Abstract
We have generated 2 zebrafish lines carrying inactivating germline mutations in the von Hippel-Lindau (VHL) tumor suppressor gene ortholog vhl. Mutant embryos display a general systemic hypoxic response, including the up-regulation of hypoxia-induced genes by 1 day after fertilization and a severe hyperventilation and cardiophysiologic response. The vhl mutants develop polycythemia with concomitantly increased epo/epor mRNA levels and erythropoietin signaling. In situ hybridizations reveal global up-regulation of both red and white hematopoietic lineages. Hematopoietic tissues are highly proliferative, with enlarged populations of c-myb+ hematopoietic stem cells and circulating erythroid precursors. Chemical activation of hypoxia-inducible factor signaling recapitulated aspects of the vhl−/− phenotype. Furthermore, microarray expression analysis confirms the hypoxic response and hematopoietic phenotype observed in vhl−/− embryos. We conclude that VHL participates in regulating hematopoiesis and erythroid differentiation. Injections with human VHLp30 and R200W mutant mRNA demonstrate functional conservation of VHL between mammals and zebrafish at the amino acid level, indicating that vhl mutants are a powerful new tool to study genotype-phenotype correlations in human disease. Zebrafish vhl mutants are the first congenital embryonic viable systemic vertebrate animal model for VHL, representing the most accurate model for VHL-associated polycythemia to date. They will contribute to our understanding of hypoxic signaling, hematopoiesis, and VHL-associated disease progression.
Introduction
The von Hippel-Lindau tumor suppressor (VHL) plays a critical role in the adaptive cellular response to hypoxia through the negative regulation of hypoxia-inducible factors (HIFs). VHL protein (pVHL) is the substrate recognition component of the multisubunit VHL-E3 ubiquitin ligase complex. The interaction between pVHL and HIF-α subunits (HIF-1-3α) requires oxygen-dependent hydroxylation of HIF-α on either of 2 prolyl residues by the family of prolyl hydroxylases. Upon binding pVHL, HIF-α is targeted for proteasomal degradation by polyubiquitination. In the absence of oxygen or functional pVHL, HIF-α is stabilized and translocated to the nucleus, where it partners with HIF-β to mediate transcription of hypoxia-inducible genes to facilitate glucose uptake, glycolysis, and oxygen delivery by increasing angiogenesis and erythropoiesis (for review, see Kaelin1 ).
Congenital erythrocytoses or polycythemias are rare autosomal recessive disorders often found to be associated with homozygous or compound heterozygous germline mutations in VHL (for review, see Gordeuk et al2 ). The most frequently found mutation in VHL-related erythrocytosis is the C598T transition,3 inducing the Chuvash form of polycythemia (CP). The resulting Arg200Trp (R200W) substitution at the extreme C terminus of pVHL diminishes the binding affinity for hydroxylated HIF-α, thereby partially inhibiting its normoxic ubiquitination and degradation that results in the pathologic up-regulation of HIF target genes, including the following: glucose transporter member 1 (GLUT1), vascular endothelial growth factor (VEGF), and erythropoietin (EPO).3 Chuvash polycythemia is further characterized by high hemoglobin and hematocrit levels. Patients have abnormalities in respiratory and pulmonary vascular regulation. Basal ventilation and pulmonary vascular tone are elevated, and ventilatory, pulmonary vasoconstrictive, and heart rate responses to acute hypoxia are greatly increased.4 Premature mortality is observed as a result of cerebral vascular events or peripheral thrombosis.5
CP is distinct from the hereditary cancer syndrome known as VHL disease, which is associated with heterozygous fully inactivating germline mutations in VHL.1 Inactivation of the remaining allele in somatic cells predisposes VHL patients to the development of highly vascularized tumors and cysts in many organ systems, including hemangioblastomas of the retina and central nervous system, pheochromocytomas, and clear-cell renal cell carcinoma (ccRCC).1,6 Patients with VHL disease can develop secondary polycythemias in response to EPO production from these tumors (prevalence of ∼ 5%-20%).6,7 Chuvash polycythemia patients, however, are not predisposed to cancer.5
To study VHL function in vivo and to gain insight into these 2 distinct disease etiologies, several murine models have been generated (for review, see Haase).8 Vhlh knockout mice obtained by conventional homologous recombination die in utero between E11.5 and 12.5 due to hemorrhagic lesions in the placenta.8 To avoid embryonic lethality, Cre/lox site-specific recombination technology was used, and multiple tissue- or organ-specific Vhlh deletions were engineered. Whereas several VHL disease aspects were observed, conditional knockout mice do not develop human VHL disease-associated tumors or ccRCC. Deletion of Vhlh in the liver and kidney results in secondary polycythemias as a cause of increased EPO production in the liver.8 Mice homozygous for the CP R200W mutation were recently shown to develop polycythemia from 10 to 14 weeks after birth similar to the human disease; however, cardiopulmonary defects were not described.9
Although these murine models have contributed greatly to our understanding of VHL function, their use is limited because they lack important aspects of VHL-associated pathologies. To explore the underlying mechanisms of CP and VHL disease, an additional animal model is warranted. Zebrafish have become a valuable model system for vertebrate biology, human disease, and cancer.10 Importantly, the HIF pathway11,12 is highly conserved, as are other vertebrate genetic programs such as hematopoiesis13,14 and EPO15 signaling.
In this study, we report the identification and first characterization of the zebrafish VHL ortholog vhl. Using target-selected gene inactivation,16 we have generated 2 different zebrafish lines carrying early inactivating germline mutations in vhl. Homozygous and transheterozygous mutants faithfully recapitulate Chuvash polycythemia, including key aspects that were not observed in existing VHL mouse models. Unlike Vhlh knockout mice, which die during early embryonic stages, zebrafish vhl mutants complete embryogenesis and survive up to larval stages (8-11 days postfertilization [dpf]). Therefore, vhl−/− zebrafish represent a unique vertebrate animal model in which the role of VHL can be studied during both embryonic and postembryonic stages. Our results demonstrate that this novel genetic zebrafish vhl model will provide a basis for further studies to gain new insights into the role of VHL in the context of oxygen homeostasis and hematopoiesis.
Methods
Zebrafish strains and screening methods
Zebrafish were maintained as described.17 Animal experiments were conducted in accordance with the Dutch guidelines for the care and use of laboratory animals, with the approval of the Animal Experimentation Committee (Dier Experimenten Commissie [DEC]) of the Royal Netherlands Academy of Arts and Sciences (Koninklijke Nederlandse Akademie van Wetenschappen [KNAW]). An N-ethyl-N-nitrosourea (ENU)–induced mutation library was screened for vhl mutants by sequence analysis of the first 2 exons, according to the Hubrecht TILLING protocol.16 Identified mutant alleles vhlhu2117(Q23X) and vhlhu2081(C31X) were outcrossed to wild-type AB and TL, and transgenic TG(gata1:egfp),18 TG(CD41:egfp),19 and TG(cmlc2:egfp)20 lines. Unless indicated otherwise, transheterozygote embryos (vhlhu2117/vhlhu2081) were used in experimental assays. Where indicated, embryos were anesthetized with MS222 (final concentration of 0.17 mg/mL).
In situ hybridization
A full-length vhl construct and antisense digoxygenin (Roche, Almere, The Netherlands)–labeled mRNA probe were generated, as described in Document 1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). Other antisense mRNA probes used were either transcribed from expressed sequence tag (EST) clones (RZPD/Imagenes, Berlin, Germany): prolyl hydroxylase 3 (phd3; BC066699), lactate dehydrogenase A (ldha1; BC067188), reduced nicotinamide adenine dinucleotide dehydrogenase 1α subcomplex 4 (ndufa4; BC059706), epo (CK239342), epor (NM_001043334), glut1 (BI706432), lck (NM_001001596); or as previously described: vegfa,21 scl,22 gata1,21 ikaros,23 l-plastin,24 mpo,25 c-myb,26 βE1 globin,27 kdr-like,28 and kdr.28 Whole-mount in situ hybridizations were performed as described in Document 1. Embryos were imaged in 2% methylcellulose on a depression slide and imaged on a Zeiss (Sliedrecht, The Netherlands) Axioplan microscope with a 5× objective.
Agilent microarray expression profile
Total RNA from 40 vhl mutants and 40 siblings (equally pooled from 4 different clutches) at 7 dpf was isolated using TRIzol (Invitrogen, Breda, The Netherlands). A 4 × 44K zebrafish expression array (G2519F; Agilent Technologies, Amstelveen, The Netherlands) was performed according to the manufacturer's protocol (Document 1). Scanned images were processed using the Feature Extraction software (Agilent Technologies), and analyzed (P ≤ .01) using Limma in R software.29 These data have been deposited in the National Center for Biotechnology Information's Gene Expression Omnibus, accessible through GEO Series accession number GSE14866.
Bromodeoxyuridine labeling and immunohistochemistry
Seven-day-old embryos were pulsed with 3 mM bromodeoxyuridine (BrdU; Sigma-Aldrich, Zwijndrecht, The Netherlands) in embryo medium for 6 hours at 28°C and fixed in 4% paraformaldehyde overnight. BrdU incorporation and l-plastin protein levels were detected by immunohistochemistry (Document 1). Antibodies used were mouse anti-BrdU (1:100; Dako, Heverlee, Belgium), rabbit anti–l-plastin30 (1:500), anti–mouse IgG horseradish peroxidase (HRP; 1:300; Dako), and anti–rabbit Alexa488 (1:500; Invitrogen/Molecular Probes, Breda, The Netherlands). Transversal sections (7 μm) of plastic-embedded BrdU-labeled embryos were imaged on a Zeiss Axioplan microscope with a 20× objective, using a Leica (Leica Microsystems, Rijswijk, The Netherlands) DFC480 digital camera. l-plastin–stained embryos were imaged using a Leica MZ10F stereomicroscope at an 8 × 10 magnification.
Hematopoietic analyses
For blood smears, o-dianisidine and May-Grünwald-Giemsa–staining procedures, and iron injections, see Document 1. Blood cell images were collected on a Zeiss Axioskop 2 with a 40× objective, using a Leica DFC490 digital camera. CD41–green fluorescent protein (GFP)+ hematopoietic cells were imaged on a Leica DM IRE2 confocal microscope using a 40× oil objective.
Determining heart rate, stroke volume, and cardiac output
Heart rate, stroke volume, and cardiac output were determined by high speed videomicroscopy, as previously described31 (Document 1).
Generation of full-length human VHLp30 and VHL R200W rescue constructs
VHLp30 (reference Lolkema et al32 ) and VHL R200W rescue constructs and mRNA were generated as described in Document 1. For mRNA injections, 7.5 pg of VHLp30 or VHL R200W mRNA was injected into the yolk of 1-cell stage embryos, and translation of the mRNAs was verified by Western blot analysis. After phenotypic analysis, embryos were genotyped.
Western analyses
Protein samples were prepared as described in Document 1. Vhl, VHL, and R200W protein bands were visualized using supernatant from anti-VHL monoclonal hybridoma 1B3B11 (1:4).33 Other antibodies used were anti-signal transducer and activator of transcription (STAT)5 (1:500, sc-836; Santa Cruz Biotechnology, Santa Cruz, CA), anti-phosphoSTAT5 (Y694; 1:500, 9351S; NEB, Beverly, MA), beta-actin (1:20 000; Abcam), rabbit anti–mouse HRP (1:20 000; Pierce, Rockford, IL), and swine anti–rabbit HRP (1:2000; Dako).
Dimethyloxaloylglycine treatment
Wild-type embryos were treated with 100 μM dimethyloxaloylglycine (DMOG; Frontier Scientific, Logan, UT) or a 0.1% dimethylsulfoxide (DMSO; Sigma-Aldrich) vessel control in 1 mL embryo medium at 28°C in 24-well culture plates, containing 12 embryos per well. Treatments were started at 3 dpf and continued for 4 days with a daily refreshment of the medium. Experiments were performed in triplicate samples and repeated at least 3 independent times.
Results
Identification of zebrafish VHL ortholog vhl
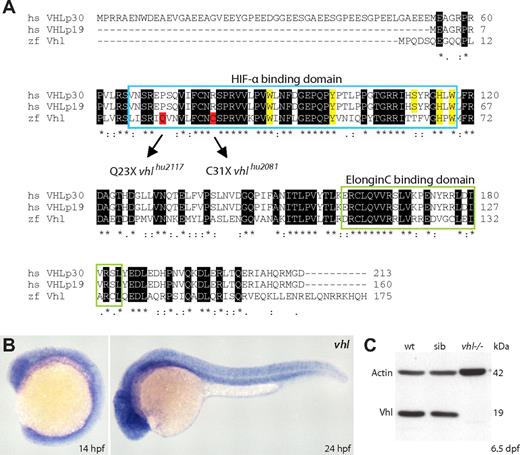
The zebrafish VHL ortholog was determined in silico by blast search analysis of the human VHL (p30) protein against the zebrafish translated database (tblastn) (http://www.ncbi.nlm.nih.gov/BLAST/). Identified EST clone CK360574 showed the highest sequence similarity with VHL. A blastx query against the human nonredundant protein database confirmed this coding sequence to represent the best reciprocal hit. Phylogenetic analysis showed the zebrafish clone to cluster with VHL homologs from fish to mammals, confirming its orthology (Figure S2). Zebrafish vhl (zgc:158722) is located on chromosome 6, around position 27.3 Mb on the current Zv7 Ensembl genome assembly. This region is syntenic with the location of human VHL on chromosome band 3p25.3 (Figure S3). vhl consists of 3 exons that encode a 175-amino-acid protein that is 52% identical and 70% similar to human pVHL. Zebrafish vhl has 1 transcript, and protein sequence alignment reveals high conservation of the Elongin C binding domain involved in the polyubiquitination of HIF-α subunits and the HIF-α binding domain. Furthermore, 4 of the 5 residues binding the HIF-1α core hydroxyproline Pro564 within the oxygen-dependent degradation domain (W88, Y98, H115, W117) are conserved (Figure 1A, highlighted in yellow). Zebrafish Vhl lacks the N-terminal acidic domain, found in the first 53 amino acids of VHLp30. Notably, comparative genomics shows this domain of unknown function to be mammal specific, shorter in rodents, and absent in the fly and worm.34 During embryonic development, vhl is ubiquitously expressed, as shown by whole-mount in situ hybridization on wild-type embryos at 14 and 24 hours after fertilization (hpf; Figure 1B).
Identification of zebrafish vhl and loss-of-function alleles. (A) The zebrafish VHL ortholog vhl encodes a single 175-amino-acid protein that is 52% identical and 70% similar to human pVHL. ClustalW alignment shows high conservation of the pVHL Elongin C and HIF-α binding domains (HIF-1α core hydroxyproline Pro564 binding sites are highlighted in yellow). The VHLp30 isoform-specific acidic domain (residues 1-53) is not present. By screening the Hubrecht target-selected ENU-mutagenized library, we identified 2 different zebrafish lines carrying inactivating germline vhl mutations within the HIF-α binding domain (Q23X and C31X; highlighted in red). (B) Whole-mount in situ hybridizations show ubiquitous vhl expression in wild-type embryos at 14 and 24 hpf. Original magnification ×5. (C) Vhl protein (∼ 19 kDa) is not detected in 6.5 dpf vhl mutant zebrafish. One embryo equivalent is loaded per lane. β-Actin is used as a loading control. Wt, wild-type; sib, sibling.
Identification of zebrafish vhl and loss-of-function alleles. (A) The zebrafish VHL ortholog vhl encodes a single 175-amino-acid protein that is 52% identical and 70% similar to human pVHL. ClustalW alignment shows high conservation of the pVHL Elongin C and HIF-α binding domains (HIF-1α core hydroxyproline Pro564 binding sites are highlighted in yellow). The VHLp30 isoform-specific acidic domain (residues 1-53) is not present. By screening the Hubrecht target-selected ENU-mutagenized library, we identified 2 different zebrafish lines carrying inactivating germline vhl mutations within the HIF-α binding domain (Q23X and C31X; highlighted in red). (B) Whole-mount in situ hybridizations show ubiquitous vhl expression in wild-type embryos at 14 and 24 hpf. Original magnification ×5. (C) Vhl protein (∼ 19 kDa) is not detected in 6.5 dpf vhl mutant zebrafish. One embryo equivalent is loaded per lane. β-Actin is used as a loading control. Wt, wild-type; sib, sibling.
Careful analysis of the tblastn hits of the human VHLp30 against the zebrafish-translated database revealed another vhl-like predicted sequence (XM_001335821), 35% and 33% identical and 52% and 56% similar to the human and zebrafish Vhl protein, respectively. A blast search of the putative vhl-like protein sequence against the EST database (National Center for Biotechnology Information) revealed the presence of a homolog only in some other fishes (minnow, rainbow trout, and dogfish). Phylogenetic analysis showed Vhl-like homologs to cluster together in a separate group from Vhl (Figure S2), suggesting this gene to be fish specific. vhl-like, or Dr.19805, is located on chromosome 4, around position 13.5 Mb on Zv7 in a region that is not syntenic with human 3p25.3 (data not shown). Intriguingly, alignment of Vhl-like and VHL proteins revealed the presence of several amino acids in Vhl-like that have been reported in the VHL Mutations Database (http://www.umd.be/VHL/, consulted by E.v.R.) as loss-of-function mutations in human VHL disease (P86A [within the HIF-α domain], T133S, V155M, and Q164R [within the Elongin C binding domain]). Furthermore, whereas the arginine at position 200 is conserved in zebrafish Vhl, in Vhl-like it is substituted for a threonine residue. In humans, the only mutation described at this position is CP R200W; however, using PolyPhen (http://tux.embl-heidelberg.de/ramensky/polyphen.cgi), the impact of an R200T substitution was predicted to be possibly damaging to the structure and function of VHL (PSIC score > 2). We have not yet been able to generate genetic vhl-like mutant zebrafish, and therefore restrict our analysis in this study to the pathophysiologic implications of vhl loss.
By screening the Hubrecht target-selected ENU-mutagenized F1 zebrafish library,16 we identified 2 different lines carrying early nonsense mutations in the Hif-α binding domain of vhl (Figure 1A, highlighted in red). Mutations were confirmed by resequencing, and identified mutant alleles, designated vhlhu2117(C/T, Q23X) and vhlhu2081(C/A, C31X), were successively outcrossed 3 to 4 times into wild-type lines to reduce the chance of background mutations. We verified Mendelian cosegregation of the developmental defects in F3 progeny of single and complemented mutant alleles, and did not observe any phenotypical differences between alleles. Unless indicated otherwise, vhlhu2117/vhlhu2081 transheterozygous mutant embryos are presented (hereafter called vhl−/−). We have not observed phenotypes in embryonic or adult vhl heterozygotes. Because Western blot analysis using an anti–human VHL antibody33 could not detect Vhl protein (∼19 kDa) in 6.5-day-old vhl mutants (Figure 1C), we assume both vhl alleles to represent a loss-of-function situation.
vhl mutants display a systemic hypoxic response
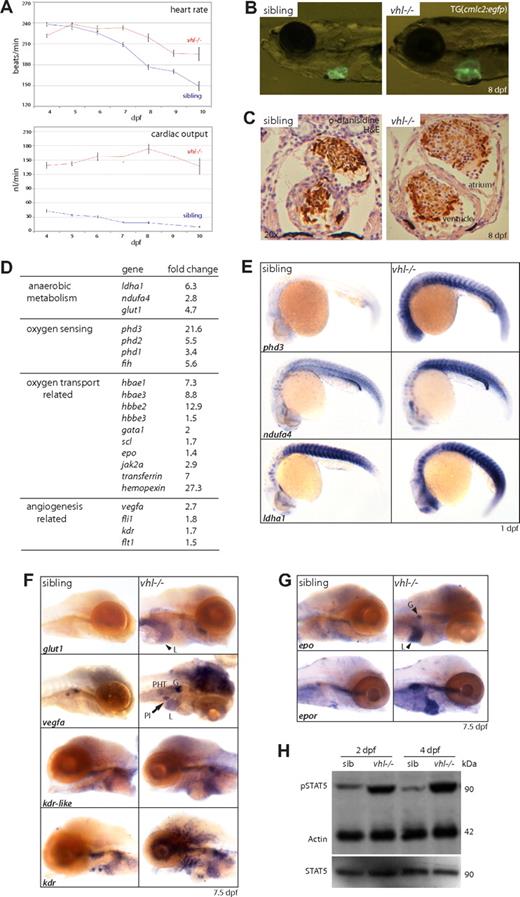
vhl mutants show a striking behavioral and physiologic hypoxic response. From 3 dpf, frequent movements of the pectoral fins, operculum, and gills are observed. When the mouth is fully developed by 4 to 5 dpf, vhl−/− embryos begin to hyperventilate, displaying up to 80 buccal movements per minute versus 1 per minute in siblings at 8 dpf (Movie S1). Similar to CP patients, time course analysis of the heart rate and cardiac output revealed an increased cardiophysiologic response in vhl mutants (Figure 2A). From 7 dpf, the vhl−/− heart rate was significantly elevated (Figure 2A) and the stroke volume was increased (data not shown). This resulted in a significantly elevated cardiac output (heart rate × stroke volume) from a 3.2-fold increase at 4 dpf (139 ± 7.6 vs 43.5 ± 4.1 nL/min) to a 15-fold increase (137.3 ± 17.5 vs 9.4 ± 1.5 nL/min) in 10-day-old vhl mutants. Whereas overall cardiac output in mutants did not significantly alter from 4 to 10 dpf (∼152 ± 10.6 nL/min), siblings showed a significant reduction from 43.5 to 9.4 nL/min, according to normal developmental trends, as described previously.35 In response to the increased cardiac output, vhl mutants developed progressive cardiac dilation that is characterized by cardiomegaly and stretched cardiomyocytes at 8 dpf (Figure 2B,C). Vhl−/− larvae survive up to 8 to 11 days, when more frequent arrhythmias and atrial shape defects were observed, suggesting the onset of congestive heart failure. Vhl mutants ultimately develop severe edema and die.
vhl mutants display a systemic hypoxic response. (A) From 7 dpf onward, vhl mutants display a significantly increased heart rate. Cardiac output (stroke volume × heart rate) is significally up-regulated 3.2-fold at 4 dpf (139 ± 7.6 vs 43.5 ± 4.1 nL/min) to 15-fold (137.3 ± 17.5 vs 9.4 ± 1.5 nL/min) in 10 dpf vhl−/− larvae. (B) vhl mutants develop progressive cardiac dilation, as shown by cardiomegaly at 8 dpf (TG(cmlc2:egfp background)). (C) Sectioning (7 μm) through the heart reveals vhl−/− cardiomyocytes to be stretched (arrow), suggestive of dilated cardiomyopathy. Sibling and mutant hearts are presented with the same magnification (×20). Blood cells are stained with o-dianisidine (brown). (D) As determined by microarray expression profiling of 7 dpf vhl mutants compared with siblings, loss of vhl leads to a general overexpression of hypoxia response genes, which was confirmed by whole-mount in situ hybridizations. (E) This includes the up-regulation of genes involved in oxygen sensing (phd3) and anaerobic metabolism (ndufa4, ldha1) as early as 1 dpf. (F) As shown at 7.5 dpf, glut1 (liver), vegfa (glomerulus, pancreatic islet, brain, and PHT), and its receptors kdr-like and kdr (liver, glomerulus, and blood vessels) are up-regulated. (G) Furthermore, epo (liver and glomerulus) and its receptor epor are highly up-regulated in 7.5 dpf vhl mutants. (H) Western blot analysis of STAT5/phosphoSTAT5 levels at 2 and 4 dpf. At both time points, phosphoSTAT5 levels are highly increased in vhl mutants, whereas STAT5 levels remain comparable with siblings, indicating increased EPO signaling. One lane represents 1.5 embryo equivalents. β-Actin is used as a loading control. Original magnifications, ×5 (B,D-F), ×20 (C). L indicates liver; G, glomerulus; PI, pancreatic islet; and H&E, hematoxylin and eosin.
vhl mutants display a systemic hypoxic response. (A) From 7 dpf onward, vhl mutants display a significantly increased heart rate. Cardiac output (stroke volume × heart rate) is significally up-regulated 3.2-fold at 4 dpf (139 ± 7.6 vs 43.5 ± 4.1 nL/min) to 15-fold (137.3 ± 17.5 vs 9.4 ± 1.5 nL/min) in 10 dpf vhl−/− larvae. (B) vhl mutants develop progressive cardiac dilation, as shown by cardiomegaly at 8 dpf (TG(cmlc2:egfp background)). (C) Sectioning (7 μm) through the heart reveals vhl−/− cardiomyocytes to be stretched (arrow), suggestive of dilated cardiomyopathy. Sibling and mutant hearts are presented with the same magnification (×20). Blood cells are stained with o-dianisidine (brown). (D) As determined by microarray expression profiling of 7 dpf vhl mutants compared with siblings, loss of vhl leads to a general overexpression of hypoxia response genes, which was confirmed by whole-mount in situ hybridizations. (E) This includes the up-regulation of genes involved in oxygen sensing (phd3) and anaerobic metabolism (ndufa4, ldha1) as early as 1 dpf. (F) As shown at 7.5 dpf, glut1 (liver), vegfa (glomerulus, pancreatic islet, brain, and PHT), and its receptors kdr-like and kdr (liver, glomerulus, and blood vessels) are up-regulated. (G) Furthermore, epo (liver and glomerulus) and its receptor epor are highly up-regulated in 7.5 dpf vhl mutants. (H) Western blot analysis of STAT5/phosphoSTAT5 levels at 2 and 4 dpf. At both time points, phosphoSTAT5 levels are highly increased in vhl mutants, whereas STAT5 levels remain comparable with siblings, indicating increased EPO signaling. One lane represents 1.5 embryo equivalents. β-Actin is used as a loading control. Original magnifications, ×5 (B,D-F), ×20 (C). L indicates liver; G, glomerulus; PI, pancreatic islet; and H&E, hematoxylin and eosin.
At a molecular level, loss of VHL stabilizes HIF-α subunits and leads to expression of a suite of hypoxia response genes. To determine whether HIF-α target genes are up-regulated in vhl mutants, we performed a microarray (44K; Agilent Technologies) expression profile of 7 dpf vhl mutants and siblings, revealing enrichment of genes related to the anaerobic metabolism, oxygen sensing and transport, and angiogenesis (Figure 2D; for the complete microarray gene list, see Table S4). Gene expression was verified by whole-mount in situ hybridizations. Figure 2E shows that genes involved in oxygen sensing (phd3) and anaerobic metabolism (ndufa4 and ldha1) are overexpressed in vhl−/− already from 1 dpf, indicating a general and early molecular hypoxic response. At 7.5 dpf, glut1 (liver), vegfa (glomerulus, pancreatic islet, brain, and pronephric hematopoietic tissue [PHT]), and its receptors kdr and kdr-like28 (liver, glomerulus, and blood vessels) are up-regulated (Figure 2F).
Furthermore, mRNA levels of epo (liver and glomerulus) and its receptor epor (heart, PHT) are elevated in vhl mutants, as shown at 7.5 dpf (Figure 2G). EPO signaling is mediated via the Janus kinase (JAK)/STAT signaling pathway. Upon binding of EPO to its receptor EpoR, Jak2a is recruited, leading to the phosphorylation and activation of transcription factor STAT5. Because dysregulated JAK/STAT signaling is a key feature of polycythemia in patients, we performed Western analysis of STAT5/phosphoSTAT5 levels to investigate whether elevated epo/epor mRNA levels would lead to increased Epo signaling in vhl mutants. Already at 2 dpf, phosphoSTAT5 levels were highly increased in vhl mutants, whereas STAT5 levels remained comparable with age-matched siblings (Figure 2H). We conclude that Epo signaling is increased in vhl mutants.
vhl mutants develop severe polycythemia
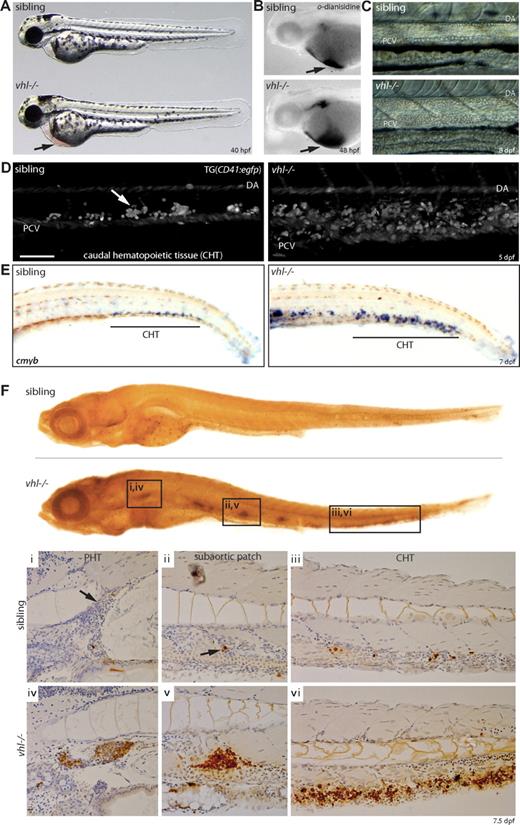
vhl−/− embryos develop a marked increase in circulating red blood cells (polycythemia). This becomes apparent by 40 hpf, when blood accumulation on the yolk sack is observed (Figure 3A,B). As development progresses, total blood volume further rises. vhl mutants expressing the gata1:egfp transgene showed increased blood flow at 6 dpf in all blood vessels, including the intersegmental and parachordal vessels, through which normally only occasionally blood cells pass (Movie S2). Furthermore, whereas in siblings a single row of blood cells is transported through the dorsal aorta and posterior cardinal vein at 8 dpf, several rows of circulating blood cells were observed in vhl mutants (Figure 3C and Movies S3 and S4).
vhl mutants develop severe polycythemia. (A) vhl mutants develop a severe increase in circulating red blood cells (polycythemia), apparent by 40 hpf by blood accumulation under the heart on the yolk sack ( ). (B) o-Dianisidine blood staining at 48 hpf. (C) Circulating blood cells imaged just anterior to the cloaca. Whereas normally a single row of blood cells is transported through the dorsal aorta (DA) and posterior cardinal vein (PCV) at 8 dpf, several rows of circulating blood cells are observed in vhl mutants. Anterior is left. (D) Confocal analysis through the CHT (posterior to cloaca) of 5 dpf embryos expressing the CD41:egfp transgene reveals an enlarged population of CD41-GFP+ hematopoietic cells between the DA and PCV in vhl mutants. Anterior is left. Scale bar indicates 100 μm. (E) Whole-mount in situ hybridization for c-myb mRNA reveals an increase number of c-myb+ HSCs in the vhl−/− CHT at 7 dpf. (F) BrdU incorporation assays demonstrate increased proliferation in all hematopoietic tissues in 7.5 dpf vhl−/−, as shown in whole-mount and cross-sections (i-iii). Original magnifications, ×5 (A,B,E), ×20 (C,Fi-iii), and ×40 (D).
). (B) o-Dianisidine blood staining at 48 hpf. (C) Circulating blood cells imaged just anterior to the cloaca. Whereas normally a single row of blood cells is transported through the dorsal aorta (DA) and posterior cardinal vein (PCV) at 8 dpf, several rows of circulating blood cells are observed in vhl mutants. Anterior is left. (D) Confocal analysis through the CHT (posterior to cloaca) of 5 dpf embryos expressing the CD41:egfp transgene reveals an enlarged population of CD41-GFP+ hematopoietic cells between the DA and PCV in vhl mutants. Anterior is left. Scale bar indicates 100 μm. (E) Whole-mount in situ hybridization for c-myb mRNA reveals an increase number of c-myb+ HSCs in the vhl−/− CHT at 7 dpf. (F) BrdU incorporation assays demonstrate increased proliferation in all hematopoietic tissues in 7.5 dpf vhl−/−, as shown in whole-mount and cross-sections (i-iii). Original magnifications, ×5 (A,B,E), ×20 (C,Fi-iii), and ×40 (D).
vhl mutants develop severe polycythemia. (A) vhl mutants develop a severe increase in circulating red blood cells (polycythemia), apparent by 40 hpf by blood accumulation under the heart on the yolk sack ( ). (B) o-Dianisidine blood staining at 48 hpf. (C) Circulating blood cells imaged just anterior to the cloaca. Whereas normally a single row of blood cells is transported through the dorsal aorta (DA) and posterior cardinal vein (PCV) at 8 dpf, several rows of circulating blood cells are observed in vhl mutants. Anterior is left. (D) Confocal analysis through the CHT (posterior to cloaca) of 5 dpf embryos expressing the CD41:egfp transgene reveals an enlarged population of CD41-GFP+ hematopoietic cells between the DA and PCV in vhl mutants. Anterior is left. Scale bar indicates 100 μm. (E) Whole-mount in situ hybridization for c-myb mRNA reveals an increase number of c-myb+ HSCs in the vhl−/− CHT at 7 dpf. (F) BrdU incorporation assays demonstrate increased proliferation in all hematopoietic tissues in 7.5 dpf vhl−/−, as shown in whole-mount and cross-sections (i-iii). Original magnifications, ×5 (A,B,E), ×20 (C,Fi-iii), and ×40 (D).
). (B) o-Dianisidine blood staining at 48 hpf. (C) Circulating blood cells imaged just anterior to the cloaca. Whereas normally a single row of blood cells is transported through the dorsal aorta (DA) and posterior cardinal vein (PCV) at 8 dpf, several rows of circulating blood cells are observed in vhl mutants. Anterior is left. (D) Confocal analysis through the CHT (posterior to cloaca) of 5 dpf embryos expressing the CD41:egfp transgene reveals an enlarged population of CD41-GFP+ hematopoietic cells between the DA and PCV in vhl mutants. Anterior is left. Scale bar indicates 100 μm. (E) Whole-mount in situ hybridization for c-myb mRNA reveals an increase number of c-myb+ HSCs in the vhl−/− CHT at 7 dpf. (F) BrdU incorporation assays demonstrate increased proliferation in all hematopoietic tissues in 7.5 dpf vhl−/−, as shown in whole-mount and cross-sections (i-iii). Original magnifications, ×5 (A,B,E), ×20 (C,Fi-iii), and ×40 (D).
In zebrafish, the first known marker distinguishing between hematopoietic and endothelial stem cells is CD41 (reference Murayama et al36 ). In the CD41:egfp transgenic line,19 CD41-GFPlow–expressing cells have been shown to represent hematopoietic stem cells (HSCs), whereas the CD41-GFPhigh population represents thrombocytes and thrombocyte precursors.36 The caudal hematopoietic tissue (CHT) is thought to exert a similar hematopoietic function as the mammalian fetal liver.36 To investigate whether HSC numbers were altered, we performed confocal analysis through the CHT of 5 dpf vhl mutants expressing the CD41:egfp transgene and observed a larger population of CD41-GFP+ hematopoietic cells (Figure 3D). Whereas CD41-GFP intensities were difficult to discern unambiguously, we performed a whole-mount in situ hybridization for the HSC marker c-myb at 7 dpf (Figure 3E). Our results indicate that vhl mutants display an increased c-myb+ HSC population in the CHT compared with age-matched siblings.
Enhanced proliferation of the hematopoietic compartments
To determine whether vhl−/−-induced polycythemia is attributable to increased hematopoietic proliferation, BrdU incorporation assays were performed. Whereas in siblings a general low proliferation rate was observed at 7.5 dpf, vhl mutants displayed specific hyperproliferation in hematopoietic tissues. The PHT (site of definitive hematopoiesis36 ), CHT, and subaortic hematopoietic patches could be clearly discerned (Figure 3F).
Up-regulation of red and white hematopoietic lineages
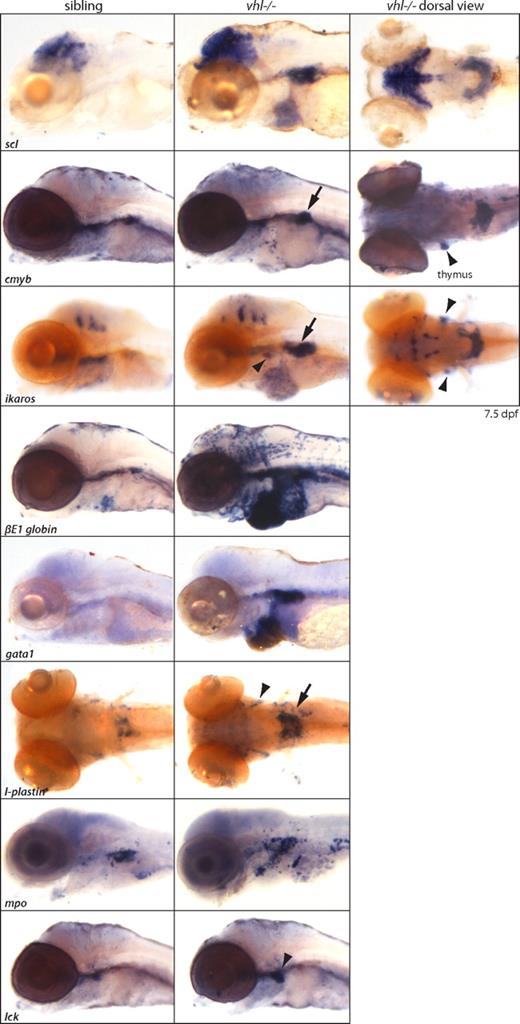
In situ hybridizations revealed a global up-regulation of both red and white hematopoietic lineages, as shown at 7.5 dpf (Figure 4). Increased expression of red lineage markers scl/tal-1 (HSC, erythrocytes), c-myb (HSC), gata1 (immature erythrocytes), and βE1 globin (differentiated erythrocytes), and white lineage markers ikaros (immature lymphocytes), lck (differentiated lymphocytes), mpo (neutrophils), and l-plastin (monocytes/early macrophages) was observed, predominantly in the PHT. Ikaros, lck, and l-plastin were also expressed in the bilateral thymus. To investigate whether mRNA up-regulation of the white lineages subsequently leads to increased protein levels, we performed a whole-mount immunohistochemical analysis for l-plastin. In Figure S5, we show that at 7 dpf, vhl mutants clearly contain an increased population of l-plastin+ early macrophages in the trunk compared with age-matched siblings.
Global up-regulation of red and white hematopoietic lineages in vhl mutants. In situ hybridizations reveal a global up-regulation of both red and white hematopoietic lineages, as shown at 7.5 dpf. Increased expression of red lineage markers scl/tal-1 (HSC, erythrocytes), c-myb (HSC), gata1 (immature erythrocytes), and βE1 globin (differentiated erythrocytes), and white lineage markers ikaros (immature lymphocytes), lck (differentiated lymphocytes), mpo (neutrophils), and l-plastin (monocytes) is observed, predominantly in the horseshoe-like PHT (→) adjacent to the glomerulus. c-myb, ikaros, lck, and l-plastin are also expressed in the bilateral thymus ( ). Accumulated blood cells in the heart remain scl, ikaros, and βE1 globin positive. Original magnification ×5.
). Accumulated blood cells in the heart remain scl, ikaros, and βE1 globin positive. Original magnification ×5.
Global up-regulation of red and white hematopoietic lineages in vhl mutants. In situ hybridizations reveal a global up-regulation of both red and white hematopoietic lineages, as shown at 7.5 dpf. Increased expression of red lineage markers scl/tal-1 (HSC, erythrocytes), c-myb (HSC), gata1 (immature erythrocytes), and βE1 globin (differentiated erythrocytes), and white lineage markers ikaros (immature lymphocytes), lck (differentiated lymphocytes), mpo (neutrophils), and l-plastin (monocytes) is observed, predominantly in the horseshoe-like PHT (→) adjacent to the glomerulus. c-myb, ikaros, lck, and l-plastin are also expressed in the bilateral thymus ( ). Accumulated blood cells in the heart remain scl, ikaros, and βE1 globin positive. Original magnification ×5.
). Accumulated blood cells in the heart remain scl, ikaros, and βE1 globin positive. Original magnification ×5.
Increased number of circulating erythroid progenitors
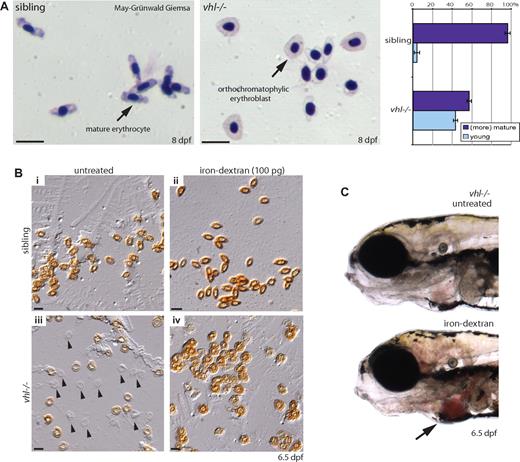
We interrogated whether blood composition was altered by May-Grünwald-Giemsa–stained blood smears at 8 dpf. By analyzing approximately 100 blood cells per embryo, we observed a marked increase in circulating orthochromatophilic erythroblasts in vhl mutants (40%, n = 9) compared with siblings (5%, n = 9), indicating an increased number of erythroid precursors (Figure 5A). Furthermore, the cytoplasm of the morphologically more mature vhl−/− erythrocytes appeared paler or hypochromic. To assess whether this could be attributed to anemia (iron deficiency), blood cells were stained with o-dianisidine, which reacts with iron in heme. Whereas erythrocytes uniformly stained dark brown in siblings, variable heme levels were detected in vhl−/− erythrocytes at 6.5 dpf (Figure 5Bi,iii) and 8 dpf (Figure 2C, visible in the accumulated blood in the heart). Injection of 100 mM iron-dextran at 2 dpf rescued the observed anemic phenotype (Figure 5Biv). Rescue could even be observed in live vhl mutants by darker red circulating blood cells (Figure 5C). Because zebrafish embryos in this study were not fed and thus iron stores are limited, the observed late anemia is most likely secondary to the constant overproduction of red blood cells. Similarly, CP patients undergoing venesection display lower iron levels, and as a consequence often develop secondary anemia.4
vhl mutants display an altered blood composition and develop anemia. (A) May-Grünwald-Giemsa–stained blood smears of 8 dpf vhl mutants and siblings. Relative to the number of (more) mature erythrocytes, vhl mutants display a marked increase in circulating orthochromatophilic erythroblasts (40%, n = 9) compared with siblings (5%, n = 9). Similar to human embryonic blood, zebrafish erythrocytes are nucleated. The cytoplasm of the morphologically more mature vhl−/− erythrocytes appears paler (hypochromic). (B) To investigate whether vhl mutants are iron deficient, blood cells were stained with o-dianisidine. Whereas in siblings uniformly stained dark brown erythrocytes are observed (Bi), in vhl mutants no ( ) or variable heme levels are detected at 6.5 dpf (Biii). Injection of 100 mM iron-dextran at 2 dpf rescued the observed anemic phenotype (Biv). (C) Rescue is even observed in live vhl mutants by darker red circulating blood cells. Original magnifications, ×40 (A,B) and ×5 (C). Scale bar indicates 25 μm.
) or variable heme levels are detected at 6.5 dpf (Biii). Injection of 100 mM iron-dextran at 2 dpf rescued the observed anemic phenotype (Biv). (C) Rescue is even observed in live vhl mutants by darker red circulating blood cells. Original magnifications, ×40 (A,B) and ×5 (C). Scale bar indicates 25 μm.
vhl mutants display an altered blood composition and develop anemia. (A) May-Grünwald-Giemsa–stained blood smears of 8 dpf vhl mutants and siblings. Relative to the number of (more) mature erythrocytes, vhl mutants display a marked increase in circulating orthochromatophilic erythroblasts (40%, n = 9) compared with siblings (5%, n = 9). Similar to human embryonic blood, zebrafish erythrocytes are nucleated. The cytoplasm of the morphologically more mature vhl−/− erythrocytes appears paler (hypochromic). (B) To investigate whether vhl mutants are iron deficient, blood cells were stained with o-dianisidine. Whereas in siblings uniformly stained dark brown erythrocytes are observed (Bi), in vhl mutants no ( ) or variable heme levels are detected at 6.5 dpf (Biii). Injection of 100 mM iron-dextran at 2 dpf rescued the observed anemic phenotype (Biv). (C) Rescue is even observed in live vhl mutants by darker red circulating blood cells. Original magnifications, ×40 (A,B) and ×5 (C). Scale bar indicates 25 μm.
) or variable heme levels are detected at 6.5 dpf (Biii). Injection of 100 mM iron-dextran at 2 dpf rescued the observed anemic phenotype (Biv). (C) Rescue is even observed in live vhl mutants by darker red circulating blood cells. Original magnifications, ×40 (A,B) and ×5 (C). Scale bar indicates 25 μm.
Human VHLp30 mRNA rescues vhl−/− polycythemia
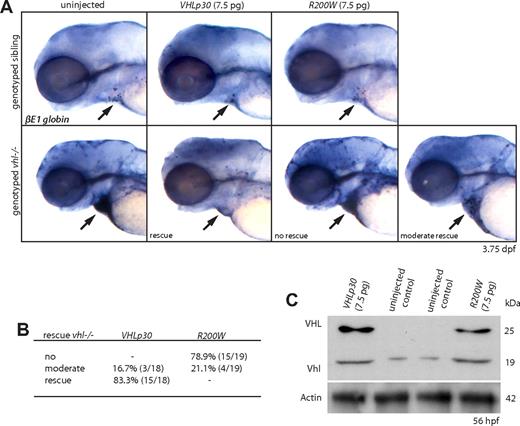
To confirm the described polycythemic phenotype to reflect loss of vhl, we injected vhl mutants with 7.5 pg mRNA of either the full-length human VHLp30 or the CP VHL R200W allele at the 1-cell stage (Figure 6A,B), and verified translation by Western analysis (Figure 6C). Whereas at 3.75 dpf only a few βE1 globin+ blood cells are observed in siblings, uninjected vhl mutants show a strong overexpression of βE1 globin mRNA that is most clearly visible in the blood cells accumulated in the heart (arrow). After VHLp30 mRNA injection, this was no longer observed in 83.3% (15 of 18) of the genotyped vhl mutants, indicating a near complete rescue of the polycythemic phenotype by the human protein. Accordingly, injection of VHL R200W mRNA did not rescue the zebrafish mutant phenotype in 78.9% (15 of 19) of the genotyped vhl mutants, suggesting a conserved function for this residue between the 2 species. Notably, in 21.1% (4 of 19) of the R200W-injected vhl mutants, a moderate rescue was observed (Figure 6A,B), most likely reflecting the diminished, but not completely impaired, VHL function induced by the CP R200W mutation.3 Importantly, our results show the potential power of our novel in vivo vhl model as a tool to assess the possible impact of VHL mutations on different disease aspects, and permitting investigation of protein functionality at the single amino acid level.
Human VHLp30 mRNA rescues vhl−/− polycythemia. (A,B) Embryos were injected with 7.5 pg of either full-length human VHLp30 or VHL R200W mRNA at the 1-cell stage. Whereas at 3.75 dpf only a few βE1 globin+ blood cells are observed in siblings, uninjected vhl mutants show a strong overexpression of βE1 globin mRNA that is most clearly visible in the blood cells accumulated in the heart ( ). After VHLp30 mRNA injection, this is not observed in 83.3% (15 of 18) of the genotyped vhl mutants, indicating a nearly complete rescue of the polycythemic phenotype by the human protein. Injection of VHL R200W mRNA could not restore the vhl−/− βE1 globin expression to wild-type levels. (C) Translation of VHLp30 and R200W mRNA is verified by Western analysis at 56 hpf. One lane represents 3 embryo equivalents. β-Actin is used as a loading control. Original magnification, ×5 (A).
). After VHLp30 mRNA injection, this is not observed in 83.3% (15 of 18) of the genotyped vhl mutants, indicating a nearly complete rescue of the polycythemic phenotype by the human protein. Injection of VHL R200W mRNA could not restore the vhl−/− βE1 globin expression to wild-type levels. (C) Translation of VHLp30 and R200W mRNA is verified by Western analysis at 56 hpf. One lane represents 3 embryo equivalents. β-Actin is used as a loading control. Original magnification, ×5 (A).
Human VHLp30 mRNA rescues vhl−/− polycythemia. (A,B) Embryos were injected with 7.5 pg of either full-length human VHLp30 or VHL R200W mRNA at the 1-cell stage. Whereas at 3.75 dpf only a few βE1 globin+ blood cells are observed in siblings, uninjected vhl mutants show a strong overexpression of βE1 globin mRNA that is most clearly visible in the blood cells accumulated in the heart ( ). After VHLp30 mRNA injection, this is not observed in 83.3% (15 of 18) of the genotyped vhl mutants, indicating a nearly complete rescue of the polycythemic phenotype by the human protein. Injection of VHL R200W mRNA could not restore the vhl−/− βE1 globin expression to wild-type levels. (C) Translation of VHLp30 and R200W mRNA is verified by Western analysis at 56 hpf. One lane represents 3 embryo equivalents. β-Actin is used as a loading control. Original magnification, ×5 (A).
). After VHLp30 mRNA injection, this is not observed in 83.3% (15 of 18) of the genotyped vhl mutants, indicating a nearly complete rescue of the polycythemic phenotype by the human protein. Injection of VHL R200W mRNA could not restore the vhl−/− βE1 globin expression to wild-type levels. (C) Translation of VHLp30 and R200W mRNA is verified by Western analysis at 56 hpf. One lane represents 3 embryo equivalents. β-Actin is used as a loading control. Original magnification, ×5 (A).
Chemically induced Hif signaling in wild-type embryos by DMOG partly mimics the vhl hypoxic phenotype
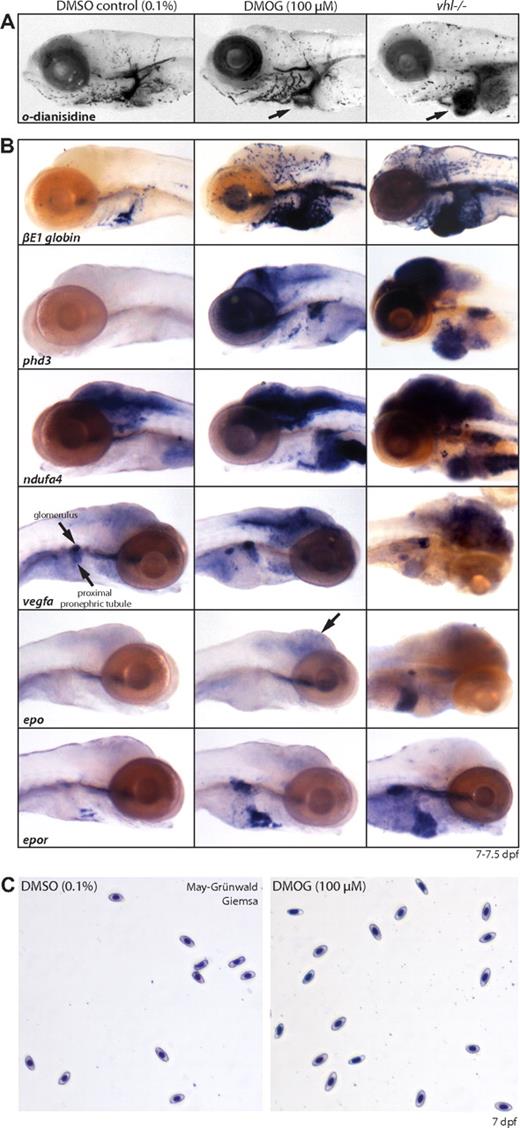
Collectively, our data support the notion that vhl mutants experience a prolonged systemic artificial hypoxic response under normoxic conditions, and that Hif targets such as Epo play a key role in the observed hematopoietic phenotype. Whereas our data suggest stabilization of Hif, evidence remains indirect because no antibodies against zebrafish Hif-α subunits are available. We therefore took a chemical approach to activate Hif signaling in wild-type embryos with the nonspecific PHD/factor inhibiting HIF (FIH)–inhibitor dimethyloxalylglycine (DMOG) to compare the resulting (molecular) phenotype with age-matched vhl mutants. In embryos younger than 2 dpf, treatment with 10 to 100 μM DMOG was lethal or severely toxic (data not shown). Three-day-old embryos treated with 100 μM DMOG for 4 days develop normally and display a strong hypoxic response. Like vhl mutants, at 7 dpf DMOG-treated embryos hyperventilate (data not shown) and have an increased number of blood cells, as shown by o-dianisidine staining in Figure 7A. Whole-mount in situ hybridization analysis shows βE1 globin, phd3, ndufa4, vegfa, epo, and epor are up-regulated compared with the 0.1% DMSO-treated controls (Figure 7B). However, compared with age-matched vhl mutants, expression levels and patterns slightly differ. The vhl−/− expression of phd3/ndufa4 in the heart, vegfa/epo in the liver, and epo in the glomerulus was not observed in 100 μM DMOG-treated embryos. In addition, DMOG-treated embryos showed strong vegfa expression in the proximal renal tubule, which is not apparent in vhl mutants. Unlike vhl mutants, May-Grünwald-Giemsa–stained blood smears of 0.1% DMSO and 100 μM DMOG-treated wild-type embryos revealed blood cells of normal maturation (Figure 7C). However, direct comparison of vhl mutants with DMOG-treated embryos is complicated, due to differences in embryonic timing of the chemical hypoxic stimulus and the level of induced hypoxia by PHD/FIH inhibition. Because a higher concentration of DMOG is lethal, no other inhibitors are commercially available, and anoxia is lethal or renders embryos in a state of suspended animation,37 it is currently not possible to study the effect of full activation of hypoxic signaling in vivo. Nonetheless, these results indicate that chemical activation of the Hif signaling pathway in DMOG-treated embryos is to a large extent comparable with the hypoxic response observed in vhl mutants, providing additional evidence that stabilization of Hif plays an important role in the vhl mutant phenotype and the pathogenesis of Chuvash polycythemia.
Chemically induced hypoxia in wild-type embryos by DMOG partly mimics the vhl hypoxic phenotype. (A) o-Dianisidine stainings and (B) whole-mount in situ hybridizations for phd3, ndufa4, βE1 globin, vegfa, epo, and epor on 7 dpf wild-type embryos treated for 4 days with 0.1% DMSO (control, left column) or 100 μM of the nonspecific PHD/FIH inhibitor DMOG (middle column). Compared with age-matched vhl mutants (right column), DMOG-treated embryos develop a similar molecular hypoxic response and an increased number of βE1 globin+ blood cells; however, expression levels are generally somewhat lower and vhl−/− expression of phd3/ndufa4 in the heart, vegfa/epo in the liver, and epo in the glomerulus was not observed in 100 μM DMOG-treated embryos. In addition, DMOG-treated embryos show strong vegfa expression in the proximal renal tubule, which is not apparent in vhl mutants. (C) Unlike vhl mutants, May-Grünwald-Giemsa–stained blood smears of 0.1% DMSO and 100 μM DMOG-treated wild-type embryos reveal blood cells of normal maturation. Original magnifications, ×5 (A,B) and ×40 (C).
Chemically induced hypoxia in wild-type embryos by DMOG partly mimics the vhl hypoxic phenotype. (A) o-Dianisidine stainings and (B) whole-mount in situ hybridizations for phd3, ndufa4, βE1 globin, vegfa, epo, and epor on 7 dpf wild-type embryos treated for 4 days with 0.1% DMSO (control, left column) or 100 μM of the nonspecific PHD/FIH inhibitor DMOG (middle column). Compared with age-matched vhl mutants (right column), DMOG-treated embryos develop a similar molecular hypoxic response and an increased number of βE1 globin+ blood cells; however, expression levels are generally somewhat lower and vhl−/− expression of phd3/ndufa4 in the heart, vegfa/epo in the liver, and epo in the glomerulus was not observed in 100 μM DMOG-treated embryos. In addition, DMOG-treated embryos show strong vegfa expression in the proximal renal tubule, which is not apparent in vhl mutants. (C) Unlike vhl mutants, May-Grünwald-Giemsa–stained blood smears of 0.1% DMSO and 100 μM DMOG-treated wild-type embryos reveal blood cells of normal maturation. Original magnifications, ×5 (A,B) and ×40 (C).
Discussion
In this study, we report the first systemic embryonic viable vertebrate animal model for VHL, and demonstrate VHL function to be conserved between mammals and zebrafish. Zebrafish mutants carrying early inactivating mutations in vhl faithfully recapitulate Chuvash polycythemia, including aspects that have not been observed in a mouse model homozygous for the VHL R200W mutation, Vhl(R/R).9 Similarly, both mutants display enhanced epo and vegf mRNA levels, as well as an up-regulation of other HIF-target genes. Severe polycythemia is observed that becomes progressively worse during development. However, in zebrafish, vhl−/− defects become eminent at early embryonic stages (40 hpf), whereas mutant mice develop polycythemia from 10 to 14 weeks after birth due to splenic erythropoiesis.9 Zebrafish vhl mutants display a general hyperproliferation of the primitive and definitive hematopoietic tissues throughout development, suggesting a more general contribution to the polycythemic phenotype. Splenic erythropoiesis does not yet occur during these embryonic and early larval stages.
In vhl mutants, the number of circulating erythrocyte precursors was increased 8-fold, and blood cells appeared to remain immature, retaining scl, gata1, and ikaros expression. Similarly, Vhl(R/R) mice displayed an increase in CD17+Ter119+ erythroid precursors in circulation (2-fold) and in the spleen (6-fold).9 Bone marrow defects were not observed, and Hickey et al9 show the polycythemic mouse phenotype to result from HIF-2α–induced splenic erythropoiesis. Because transgenic mice constitutively overexpressing human EPO cDNA develop severe polycythemia with normal erythrocyte maturation,38,39 it is less likely that the increase in erythroid precursors is secondary to an increased demand. Vhl might also exert a role in erythroid maturation. Whereas DMOG-treated wild-type embryos have an increased number of βE1 globin+ red blood cells, we show maturation of these cells to be normal, which might indicate a direct role for Vhl in this respect. However, because direct comparison of vhl mutants with DMOG-treated embryos is complicated, due to differences in embryonic timing and intensity of the chemical hypoxic stimulus, a role for Hif cannot be excluded. HIF-1α has been shown to play an important role in the expansion of committed erythroid progenitors and in terminal differentiation of erythroid cells; however, it is not essential for the formation of multipotential hematopoietic progenitors.40 Furthermore, hypoxia has been described to promote the undifferentiated cell state in various stem and precursor cells via notch1a–intracellular domain.41
In CP patients, white blood cell numbers are not affected.42 Interestingly, vhl mutants display a global up-regulation of white hematopoietic lineage markers. We show an increased population of early macrophages in the vhl−/− trunk at 7 dpf, indicating that in the case of l-plastin, increased mRNA levels translate into elevated protein levels. Therefore, our data demonstrate that the general hyperproliferation of the hematopoietic tissues and the increased number of c-myb+ HSCs can contribute to the expansion of both red and white hematopoietic lineages. Also in Vhl(R/R) mice, circulating white blood cell numbers were slightly higher (tending toward significance).9 Because overexpression of Epo in zebrafish induced increased expression of red lineages only, and mpo, pu.1, rag1, and lck mRNA levels remained unchanged,15 vhl might play a more general role in hematopoietic differentiation. This is further supported by a study in which inactivation of Vhlh in myeloid cells resulted in a hyperinflammatory response in one model of acute inflammation, as measured by myeloperoxidase activity.43 Because inactivation of vhl in this study resulted in a stronger response than systemic R200W substitution in Vhl(R/R) mice, this might indicate genotype-phenotype correlations to be important. The CP R200W mutation allows partial VHL function; as a hypomorphic allele, it might not induce a strong inflammatory response in patients. However, interspecies differences cannot be excluded.
Similar to CP patients and unique to our zebrafish model, vhl mutants have striking abnormalities in heart and basal ventilation rates, characteristic of acclimatization to hypoxia at high altitude. Induced hypoxia in zebrafish has been shown to increase heart rate from 4 to 12 dpf (at PO2 ≤ 10 kPa);31,44 however, enlargement of the heart was not described. Recently, it was reported that specific deletion of Vhlh in mouse cardiomyocytes results in HIF-1α-dependent cardiac dilation, increased cardiac weight, and heart failure from 5 months of age.45 Malignant cardiac transformations were frequently found that have not been observed in human VHL patients. Because cardiophysiologic parameters were not altered, this study shows that chronic cardiac activation of HIF-α alone does not affect heart rate and cardiac output. Interestingly, adult transgenic mice overexpressing human EPO develop an increased heart size and weight, whereas blood pressure was not elevated and cardiac output was not decreased (which is normally observed during prolonged hypoxia).39 However, other studies using similar transgenic mouse strains did not observe changes in heart size and cardiac output.46 EPO has been shown to be a key factor in modulating neural respiratory control in hypoxia by acting on both the central nervous system via EPO receptors in respiratory neurons in the brainstem and peripheral chemoreceptors (carotid bodies). Transgenic mice overexpressing human EPO in neuronal cells display an increased ventilation response to acute reduction of environmental oxygen.47 EPO is thus a multitarget factor that modulates hyperventilation response and carotid body activity, but above all increases red cell mass. Our results indicate vhl to play a mayor role in underlying calibration and homeostasis of the respiratory and cardiovascular systems via Epo.
It is currently debated to what extent stabilization of HIF-α subunits plays a role in the pathogenesis of VHL disease and Chuvash polycythemia, because several HIF-independent functions for pVHL have recently been reported.48 We have shown by microarray analysis that the vhl−/− gene expression profile is enriched for genes related to hypoxic regulation. However, other known HIF-independent genes,48 such as genes involved in extracellular matrix formation (eg, fibronectin,49 collagen IV50 ), are also found to be up-regulated in vhl mutants (Table S4). This indicates that careful analysis of the array will be of great value to gain insight into the Hif-independent processes regulated by vhl. We are currently screening for hif1-β mutants to facilitate this process. Furthermore, we are currently investigating other VHL disease aspects in our vhl mutant, including late pronephric tubule abnormalities that are not observed in wild-type DMOG-treated embryos (data not shown) and possibly share similarities with ccRCC.
Because Vhlh knockout mice die in utero during early embryogenesis, zebrafish vhl mutants represent the first embryonic viable systemic vertebrate animal model in which the role of Vhl can be studied even at larval stages. Our results demonstrate that this novel genetic zebrafish vhl model will contribute to our understanding of hypoxic signaling, hematopoiesis, and VHL-associated disease progression. Because we have shown VHLp30 mRNA to rescue the vhl−/− polycythemic phenotype, whereas the CP R200W mutant form could not, our model provides a powerful tool in which genotype-phenotype correlation studies could be performed to decipher VHL function at the single amino acid level. In addition, small molecule screens in the vhl mutant background could identify novel compounds for the intervention and treatment of different aspects of VHL disease.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We gratefully acknowledge the Hubrecht target-selected ENU-mutagenesis screen team, Martine Paquay for performing BrdU labeling, Elsbeth Römer (Cytomorphology, University Medical Center, Utrecht) for expertise and help on blood smear analysis, Ies Nijman and Joram Mul for help with the microarray analysis, Miranda Buitenhuis (Molecular Immunology, University Medical Center, Utrecht) for providing the STAT5 antibodies, Paul Martin (Bristol, United Kingdom) for providing the l-plastin antibody, Rui Monteiro (Oxford, United Kingdom) for the βE1 globin probe, and Stefano Volpi for his input on the initial characterization of the vhl mutant.
This work was supported by the Dutch Cancer Association (UU-2006-3565). R.H.G. is supported by a VIDI award (Netherlands Organization for Scientific Research). F.J.v.E. is supported by the Medical Research Council Center for Developmental and Bio-medical Genetics (G0700091).
Authorship
Contribution: E.v.R., I.L., R.H.G., F.J.v.E., and J.K. performed experiments. T.S. designed an experiment. E.v.R., S.S.-M., E.E.V., R.H.G., and F.J.v.E. designed the research, analyzed data, and checked or improved the manuscript. E.v.R. drafted the manuscript.
Conflict-of-interest disclosure: All the authors declare no competing financial interests.
Correspondence: Dr Fredericus J. van Eeden, Department of Biomedical Science, Sheffield University, Firth Court, Western Banks, Sheffield S10 2TN, United Kingdom; e-mail: f.j.vaneeden@sheffield.ac.uk or Dr Rachel H. Giles, Department of Medical Oncology, University Medical Center Utrecht, Universiteitsweg 100 (STR. 2.118), 3584 CG Utrecht, The Netherlands; e-mail: r.giles@umcutrecht.nl.
References
Author notes
*R.H.G. and F.J.v.E. contributed equally to this study.