMXR3 cells expressed perforin and granzyme B (Figure 4D right panels), and displayed equivalent lytic activity regardless of whether they were restimulated with anti-CD3 after sorting (Figure 4E right panel). In contrast, TCMR7 cells expressed low levels of perforin and granzyme B (Figure 4D left panels) and displayed poor lytic activity if they were not restimulated after sorting (Figure 4E left panels). However, in response to reactivation, TCMR7 cells gave rise to perforin- and granzyme-expressing cells. The induction of perforin and granzyme in these TCM cells correlated directly to the acquisition of lytic activity (Figure 4D,E left panels), demonstrating a high degree of plasticity in their ability to reconstitute effector cell populations. Taken together, these data demonstrate that IL-12–driven TEMXR3 cells function in a more terminally differentiated manner, with poor survival and proliferation and an inability to give rise to heterogenous populations upon secondary activation. In contrast, IFN-α–regulated TCMR7 cells displayed strong survival and division to secondary activation and were endowed with the ability to give rise to functional effector populations.
It was possible that CCR7high/CXCR3low TCM cells were derived from populations that were not efficiently activated and remained naive during the primary activation. However, naive CCR7high cells derived from cultures, which did not receive primary TCR/costimulatory activation, displayed a resting lymphocyte profile, failed to divide, and did not express granzyme B in response to anti-CD3 alone (Figure S5). In contrast, memory CCR7high cells, which received primary activation, proliferated and expressed granzyme B as a function of division in response to secondary anti-CD3 stimulation (Figure S5). Therefore, the memory CCR7high cells are a unique population of memory cells and not simply naive cells that failed to receive primary activation.
Reciprocal regulation of the IL-12R and IFNAR in TEM and TCM
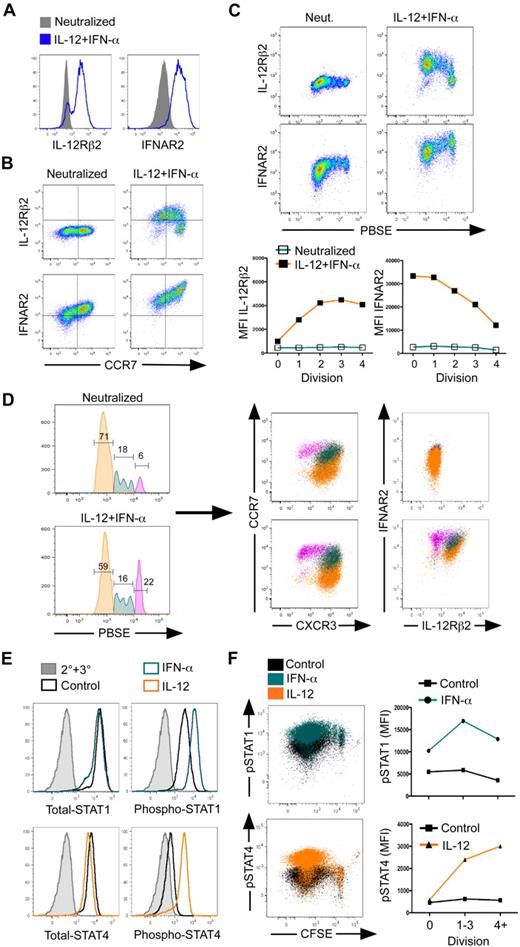
In CD4+ T cells, Th1 commitment is regulated by IL-12 through the induction of the IL-12Rβ2 subunit.17 However, IFNAR is thought to be constitutively expressed on all cells, enabling them to respond in an autocrine fashion to IFN-α that is secreted during viral infections.6 Thus, selective IFNAR expression has not been examined during T-cell differentiation. It was possible that differential sensitivities to IL-12 and IFN-α may account for the concomitant development of TEM and TCM in response to combined activation with IL-12 and IFN-α, allowing for selective outgrowth or differential programming of these 2 subpopulations. To address this possibility, we measured both IL-12Rβ2 and IFNAR2 expression in response to TCR and cytokine activation. As expected, IL-12 plus IFN-α stimulation dramatically enhanced IL-12Rβ2 expression by day 3 of culture (Figure 5A). Surprisingly, we also found that the IFNAR2 was markedly induced by IL-12 plus IFN-α compared with the neutralized control. Furthermore, analysis of coexpression with CCR7 demonstrated the development of a distinct subpopulation of cells in which the IL-12Rβ2 was inversely expressed with CCR7 (Figure 5B). In contrast, CCR7 expression was directly correlated with induction of IFNAR2 on a subpopulation of cells. These data demonstrate that IL-12 and IFN-α differentially regulate the expression of their surface receptors, implicating a potential role for this response in determining effector or memory development.
Reciprocal responsiveness to IL-12 and IFN-α/β correlates to development of TEM and TCM cells. (A) Day 3, neutralized or IL-12 plus IFN-α–activated cells were assessed for surface expression of IL-12Rβ2 or IFNAR2 or (B) expression of CCR7, IL-12Rβ2, and IFNAR2. (C) Day 5, PBSE-labeled cells were assessed for IL-12Rβ2 and IFNAR2 expression as a function of division (top), and relative mean fluorescence intensity was quantified (bottom). (D) Day 5, PBSE-labeled cells were gated on division 0 (magenta), division 1-3 (teal), or division 4+ (orange), and chemokine receptor (middle panels) and cytokine receptor (right panels) expression was measured. (E,F) Day 5 cells were polarized with IL-12 + IFN-α, reactivated with cytokines for 30 minutes, and assessed for intracellular STAT or phospho-STAT protein expression. (E) Total STAT, STAT4 (left panels), or phospho-STAT1, phospho-STAT4 (right panels) expression in live CD8+ gated cells. The 2° + 3° antibody alone (gray), unstimulated (black), IFN-α (teal), or IL-12 treated (orange). (F) Dot plot overlays of phospho-STAT1 (top panel, right) or phospho-STAT4 (bottom panel, left) expression as a function of CFSE dilution. Unstimulated (black), IFN-α treated (teal), or IL-12 treated (orange). Quantification of mean fluorescence intensity as a function of CFSE dilution (right panels); phopho-STAT1 (top), phospho-STAT4 (bottom).
Reciprocal responsiveness to IL-12 and IFN-α/β correlates to development of TEM and TCM cells. (A) Day 3, neutralized or IL-12 plus IFN-α–activated cells were assessed for surface expression of IL-12Rβ2 or IFNAR2 or (B) expression of CCR7, IL-12Rβ2, and IFNAR2. (C) Day 5, PBSE-labeled cells were assessed for IL-12Rβ2 and IFNAR2 expression as a function of division (top), and relative mean fluorescence intensity was quantified (bottom). (D) Day 5, PBSE-labeled cells were gated on division 0 (magenta), division 1-3 (teal), or division 4+ (orange), and chemokine receptor (middle panels) and cytokine receptor (right panels) expression was measured. (E,F) Day 5 cells were polarized with IL-12 + IFN-α, reactivated with cytokines for 30 minutes, and assessed for intracellular STAT or phospho-STAT protein expression. (E) Total STAT, STAT4 (left panels), or phospho-STAT1, phospho-STAT4 (right panels) expression in live CD8+ gated cells. The 2° + 3° antibody alone (gray), unstimulated (black), IFN-α (teal), or IL-12 treated (orange). (F) Dot plot overlays of phospho-STAT1 (top panel, right) or phospho-STAT4 (bottom panel, left) expression as a function of CFSE dilution. Unstimulated (black), IFN-α treated (teal), or IL-12 treated (orange). Quantification of mean fluorescence intensity as a function of CFSE dilution (right panels); phopho-STAT1 (top), phospho-STAT4 (bottom).
Recent studies have suggested that increased proliferation during the primary expansion leads to more terminally differentiated phenotypes of CD8+ T cells that acquire a TEM phenotype.31,32 If this observation is related to IL-12 responsiveness, then the development of effector and memory cells may hinge on the differential acquisition of cytokine responsiveness over the course of division. To examine this, we monitored expression of IL-12Rβ2 and IFNAR2 as a function of division on day 3 of culture. First, we observed that IFN-α slowed the progression of cell division compared with activation of cells with either neutralizing conditions or IL-12, and this effect was evident even in the presence of IL-12 (Figure S6A). Furthermore, IL-12Rβ2 expression was enhanced in response to IL-12 and IFN-α alone at each progressive division and even more dramatically induced in the presence of both IL-12 and IFN-α, indicating a cooperative role for IL-12 and IFN-α in regulating IL-12 responsiveness as a function of cell division (Figure S6A,B). In contrast, expression of IFNAR2 was progressively diminished at each cell division, and this effect was marginally influenced by IL-12 and IFN-α on day 3 of culture.
At each division, we observed that cells progressively gained expression of IL-12Rβ2 while losing expression of IFNAR2. Thus, cells that had progressed through fewer divisions had the potential to be more responsive to IFN-α and less responsive to IL-12. We compared the expression of multiple effector and memory markers in the context of cytokine receptors on day 5 of culture in response to IL-12 plus IFN-α stimulation (Figure 5C,D). On day 5 of culture, we observed a greater induction of IL-12Rβ2 and IFNAR2 by IL-12 plus IFN-α than on day 3 (Figure 5C). At this time point, the magnitude of modulation of IL-12Rβ2 and IFNAR2 was dramatically increased, clearly demonstrating the induction of IL-12Rβ2 as a function of division and the retention of IFNAR2 on less divided cells. In addition, IL-12 plus IFN-α preserved a subpopulation of cells that remained undivided compared with neutralizing conditions (Figure 5D left panels). Strikingly, cells that had undergone extensive division displayed a TEM phenotype marked by high expression of CXCR3 and IL-12Rβ2 and low levels of CCR7 and IFNAR (Figure 5D), and correlated with expression and secretion of perforin, granzyme B, and IFN-γ (data not shown). Those cells that were retained in the undivided population in response to IL-12 plus IFN-α displayed characteristic TCM phenotypes, including low CXCR3 and high CCR7 expression and high IFNAR (Figure 5D), with lower levels of perforin, granzyme B, and IFN-γ (data not shown). Cytokine titration revealed that at lower concentrations of IFN-α (10-100 U/mL), the effects of IL-12 signaling dominate, promoting the development of TEMXR3 cells over that of TCMR7 cells (Figure S7). However, even in the context of IL-12, as the concentration of IFN-α was increased, the development of TCMR7 cells was enhanced. This result suggests that IL-12 and IFN-α work independently to induce TEM and TCM phenotypes. As the ratios of these cytokines are shifted, the development of TCM and TEM follows accordingly. Collectively, these data demonstrate reciprocal regulation of IL-12Rβ2 and IFNAR2 on cells that commit to TEM and TCM fates. TEM cells are derived from rapidly dividing cells and are regulated by IL-12 through the progressive acquisition of IL-12 responsiveness at each cell division. In contrast, TCM cells developing in response to IFN-α are retained at earlier divisions, have the greatest sensitivity to IFN-α, and express the lowest levels of IL-12Rβ2.
The reciprocal regulation of the IL-12R and IFNAR suggested that TCM and TEM development was balanced by differential responsiveness to cytokines. Thus, we measured cytokine-driven STAT phosphorylation as a function of division. First, total STAT1 and STAT4 protein was not altered in response to IFN-α and IL-12, respectively (Figure 5E). Furthermore, we observed strong induction of phospho-STAT1 in response to IFN-α signaling, as well as phospho-STAT4 in response to IL-12 in the total population (Figure 5E), demonstrating a clear responsiveness to cytokine treatment. Interestingly, IFN-α–mediated STAT1 phosphorylation was observed at various levels in all cells, including cells that had progressed through more than 4 divisions (Figure 5F). In contrast, IL-12–driven STAT4 phosphorylation was not observed in division 0. However, as cell division progressed, levels of phospho-STAT4 increased 5- to 6-fold over that of the control cells. Taken together, the ratio of IL-12:IFN-α responsiveness increased progressively with each cell division, correlating directly with the expression of the respective receptor ratios.
In addition to cell division, the strength of signal delivered through TCR engagement has also been implicated in the regulation of memory cell development.31 Some studies have suggested that a strong and prolonged antigen signal promotes efficient generation of TEM, leading to the eventual development of TCM.29 An alternative view posits that TEM and TCM develop in parallel and are balanced by TCR signal strength in which some clones receive a strong signal leading to rapid proliferation and TEM development, whereas other clones receive a weaker or less sustained signal leading to TCM.33 In the present study, the in vitro priming conditions were based on concentrations of anti-CD3 traditionally used to promote efficient proliferation and effector cell development.34 However, this particular culture condition may only provide a single view of how cells interpret IL-12 and IFN-α signals as they divide and differentiate. We wished to determine whether the strength of TCR engagement altered the balance between TEM and TCM as cells develop in response to IL-12 plus IFN-α. To address this, we examined the effect of increasing concentrations of anti-CD3/anti-CD28 under either neutralizing conditions or with IL-12 plus IFN-α.
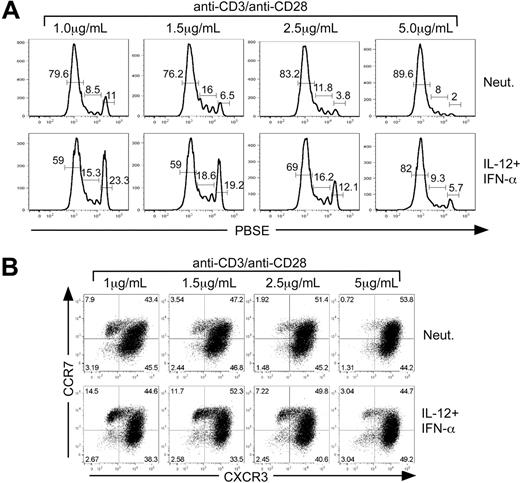
Analysis of cell division revealed that increasing TCR stimulation promoted more efficient and rapid cell division (Figure 6A). However, IL-12 plus IFN-α treatment slowed cell division at each concentration of anti-CD3 compared with the neutralized control. Although each culture condition induced proliferation, twice as many cells were retained within divisions 0-3 when activated with the lowest concentration of anti-CD3/anti-CD28 in the presence of IL-12 plus IFN-α compared with cells activated under neutralizing conditions (Figure 6A). As described above, cells that remained in the undivided population exhibited all of the cell surface phenotypes and functional characteristics of TCM. Despite the pronounced acceleration of cell division driven by increased TCR signal strength, IL-12 plus IFN-α signaling slowed the progression of cell division and enhanced TCMR7 cells even at the highest concentration of anti-CD3/anti-CD28 (Figure 6B).
TCR signal strength regulates cytokine-dependent TCM development. CD8+ CD45RA+ sorted cells were labeled with PBSE and polarized under either neutralizing or IL-12 + IFN-α conditions with 1 μg/mL, 1.5 μg/mL, 2.5 μg/mL, or 5 μg/mL anti-CD3/anti-CD28 for 5 days. (A) Assessment of division by PBSE dilution as a function of primary activation strength. The percentages of cells that are contained within each gate are indicated above the gate. (B) Cells were analyzed for expression of CXCR3 and CCR7 by bivariant dot plot analysis.
TCR signal strength regulates cytokine-dependent TCM development. CD8+ CD45RA+ sorted cells were labeled with PBSE and polarized under either neutralizing or IL-12 + IFN-α conditions with 1 μg/mL, 1.5 μg/mL, 2.5 μg/mL, or 5 μg/mL anti-CD3/anti-CD28 for 5 days. (A) Assessment of division by PBSE dilution as a function of primary activation strength. The percentages of cells that are contained within each gate are indicated above the gate. (B) Cells were analyzed for expression of CXCR3 and CCR7 by bivariant dot plot analysis.
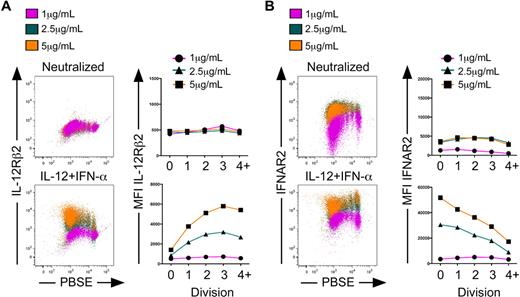
We next assessed expression of IL-12Rβ2 and IFNAR2 at each concentration of anti-CD3/anti-CD28. Under neutralizing conditions, IL-12Rβ2 was expressed at low levels and remained constant at each concentration of anti-CD3/anti-CD28 and at each cell division (Figure 7A top panels). A similar trend was observed with IFNAR2 expression, although a 2- to 3-fold increase in expression was observed at 2.5 and 5 μg/mL compared with 1 μg/mL anti-CD3/anti-CD28 (Figure 7B top panels). However, in the presence of IL-12 plus IFN-α, we observed a striking regulation of both IL-12Rβ2 and IFNAR2 as a function of anti-CD3 concentration. First, IL-12Rβ2 remained low on cells that did not progress into the first division regardless of anti-CD3/anti-CD28 concentration (Figure 7A bottom panels). As cells divided, IL-12Rβ2 was markedly induced up to division 3 in the presence of 2.5 and 5 μg/mL, but not 1 μg/mL anti-CD3/anti-CD28. In stark contrast, IFNAR2 was most highly induced in response to IL-12 plus IFN-α on cells that were retained in the undivided population, and this effect was amplified in response to increasing anti-CD3/anti-CD28 (Figure 7B bottom panels). IFNAR2 levels then declined precipitously at each cell division. Taken together, we found that at division 0, IL-12Rβ2 remained low, whereas IFNAR2 expression increased dramatically in response to IL-12 plus IFN-α stimulation and as a function of increased TCR signal strength. These TCM cells bear the highest potential for IFN-α sensitivity and the lowest potential for IL-12 responsiveness. As cells divided in response to TCR stimulation, they rapidly induced IL-12Rβ2 while simultaneously down-regulating the IFNAR2. Cells in later divisions lose sensitivity to IFN-α while gaining responsiveness to IL-12, and these cells are characterized phenotypically and functionally as TEM. These results demonstrate that the strength of the TCR signal dictates the level at which cells become responsive to IL-12 and IFN-α. Thus, as the TCR signal threshold is altered, the reciprocal regulation of IL-12R and IFNAR allows for the simultaneous commitment of precursors to the TEM and TCM fates.
The development of human CD8+ TEM and TCM cells is regulated by both cytokine signaling and strength of primary activation. CD8+ CD45RA+ sorted cells were labeled with PBSE and polarized under neutralizing or IL-12 + IFN-α conditions with 1 μg/mL, 2.5 μg/mL, or 5 μg/mL anti–human CD3 and anti–human CD28 for 5 days. Cells were assessed for expression of IL-12Rβ2 (A) or IFNAR2 (B) as a function of division. Quantification of mean fluorescence intensity is displayed as a function of division for IL-12Rβ2 (A right panels) or IFNAR2 (B right panels) in neutralized (top panels) or IL-12 + IFN-α (bottom panels) cells.
The development of human CD8+ TEM and TCM cells is regulated by both cytokine signaling and strength of primary activation. CD8+ CD45RA+ sorted cells were labeled with PBSE and polarized under neutralizing or IL-12 + IFN-α conditions with 1 μg/mL, 2.5 μg/mL, or 5 μg/mL anti–human CD3 and anti–human CD28 for 5 days. Cells were assessed for expression of IL-12Rβ2 (A) or IFNAR2 (B) as a function of division. Quantification of mean fluorescence intensity is displayed as a function of division for IL-12Rβ2 (A right panels) or IFNAR2 (B right panels) in neutralized (top panels) or IL-12 + IFN-α (bottom panels) cells.
Discussion
In this study, we have systematically examined the independent and combined roles of IL-12 and IFN-α in the regulation of human CD8+ T-cell differentiation. For the first time, we report nonredundant roles for IL-12 and IFN-α/β in the development of human CD8+ T responses. Together, our data support a model in which IL-12 and IFN-α/β act in concert with signals 1 and 2 to promote the variegated development of effector and memory populations of human CD8+ T cells (Figure S8).
Recently, IL-12 and IFN-α were proposed to act in a redundant fashion to promote effector cell development in murine CD8+ T cells.2,3,35 However, our examination of IL-12– and IFN-α–driven cytokine secretion and lytic activity in human CD8+ T cells revealed striking dissimilarities. IL-12, but not IFN-α, drove effector cell development characterized by marked secretion of IFN-γ and TNF-α and enhanced lytic activity. Surprisingly, whereas IFN-α did not inhibit IL-12–regulated effector cell development, IFN-α signaling was not sufficient to promote this response in the absence of IL-12. Thus, IL-12 remains unique in its ability to drive effector functions, and suggests that IL-12 and IFN-α are not redundant signals in this regard.
Whereas human CD8+ T cells clearly do not adopt effector functions in response to IFN-α, we found a remarkable role for IFN-α in driving memory cell development. In line with observations that IFNAR−/− CD8+ T cells develop effector phenotypes, but lack functional memory,13,36 we found that IFN-α markedly enhanced human CD8+ T cells displaying a TCM surface phenotype. Several models have been proposed to explain the development of effector and memory cells from the same pool of naive precursors.33 For example, one model proposes that TCM cells develop in a linear manner from a pool of rested effector cells.29,37 Alternatively, multiple studies suggest that effector and memory cells develop from distinct lineages that may arise as early as the first division after antigen encounter.38 In the present study, we found that cells activated with both IL-12 and IFN-α simultaneously segregated to both the TCM and TEM fates, suggesting that signaling by both cytokines regulates their parallel development rather than sequential development (Figure S8). The first evidence for this model comes from our observation that signals derived from IFN-α program the development of a population of TCM cells. These cells express high levels of the lymphoid homing receptor CCR7, lack immediate effector function, and display the hallmark characteristics of TCM cells upon a secondary activation. Importantly, development of TCM in response to IFN-α/β occurs concomitantly with the generation of TEM cells that develop in response to IL-12 when both cytokines are present.
The data presented in this study support a model of colinear commitment to TEM and TCM that is regulated independently by IL-12 and IFN-α, respectively (Figure S8). Importantly, IL-12 and IFN-α drove these divergent pathways through the reciprocal regulation of IL-12R and IFNAR. In the absence of innate cytokines, we clearly observed the outgrowth of cells that phenotypically resembled TEM by their selective expression of CXCR3 and low expression of CCR7. However, in the absence of IL-12, these cells were incapable of effector functions. Thus, IL-12 acted in an instructive manner to regulate increased cytokine expression and lytic activity. This correlated precisely with the induction of the IL-12Rβ2, as its expression was markedly increased at each cell division in response to innate cytokines. Furthermore, we observed an even greater enhancement of IL-12Rβ2 expression in response to increasing concentrations of anti-CD3. In contrast, IFN-α–regulated TCM cells were derived primarily from subpopulations that either were retained in the undivided population or had undergone only 1 or 2 divisions, rather than TEM cells that divided extensively. As the initial TCR signal strength was increased, far fewer cells were retained at earlier divisions, giving rise to TEM phenotypes at later divisions. Importantly, IFN-α enhanced the proportion of TCM at every concentration of anti-CD3 tested. These TCM cells expressed low levels of IL-12R and high levels of IFNAR, endowing them with the greatest sensitivity to IFN-α.
TCR signal strength has been implicated as a regulatory component for effector and memory cell development.31 In some models, low TCR engagement, either through decreased antigen concentration or an acute TCR activation, favors the development of memory over effector cells, whereas cells receiving strong or prolonged activation develop primarily into effector cells.39 These observations have been extended to in vivo infection models, in which acute infection promotes stronger memory responses than that of chronic infection.40,41 Indeed, our results support this model as cells that map to the TCM phenotype were primarily derived from subpopulations of cells that were either retained in the undivided population or had undergone only 1 or 2 divisions compared with TEM cells that divided extensively. As the initial TCR strength was increased, far fewer cells were retained at earlier divisions, giving rise to TEM phenotypes at later divisions. Importantly, IFN-α enhanced the proportion of TCM by modulating the TCR signal strength and slowing the progression of cell division in some cells. The cyclin-dependent kinase (CDK) family members CDK2 and CDK6 have been implicated in the rapid division of memory cells to secondary activation.42 In addition, the CDK inhibitor, p27kip1, has been shown to be highly expressed in cells that do not actively divide.42,43 Thus, differential regulation of these factors by IL-12 and IFN-α may explain the variegated behavior of cells as they commit to TEM and TCM phenotypes.
This study provides new and important insight into the development of effector and memory human CD8+ T cells. Although these responses were derived from cells developing in response to signals 1 and 2 in vitro, this approach allowed us to methodically examine the direct and independent roles of IL-12 and IFN-α/β in the generation of CD8+ T-cell effector and memory responses. Our data support a cooperative model in which TCR strength and innate cytokines act as rheostats to fine-tune the balance between effector and memory cell development. If the demands are great and antigen levels are high, IL-12 dominates, and effector cells develop at the expense of memory cells. Alternatively, when antigen levels wane, cytokines then act to modulate the development of both effector and memory subpopulations. Whereas this study suggests that TEM and TCM development can occur in parallel, it does not rule out the possibility that TCM can be derived from rested TEM cells, as many recent studies have suggested.37,44,45 Nonetheless, the present study has broad implications to the field of CD8+ T-cell biology and suggests that optimal memory generation requires a precise balance of TCR signals and innate cytokines. This study marks the first discovery of independent roles for IL-12 and IFN-α/β in the development of human CD8+ T-cell responses and underscores the importance of these 2 cytokines in regulating effector and memory responses to infection.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank A. Mobley for cell-sorting assistance and R. Munford, C. Pasare, F. Yarovinsky, and L. Hooper for helpful discussions.
This work was supported by the National Institutes of Health (Bethesda, MD; H.J.R. supported by AI68622, A.M.D. supported by GM820317, AI45764 awarded to J.F., and AI56222 awarded to J.D.F.).
National Institutes of Health
Authorship
Contribution: H.J.R. performed research, analyzed data, and wrote the paper; A.M.D. performed research and analyzed data; A.G.C. performed research; J.D.S. designed research and contributed reagents; J.F. designed research and analyzed data; and J.D.F. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr J. David Farrar, Department of Immunology, University of Texas Southwestern Medical Center, 5323 Harry Hines Blvd, Dallas, TX 75390-9093; e-mail: David.Farrar@UTSouthwestern.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal