Abstract
In this issue of Blood, Swann and colleagues show that type I NKT cells mediate protection against tumor development in p53-deficient mice.
Tumor suppressor p53 serves as a guardian of the genome and plays a key role in preserving genomic integrity in response to several stressors including DNA damage and oncogene activation.1 Not surprisingly, malfunction of the p53 pathway is an almost universal feature of human cancers. However, when this cell-intrinsic control fails, the immune system may provide an additional barrier to the develop-ment of cancers.
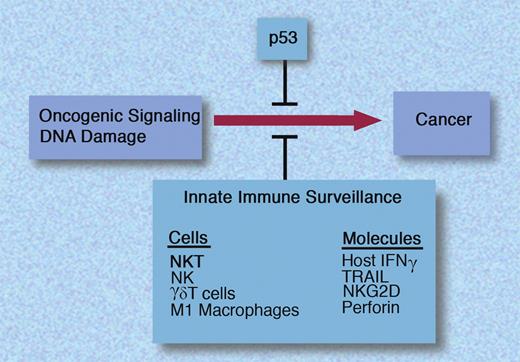
Intrinsic and innate immune barriers to tumorigenesis. INF indicates interferon-gamma; TRAIL, tumor necrosis factor–related apoptosis-inducing ligand; and NK, natural killer. Professional illustration by Marie Dauenheimer.
Intrinsic and innate immune barriers to tumorigenesis. INF indicates interferon-gamma; TRAIL, tumor necrosis factor–related apoptosis-inducing ligand; and NK, natural killer. Professional illustration by Marie Dauenheimer.
Natural killer T (NKT) cells are innate lymphocytes that recognize glycolipid ligands in the context of CD1d.2 At least 2 broad groups of NKT cells have been described. Type I NKT cells express an invariant T-cell receptor (Vα24+ in humans), and have been extensively studied in the context of response to glycolipid, α-galactosylceramide. In contrast, type II NKT cells express diverse T-cell receptors and ligands recognized by these cells are just beginning to emerge.3 NKT cells have been implicated as conductors of the immune system and can have several effects, including the activation of NK cells and dendritic cells (DCs), ultimately leading to the activation of adaptive immunity.4 Type I NKT cells can mediate antitumor effects by several mechanisms including NK activation, direct cytotoxicity, and antiangiogenesis. In this issue, Swann et al demonstrate a critical role for type I NKT cells in protection against diverse tumor types in a clinically relevant model of p53-deficient mice.5 These findings set the stage for studies to better understand the mechanism of tumor immune surveillance by NKT cells. It would be important to discern the overlap/synergy with other pathways such as host interferon-γ, tumor necrosis factor–related apoptosis-inducing ligand (TRAIL), and perforin, also known to regulate tumorigenesis in this setting6 (see figure). Better understanding of the biology of tumors that escape protection by NKT cells is also needed. Stressors, such as DNA damage, that activate intrinsic defenses (such as p53) may sensitize tumor cells for innate immune defense.6 Therefore, the 2 pathways may provide a synergistic defense against tumorigenesis.
The data in this paper suggest that the effects of type I NKT cells may dominate over those of type II NKT cells in these mice. However, improved understanding of type II NKT cells is needed to elucidate their role in tumor immunity and cross-talk with type I NKT cells. It is also critical to understand the nature of tumor-derived ligands recognized by both type I and II NKT cells in mice and humans.
However, in contrast to mice, the proportion of type I NKT cells in human blood and tissues is much lower, and there may be additional differences in NKT subsets and biology between the 2 species.2 Nonetheless, these findings provide strong support for systematic studies to manipulate NKT cell function in humans and harness the antitumor properties in the clinic. Availability of novel glycolipid ligands, as well as the development of combination approaches to enhance NKT cell function are therefore of considerable interest and potential in immunotherapy of cancer.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■
REFERENCES
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal