Abstract
Natural killer (NK) cells serve as important effectors for antitumor immunity, and CD56+CD45+ NK cells can be routinely derived from human embryonic stem cells (hESCs). However, little is know about the ability of hESC-derived NK cells to mediate an effective in vivo antitumor response. Using bioluminescent imaging, we now demonstrate that H9 line hESC-derived NK cells mediate effective clearance of human tumor cells in vivo. In addition to increased in vitro killing of diverse tumor targets, the in vivo tumor clearance by H9 hESC-derived NK cells was more effective compared with NK cells derived from umbilical cord blood (UCB). Phenotypic analysis demonstrates the hESC-derived NK cells are uniformly CD94+CD117low/−, an NK-cell population characterized by potent cytolytic activity and thus more competent to mediate tumor clearance. These studies demonstrate that hESCs provide an important model to study human lymphocyte development and may serve as a novel source for antitumor immunotherapy.
Introduction
Many cell-based therapies against malignancies are currently in clinical practice, including hematopoietic stem cell transplantation, as well as T and natural killer (NK) cell–adoptive immunotherapy. Whereas studies have shown that T cells specific for tumor antigens can mediate tumor regression,1-3 cell-based therapies using cells of the adaptive immune system typically have a limited clinical response. Multiple mechanisms that allow tumor cells to evade T cell–mediated immune recognition have been identified. These escape mechanisms include loss of antigen expression by the tumor, loss of major histocompatibility complex class I expression, local presence of immunosuppressive factors and/or cells, and the inability of the tumor to activate an effective adaptive antitumor immune response.4 Unlike their antigen-specific lymphoid counterparts, NK cells belong to the innate immune system and do not require prestimulation to perform their effector functions.5,6 Cytolytic activity of NK cells is regulated by the signals derived from activating and inhibitory receptors expressed on the NK-cell surface. NK cells can recognize and kill many transformed cell lines, and recent clinical studies have evaluated the effect of NK-cell infusion in patients with cancer. In some cases, adoptive NK-cell infusions can provide safe and effective immunotherapy against tumor relapse.7-9 However, as with T cell–mediated antitumor therapy, NK cells are limited to specific cancer types, and not all patients demonstrate optimal response.9-12 Although immunotherapy against malignancies remains very promising, alternative strategies and cell sources to mediate antitumor immunity are still needed.
Human embryonic stem cells (hESCs) are pluripotent stem cells capable of unlimited self-renewal while retaining the ability to derive myeloid, erythroid, and megakaryocytic hematopoietic cell lineages when induced to differentiate under appropriate conditions.13-15 However, relatively little has been done to demonstrate functional lymphocytes derived from hESCs. Although one study did analyze hESC-derived T cells, the number of cells produced were limited, and no functional studies were done.16 Previously, we have shown that hESCs can efficiently generate NK cells using a 2-step in vitro differentiation scheme.17 These initial studies demonstrate hESC-derived NK (hESC-NK) cells acquire the ability to lyse leukemia cell lines in vitro by both direct and antibody-dependent cell-mediated cytotoxicity, as well as produce cytokines, such as interferon-γ (IFN-γ), similar to NK cells generated from umbilical cord blood (UCB) progenitor cells cultured in identical conditions. However, no studies have investigated the ability of hESC-NK cells (or any hESC-derived cell population) to mediate an effective in vivo antitumor response and provide an alternative source of cells for cancer immunotherapy. Here we more thoroughly investigated the phenotypic profile and functional abilities of hESC-NK cells. These studies find hESC-NK cells as a more homogeneous and competent population of effector cells compared with UCB-NK cells, with potent ability to kill human tumor cells both in vitro and in vivo. These studies include use of a novel bioluminescent imaging model to enable long-term evaluation of in vivo antitumor activity in individual tumor-bearing animals treated with hESC-NK cells. All studies were performed with the H9 hESC line.
Methods
Differentiation of hESC cells
H9 hESC line (obtained from WiCell, Madison, WI) was maintained as undifferentiated cells as previously described.13 To induce differentiation, hESCs were transferred to coculture with murine bone marrow stromal cell line M210-B4 (ATCC, Manassas, VA) in medium containing RPMI 1640 (Invitrogen, Carlsbad, CA), 15% defined fetal bovine serum (FBS; HyClone Laboratories, Logan, UT), 2 mM L-glutamine (Cellgro/Mediatech, Herndon, VA), 1% nonessential amino acids (Invitrogen), 1% penicillin/streptomyocin (Invitrogen), and 0.1 mM β-mercaptoethanol (Invitrogen) with medium changes every 2 to 3 days as previously described.13,17,18 After 17 to 20 days, single-cell suspension was prepared and CD34+CD45+ cells were isolated, as previously described.17 Isolated cells were transferred to a second coculture with the murine fetal liver–derived stromal cell line AFT024 (kindly provided by Drs K. Moore and I. Lemischka, Princeton University, Princeton, NJ) in medium containing a 1:2 mixture of Dulbecco modified Eagle medium/Ham F12 (Cellgro/Mediatech), 20% heat-inactivated human serum AB (Valley Biomedicals, Winchester, VA), 2 mM L-glutamine, 1% penicillin/streptomyocin, 5 ng/mL sodium selenite (Sigma-Aldrich, St Louis, MO), 50 μM ethanolamine (MP Biomedicals, Irvine, CA), 25 μM β-mercaptoethanol, 20 mg/mL ascorbic acid (Sigma-Aldrich), interleukin-3 (IL-3; PeproTech, Rocky Hill, NJ; for first week only), stem cell factor (PeproTech), IL-15 (PeproTech), Fms-like tyrosine kinase 3 ligand (PeproTech), and IL-7 (National Cancer Institute [NCI], Bethesda, MD). Cells were fed with fresh medium by half medium changes every 5 to 6 days. After 30 to 35 days in culture, cells were harvested, filtered through 70-μm filter, and used for further analysis. All stromal cells were maintained as previously described.17,18
In vitro cytotoxicity analysis
Direct cytotoxicity assays were performed by standard 4-hour 51Cr-release assay using the K562, MCF7, U87, PC3, and NTERA2 (ATCC) as target cells. Effector cells were added in limiting dilution starting at 10:1 effector-to-target ratio. Specific 51Cr lysis was calculated using the equation: % specific lysis = 100 × (test release − spontaneous release)/(maximal release − spontaneous release).
Flow cytometry
NK cells were stained with allophycocyanin (APC), phycoerythrin (PE), peridinin chlorophyll protein (PerCP)–Cy5.5, and fluorescein isothiocyanate (FITC)–coupled control immunoglobins or specific antibodies against human blood surface antigens: CD45-FITC/-PE, CD56-APC, CD158b-FITC (killer-immunoglobulin-like receptor [KIR]2DL2/2DL3), CD158e1-FITC (KIR3DL1), CD16-FITC, CD94-FITC, CD161-PE, NKp44-PE, CD117-PerCP-Cy5.5, CXCR3-PE, LFA-1-PE, CD62L-PE, and CCR7-PE (all from BD Biosciences PharMingen, San Diego, CA); CD16-PerCP-Cy5.5 (BD Biosciences, San Jose, CA); CD158a/h-PE (KIR2DL1/2DS1), CD158i-PE (KIR2DS4), and NKp46-PE (Immunotech/Beckman Coulter, Fullerton, CA); CXCR4-PE (eBioscience, San Diego, CA); and NKG2D-PE (R&D Systems, Minneapolis, MN). All analyses were performed with a FACSCalibur (BD Biosciences) and FlowJo analysis software (TreeStar, Ashland, OR). Perforin and granzyme were detected by intracellular staining using perforin-PE and granzyme-PE antibodies (BD Biosciences PharMingen).
To isolate CD117−/lowCD94+ NK cells, cells generated from hESC- and UCB-derived progenitors cultured in NK conditions for 30 days were harvested, stained with antibodies: CD94-FITC, CD56-PE, CD117-PerCP-Cy5.5, and CD45-APC, and isolated by fluorescent-activated cell sorting on FACSAria (BD Biosciences). Cell purity was greater than 90% after sorting.
Generation of luc+ K562 cells
Luciferase positive (Luc+) K562 cells were generated by Sleeping Beauty transposon-mediated stable integration. Briefly, 106 K562 cells were nucleofected with 10 μg of the bicistronic pKT2 vector containing GFP reporter and blasticidin resistance genes transcriptionally regulated by PGK promoter and firefly luciferase gene transcriptionally regulated a CpG-free promoter, and 5 μg of transposase DNA (pPGK-SB11) resuspended in 100 μL nucleofector solution (Solution V) using program T16 according to the manufacturer's protocol (Amaxa Biosystems, Gaithersburg, MD). Sleeping Beauty constructs kindly provided by Dr Scott McIvor, University of Minnesota. Cells with stable integration were selected by adding 10 μg/mL blasticidin (MP Biomedicals) to the K562 cell-culture medium. Thirty days after nucleofection, more than 95% of cells demonstrated GFP expression by flow cytometry with luciferase expression of 957 RLU/15 seconds per cell by enzymatic assay as previously described.19
In vivo mouse model for human leukemia
At 24 hours before tumor inoculation, 4- to 6-week-old nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice were given a sublethal irradiation (300 cGy) by Cs-source. Luc+ K562 cells in exponential growth face were used for injection. A total of 200 000 luc+ K562 cells resuspended in 200 μL Iscove modified Dulbecco medium (Cellgro/Mediatech) supplemented with 20% FBS (HyClone Laboratories) were injected subcutaneously into the lower thorax of sedated mice. After 3 days, allowing the tumor to engraft, mice were given intravenous injection of 2 × 106 hESC-NK cells or 2 × 106 UCB-NK cells resuspended in 300 μL Iscove modified Dulbecco medium supplemented with 20% FBS. For all experiments, mice receiving no NK-cell infusion were included as a negative control. All mice received intraperitoneal injections of IL-15 every day for the first 7 days after treatment and IL-2 every 2 to 3 days for 3 weeks. Immediately after tumor injection (day 0) and on days 4, 7, 14, and 21, mice were analyzed for the presence of tumor cells by bioluminescent imaging. Briefly, anesthetized mice were injected intraperitoneally with D-luciferin substrate (120 μL of 25.0 mg/mL in PBS; Xenogen, Alameda, CA). At 15 to 20 minutes after injection, luc activity was detected as emitted light by exposure to an intensified, charge-coupled device camera for 1 minute using the Xenogen imaging system (series 50). All images were analyzed by Living Image software (version 2.50; Lake Oswago, OR). Equal sized gates were drawn around the tumor area to quantify the luciferase intensity and used as a surrogate measure for tumor cell growth in vivo. Total flux indicating emitted photons per second (p/s) was used as units.
To detect the presence of micrometastasis, individual organs were collected at the time of death. Organs were washed in PBS and transferred to individual wells of 24-well plate (spleen and kidneys) or 12-well plate (liver and lungs) in Iscove modified Dulbecco medium supplemented with 5% FBS. D-Luciferin was added, and plates were imaged for the presence of luciferase after 15 minutes of incubation at 37°C. To quantify the luciferase activity (p/s), a gate was drawn around each well and adjusted to signal from a well without any organ added. For analysis, measurements of spleen and kidneys were combined, whereas liver and lungs were kept in separate groups. All mice were housed, treated, and handled in accordance with the guidelines set forth by the University of Minnesota Institutional Animal Care and Use Committee and the Institute of Laboratory Animal Resources of the National Academy of Sciences Guide for the Care and Use of Laboratory Animals.20
Statistical analysis
Differences between groups were compared using Student t test post hoc analysis using Prism 4 (GraphPad Software, San Diego, CA), and differences between proportions were analyzed using z test of proportions. Results were considered significant at P values of .05 or less.
Results
NK cells derived from hESCs mediate direct cytolysis of diverse tumor types in vitro
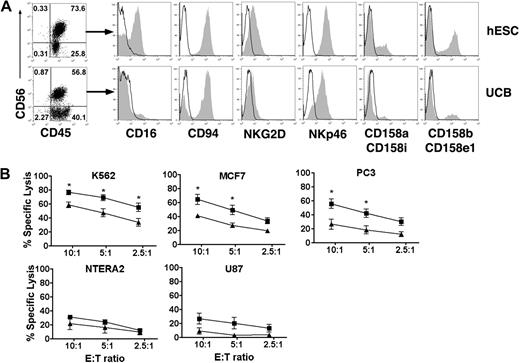
To generate NK cells from hESCs, a 2-step in vitro differentiation was used as previously described with slight modifications.17 Briefly, undifferentiated hESCs were induced to differentiate by coculture with the mouse bone marrow–derived stromal cell line M210-B4. Although S17 has previously been used for this purpose and is able to support hESC differentiation, we have more recently found M210-B4 cells to be more efficient and reproducible at generating CD34+CD45+ cells. After 17 to 20 days, CD34+CD45+ hematopoietic progenitor cells were isolated and transferred to a second culture that supports NK-cell development. On 3 to 5 weeks in culture, an approximately 2 log expansion of the hESC-derived progenitor cells is observed and the majority of the cells derived under these conditions are CD56+CD45+ NK cells, which also express inhibitory and activating receptors typically found on adult NK cells (Figure 1A).17 Interestingly, many of these receptors are expressed at higher levels in hESC-derived NK cells compared with UCB-NK cells, including CD16, NKG2D, and KIRs (Figure 1A).
Derivation of NK cells from hESC capable of direct cytolysis of diverse human tumor cell lines. (A) Flow cytometric identification of CD56+CD45+ NK cells derived from hESC and UCB progenitor cells cultured in NK-cell conditions for 35 days. Histograms demonstrate that CD56+CD45+-gated NK cells express a repertoire of receptors important for regulating NK-cell activity, including CD16, C-type lectin-like receptors (CD94 and NKG2D), natural cytotoxicity receptors (NKp46), and killer-cell Ig-like receptors (CD158). (B) In vitro direct cytolysis was evaluated by standard 4-hour 51Cr-release assay after 35 days in NK-cell culture. NK cell–mediated killing was assessed by incubating effector cells with tumor targets from K562 (n = 7), MCF7 (n = 3), NTERA2 (n = 3), PC3 (n = 3), and U87 (n = 3) at indicated effector-to-target cell ratios. hESC-derived NK cells (■) demonstrate a significantly higher cytolytic activity against K562, MCF7, and PC3 tumor cells compared with UCB-derived NK cells (▲). Mean ± SEM is shown. *P < .05.
Derivation of NK cells from hESC capable of direct cytolysis of diverse human tumor cell lines. (A) Flow cytometric identification of CD56+CD45+ NK cells derived from hESC and UCB progenitor cells cultured in NK-cell conditions for 35 days. Histograms demonstrate that CD56+CD45+-gated NK cells express a repertoire of receptors important for regulating NK-cell activity, including CD16, C-type lectin-like receptors (CD94 and NKG2D), natural cytotoxicity receptors (NKp46), and killer-cell Ig-like receptors (CD158). (B) In vitro direct cytolysis was evaluated by standard 4-hour 51Cr-release assay after 35 days in NK-cell culture. NK cell–mediated killing was assessed by incubating effector cells with tumor targets from K562 (n = 7), MCF7 (n = 3), NTERA2 (n = 3), PC3 (n = 3), and U87 (n = 3) at indicated effector-to-target cell ratios. hESC-derived NK cells (■) demonstrate a significantly higher cytolytic activity against K562, MCF7, and PC3 tumor cells compared with UCB-derived NK cells (▲). Mean ± SEM is shown. *P < .05.
Previously, we evaluated the ability of hESC-NK cells to mediate direct cell-mediated cytolytic activity against the erythroleukemia cell line K562. We have now investigated the ability of hESC-NK cells to lyse more diverse human tumor cells, including both leukemia and solid tumors, in a standard 4-hour 51Cr-release assay (Figure 1B). The hESC-NK cells not only demonstrated efficient lysis of leukemic cells, K562 (erythroleukemia), but also several solid tumors, including breast cancer (MCF7), testicular embryonal carcinoma (NTERA2), prostate cancer (PC3), and glioma (U87) cell lines. Remarkably, hESC-NK cells demonstrated a significantly increased cytolytic activity against K562, MCF7, and PC3 tumor cells compared with NK cells derived from UCB progenitor cells (UCB-NK). In addition, there was a trend toward higher hESC–NK-cell cytolytic activity against NTERA2 and U87 cells compared with UCB-NK cells. These results demonstrate that hESC-NK cells have ability to kill a wide variety of tumor cells and can be more potent in killing both leukemia and solid tumors compared with UCB-NK cells.
Increased in vivo antitumor activity of hESC-NK cells compared with UCB-NK cells
To test these hESC-NK cells in a more stringent preclinical model, we next investigated the in vivo activity of hESC-NK cells using the K562 erythroleukemia cells engineered to stably express Firefly luciferase (luc) and xenotransplanted into immunocompromised mice. This model allows serial bioluminescent imaging to noninvasively monitor growth or regression of the tumor cells in individual animals over a prolonged time course.21 To avoid viral transfection that could make K562 cells more susceptible to NK cell–mediated lysis,19,22 we chose to generate luc+ cells by Sleeping Beauty transposon-mediated transfection as described in “Methods” in “Generation of luc+ K562 cells” (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Luc+ K562 cells were injected subcutaneously into sublethally irradiated NOD/SCID mice and allowed to grow for 3 days to provide an established tumor before NK-cell immunotherapy. Three days after tumor inoculation, mice were divided into 3 treatment courses: no NK-cell infusion (control group), or 1 systemic (intravenous) infusion of 2 × 106 NK cells derived from hESCs, or 2 × 106 NK cells derived from UCB. No additional cells were given after this initial treatment. Based on previous studies demonstrating improved NK-cell growth and stimulation of NK-cell activity in vivo by administration of IL-2 and IL-15,23-27 each group received intraperitoneal injections of IL-15 (10 ng/mouse) every day for the first 7 days after treatment and IL-2 (10 000 U/mouse) every 2 to 3 days for 3 weeks. In this model, almost all mice (18 of 20) that received cytokine treatment but did not receive NK cells developed large tumors within 3 weeks (Figure 2A,B). Remarkably, all mice (13 of 13) treated with hESC-NK cells demonstrated rapid and complete clearance of the primary tumor within 2 weeks after tumor inoculation. In contrast, mice treated with UCB-NK cells had significantly less antitumor activity in vivo, with only 5 of 13 tumor-free animals treated with UCB-NK cells. Animals treated with UCB-NK cells had a trend toward slower tumor growth as indicated by lower luciferase activity at early time points compared with no NK-cell control animals, although not statistically significant (Figure 2B). To more stringently demonstrate the antitumor activity, we monitored mice showing tumor regression after hESC- and UCB–NK-cell treatment for up to 8 weeks and found no evidence of tumor recurrence, demonstrating stable clearance of tumor cells, despite no additional treatments given after the first 4 weeks (Figure 2B).
hESC-derived NK cells demonstrate clearance of established K562 tumors xenografted in mice with higher efficacy compared with NK cells generated from UCB. NOD/SCID mice were inoculated with luc+ K562 tumor cells, and cells were allowed to engraft for 3 days before animals were given 1 systemic (intravenous) infusion of NK cells. All hESC- and UCB-derived NK cells were injected after 30 to 35 days of culture. Mice were monitored by bioluminescent imaging at days 0, 4, 7, 14, and 21. In addition, some mice demonstrating tumor regression were monitored long-term (up to 8 weeks) for tumor recurrence. (A) Representative in vivo bioluminescent images of animals at the day of tumor inoculation and 21 days after tumor inoculation. Non-NK cell–treated control animals did receive the IL-2/IL-15 cytokine regimen and typically developed large tumors as indicated by luciferase expression. Mice treated with UCB-derived NK (UCB-NK) cells typically display slower tumor progression. All mice treated with hESC-derived NK (hESC-NK) cells displayed a complete clearance of tumor cells. (B) Analysis of luciferase activity (photons/second) of individual mice and the mean activity in each treatment group. Luciferase activity was analyzed from the site of tumor inoculation in control (n = 20, ■), UCB-NK treated (n = 13, [ ]), and hESC-NK–treated (n = 13, ●) mice. Error bars indicated SEM. *P < .01 for hESC-NK vs UCB-NK and control. (C) CD56+ NK cells were magnetically sorted and injected intravenously into tumor-bearing mice. Mice treated with either sorted CD56+ hESC-NK cells (n = 3, —) or sorted CD56+ UCB-NK cells (n = 2,
]), and hESC-NK–treated (n = 13, ●) mice. Error bars indicated SEM. *P < .01 for hESC-NK vs UCB-NK and control. (C) CD56+ NK cells were magnetically sorted and injected intravenously into tumor-bearing mice. Mice treated with either sorted CD56+ hESC-NK cells (n = 3, —) or sorted CD56+ UCB-NK cells (n = 2,  ) demonstrate tumor regression similar to mice treated with unsorted hESC- or UCB-NK cells.
) demonstrate tumor regression similar to mice treated with unsorted hESC- or UCB-NK cells.
hESC-derived NK cells demonstrate clearance of established K562 tumors xenografted in mice with higher efficacy compared with NK cells generated from UCB. NOD/SCID mice were inoculated with luc+ K562 tumor cells, and cells were allowed to engraft for 3 days before animals were given 1 systemic (intravenous) infusion of NK cells. All hESC- and UCB-derived NK cells were injected after 30 to 35 days of culture. Mice were monitored by bioluminescent imaging at days 0, 4, 7, 14, and 21. In addition, some mice demonstrating tumor regression were monitored long-term (up to 8 weeks) for tumor recurrence. (A) Representative in vivo bioluminescent images of animals at the day of tumor inoculation and 21 days after tumor inoculation. Non-NK cell–treated control animals did receive the IL-2/IL-15 cytokine regimen and typically developed large tumors as indicated by luciferase expression. Mice treated with UCB-derived NK (UCB-NK) cells typically display slower tumor progression. All mice treated with hESC-derived NK (hESC-NK) cells displayed a complete clearance of tumor cells. (B) Analysis of luciferase activity (photons/second) of individual mice and the mean activity in each treatment group. Luciferase activity was analyzed from the site of tumor inoculation in control (n = 20, ■), UCB-NK treated (n = 13, [ ]), and hESC-NK–treated (n = 13, ●) mice. Error bars indicated SEM. *P < .01 for hESC-NK vs UCB-NK and control. (C) CD56+ NK cells were magnetically sorted and injected intravenously into tumor-bearing mice. Mice treated with either sorted CD56+ hESC-NK cells (n = 3, —) or sorted CD56+ UCB-NK cells (n = 2,
]), and hESC-NK–treated (n = 13, ●) mice. Error bars indicated SEM. *P < .01 for hESC-NK vs UCB-NK and control. (C) CD56+ NK cells were magnetically sorted and injected intravenously into tumor-bearing mice. Mice treated with either sorted CD56+ hESC-NK cells (n = 3, —) or sorted CD56+ UCB-NK cells (n = 2,  ) demonstrate tumor regression similar to mice treated with unsorted hESC- or UCB-NK cells.
) demonstrate tumor regression similar to mice treated with unsorted hESC- or UCB-NK cells.
To confirm that this antitumor activity is specific to CD56+CD45+ NK cells derived from in vitro culture and not facilitated by CD56− cells also present in these cultures, we injected NK cells derived from both hESCs and UCB sorted to more than 99% purity (Figure S2). Similar to studies with unsorted cells, all mice treated with CD56+-sorted hESC-NK cells demonstrated complete clearance of K562 tumor cells (3 of 3; Figure 2C). For mice treated with CD56+-sorted UCB-NK cells, tumor regression was observed in one mouse, whereas continued tumor growth was observed in another, complementing results observed for mice treated with unsorted cells. As shown previously, this demonstrates that the in vitro and in vivo cytolytic activity resides in the CD56+ NK cells.17
hESC-NK cells protect against tumor metastasis
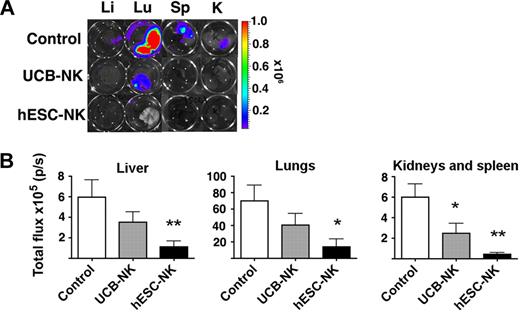
As the luc+ K562 cells allow sensitive detection of metastasis, we isolated liver, lungs, spleen, and kidneys from animals receiving the different therapies and analyzed these tissues for presence of micrometastasis by detection and quantification of luc (Figure 3). As a baseline analysis, 73% mice without NK-cell infusion demonstrated some presence of metastasis as indicated by foci of luc+ cells in liver, lungs, spleen, or kidneys (Table 1). Of the mice treated with hESC-NK cells, only one mouse (7%) displayed the presence of luc+ cells in the lungs, whereas 53% of mice treated with UCB-NK cells displayed the presence of metastatic cells (Table 1). Quantification of the luc from each individual organ demonstrated a significantly reduced presence of luc in liver, lungs, spleen, and kidneys in animals treated with hESC-NK cells compared with noninjected control animals (Figure 3B). Animals treated with hESC-NK cells also had a significantly reduced presence of luc in liver and kidneys/spleen compared with animals treated with UCB-NK cells. When combined, the total number of organs from each group demonstrates that mice treated with UCB-NK cells have fewer organs positive for metastasis compared with control (20% compared with 63% in UCB-NK treated vs control animals, respectively, P < .05; Table 1). In addition, there was a significantly reduced presence of luc in spleen/kidneys in UCB-NK–treated animals compared with control (Figure 3B), demonstrating that UCB-NK cells also have efficacy in protecting against metastasis (Figure 3B; Table 1). Together, these results demonstrate hESC-NK cells are more potent in clearing human tumor cells in vivo than UCB-NK cells.
hESC-derived NK cells protect against K562 metastasis. (A) Representative images of micrometastasis foci in liver, lungs, spleen, and kidneys as identified by bioluminescent imaging. (B) Quantification of luciferase activity (p/s) from isolated organs. Luciferase intensity from each well was quantified using LivingImage software. Mean ± SEM from indicated organs is shown.
hESC-derived NK cells protect against K562 metastasis. (A) Representative images of micrometastasis foci in liver, lungs, spleen, and kidneys as identified by bioluminescent imaging. (B) Quantification of luciferase activity (p/s) from isolated organs. Luciferase intensity from each well was quantified using LivingImage software. Mean ± SEM from indicated organs is shown.
Evaluation of K562 micrometastasis
| . | Mice with micrometastasis, no. (%) . | Total metastasis- positive organs, no. (%) . |
|---|---|---|
| Control | 14/19 (73) | 46/72 (63)* |
| UCB-NK | 7/13 (53) | 10/48 (20) |
| hESC-NK | 1/13 (7)† | 1/36 (3) |
| . | Mice with micrometastasis, no. (%) . | Total metastasis- positive organs, no. (%) . |
|---|---|---|
| Control | 14/19 (73) | 46/72 (63)* |
| UCB-NK | 7/13 (53) | 10/48 (20) |
| hESC-NK | 1/13 (7)† | 1/36 (3) |
The presence of micrometastasis was evaluated macroscopically by detection of luc+ foci after bioluminescent imaging.
P < .05 versus control versus UCB-NK and hESC-NK by z test of proportions.
P < .05 for hESC-NK versus control and UCB-NK by z test of proportions.
Homogeneously mature NK cells are generated from hESCs
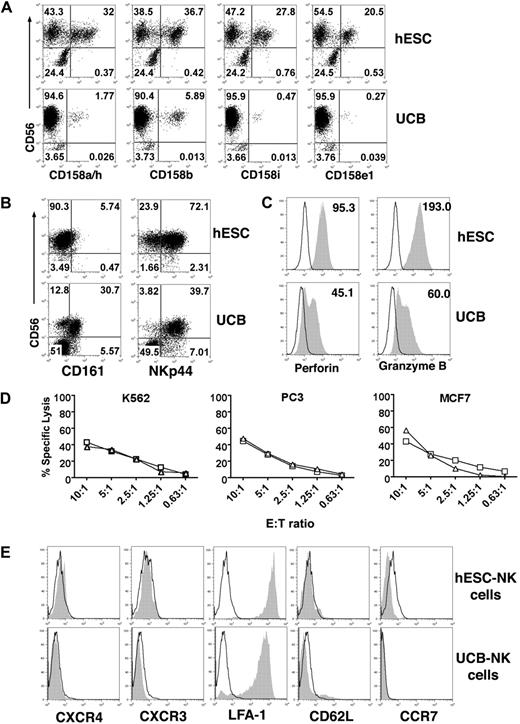
Next, we investigated underlying mechanisms that could explain the more effective in vivo antitumor activity of hESC-NK cells. Flow cytometric analysis demonstrated a notable difference between the hESC- and UCB-derived NK cells in cell surface antigen expression known to define NK-cell maturation stages. CD117+CD94−CD56+ NK-cells have been identified both in vivo and in vitro as a developmentally immature NK-cell population, whereas expression of CD94 combined with low (or no) expression of CD117 defines transition into mature NK cells with more competent effector function.28,29 CD117−/lowCD94+CD56+ NK cells acquire expression of molecules important for NK-cell function, including KIRs, CD16, perforin, granzyme, and FasL, which are not found on CD117+CD94−CD56+ NK cells.28,29 Indeed, as previously demonstrated, UCB-NK cells contain both of the CD117−/lowCD94+CD56+ and CD117+CD94−CD56+ populations (Figure 4A). Strikingly, essentially all hESC-NK cells are CD117−/lowCD94+, and the majority of these cells express CD16 (Figure 4A), equivalent of mature NK cells.30 Even at the earliest time point (day 14) that can be analyzed in the NK cultures (the time when the first CD56+ NK cells are observed), the majority of hESC-NK cells have a CD117−/lowCD94+ phenotype, whereas UCB-NK cells are virtually all CD117+CD94− (Figure 4B).
Development of homogeneously mature NK cells from hESCs. (A) Phenotypic analysis of hESC-NK cells compared with UCB-NK cells. hESC- and UCB-derived progenitors cultured in NK-cell conditions for 30 days were evaluated by flow cytometry for surface antigen expression. Two distinct populations, CD117+CD94− and CD117−/lowCD94+, are found in UCB-derived cells, whereas almost all hESC-derived cells are CD117−/lowCD94+. Additional analysis for CD94 and CD16 expression on the CD56+ cell population also demonstrates distinct differences between the hESC and UBC-NK cell populations. (B) Time-course analysis for expression of CD117 and CD94 on CD56+ NK cells. Cells were harvested after 14, 23, and 30 days in NK culture. FSC indicates forward scatter; and SSC, side scatter.
Development of homogeneously mature NK cells from hESCs. (A) Phenotypic analysis of hESC-NK cells compared with UCB-NK cells. hESC- and UCB-derived progenitors cultured in NK-cell conditions for 30 days were evaluated by flow cytometry for surface antigen expression. Two distinct populations, CD117+CD94− and CD117−/lowCD94+, are found in UCB-derived cells, whereas almost all hESC-derived cells are CD117−/lowCD94+. Additional analysis for CD94 and CD16 expression on the CD56+ cell population also demonstrates distinct differences between the hESC and UBC-NK cell populations. (B) Time-course analysis for expression of CD117 and CD94 on CD56+ NK cells. Cells were harvested after 14, 23, and 30 days in NK culture. FSC indicates forward scatter; and SSC, side scatter.
More specific phenotypic analysis also demonstrates a consistently higher percentage of the hESC-NK cells express KIRs compared with UCB-NK cells, even comparing against NK cells derived from genetically diverse UCB progenitor cells (Figure 5A).17 In addition, the mature hESC-NK cells do not express CD161 but express the NK cytotoxocity receptor NKp44 (Figure 5B).17 More importantly, hESC-NK cells demonstrate a homogeneous expression of both perforin and granzyme B, 2 key effector molecules (Figure 5C). In contrast, UCB-NK cells have a bimodal expression of perforin and granzyme B, and the expression does not reach the level observed in hESC-NK cells. This is consistent with other studies that suggest CD117+CD94−CD56+ cells are immature NK precursors, which do not express perforin or granzyme B and lack cytolytic activity.29 Importantly, such cells were very few (< 2%) in hESC–NK-cell cultures (Figure 4). To confirm the functional relevance of this phenotype, we isolated CD117−/lowCD94+ hESC- and UCB-derived NK cells by cell sorting and directly compared their ability to lyse tumor cells in vitro. As expected, directly comparing phenotypically mature CD117−/lowCD94+ NK cells derived from hESCs and UCB demonstrates equivalent cytolytic activity against K562, PC3, and MCF7 tumor cells (Figure 5D).
Phenotypic and functional analysis of hESC-NK cells compared with UCB-NK cells. (A) Flow cytometric analysis for 4 individual KIRs demonstrates a higher percentage of hESC-NK cells expressing these regulatory receptors compared with what is found on UCB-NK cells. (B) Expression of CD161 and NKp44 on hESC- and UCB-NK cells. (C) Expression of perforin and granzyme B on CD56+ NK cells was evaluated by intracellular flow cytometric straining. Histograms of CD56+-gated cells are shown. Mean fluorescent intensity (MFI) was analyzed from all hESC-derived CD56+ cells and from perforin and granzyme B-positive CD56+ cells from UCB. Isotype control is indicated in open histograms. (D) Cytolytic activity against K562, PC3, and MCF7 tumor cells was compared between purified CD117−/lowCD94+ NK cells derived from hESCs (□) and UCB ( ) at the indicated effector-to-target cell ratios. Representative results from 2 separate experiments are shown. (E) Flow cytometric analysis of cell trafficking molecules on hESC- and UCB-derived NK cells. Histograms of CD56+-gated NK cells are shown. Open histogram indicates isotype control.
) at the indicated effector-to-target cell ratios. Representative results from 2 separate experiments are shown. (E) Flow cytometric analysis of cell trafficking molecules on hESC- and UCB-derived NK cells. Histograms of CD56+-gated NK cells are shown. Open histogram indicates isotype control.
Phenotypic and functional analysis of hESC-NK cells compared with UCB-NK cells. (A) Flow cytometric analysis for 4 individual KIRs demonstrates a higher percentage of hESC-NK cells expressing these regulatory receptors compared with what is found on UCB-NK cells. (B) Expression of CD161 and NKp44 on hESC- and UCB-NK cells. (C) Expression of perforin and granzyme B on CD56+ NK cells was evaluated by intracellular flow cytometric straining. Histograms of CD56+-gated cells are shown. Mean fluorescent intensity (MFI) was analyzed from all hESC-derived CD56+ cells and from perforin and granzyme B-positive CD56+ cells from UCB. Isotype control is indicated in open histograms. (D) Cytolytic activity against K562, PC3, and MCF7 tumor cells was compared between purified CD117−/lowCD94+ NK cells derived from hESCs (□) and UCB ( ) at the indicated effector-to-target cell ratios. Representative results from 2 separate experiments are shown. (E) Flow cytometric analysis of cell trafficking molecules on hESC- and UCB-derived NK cells. Histograms of CD56+-gated NK cells are shown. Open histogram indicates isotype control.
) at the indicated effector-to-target cell ratios. Representative results from 2 separate experiments are shown. (E) Flow cytometric analysis of cell trafficking molecules on hESC- and UCB-derived NK cells. Histograms of CD56+-gated NK cells are shown. Open histogram indicates isotype control.
Expression of several cell surface antigens important for cell trafficking and homing, including CXCR4, CXCR3, CD62L, and CCR7, were also evaluated (Figure 5E). We observed no difference in expression of these cell surface antigens comparing hESC- and UCB-derived NK cells. Furthermore, very few human NK cells were found in peripheral blood, bone marrow, or spleen in mice at all time points analyzed after injection of both UCB- and hESC-NK cells, arguing against preferential in vivo survival and/or homing of either group. Taken together, these results suggest that NK cells derived from hESCs are significantly more homogeneous and more developmentally competent to mediate tumor killing compared with UCB-NK cells.
Discussion
Multiple hematopoietic cell lineages, including lymphoid and myeloid cells, can be efficiently and routinely derived from hESCs by in vitro differentiation, establishing these cells as an important model system to study human hematopoietic ontogeny.13-15,17 In addition to phenotypic analysis of the hematopoietic cells generated from hESCs, rigorous functional analyses, as well as direct comparison with hematopoietic cells derived or isolated from more conventional sources, such as peripheral blood, bone marrow, and UCB, are needed to better define the clinical potential of these cells. Here we have shown that H9 line hESC-derived NK cells demonstrate the ability to mediate clearance of established human tumors in immune-deficient mice (Figure 2). This ability of NK cells to kill an established tumor in vivo after systemic injection requires several critical functional characteristics in addition to tumor cytolysis. Appropriate migration in the blood circulation, homing and chemotaxis to the tumor, and in vivo survival and proliferation are all critical for an effective antitumor response.31 Our studies find that, compared with NK cells derived from UCB stem and progenitor cells cultured in identical conditions, hESC-NK cells demonstrate a substantially increased ability to mediate in vivo clearance of tumor cells. Indeed, all mice treated with hESC-NK cells had complete clearance of tumor cells 3 to 4 weeks after NK-cell treatment, whereas less than half of the UCB-NK cell–treated animals demonstrated tumor clearance. These results suggest the intriguing potential of hESC-NK cells to provide a novel and highly potent resource for cell-mediated adoptive cancer immunotherapy. It is important to emphasize that these studies only used the H9 hESC line. Further studies with more diverse hESC lines are needed to confirm that this is not a characteristic unique to the H9 line, but also other hESC lines in general.
We investigated several potential mechanisms to account for the increased in vivo antitumor activity of the hESC-NK cells compared with the UCB-NK cells. Analysis of homing receptors on the 2 cell populations did not demonstrate any significant differences (Figure 5E), and studies to determine whether there was increased in vivo survival of the hESC-NK cells were inconclusive. Instead, there was a striking difference in the developmental status of the NK cells. Recently, expression of the receptor tyrosine kinase CD117 and the c-type lectin receptor CD94 have been shown to discriminate between immature and mature NK cells, both in vivo and in vitro.28,29 CD117+CD94− cells represent an immature stage where the cells have committed to the NK-cell lineage but lack expression of NK-cell receptors and have no cytotoxic capacity. These cells give rise to CD117−/lowCD94+ cells, which coincide with acquisition of NK-cell receptors, cytolytic activity, and IFN-γ production. Culture of CD34+ UCB stem and progenitor cells results in a heterogeneous population with both immature CD117+CD94− and mature CD117−/lowCD94+ NK cells (Figure 4). This is in contrast to CD34+CD45+ hESC-derived progenitors cultured in the same conditions, which generates a homogeneous population of mature CD117−/lowCD94+ NK cells. The functional relevance of this phenotype is confirmed by homogeneous expression of the effector molecules perforin and granzyme B, demonstrating increased cytolytic competence (Figure 5B). This is further demonstrated by equivalent high cytolytic activity against K562, PC3, and MCF7 tumor cells when directly comparing the cytolytic activity of purified CD117−/lowCD94+ NK cells from both hESCs and UCB (Figure 5D). This suggests that, although cytolytic cells are generated from UCB progenitors, the proportion of mature and armed NK cells is higher from the hESC-derived progenitor cells. Taken together, NK-cell differentiation of hESC-derived progenitors generates NK cells at a “more mature” developmental stage with more homogeneous expression of effector molecules and increased cytolytic activity. NOD/SCID mice used for these studies do have residual NK-cell activity32 and both UCB- and hESC-derived NK cells have been previously shown to produce IFN-γ.17 Therefore, although we cannot exclude the possibility that these injected NK cells activate some endogenous mouse NK cells to facilitate the antitumor response, it would be expected this response to be similar in both groups of NK cell–treated mice. In addition, although it is possible that the IL2 and/or IL15 given to the mice after NK-cell injection could preferentially activate hESC-NK cells more than UCB-NK cells, we know of no information to suggest this is the case. Indeed, because the UCB-NK cells respond as well or better than the hESC-NK cells during in vitro culture with IL-2 or IL-15 based on proliferation and survival, we would not expect the UCB-NK cells to have a deficient response when stimulated by these cytokines in vivo.
There are several potential mechanisms that could account for the observed difference in terminal NK-cell differentiation between hESC- and UCB-derived hematopoietic stem and progenitor cells. First, hESC-derived progenitors may go through the NK-cell developmental pathway faster compared with UCB cells. A second explanation could be that there are alternative developmental pathways used by hESC- and UCB-progenitor cells. Third, fundamental developmental differences between the hESC- and UCB-progenitor cells could facilitate different responses to the in vitro differentiation conditions used to derive NK cells. Understanding the developmental pathways that lead to this development of homogeneous and highly potent hESC-derived NK cells, as well as better characterization of the lymphoid progenitor cell population derived from hESCs, will help to improve current clinical protocols using NK cells, whether these cells come from peripheral blood, UCB, or hESCs.7,8 Finally, as KIRs important for regulation of NK-cell function are associated with haplotypic variation and allelic polymorphisms, it will be important to investigate the phenotypic and functional characteristics of NK cells derived from genetically diverse hESC lines.
hESCs have been proposed as an alternative source of cells for clinical therapy.33 However, these cells are like other cells used for clinical transplantation, limited by their potential to evoke an allogeneic immune response. Development of hESC stem cell banks has been proposed as a measure to increase the coverage of the genetic diversity in the general population, but these would require substantial effort to maintain and would not cover all patients in need of cell therapy. The recent development of induced pluripotent stem cells (iPS) from human somatic cells resulting in reprogrammed cells with ES cell characteristics opens the potential for derivation of patient-specific cells for autologous cell transplantation.34-36 Indeed, iPS cells have been shown to differentiate into hematopoietic cells by applying similar in vitro differentiation protocols as used to generate hematopoietic cells from hESCs.36 However, continued studies using hESCs will remain critical for studies of iPS differentiation. In addition, genetic modification may allow patient-specific immune effector cells that are capable of killing specific tumor cells,10,37,38 a process that may be more readily accomplished starting with hESCs and iPS to derive NK cells. Taken together, the results presented here provide an important advance in use of hESCs as a novel source for effective antitumor immunotherapy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Jeff Miller for technical assistance and for sharing NK-cell reagents; Dr R. Scott McIvor and Dr Andrew Wilber for SB constructs and assistance with generating luciferase-expressing K562 cells; Dr Doug Yee, Dr Paul Marker, Dr Yoji Shimizu, and Dr John Ohlfest for providing tumor cell lines; and Dr Juan Carlos Zúñiga-Pflücker and Dr William Kerr for critical reading of the manuscript.
This work was supported by the National Heart, Lung, and Blood Institute (grant R01) and the University of Minnesota (doctoral dissertation fellowship).
National Institutes of Health
Authorship
Contribution: P.S.W. designed and performed experiments and wrote the manuscript; B.G. designed and performed experiments; X.T., R.K.M., and D.A.K. performed experiments; M.R.V. provided essential reagents and assisted with experimental design; D.S.K. designed experiments and wrote and edited the manuscript; and all authors read and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dan S. Kaufman, Stem Cell Institute and Department of Medicine, University of Minnesota, Translational Research Facility, 2001 6th St SE, Minneapolis, MN 55455; e-mail: kaufm020@umn.edu.

![Figure 2. hESC-derived NK cells demonstrate clearance of established K562 tumors xenografted in mice with higher efficacy compared with NK cells generated from UCB. NOD/SCID mice were inoculated with luc+ K562 tumor cells, and cells were allowed to engraft for 3 days before animals were given 1 systemic (intravenous) infusion of NK cells. All hESC- and UCB-derived NK cells were injected after 30 to 35 days of culture. Mice were monitored by bioluminescent imaging at days 0, 4, 7, 14, and 21. In addition, some mice demonstrating tumor regression were monitored long-term (up to 8 weeks) for tumor recurrence. (A) Representative in vivo bioluminescent images of animals at the day of tumor inoculation and 21 days after tumor inoculation. Non-NK cell–treated control animals did receive the IL-2/IL-15 cytokine regimen and typically developed large tumors as indicated by luciferase expression. Mice treated with UCB-derived NK (UCB-NK) cells typically display slower tumor progression. All mice treated with hESC-derived NK (hESC-NK) cells displayed a complete clearance of tumor cells. (B) Analysis of luciferase activity (photons/second) of individual mice and the mean activity in each treatment group. Luciferase activity was analyzed from the site of tumor inoculation in control (n = 20, ■), UCB-NK treated (n = 13, []), and hESC-NK–treated (n = 13, ●) mice. Error bars indicated SEM. *P < .01 for hESC-NK vs UCB-NK and control. (C) CD56+ NK cells were magnetically sorted and injected intravenously into tumor-bearing mice. Mice treated with either sorted CD56+ hESC-NK cells (n = 3, —) or sorted CD56+ UCB-NK cells (n = 2, ) demonstrate tumor regression similar to mice treated with unsorted hESC- or UCB-NK cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/24/10.1182_blood-2008-06-165225/4/m_zh89990937190002.jpeg?Expires=1769095998&Signature=Rl087pGqy34W7Fvf1Eu58pmOwTA16-WddSj8knltEvqTaHDZsx2TGasRHMIyjlVxzjf9WVkZHWO4LVHbtCuIgRxysLPLB2tQ2a2SCwTjVF77OPM-C7rhqQhT8sN4KSoJRFe-dvGgms0mcUJeyS-SQFaT7efsnr1WJe1cuYBHxD0MjnwSjGKSW9vgDdeeDYn3P3BbVuBvUpIJNGppgLhwPAN~1ojP0Qafjz5SDfnDfyBgUiYDfYG5Goe95A8X01bDKT8fPS7DMUXmjfKLF9K-4794i1KvVAaA~iQIo7j9M4HH0I9IhBMnksfJ5Ib9sIsaC1lmP4-gAVR6GtgKvcVT~g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal