Abstract
SDF-1α/CXCR4 signaling plays a key role in leukemia/bone marrow microenvironment interactions. We previously reported that bone marrow–derived stromal cells inhibit chemotherapy-induced apoptosis in acute myeloid leukemia (AML). Here we demonstrate that the CXCR4 inhibitor AMD3465 antagonized stromal-derived factor 1α (SDF-1α)–induced and stroma-induced chemotaxis and inhibited SDF-1α–induced activation of prosurvival signaling pathways in leukemic cells. Further, CXCR4 inhibition partially abrogated the protective effects of stromal cells on chemotherapy-induced apoptosis in AML cells. Fetal liver tyrosine kinase-3 (FLT3) gene mutations activate CXCR4 signaling, and coculture with stromal cells significantly diminished antileukemia effects of FLT3 inhibitors in cells with mutated FLT3. Notably, CXCR4 inhibition increased the sensitivity of FLT3-mutated leukemic cells to the apoptogenic effects of the FLT3 inhibitor sorafenib. In vivo studies demonstrated that AMD3465, alone or in combination with granulocyte colony-stimulating factor, induced mobilization of AML cells and progenitor cells into circulation and enhanced antileukemic effects of chemotherapy and sorafenib, resulting in markedly reduced leukemia burden and prolonged survival of the animals. These findings indicate that SDF-1α/CXCR4 interactions contribute to the resistance of leukemic cells to signal transduction inhibitor– and chemotherapy-induced apoptosis in systems mimicking the physiologic microenvironment. Disruption of these interactions with CXCR4 inhibitors represents a novel strategy of sensitizing leukemic cells by targeting their protective bone marrow microenvironment.
Introduction
Normal and leukemic hematopoietic cells and stem cells reside in the bone marrow in specialized areas (“niches”) that provide the structural and physiologic conditions for their growth and survival.1 Subpopulations of leukemic cells can be sequestered in niches and thereby evade chemotherapy-induced death.2 We and others have reported that stromal cells protect acute myeloid leukemia (AML) and chronic lymphocytic leukemia cells from the apoptosis induced by chemotherapy.3-6 While the mechanisms of stroma-mediated protection are pleiotropic and involve a complex interplay of stroma-produced cytokines, chemokines, and adhesion molecules, the stroma-secreted chemokine stromal-derived factor 1α (SDF-1α) and its cognate receptor CXCR4 have recently emerged as critical mediators of stromal/leukemic cell interactions.7,8 SDF-1α and CXCR4 primarily regulate the migration, homing, and mobilization of hematopoietic cells.9,10 Binding of SDF-1α to CXCR4 causes CXCR4 to be incorporated into lipid rafts11 and increases its phosphorylation.12 The latter leads to prolonged activation of the extracellular signaling–regulated kinase (ERK) and phosphoinositol 3-kinase (PI3K) pathways,13 which are key signaling pathways that promote leukemia cells survival.14,15 Both surface and intracellular16 CXCR4 levels were found to be elevated in a subset of AML cases. Further, CXCR4 has been shown to mediate the homing and engraftment of AML cells to the bone marrow of nonobese diabetes (NOD)/severe combined immunodeficiency (SCID) mice.17,18 Finally, CXCR4 was recently reported to be expressed at higher levels in cases of AML associated with an internal tandem duplication (ITD) type of mutation of the gene that encodes fetal liver tyrosine kinase-3 (FLT3).19 This is one of the most frequent mutations in AML, which confers poor response to chemotherapy and only transient response to FLT3 inhibitors.20,21 Our recent studies, in addition, indicated that CXCR4 expression is associated with poor prognosis in patients with diploid AML regardless of FLT3 mutation status.22,23 Altogether, these findings suggest that disruption of these interactions by SDF-1α/CXCR4 antagonists represents a novel strategy for targeting leukemia/bone marrow microenvironment interactions. We have reported that inhibition of CXCR4 by specific synthetic peptides (ie, RCP168) interferes with stromal/leukemic cell interactions and increases the sensitivity of leukemic cells to chemotherapy.24 In this study, we used AMD3465 (Anormed and Genzyme, Cambridge, MA), a second-generation small-molecule reversible inhibitor of SDF-1α/CXCR4 with a half maximal inhibitory concentration (IC50) for SDF-1α binding of 42 plus or minus 2 nM.25 Its analog plerixafor (AMD3100) has recently demonstrated remarkable clinical activity in mobilizing normal progenitor cells.26,27 AMD3465 antagonized SDF-1α–induced and stroma (MS-5 cells)–induced migration of AML cells and inhibited SDF-1α/CXCR4 signaling. In AML cells harboring FLT3 mutations AMD3465 down-regulated CXCR4 phosphorylation and suppressed stroma-activated PI3K/AKT and MEK/ERK survival pathways. Further, CXCR4 inhibition partially abrogated the protection conferred by stromal cells and enhanced the sensitivity of leukemic cells to chemotherapy and to FLT3 inhibitors in an in vitro coculture system. Finally, we report that in murine in vivo xenograft models, AMD3465 effectively mobilized leukemia cells and stem cells into the circulation and made them more susceptible to chemotherapy-induced or FLT3 inhibitor-induced cell death. These findings strongly support the notion that blockade of SDF-1α/CXCR4 interactions may have utility in eliminating leukemic cells that are otherwise protected by the bone marrow microenvironment.
Methods
Cell culture
MOLM13, U937, and Jurkat cells were purchased from ATCC (Manassas, VA) and maintained in RPMI-1640 medium containing 10% fetal bovine serum (FBS; Gemini Bio-Products, West Sacramento, CA) and 1% penicillin-streptomycin (Gibco Laboratories, Grand Island, NY). The murine pro-B lymphocyte line transfected with wild-type FLT3 (Ba/F3-FLT3) and its subvariant transfected with FLT3-ITD (Ba/F3-ITD) were generated as described elsewhere.28 Ba/F3-FLT3 cells were maintained in RPMI and 10% FBS supplemented with murine interleukin 3 at a concentration of 2 ng/mL. In the indicated experiments, cells were cultured in serum-containing medium supplemented with recombinant Flt3 ligand (FL; R&D Systems, Minneapolis, MN) at a concentration of 25 to 50 ng/mL. The murine stromal cell line (MS-5) was kindly provided by Dr Itoh from Niigata University in Japan.29 The murine A20 cell line was purchased from ATCC, and the A20-luc/yellow fluorescent protein (YFP) B-cell leukemia cell line was described previously.30 Both cell lines were cultured in ATCC complete growth medium (ATCC-30-2001).
Patient sample processing
Peripheral blood and bone marrow samples from patients with AML were collected during routine diagnostic procedures after informed consent was obtained in accordance with Institutional Review Board (IRB) regulations of The University of Texas M. D. Anderson Cancer Center and the Declaration of Helsinki. Mononuclear cells were separated by Ficoll-Hypaque (Sigma-Aldrich, St Louis, MO) density gradient centrifugation.
Chemotaxis studies
For the chemotaxis studies, cells from leukemia cell lines and primary samples were treated with the indicated concentrations of CXCR4 inhibitor AMD3465 (provided by Anormed and Genzyme) in 10% FBS-containing RPMI at 37°C for 30 minutes. A total of 0.5 × 106 treated cells in a volume of 200 μL were added to the top chamber of 6.5-mm diameter Transwell culture inserts (Costar, Corning, NY) with a pore size of 5 μm. Inserts were placed in wells containing 800 μL 10% FBS RPMI or 10% FBS RPMI with the indicated concentrations of SDF-1α (R&D Systems) or the indicated number of MS-5 cells. Chemotaxis assays were performed at 37°C for 4 or 24 hours. Cells that migrated were counted in triplicates by a Vi-CELL XR 2.03 (Beckman Coulter, Fullerton, CA) using trypan blue. Results are expressed as the percentage of migrated cells.
Cell viability and apoptosis assays
For the cell viability assay, 0.1 × 106 MS-5 cells were plated in 6-well plates in α-minimum essential (α-ME) medium supplemented with 10% FBS and penicillin-streptomycin at 37°C in 5% CO2 in a humidified incubator for 3 hours. For the experiments performed under hypoxic conditions, cells grown in the same medium were flushed for 5 minutes with 1% to 3% oxygen, 5% carbon dioxide, and 87% to 89% nitrogen. Leukemic cells were incubated for 1 hour at 37°C either in 10% FBS RPMI alone or in the same medium containing the indicated concentrations of AMD3465 or with anti-CD49d (VLA-4) antibody (NA/LE CD49d, clone 9F10; BD Pharmingen, San Diego, CA) at a density of 0.5 × 106 cells/mL; seeded on top of MS-5 stromal cells that had been washed to remove 10% FBS α-ME medium; and cultured with MS-5 cells in 10% FBS RPMI for 3 hours before the addition of cytarabine (ara-C; Skye Pharmaceutical, San Diego, CA) or FLT3 inhibitors (AG1296; Sigma-Aldrich; and sorafenib; Custom Chemicals, San Diego, CA) at the indicated concentrations. After 24 or 48 hours of incubation at 37°C in a humidified atmosphere containing 5% CO2, cells were harvested with trypsin/ethylenediaminetetraacetic acid (EDTA; Invitrogen, Carlsbad, CA), washed, and resuspended in binding buffer containing annexin V (Roche Diagnostic, Indianapolis, IN). Cells were counterstained with CD45 or CD34 (BD Pharmingen) or with respective isotype control antibody and analyzed by flow cytometry after electronic gating on CD45+ (for cell lines) or CD34+ (for primary AML) leukemic progenitor cells. The specific apoptosis was calculated by the formula: % specific apoptosis = (test – control) × 100/(100 – control).
Western blot analysis
For Western blot analyses, cells were lysed in phosphoprotein lysis buffer (150 mM NaCl, 1 mM MgCl2, 1 mM CaCl2, 10 mM NaF, 5 mM sodium pyrophosphate, 10 mM β-glycerophosphate, 1% Triton X-100, 10 mM iodoacetamide, 1 mM Na3VO4, 0.1% NaN3, and 3 mM phenylmethyl sulfonyl fluoride). Lysis buffer was supplemented with a protease inhibitor cocktail (Roche Diagnostic). Lysates were then separated on a 12% polyacrylamide gel (Bio-Rad Laboratories, Hercules, CA), transferred to Hybond-P membranes (GE Healthcare, Little Chalfont, United Kingdom), probed with the appropriate antibodies (anti–human AKT, Ser 473–phosphorylated AKT, phosphorylated ERK from Cell Signaling Technologies [Beverly, MA]; ERK from Santa Cruz Biotechnology [Santa Cruz, CA]; phosphorylated CXCR4 described previously12 ), and visualized using an enhanced chemiluminescence (ECL) plus kit (GE Healthcare). Western blot analyses were analyzed on a STORM-860 system using ImageQuant software (Molecular Dynamics, Sunnyvale, CA).
CXCR4 and VLA-4 expression studies
For CXCR4 and VLA-4 expression studies, AML cell lines or primary AML cells were adjusted to a density of 0.5 × 106/mL in RPMI-1640 with 0.5% bovine serum albumin. The cells were cultured with CXCR4 inhibitors either at various concentrations for 1 hour or at a constant concentration for the indicated time in the humidified incubator at 37°C with 5% CO2. Cells were then washed with a 20-fold volume of ice-cold buffer without FBS, stained at 4°C with saturating concentrations of phycoerythrin (PE)–conjugated anti-CXCR4 monoclonal antibody (12G5; R&D Systems) or allophycocyanin (APC)–conjugated anti-CD49d monoclonal antibody (9F10; BD Pharmingen), and then analyzed by flow cytometry.
A20-luc/YFP leukemia murine model
All animal work was done in accordance with a protocol approved by the institutional animal care and use committee of the M. D. Anderson Cancer Center (MDACC). BALB/c mice were intravenously injected with A20-luc/YFP cells at a concentration of 0.5 × 106/100 μL 6 hours after irradiation (6 Gy). Bioluminescence imaging, as described elsewhere,31 was used to monitor tumor burden. Briefly, animals were noninvasively imaged using the In Vivo Imaging System (IVIS-200; Xenogen, Hopkinton, MA) after injection with the luciferase substrate colenterazine (native; Biotium, Hayward, CA). Total body bioluminescence was quantified in a region of interest drawn around each mouse. Mice (5 mice/group) were treated on days 7 and 8 after A20 cell injection as follows: AMD3465 10 mg/kg was administered subcutaneously 1 hour before and 1 hour after ara-C administration; ara-C as a single injection intraperitoneally at a dose of either 50 or 100 mg/kg; or their combination. To examine the AMD3465-induced mobilization of the A20-luc/YFP cells, peripheral blood was collected before and 2 hours after AMD3465 administration, and the mobilized cells were detected by flow cytometry. Mice were killed on day 14, and the extent of leukemic infiltration of different organs was assessed by hematoxylin and eosin (H & E) staining.
Ba/F3-ITD/luc/GFP murine tumor model
Ba/F3-ITD/luc/GFP cells (0.5 × 106) were suspended in 10 μL phosphate-buffered saline (PBS) and transplanted directly into the right tibia of C.B-17 SCID (4- to 6-week-old) female mice (Harlan Sprague-Dawley, Madison, WI) using a Hamilton syringe equipped with a 31-gauge needle.32 Mice (7 mice/group) were treated with sorafenib alone (2.5 mg/kg, by mouth, every other day), with AMD3465 (5 mg/kg, subcutaneously, every other day) and granulocyte colony-stimulating factor (G-CSF; Neupogen, AMGEN, Thousand Oaks, CA; 10 μg/mouse, intraperitoneally, every other day), or with sorafenib given 1 hour after AMD3465 and G-CSF administration. The percentage of the circulating Ba/F3-ITD/luc/GFP cells was determined by flow cytometry. Tumor infiltration was monitored by bioluminescence imaging described previously. Selected mice from each group were killed on day 13 or 14, and the extent of leukemic infiltration of different organs was assessed by H & E staining or anti-GFP staining. Overall survival of the remaining mice in each group was estimated by the Kaplan-Meier method.
Immunohistochemistry
Mouse organs were harvested and fixed by immersion in 4% paraformaldehyde. The sections (11 μM) were stained with H & E (×5000; Sigma-Aldrich) and analyzed by light microscopy. For GFP+ staining, the tissue sections were incubated for 1 hour in blocking solution (1× PBS, 0.5% Tween-20, 0.1% BSA) and 10% FBS followed by the incubation overnight with the anti-GFP antibody (Santa Cruz Biotechnology). After washing, sections were incubated for 1 hour with secondary antibody, washed in PBS 3 times, and coverslips were mounted with Fluoromount-G (Electron Microscope Science, Hatfield, PA). The slides were analyzed under a 60×/1.40 PlanApo objective lens on an Olympus FV500 confocal microscope with Fluoview version 4.3 software (Olympus, Melville, NY).
Mobilization of FLT3-ITD cells in vivo
To test the ability of AMD3465 to mobilize leukemic cells harboring FLT3 mutation, 2 types of cells were used to engraft mice: (1) CB.17 (SCID) female mice (5-6 weeks old; purchased from Harlan Sprague-Dawley) were injected intravenously with 0.5 × 106 of Ba/F3-ITD/luc/GFP cells. Two weeks after cell injection, AMD3465 was administrated subcutaneously at a dose of 20 mg/kg per day for 4 days. The percentage of mobilized Ba/F3-ITD/Luc/GFP cells was detected in peripheral blood on day 4 by GFP flow cytometry. (2) Cells from primary AML samples carrying FLT3-ITD mutation were intravenously injected (106/mouse) into NOD/SICD mice 8 hours after irradiation (300 rad). AMD3465 was administered subcutaneously at a dose of 10 mg/kg after leukemia cell engraftment was confirmed by killing 2 randomly selected mice at week 4. Mobilization of human AML cells was detected by anti–human CD45-APC antibody staining after exclusion of nonviable cells by diamidino-2-phenylindole (DAPI; Sigma-Aldrich) 1 hour after AMD3465 administration. The immunophenotypic subset analysis of AML cells was performed by multicolor flow cytometry using anti–human CD38-APC (BD Pharmingen), CD34-fluorescein isothiocyanate (FITC), and CD123-PE (BD Pharmingen).
Statistical analysis
Results are shown as the mean plus or minus the SD or SEM of the results of at least 3 experiments each. The Student paired t test was used for statistical comparison between groups. P values less than .05 were considered statistically significant. Flow cytometry data were analyzed using WinMDI flow software (version 2.8; http://facs.scripps.edu/software.html).
Results
AMD3465 blocks binding of anti-CXCR4 antibody 12G5 to surface CXCR4 and inhibits SDF-1α–induced and stroma-induced chemotaxis of leukemic cells
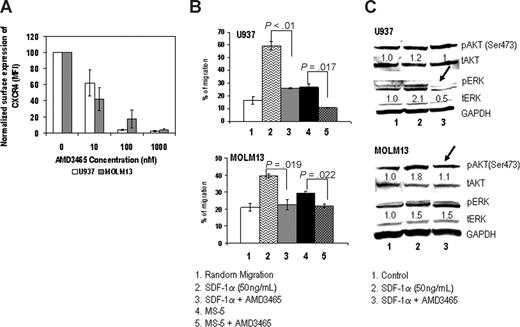
To determine the functional consequences of CXCR4 blockade by AMD3465, we used 2 AML cell lines, U937 and MOLM13, which express high levels of CXCR4 on their cell surface (MFI of U937, 172 ± 7.7; MOLM13, 98 ± 2.1 not shown). First we examined the ability of AMD3465 to compete for CXCR4 binding using the anti-CXCR4 antibody 12G5.33 AMD3465 resulted in concentration-dependent decrease in surface staining of CXCR4 (Figure 1A). Specifically, at 100 nM, AMD3465 reduced the surface staining of CXCR4 to 3.7 plus or minus 0.6% on U937 and 17.2 plus or minus 11% on MOLM13. At 1 μM, it completely suppressed surface staining of CXCR4 in all cell lines tested, compared with 100% in controls. Cell migration experiments demonstrated that SDF-1α and MS-5, a murine stromal cell line known to produce SDF-1α and support growth of human hematopoietic progenitor cells,34 induced the migration of leukemic cells, and these effects were completely abolished by 1 μM AMD3465 (Figure 1B). We next performed Western blot analysis to analyze the activation of ERK and AKT in leukemic cells treated with SDF-1α, alone or in combination with the CXCR4 inhibitor. The results showed that AMD3465 inhibited SDF-1α–induced ERK phosphorylation in U937 cells, and reduced AKT activation in MOLM13 cells (Figure 1C). The similar inhibitory effects of AMD3465 on stroma-induced or SDF-1α–induced migration, and signaling was observed in Jurkat cells, a T-acute lymphocytic leukemia cell line with high expression of CXCR4 (data not shown).
AMD3465 inhibits migration and intracellular signaling in AML cell lines. (A) Surface expression of CXCR4 was measured by flow cytometry, and the results are expressed as percent change in the mean fluorescent intensity (MFI) compared with control (untreated) cells. (B) U937 and MOLM13 cells (0.5 × 106) were plated onto the upper chamber of transwell plates and exposed to 50 ng/mL SDF-1α in the lower chamber or to 0.1 × 106 MS-5 cells preplated in the lower chamber with or without 1 μM AMD3465 for 24 hours. Migrating cells were counted after 24 hours of incubation. The results are expressed as a percentage of the migrating cells relative to the numbers of input cells. (C) Cells were pretreated with or without SDF-1α for 30 minutes, followed by exposure to 1 μM AMD3465 for 4 hours. Phosphorylation of Akt (pAkt) and Erk (pErk) was detected by Western blot analysis, and the intensity of the bands was quantified by densitometry and displayed as ratios of either phospho-proteins to total proteins. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control.
AMD3465 inhibits migration and intracellular signaling in AML cell lines. (A) Surface expression of CXCR4 was measured by flow cytometry, and the results are expressed as percent change in the mean fluorescent intensity (MFI) compared with control (untreated) cells. (B) U937 and MOLM13 cells (0.5 × 106) were plated onto the upper chamber of transwell plates and exposed to 50 ng/mL SDF-1α in the lower chamber or to 0.1 × 106 MS-5 cells preplated in the lower chamber with or without 1 μM AMD3465 for 24 hours. Migrating cells were counted after 24 hours of incubation. The results are expressed as a percentage of the migrating cells relative to the numbers of input cells. (C) Cells were pretreated with or without SDF-1α for 30 minutes, followed by exposure to 1 μM AMD3465 for 4 hours. Phosphorylation of Akt (pAkt) and Erk (pErk) was detected by Western blot analysis, and the intensity of the bands was quantified by densitometry and displayed as ratios of either phospho-proteins to total proteins. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control.
AMD3465 enhances the sensitivity of leukemic cells to chemotherapy in vitro and in vivo
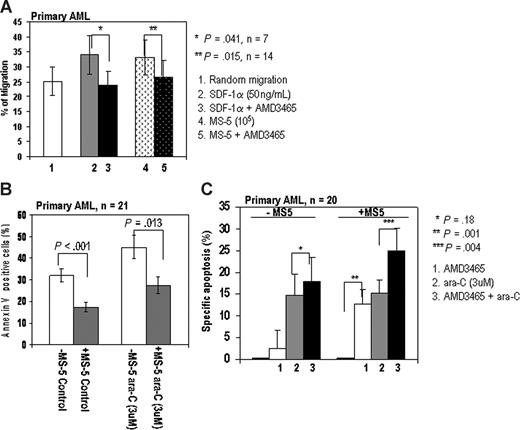
Next, we examined the inhibitory effects of AMD3465 on the SDF-1α–induced or MS-5–induced migration of primary AML cells. AMD3465 significantly inhibited the SDF-1α–induced migration of leukemic cells in all 7 AML samples tested. While random migration was observed in 25% plus or minus 4.8% of the AML cells, SDF-1α induced 35% plus or minus 6.4% of AML cells to migrate, and AMD3465 significantly inhibited this effect in all samples tested. Similarly, MS-5 induced 33% plus or minus 5.8% of AML cells to migrate, and AMD3465 inhibited MS-5-induced migration in 12 of 14 samples examined (Figure 2A). These results suggest that AMD3465 effectively inhibits both SDF-1α–induced and MS-5-induced migration of primary AML cells.
AMD3465 inhibits migration and enhances proaptotic effect of ara-C in primary AML.(A) AMD3465 suppresses SDF-1α–induced (n = 7 samples) and MS-5-induced (n = 14 samples) migration of primary AML cells. The error bars represent SEM. (B) Stromal cells protect primary AML cells (n = 21) from spontaneous and chemotherapy-induced apoptosis. Primary AML cells cultured alone (□) or cocultured with stroma ( , ■) were treated with 3 μM ara-C for 24 hours. The percentage of the apoptotic cells (annexin V–positive cells) were analyzed by flow cytometry. (C) AMD3465 sensitized primary AML cells (n = 20) cocultured with stromal MS-5 cells to ara-C–induced apoptosis (24 hours). Apoptotic cells were detected by annexin V flow cytometry after gating on CD34+ leukemic cells. The specific apoptosis was calculated by the formula: % specific apoptosis = (test – control) × 100/(100 – control).
, ■) were treated with 3 μM ara-C for 24 hours. The percentage of the apoptotic cells (annexin V–positive cells) were analyzed by flow cytometry. (C) AMD3465 sensitized primary AML cells (n = 20) cocultured with stromal MS-5 cells to ara-C–induced apoptosis (24 hours). Apoptotic cells were detected by annexin V flow cytometry after gating on CD34+ leukemic cells. The specific apoptosis was calculated by the formula: % specific apoptosis = (test – control) × 100/(100 – control).
AMD3465 inhibits migration and enhances proaptotic effect of ara-C in primary AML.(A) AMD3465 suppresses SDF-1α–induced (n = 7 samples) and MS-5-induced (n = 14 samples) migration of primary AML cells. The error bars represent SEM. (B) Stromal cells protect primary AML cells (n = 21) from spontaneous and chemotherapy-induced apoptosis. Primary AML cells cultured alone (□) or cocultured with stroma ( , ■) were treated with 3 μM ara-C for 24 hours. The percentage of the apoptotic cells (annexin V–positive cells) were analyzed by flow cytometry. (C) AMD3465 sensitized primary AML cells (n = 20) cocultured with stromal MS-5 cells to ara-C–induced apoptosis (24 hours). Apoptotic cells were detected by annexin V flow cytometry after gating on CD34+ leukemic cells. The specific apoptosis was calculated by the formula: % specific apoptosis = (test – control) × 100/(100 – control).
, ■) were treated with 3 μM ara-C for 24 hours. The percentage of the apoptotic cells (annexin V–positive cells) were analyzed by flow cytometry. (C) AMD3465 sensitized primary AML cells (n = 20) cocultured with stromal MS-5 cells to ara-C–induced apoptosis (24 hours). Apoptotic cells were detected by annexin V flow cytometry after gating on CD34+ leukemic cells. The specific apoptosis was calculated by the formula: % specific apoptosis = (test – control) × 100/(100 – control).
We then tested the ability of CXCR4 inhibition to overcome stroma-mediated chemoresistance of primary AML cells in vitro. Stromal cells significantly protected primary AML cells from spontaneous (32.1% ± 3.1% vs 17.4% ± 2.4%, P < .001, n = 21) and ara-C–induced apoptosis (45% ± 5.6% vs 27.3% ± 3.9%, P = .013; Figure 2B) consistent with our previous report.24 The effects of AMD3465 in combination with chemotherapy were examined in 20 primary AML samples cultured with or without stromal support (Figure 2C). To account for differences in spontaneous apoptosis in cells cultured alone versus cells cocultured with stroma, we expressed the data as percentage of specific apoptosis.35 AMD3465 alone ameliorated stroma-induced protection of AML cells from spontaneous apoptosis (P = .001), with no significant effect on the leukemic cells in the absence of stromal cells. Further, primary AML cells cultured with stromal cells and pretreated with AMD3465 were more sensitive to ara-C than cells treated with either ara-C or AMD3465 alone (P = .004). AMD3465 failed to increase the sensitivity of primary AML cells to ara-C in the absence of stromal cells (P = .18), indicating that the effects of AMD3465 are specific to the interaction of leukemic cells with the stromal layer.
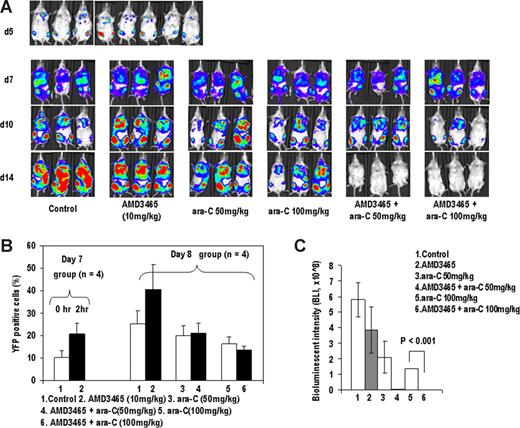
To test if the in vitro findings are applicable to the in vivo systems, we used CXCR4-expressing A20-luc/YFP leukemic cells known to engraft primarily in the bone marrow of BALB/c mice with secondary dissemination to the spleen and other organs.30 Taking into consideration the short half-life of AMD3465 in mice, AMD3465 was injected 1 hour before and 1 hour after ara-C administration on days 7 and 8, after dissemination of leukemic cells initially seeded in the bones (Figure 3A 1st row) into spleen, liver and lungs (Figure 3A 2nd row). The ability of AMD3465 to mobilize leukemic cells was monitored by YFP flow cytometry. AMD3465 induced mobilization of leukemic cells into circulation, with doubling of the percentage of YFP(+) cells in the peripheral blood on days 7 and 8 (Figure 3B). Ara-C given on day 7 resulted in the decrease in the percentage of the circulating leukemic cells measured on day 8, and this was unchanged in mice mobilized with AMD3465 followed by ara-C. Bioluminescence imaging (BLI) on days 10 and 14 showed dose-dependent antitumor effects of ara-C. Strikingly, combination of AMD3465 and ara-C resulted in lack of detectable leukemia by BLI on day 14 (Figure 3A 4th row, and 3C). H & E staining was then performed to confirm that the signal loss was due to tumor cell clearance after mice were killed on day 14. As shown in Figure S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article), A20-luc/YFP leukemic cells homogeneously infiltrated the bone marrow, liver, and spleen of untreated mice, whereas there was no evidence of leukemic cell infiltration in the organs of mice treated with AMD3465 and ara-C.
AMD3465 induces mobilization of A20 cells in vivo and enhances antitumor effects of ara-C. (A) A20-luc/YFP cells were injected IV into BALB/c mice, and bone marrow engraftment was confirmed by bioluminescence imaging on day 5 (top row). Mice were injected with ara-C, AMD3465, or ara-C plus AMD3465 on day 7 and 8 at the indicated doses described in “A20-luc/YFP leukemia murine model.” On days 5, 7, 10, and 14, mice were imaged after D-luciferin injection. Serial images of 3 representative mice are shown on day 7, 10, and 14. (B) AMD3465 was administered on day 7 after tumor cell injection, and percentages of circulating A20-luc/YFP cells in peripheral blood before and after 1 hour of AMD3465 were examined by flow cytometry (left panel). Percentage of circulating A20-luc/YFP-positive cells in control mice, in mice mobilized with AMD3465, or in mice treated with ara-C ± AMD3465 was detected by flow cytometry on day 8 after tumor cell injection (right panel). (C) Bioluminescence imaging results on day 14 were averaged from the peak light-emitting exposure from each group and displayed as photons per second. Error bars represent the SEM of each group.
AMD3465 induces mobilization of A20 cells in vivo and enhances antitumor effects of ara-C. (A) A20-luc/YFP cells were injected IV into BALB/c mice, and bone marrow engraftment was confirmed by bioluminescence imaging on day 5 (top row). Mice were injected with ara-C, AMD3465, or ara-C plus AMD3465 on day 7 and 8 at the indicated doses described in “A20-luc/YFP leukemia murine model.” On days 5, 7, 10, and 14, mice were imaged after D-luciferin injection. Serial images of 3 representative mice are shown on day 7, 10, and 14. (B) AMD3465 was administered on day 7 after tumor cell injection, and percentages of circulating A20-luc/YFP cells in peripheral blood before and after 1 hour of AMD3465 were examined by flow cytometry (left panel). Percentage of circulating A20-luc/YFP-positive cells in control mice, in mice mobilized with AMD3465, or in mice treated with ara-C ± AMD3465 was detected by flow cytometry on day 8 after tumor cell injection (right panel). (C) Bioluminescence imaging results on day 14 were averaged from the peak light-emitting exposure from each group and displayed as photons per second. Error bars represent the SEM of each group.
Combined CXCR4 and FLT3 inhibition reduce SDF-1α–induced and stroma-induced migration and prosurvival signaling pathways in AML cells harboring FLT3 mutations
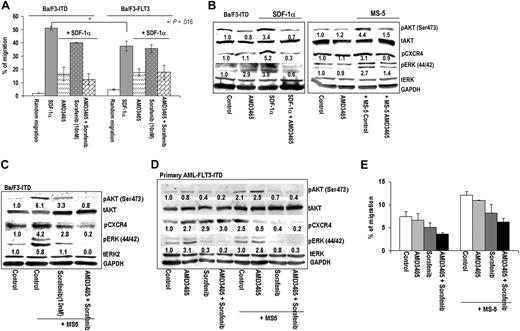
Mutation in the FLT3 receptor was shown to enhance CXCR4 expression and promote SDF-1α–mediated signaling,19 which may in turn limit the efficacy of FLT3 tyrosine kinase inhibitors. We first characterized chemotactic effects in response to SDF-1α in murine Ba/F3 cells transfected with wild-type FLT3 (Ba/F3-FLT3) or with FLT3-ITD (Ba/F3-ITD) cells. SDF-1α induced the migration of both Ba/F3-ITD and Ba/F3-FLT3 cells, with a higher number of FLT3-ITD than Ba/F3-FLT3 cells migrating consistent with published report19 (Figure 4A). FLT3 inhibitor sorafenib alone modestly inhibited the migration of Ba/F3-ITD (from 51% ± 1.4% vs 40% ± 0.1%, P = .057) but not of Ba/F3-FLT3 (from 37.6% ± 3.6% vs 35.6% ± 2.98%, P = .23) cells, indicating partial dependence of SDF-1α–induced migration on FLT3 kinase activity. In turn, AMD3465 significantly inhibited SDF-1α–induced migration of Ba/F3-ITD cells from 51% plus or minus 1.4% to 16.4% plus or minus 5.1% and of Ba/F3-FLT3 cells from 37.6% plus or minus 3.5% to 17.5% plus or minus 2.7%.
AMD3465 inhibits SDF-1α– or stroma-induced migration and suppresses prosurvival signaling pathways in FLT3-mutated cells. (A) Migration of either Ba/F3-ITD or Ba/F3-FLT3 cells in response to 4 hours SDF-1α was examined as described in “Chemotaxis studies” in the presence or absence of 1 μM AMD3465 and/or 10 nM sorafenib. (B) The effects of AMD3465 on the SDF-1α–induced or MS-5–induced up-regulation of pAKT, pERK, and pCXCR4 were analyzed by Western blot analysis in Ba/F3-ITD cells. (C) The combined effects of sorafenib and AMD3465 on AKT, ERK, and CXCR4 phosphorylation were examined in Ba/F3-ITD cells in the presence of MS-5 cells. (D) Primary AML cells with FLT3-ITD mutation grown alone or cocultured with MS-5 cells were exposed to the indicated concentration of AMD3465 alone, 1 μM sorafenib alone, or AMD3465 combined with sorafenib. Phosphorylation of AKT, ERK, and CXCR4 was analyzed by immunoblotting after 24 hours of treatment, and (E) the inhibitory effects of AMD3465 with or without sorafenib on cell migration was measured after 4 hours. The intensity of the phosphorylation bands was quantified by densitometry and displayed as ratios of phosphoproteins either to total proteins or to the loading control GAPDH.
AMD3465 inhibits SDF-1α– or stroma-induced migration and suppresses prosurvival signaling pathways in FLT3-mutated cells. (A) Migration of either Ba/F3-ITD or Ba/F3-FLT3 cells in response to 4 hours SDF-1α was examined as described in “Chemotaxis studies” in the presence or absence of 1 μM AMD3465 and/or 10 nM sorafenib. (B) The effects of AMD3465 on the SDF-1α–induced or MS-5–induced up-regulation of pAKT, pERK, and pCXCR4 were analyzed by Western blot analysis in Ba/F3-ITD cells. (C) The combined effects of sorafenib and AMD3465 on AKT, ERK, and CXCR4 phosphorylation were examined in Ba/F3-ITD cells in the presence of MS-5 cells. (D) Primary AML cells with FLT3-ITD mutation grown alone or cocultured with MS-5 cells were exposed to the indicated concentration of AMD3465 alone, 1 μM sorafenib alone, or AMD3465 combined with sorafenib. Phosphorylation of AKT, ERK, and CXCR4 was analyzed by immunoblotting after 24 hours of treatment, and (E) the inhibitory effects of AMD3465 with or without sorafenib on cell migration was measured after 4 hours. The intensity of the phosphorylation bands was quantified by densitometry and displayed as ratios of phosphoproteins either to total proteins or to the loading control GAPDH.
Similar to the findings in other leukemic cell lines (Figure 1C), SDF-1α or MS-5 coculture induced phosphorylation of AKT, ERK, and CXCR4 itself in Ba/F3-ITD cells, and these effects were inhibited by AMD3465 (Figure 4B). In turn, combined blockade of CXCR4 and mutant FLT3 completely suppressed the expression of pAKT, pEKR, and pCXCR4 stimulated by stroma in Ba/F3-ITD cells (Figure 4C) and in primary AML cells (Figure 4D). Likewise, analysis of cell migration showed that the combination of AMD3465 and sorafenib significantly inhibited the MS-5-induced chemotaxis of primary AML blasts expressing FLT3-ITD (Figure 4E).
AMD3465 facilitates FLT3 inhibitor—induced apoptosis in cells with FLT3 mutation
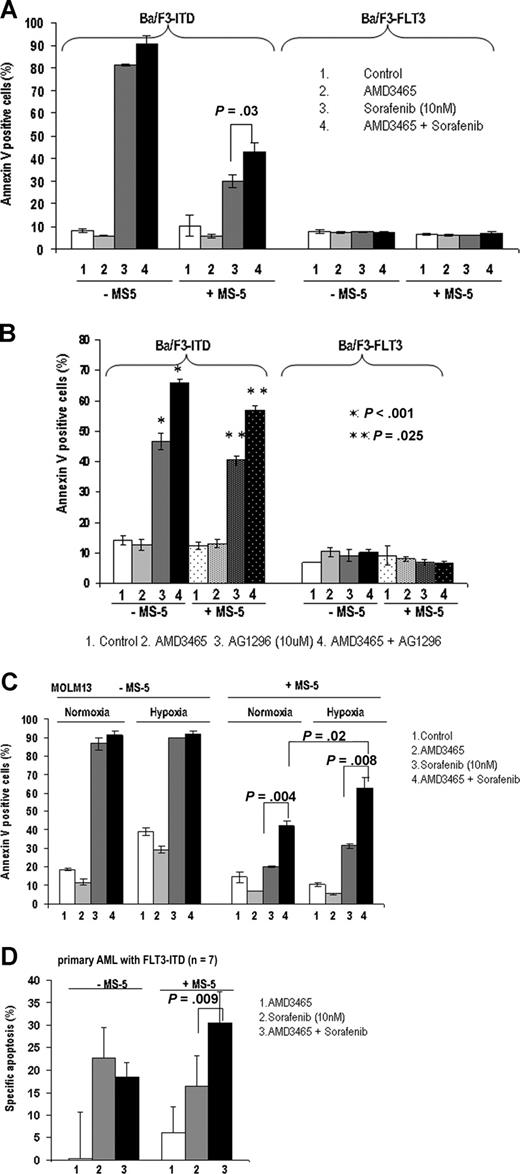
The effect of AMD3465 on FLT3 inhibitor-induced apoptosis was first examined in cells with FLT3-ITD mutation cultured with stromal (MS-5) cells. As indicated in Figure 5A, coculture with stromal cells significantly protected Ba/F3-ITD cells from sorafenib-induced apoptosis (annexin V positivity of 81.3% ± 0.3% vs 30.0% ± 2.7%). CXCR4 blockade with AMD3465 partially abrogated these protective effects (42.9% ± 3.9%, P = .03). In contrast, sorafenib alone or in combination with AMD3465 had no effect on the Ba/F3-FLT3 cells. We further tested the ability of AMD3465 to sensitize cells with FLT3 mutation to the FLT3 inhibitor AG1296. AMD3465 significantly enhanced AG1296-induced apoptosis in FLT3-ITD cells both in the presence or absence of MS-5 (Figure 5B).
AMD3465 sensitizes FLT3-mutated cells to FLT3 inhibitor–induced apoptosis. (A) The average percentage of annexin V+ Ba/F3-ITD and Ba/F3-FLT3 cells after exposure to AMD3465 alone, sorafenib alone, or sorafenib in combination with AMD3465 in the absence or presence of MS-5 cells for 24 hours. (B) Ba/F3-ITD and Ba/F3-FLT3 cells were treated with AMD3465, AG1296 (FLT3 inhibitor), or AG1296 in combination with AMD3465 in the absence or presence of MS-5 cells, and apoptotic cells were detected by annexin V flow cytometry. (C) MOLM13 carrying FLT3-ITD cells were treated with AMD3465, sorafenib, or their combination in the absence or presence of MS-5 cells for 24 hours, under normoxic (21% O2) or hypoxic (2% O2) conditions. Induction of apoptosis was measured by annexin V flow cytometry. (D) Blasts from primary AML samples with FLT3 mutations (n = 7) were treated with sorafenib alone or in combination with AMD3465 in coculture with MS-5 cells for 96 hours, and apoptosis induction was measured by annexin V flow cytometry after gating on CD34+ cells. The specific apoptosis was calculated using the formula described above. Error bars represent the SEM of each group.
AMD3465 sensitizes FLT3-mutated cells to FLT3 inhibitor–induced apoptosis. (A) The average percentage of annexin V+ Ba/F3-ITD and Ba/F3-FLT3 cells after exposure to AMD3465 alone, sorafenib alone, or sorafenib in combination with AMD3465 in the absence or presence of MS-5 cells for 24 hours. (B) Ba/F3-ITD and Ba/F3-FLT3 cells were treated with AMD3465, AG1296 (FLT3 inhibitor), or AG1296 in combination with AMD3465 in the absence or presence of MS-5 cells, and apoptotic cells were detected by annexin V flow cytometry. (C) MOLM13 carrying FLT3-ITD cells were treated with AMD3465, sorafenib, or their combination in the absence or presence of MS-5 cells for 24 hours, under normoxic (21% O2) or hypoxic (2% O2) conditions. Induction of apoptosis was measured by annexin V flow cytometry. (D) Blasts from primary AML samples with FLT3 mutations (n = 7) were treated with sorafenib alone or in combination with AMD3465 in coculture with MS-5 cells for 96 hours, and apoptosis induction was measured by annexin V flow cytometry after gating on CD34+ cells. The specific apoptosis was calculated using the formula described above. Error bars represent the SEM of each group.
Recent data indicate that the bone marrow microenvironment in physiologic conditions is highly hypoxic,36 a condition perhaps even more pronounced in leukemias.37 We therefore compared the effect of AMD3465 under normoxic (21% O2) and hypoxic (2% O2) conditions. AMD3465 further enhanced sorafenib-induced apoptotic cell death of MOLM13 cells carrying mutated FLT3-ITD cultured under hypoxic conditions compared with normoxic conditions (Figure 5C).
Next, we examined the effects of AMD3465 on FLT3 inhibitor-induced apoptosis in primary AML samples. We found that AMD3465 minimally sensitized primary cells without FLT3 mutation cocultured with MS-5 cells to sorafenib-induced apoptosis (1.4% ± 1.5% specific apoptosis in response to sorafenib alone vs 5.7% ± 2.7% with AMD3465 plus sorafenib, n = 7, not shown). On the other hand, AMD3465 enhanced proapoptotic effects of sorafenib in stromal cocultures of AML samples with FLT3 mutations (16.4% ± 6.7% vs 30.5% ± 6.8%, P = .009, n = 7; Figure 5D). No sensitization was seen in cells grown in the absence of stromal layer, suggesting that AML cell death ensues as a result of the interrupted stroma/leukemia cross-talk.
AMD3465 induces mobilization of leukemic cells with FLT3 mutation and enhances antitumor effects of FLT3 inhibitor in vivo
To evaluate the potential of AMD3465 to mobilize AML cells harboring FLT3 mutations, we injected Ba/F3-ITD/luc/GFP cells into SCID mice, and AML blasts from an AML patient into NOD/SCID mice. Compared with mice in the control group, AMD3465 significantly increased the number of circulating leukemic cells in 3 of 4 mice engrafted with Ba/F3-ITD/luc/GFP cells (9.5% ± 0.19% vs 18.7% ± 0.5%, P = .047, Figure S2A, left panel) and in 4 of 5 mice engrafted with human AML cells carrying FLT3-ITD mutation (Figure S2A right panel). Flow cytometric analysis of mobilized human cells collected from mouse no. 1 demonstrated a more than 2-fold increase in CD34+38− and CD34+38+ AML progenitor cells, which also coexpressed the AML stem cell marker CD123 (Figure S2B). Analysis of mobilized cells in mouse no. 4 demonstrated a 3-fold increase in CD34+CD123+ cells (not shown). The white blood cell count in this mouse was 0.9 × 106/mL before mobilization and increased to 2.9 × 106/mL after mobilization; this translated into an absolute increase in CD34+CD123+ progenitor cells from 1268 to 9558 (7.5-fold). These data indicate that AMD3465 can effectively mobilize murine and human FLT3-ITD AML progenitor cells into circulation.
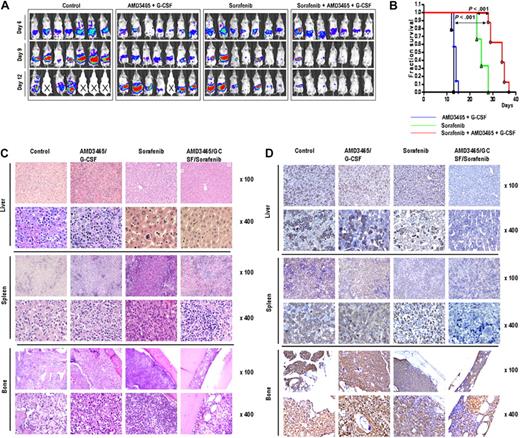
We next evaluated the ability of AMD3465 to enhance antileukemic effects of sorafenib in vivo using Ba/F3-ITD/luc/GFP mouse model. Because mobilizing effects of sorafenib are known to be promoted by G-CSF,38 we used combined G-CSF and AMD3465 administration before sorafenib exposure. Six days after intra-bone marrow injection of Ba/F3-ITD/luc/GFP cells, bioluminescence imaging showed dissemination of leukemic cells into the spleen and liver (Figure 6A top row). Mice were either left untreated or received sorafenib alone or concomitantly with AMD3465/G-CSF injection. The ability of AMD3465 and G-CSF to mobilize leukemic cells was examined in 2 randomly selected mice from AMD3465/G-CSF–treated group on day 12. Combined AMD3465 and G-CSF efficiently increased the percentage of circulating cells from 21.9% to 46.6% in one mouse and from 56.9% to 67.1% in a second mouse (data not shown). All mice from the control group and from the AMD3465-treated group died before day 15 (median survival 12.8 ± 0.4 days in the control and 13.7 ± 0.8 days in the AMD3465 groups). Sorafenib treatment suppressed leukemia progression (median survival 25.3 ± 2.2 days), and this effect was more pronounced in AMD3465/G-CSF–cotreated animals as seen by bioluminescence imaging (Figure 6A) and prolonged survival (median survival 32.6 ± 2.4 days, Figure 6B). This antitumor effect was confirmed by a dramatic decrease in the percentage of the circulating leukemia cells in 2 tested mice from AMD3465/G-CSF/sorafenib group (6.7% and 4.6% GFP+ cells on day 12) compared with sorafenib-treated mice (55.8% and 59.9% GFP+ cells; Figure S3). Consistent with the results of bioluminescence imaging, H&E staining of liver, spleen, and bone marrow demonstrated that tumor cells infiltrated the organs of mice in untreated group, AMD3465/G-CSF, and sorafenib-treated group. Remarkably, the combination of AMD3465/G-CSF and sorafenib resulted in lack of detectable tumor cells in the spleen and liver and a significant decrease in the tumor cell numbers in the bone marrow (Figure 6C). Staining using anti-GFP antibody to specifically identify leukemic cells and confirmed the dramatic reduction of leukemia infiltration in these tumor sites (Figure 6D).
In vivo effects of AMD3465/G-CSF and sorafenib in a mouse xenograft model of FLT3-ITD mutant leukemia. (A) Serial bioluminescence images of mice in the groups receiving sorafenib, AMD3465/G-CSF, sorafenib combined with AMD3465/G-CSF, or in the group without any treatment (control) were taken on days 6, 9, and 12 after tumor cell injection. Deceased mice are identified by “X” sign. (B) Overall survival in each group was estimated by Kaplan-Meier method. Statistical significance was calculated using the log-rank test. (C,D) Histologic sections of liver, spleen, and bone marrow of mice stained with H&E (C) or anti-GFP antibody (D) in untreated mice (day 13), AMD3465/G-CSF (day 14), sorafenib (day 14), or AMD3465/G-CSF + sorafenib (day 14) treated mice.
In vivo effects of AMD3465/G-CSF and sorafenib in a mouse xenograft model of FLT3-ITD mutant leukemia. (A) Serial bioluminescence images of mice in the groups receiving sorafenib, AMD3465/G-CSF, sorafenib combined with AMD3465/G-CSF, or in the group without any treatment (control) were taken on days 6, 9, and 12 after tumor cell injection. Deceased mice are identified by “X” sign. (B) Overall survival in each group was estimated by Kaplan-Meier method. Statistical significance was calculated using the log-rank test. (C,D) Histologic sections of liver, spleen, and bone marrow of mice stained with H&E (C) or anti-GFP antibody (D) in untreated mice (day 13), AMD3465/G-CSF (day 14), sorafenib (day 14), or AMD3465/G-CSF + sorafenib (day 14) treated mice.
Discussion
Identification of the factors contributing to microenvironment-mediated chemoresistance remains an unresolved challenge important for the eradication of residual chemosensitive leukemic blasts hiding in the bone marrow niches. Our findings indicate that CXCR4 inhibition interferes with stromal/leukemic cell interactions, confirming the functional significance of SDF-1α/CXCR4 cross-talk. The small molecule CXCR4 inhibitor AMD3465 inhibited the migration of AML cell lines and primary AML cells induced by SDF-1α or stroma and partially abrogated the stroma-mediated protection of primary AML blasts from chemotherapy-induced apoptosis in the stromal coculture systems. While these effects were of moderate magnitude in vitro, in the in vivo model of bone marrow–resident leukemia combination of AMD3465 with ara-C resulted in complete eradication of leukemia by bioluminescence imaging and histology. This suggests that in vitro systems do not completely mimic the in vivo interaction between leukemic cells and the bone marrow microenvironment. Monitoring of circulating leukemic cells confirmed the ability of AMD3465 to induce the mobilization of leukemic cells, which likely contributed to the enhanced killing of leukemia cells by ara-C. Notably, our group demonstrated the massive mobilization of up to 80% circulating leukemic cells from the bone marrow of patients in morphologic complete remission who received AMD3100 in combination with G-CSF with the goal of mobilizing progenitor cells for autologous stem cell transplantation.39 Similar findings were recently reported in a case report whereby AMD3100 administration resulted in a dramatic reduction of CD34+ blasts in the bone marrow of AML patient.40 These findings support the concept of mobilizing bone marrow–resident leukemic cells by CXCR4 blockade. While physical disruption of leukemia/stroma interactions with resulting mobilization is clearly one of the principal effects of CXCR4 inhibitors, other mechanisms likely contribute to their chemosensitizing effects. For example, we have reported the potential of stromal cells to activate multiple signaling cascades in leukemic cells, including PI3K/AKT and MAPK prosurvival pathways.41 The data we present here add to this understanding by showing that stroma-secreted SDF-1α is one of the contributing factors, because AMD3465 was able to inhibit not only SDF-1α, but also stroma, induced AKT and ERK phosphorylation in certain cell types. Identification of the key downstream target(s) of these and other signaling pathways activated by stromal cells may be important for combination strategies targeting microenvironment-mediated resistance.
In vitro and in vivo studies indicate that several components of the bone marrow microenvironment including endothelial cells,42 osteoblasts,43 and adipocytes44 contribute to the enhanced survival of the leukemic cells. Furthermore, several adhesion molecules, including VCAM-1, VLA-4, and CD44 have been shown to contribute to microenvironment-mediated resistance in AML.45,46 In ongoing studies, we observed synergistic sensitization of primary AML blasts to chemotherapy via simultaneous blockade of CXCR4 and VLA-4 (Figure S4). These findings indicate that disrupting several key factors involved in the leukemia/stroma interactions may be necessary for complete eradication of bone marrow–resident AML cells from bone marrow niches.
Recent characterization of the FLT3 tyrosine kinase receptor as one of the most common target for mutations in AML47 led to the development and clinical trials with small-molecule FLT3 inhibitors. Curiously, clinical observations with several structurally different FLT3 inhibitors demonstrated near-complete clearing of the circulating peripheral blood blasts, but minimal effects on bone marrow–resident blasts, suggesting a contribution of the microenvironment to the nonpharmacologic resistance to these agents. Building on the observation that FLT3-ITD activates CXCR4 signaling19 and is associated with increased CXCR4 expression in primary AML,23 we examined the role of SDF-1α/CXCR4 interactions in the resistance of AML cells to FLT3 inhibitors under the conditions mimicking bone marrow microenvironment. To this end, we took advantage of the isogenic BaF3 cells transfected with either wild-type or mutant forms of FLT3. In this system, SDF-1α induced greater migration of Ba/F3-ITD than of Ba/F3-FLT3 cells, consistent with previous reports.19 While the FLT3 inhibitor sorafenib48 did not affect CXCR4 expression (not shown), it partially inhibited the SDF-1α–induced or stroma-induced migration and AKT/ERK phosphorylation of AML cells with mutated ITD, suggesting that FLT3-ITD indeed contributes to activation of CXCR4 signaling in these cells. Most importantly, combined blockade of mutant FLT3 and of CXCR4 completely blocked prosurvival signaling pathways in stromal cocultures.
Based on these observations, we tested the ability of CXCR4 inhibitors to sensitize FLT3-mutated cells to the proapoptotic effects of FLT3 inhibitors under stroma coculture conditions. Stromal cells significantly diminished the sorafenib- and AG1296-induced apoptosis of BaF3/ITD cells, and this protection was in part attenuated by AMD3465. Notably, these effects were more pronounced under hypoxic conditions closely resembling the physiologic low-oxygen state of the bone marrow, suggesting that modulation of CXCR4 function via hypoxia-induced transcription factor HIF-1α49-51 enhances the dependence of leukemic cells on prosurvival effects of the niche. Most importantly, AMD3465 enhanced sorafenib-induced apoptosis in samples from primary AML patients with FLT3 mutation. Likewise, CXCR4 inhibition via combined use of AMD3465 and G-CSF effectively mobilized FLT3-ITD cells from the murine bone marrow and facilitated sorafenib-induced elimination of microenvironment-resident leukemic blasts in vivo. Further studies are ongoing to confirm our preliminary observation of the ability of CXCR4 blockade to cause mobilization of AML stem cells in an in vivo model of human leukemia.
In summary, these studies for the first time provide evidence for stroma-mediated resistance not only to standard chemotherapy but also to kinase inhibitors targeting specific genetic aberrations in leukemic cells. We have recently reported similar phenomenon in CML, where by CXCR4 inhibition sensitized CML cells to the proapoptotic effects of Bcr-Abl inhibitors.52 Our findings indicate that SDF-1α/CXCR4 interactions largely contribute to the protection mediated by the microenvironment, particularly in cells with mutated FLT3 that have acquired several mechanisms to promote CXCR4 signaling. Likewise, other components of bone marrow microenvironment, such as hypoxia and extracellular matrix, may modulate CXCR4 signaling in leukemic cells, hence arguing for the use of murine models as better predictors of the efficacy of these agents in vivo. Altogether, these results provide rationale for studies of CXCR4 inhibitors in combination with standard or targeted therapy with the goal to attenuate microenvironment-triggered nonpharmacologic chemoresistance. These concepts will be tested in upcoming clinical trials in AML patients.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the National Institutes of Health (AML P0I (CA55164) and a Cancer Center Support grant (CA100632).
National Institutes of Health
Authorship
Contribution: Z.Z., Y.X.S., R.-Y.W., X.L., and O.F. conducted the experiments; M.L., J.B.R., and R.R.N. provided experiment materials; I.J.S., S.K., M.A., and M.K. analyzed and interpreted the data; E.H.E., S.K., M.A., and M.K. provided clinical advice; M.A. and M.K. designed and supervised the research; and Z.Z. and M.K. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marina Konopleva, Department of Leukemia, Unit 428, 1515 Holcombe Blvd, The University of Texas M. D. Anderson Cancer Center, Houston, TX 77030; e-mail: mkonople@mdanderson.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal