Abstract
This study investigated the immunogenicity of Wilms tumor gene product 1 (WT1)–peptide vaccination in WT1-expressing acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) patients without curative treatment option. Vaccination consisted of granulocyte-macrophage colony-stimulating factor subcutaneously days 1 to 4, and WT1.126-134 peptide and 1 mg keyhole limpet hemocyanin on day 3. The initial 9 patients received 4 vaccinations biweekly, then monthly, and the subsequent 10 patients received continual biweekly vaccination. Seventeen AML patients and 2 refractory anemia with excess blasts patients received a median of 11 vaccinations. Treatment was well tolerated. Objective responses in AML patients were 10 stable diseases (SDs) including 4 SDs with more than 50% blast reduction and 2 with hematologic improvement. An additional 4 patients had clinical benefit after initial progression, including 1 complete remission and 3 SDs. WT1 mRNA levels decreased at least 3-fold from baseline in 35% of patients. In 8 of 18 patients, WT1-tetramer+ T cells increased in blood and in 8 of 17 patients in bone marrow, with a median frequency in bone marrow of 0.18% at baseline and 0.41% in week 18. This WT1 vaccination study provides immunologic, molecular, and preliminary evidence of potential clinical efficacy in AML patients, warranting further investigations.
Introduction
Cancer vaccine clinical trials using synthetic peptides have been performed for more than a decade in patients with solid tumors, mostly in patients with melanoma. The success rate in inducing specific T-cell responses was rather heterogeneous among vaccination studies using various antigens and a variety of adjuvants.1-6 The clinical efficacy has been limited, but is of interest, because in several trials minor tumor regressions and occasional objective responses were reported in patients with metastatic disease (reviewed in Mocellin et al,2 Restifo and Rosenberg,3 Schlom et al,7 and Finke et al8 ). However, objective tumor remissions were usually restricted to patients with limited disease and slow progression kinetics.
Acute leukemia has rapid progression kinetics and only recently attempts have been undertaken to investigate peptide vaccines.6,9-11 Among the few identified target antigens in acute leukemia, the transcription factor WT1 has attracted considerable interest, in particular for acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) transforming into AML.12 WT1 is highly expressed in both leukemia and various solid tumors.12-16 In contrast to differentiation antigens, which have served as vaccine antigens in vaccine trials in melanoma and other solid tumors, WT1 expression is essential for tumor proliferation, and treatment with WT1 antisense oligomers results in growth inhibition of leukemic blasts as well as WT1-expressing carcinoma cells.17,18 Thus, in contrast to the observations with melanoma differentiation antigens, development of WT1-negative tumor variants as escape mechanism was considered to be unlikely. In line with these expectations, the WT1 expression levels in leukemia relapse were found to be at least as high as at time of initial diagnosis.19,20
Two small WT1 peptide vaccination trials in AML patients in complete remission (CR) have shown evidence for immunogenicity and clinical efficacy due to decreases in WT1 mRNA expression as a marker of minimal residual disease.6,11 In the first trial, performed by the group of Sugiyama (Oka et al), 12 AML patients in CR and 2 MDS patients were vaccinated with different amounts (0.3 mg, 1.0 mg, and 3.0 mg) of HLA-A24–binding WT1 class I epitope emulsified in Montanide.6 In the second trial, reported by Rezvani et al, 5 AML patients in CR and 2 MDS patients were vaccinated with the HLA-A201–binding WT1.126-134 peptide combined with a proteinase-1 peptide and Montanide and granulocyte-macrophage colony-stimulating factor (GM-CSF).11 We here report on a phase 2 clinical trial in patients with active AML and high-risk MDS using the HLA-A201–binding WT1.126-134 peptide together with GM-CSF and KLH as adjuvants.
Methods
Patient selection criteria
Patients eligible for the study were required to have AML or high-risk MDS (International Prognostic Scoring System, IPSS > 1.5).21 Disease had to have either relapsed after chemotherapy, or responded with incomplete remission, or was untreated in patients who were not candidates for induction chemotherapy because of age and comorbidity. Further eligibility criteria included HLA-A2 positivity as assessed by serologic typing and confirmed by molecular typing and expression of WT1 mRNA in bone marrow (BM). Exclusion criteria were treatment with chemotherapy within 4 weeks prior to enrollment, concurrent chemotherapy, or eligibility for allogeneic stem cell transplantation. The study was approved by the Institutional Ethics Committee of Charité CBF, and written informed consent was obtained from each patient prior to study in accordance with the Declaration of Helsinki.
Vaccination protocol
Vaccines were injected intradermally and subcutaneously approximately 10-cm distal of the right groin. One vaccination comprised 4 days of 62.5 μg GM-CSF (Leukine; Berlex, Seattle, WA) and on day 3, 0.2 mg HLA-A201–restricted WT1.126-134 epitope peptide RMFPNAPYL (Clinalfa, Laufelfingen, Switzerland) admixed with 1 mg KLH (Immucothel; biosyn, Fellbach, Germany). The initial 9 patients (cohort 1) received 4 biweekly and subsequent monthly vaccinations; the subsequent 10 patients (cohort 2) received continual biweekly vaccination. Vaccination was continued until disease progression. During the initial 6 vaccinations, continuation of vaccination was permitted in case of disease progression, if no chemotherapy was indicated. For assessment of toxicities, a physical examination, complete blood count, routine chemistry, and urine analysis were carried out prior to each vaccination.
WT1 mRNA levels
Quantitative real-time reverse-transcription–polymerase chain reaction (RT-PCR) on a LightCycler instrument (Roche, Penzberg, Germany) was performed to assess WT1 mRNA expression levels as described previously.20 Values were provided as ratio to porphobilinogen-deaminase (PBGD) housekeeping gene expression. Samples were regarded as noninformative in case the PBGD value was less than 0.1 pg/μL.20 With 45 PCR cycles, WT1 message was undetectable in PB of 20 healthy volunteers, therefore detection of WT1 with 45 cycles in PB was judged as elevated, whereas for BM a cutoff level of 2 × 10−4/PBGD was used, which was 2 standard deviations above the mean of 20 healthy volunteer marrow samples.20
T-cell response assessment
Heparinized peripheral blood (PB) and BM samples were obtained prior to vaccination, and in weeks 10, 18, 30, and 42 or earlier at treatment termination. Serial cryopreserved mononuclear cell (MNC) samples from single patients were assessed simultaneously in one assay run. WT1-specific T cells were detected by tetramer staining as primary assay as described previously.22 The following reagents were used: WT1.126-134–binding tetramers, (Beckman Coulter, Marseille, France) fluorescence-conjugated antibodies against CD8 and CD3, and a mixture of antibodies against CD4, CD13, CD19, CD33, and CD34 (all antibodies from BD Biosciences, Heidelberg, Germany) to exclude nonspecific tetramer staining. Analysis of cytokine production as secondary assay was performed as described previously.22 The presence of baseline WT1-tetramer T cells was considered in case the frequency of tetramer-positive CD3+CD8+ T cells exceeded 0.3%, which was the mean plus or minus 2 standard deviations (0.16% ± 0.14%) observed in 12 healthy control subjects. An induction of a T-cell response to WT1 vaccination was defined as an at least 2-fold increase in frequency compared with baseline. A cytokine response was considered positive if the percentage of WT1 peptide–specific cytokine-producing CD3+CD8+ T cells was at least 2-fold the percentage of an HIV control peptide.
Clinical outcome assessment
Disease course was evaluated by analysis of PB biweekly and of BM in weeks 0, 10, 18, 30, and 42, and subsequently in case of PB changes. Modified IWG-MDS criteria were used to assess hematologic response, capturing hematologic improvement and stable disease (< 50% increase in blasts, < 50% decrement of granulocytes/platelets) for at least 8 weeks.23,24 Toxicities were graded in accordance with the National Cancer Institute Common Toxicity Criteria version 2.0 (Bethesda, MD). Since therapeutic cancer vaccines might have special clinical response characteristics, as reviewed by Hoos et al,25 a second analysis with novel response criteria was also performed for clinical outcome assessment. According to the novel, prospectively defined criteria in the vaccine protocol, early progression within the initial 6 vaccinations was accepted and vaccination was continued, if disease progression was slow and patients were without severe symptoms of clinical progression or a necessity for an alternative therapy. In those patients, who had initial progression followed by a period of subsequent response or stable disease, the modified response (mSD, modified partial response [mPR], mCR) was judged disregarding the previous transient progression, and the modified progression-free survival (mPFS) was calculated from the date of the start of therapy.
Statistical analyses
The prespecified primary immunologic trial objective was to augment the proportion of patients with a tetramer T-cell response by at least 25% in comparison of baseline and week-10 (peripheral blood) or week-18 (bone marrow) values. Student t test was used to compare baseline and week-10 and week-18 values. All other statistical comparisons have to be regarded as descriptive.
An association between T-cell response, change in WT1 mRNA levels, and clinical outcome was assessed by chi square test after construction of 2 by 2 frequency tables based on median values.
Results
Patient characteristics
Between April 2002 and June 2006, a total of 17 patients with AML and 2 patients with MDS refractory anemia with excess blasts (RAEB) I and II were enrolled. Patient characteristics are summarized in Table 1. At study entry, all 19 patients had elevated BM blasts ranging from 5% to 85% (median, 45%). Nine of the 17 AML patients had received previous chemotherapy. Eight of the AML patients had not received previous chemotherapy due to age older than 80 years and comorbidity in 4, and smoldering secondary AML in 4 that had evolved from MDS in 3 or from myeloproliferative syndrome in 1. Of the 2 MDS patients, 1 had previous immunosuppressive therapy; the other was untreated.
Patient characteristics
| Patient no. . | Age, y . | Type . | Karyotype . | Previous therapy . | Disease status baseline . | Cohort . | No. of vacc . | Blasts in BM baseline . | Best response, IWG . | PFS, d . | Best response modified criteria . | Duration of early progression, d . | mPFS, d . | Clinical response comments . | Tetramer+ T cells in pB baseline . | Tetramer response in pB following vacc . | Tetramer response in BM following vacc . | Cytokine response in pB following vacc . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 66 | M4 | Normal | 2× ind. reind. | 2. PR | 1 | 12 | 10 | PD | 21 | mCR (17 mo) | 65 | 514 | − | − | + | + | + |
| 2 | 82 | M2 | na | None | PD | 1 | 4 | 85 | SD | 136 | SD | − | 136 | Off protocol after 4 vacc due to soft tissue infectious complications | − | + | + | + |
| 3 | 64 | M6 | Complex 5q− | 2× ind. | PD | 1 | 4 | 60 | PD | 50 | PD | − | 50 | − | + | − | − | − |
| 4 | 72 | M2 | Normal | Ind. | 1. PR | 1 | 6 | 5 | PD | 37 | PD | − | 37 | − | − | + | + | + |
| 5 | 74 | M1 | 47 XX + 11 46 XX (23) | 2× ind.reind. | 2. PR | 1 | 9 | 12,5 | SD | 201 | SD | − | 201 | − | + | − | − | + |
| 6 | 45 | M7 | Normal | 3× ind reind. | PD | 1 | 4 | 60 | PD | 49 | PD | − | 49 | − | − | − | − | − |
| 7 | 73 | WHO II | Normal | None | PD | 1 | 12 | 70 | SD | 262 | SD | − | 262 | Erythroid response for 7 months | − | + | − | − |
| 8 | 63 | WHO II | Normal | None | PD | 1 | 18 | na | SD | 101 | SD | − | 101 | − | − | + | + | + |
| 9 | 80 | M4 | Normal | None | PD | 1 | 8 | 80 | PD | 60 | mSD | 82 | 144 | Decrease of peripheral blasts | + | − | + | + |
| 10 | 59 | M4 | Normal | 2× ind. reind. autoPBSCT | 3. PR | 2 | 4 | 5–10 | PD | 29 | PD | − | 29 | − | na | na | na | na |
| 11 | 65 | M4 | Normal | Ind. | PD | 2 | 12 | 10 | SD | 134 | SD | − | 134 | − | − | − | − | − |
| 12 | 67 | RAEBII | Normal | None | PD | 2 | 24 | 12 | Major neutrophil response | 112 | mSD | 231 | 339 | − | + | + | + | + |
| 13 | 61 | WHO II | Normal | None | PD | 2 | 11 | 60 | SD | 142 | SD | − | 142 | − | − | + | − | − |
| 14 | 69 | RAEB I | Normal | ATG, CsA | PD | 2 | 10 | 10 | Major neutrophil response | 69 | Major neutrophil response | − | 69 | − | − | − | − | − |
| 15 | 75 | WHO II | Complex | None | PD | 2 | 10 | 60 | SD | 132 | SD | − | 132 | Decrease of marrow blasts (30% at 3 months) | − | − | + | + |
| 16 | 82 | M4 | Normal | None | PD | 2 | 11 | 50 | PD | 66 | mSD | 112 | 137 | − | + | + | + | |
| 17 | 65 | M2 | Trisomy 8 | 1× ind. 2× cons. | 1. PR | 2 | 27+ | 5 | SD ongoing | SD ongoing | − | 571 (+) | Erythroid response after 4 months, molecular response with complete disappearance of trisomy 8 | − | + | + | + | |
| 18 | 58 | M2 | Normal | None | PD | 2 | 23+ | 40 | SD ongoing | 506 (+) | SD ongoing | − | 506 (+) | Decrease of marrow blasts (12% at 3 months) | − | − | − | − |
| 19 | 69 | WHO II | 46 XX (del5) | Low araC | 1. PR | 2 | 11 | 30 | SD | 168 | SD | 168 | Blast response at 3 months (15%) | − | + | − | + |
| Patient no. . | Age, y . | Type . | Karyotype . | Previous therapy . | Disease status baseline . | Cohort . | No. of vacc . | Blasts in BM baseline . | Best response, IWG . | PFS, d . | Best response modified criteria . | Duration of early progression, d . | mPFS, d . | Clinical response comments . | Tetramer+ T cells in pB baseline . | Tetramer response in pB following vacc . | Tetramer response in BM following vacc . | Cytokine response in pB following vacc . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 66 | M4 | Normal | 2× ind. reind. | 2. PR | 1 | 12 | 10 | PD | 21 | mCR (17 mo) | 65 | 514 | − | − | + | + | + |
| 2 | 82 | M2 | na | None | PD | 1 | 4 | 85 | SD | 136 | SD | − | 136 | Off protocol after 4 vacc due to soft tissue infectious complications | − | + | + | + |
| 3 | 64 | M6 | Complex 5q− | 2× ind. | PD | 1 | 4 | 60 | PD | 50 | PD | − | 50 | − | + | − | − | − |
| 4 | 72 | M2 | Normal | Ind. | 1. PR | 1 | 6 | 5 | PD | 37 | PD | − | 37 | − | − | + | + | + |
| 5 | 74 | M1 | 47 XX + 11 46 XX (23) | 2× ind.reind. | 2. PR | 1 | 9 | 12,5 | SD | 201 | SD | − | 201 | − | + | − | − | + |
| 6 | 45 | M7 | Normal | 3× ind reind. | PD | 1 | 4 | 60 | PD | 49 | PD | − | 49 | − | − | − | − | − |
| 7 | 73 | WHO II | Normal | None | PD | 1 | 12 | 70 | SD | 262 | SD | − | 262 | Erythroid response for 7 months | − | + | − | − |
| 8 | 63 | WHO II | Normal | None | PD | 1 | 18 | na | SD | 101 | SD | − | 101 | − | − | + | + | + |
| 9 | 80 | M4 | Normal | None | PD | 1 | 8 | 80 | PD | 60 | mSD | 82 | 144 | Decrease of peripheral blasts | + | − | + | + |
| 10 | 59 | M4 | Normal | 2× ind. reind. autoPBSCT | 3. PR | 2 | 4 | 5–10 | PD | 29 | PD | − | 29 | − | na | na | na | na |
| 11 | 65 | M4 | Normal | Ind. | PD | 2 | 12 | 10 | SD | 134 | SD | − | 134 | − | − | − | − | − |
| 12 | 67 | RAEBII | Normal | None | PD | 2 | 24 | 12 | Major neutrophil response | 112 | mSD | 231 | 339 | − | + | + | + | + |
| 13 | 61 | WHO II | Normal | None | PD | 2 | 11 | 60 | SD | 142 | SD | − | 142 | − | − | + | − | − |
| 14 | 69 | RAEB I | Normal | ATG, CsA | PD | 2 | 10 | 10 | Major neutrophil response | 69 | Major neutrophil response | − | 69 | − | − | − | − | − |
| 15 | 75 | WHO II | Complex | None | PD | 2 | 10 | 60 | SD | 132 | SD | − | 132 | Decrease of marrow blasts (30% at 3 months) | − | − | + | + |
| 16 | 82 | M4 | Normal | None | PD | 2 | 11 | 50 | PD | 66 | mSD | 112 | 137 | − | + | + | + | |
| 17 | 65 | M2 | Trisomy 8 | 1× ind. 2× cons. | 1. PR | 2 | 27+ | 5 | SD ongoing | SD ongoing | − | 571 (+) | Erythroid response after 4 months, molecular response with complete disappearance of trisomy 8 | − | + | + | + | |
| 18 | 58 | M2 | Normal | None | PD | 2 | 23+ | 40 | SD ongoing | 506 (+) | SD ongoing | − | 506 (+) | Decrease of marrow blasts (12% at 3 months) | − | − | − | − |
| 19 | 69 | WHO II | 46 XX (del5) | Low araC | 1. PR | 2 | 11 | 30 | SD | 168 | SD | 168 | Blast response at 3 months (15%) | − | + | − | + |
ATG indicates antithymocyte globulin; autoPBSCT, autologous peripheral blood stem cell transplantation; con, consolidation chemotherapy; CsA, cyclosporin A; CR, complete remission; cytokine response, intracellular cytokine response as detected by flow cytometry after stimulation with WT1 peptide in percent (%) of CD3+CD8+ T cells, a cytokine response was considered positive if the percentage of WT1 peptide–specific cytokine producing CD3+CD8+ T cells was at least 2-fold the percentage of an HIV control peptide; Ind, induction chemotherapy; low araC, low-dose cytarabin; mPFS, modified progression-free survival (in case progression occurred prior to 6th vaccination, subsequent time was estimated from first vaccination until subsequent progression); na, not available; Patient no., order of inclusion into study; PD, progressive disease; PFS, progression-free survival; PR, partial remission; reind, reinduction chemotherapy; SD, stable disease; Tetramer+ T cells, WT1-specific tetramer-positive T cells in percent (%) of CD3+CD8+ T cells, which were considered positive, if the frequency of tetramer-positive CD3+CD8+ T cells at baseline exceeded 0.3%, which was the mean + 2 SDs (0.16% + 0.14%) observed in 12 healthy control subjects; tetramer response, an increase of at least 2-fold in frequency as compared to baseline was considered as vaccine-induced response; type, AMLs were classified according to the WHO classification (patients belonging to the category ″AMLs not otherwise categorized″ are subclassified according to the old FAB classification): all other patients belonged to the second group of the WHO classification (WHOII = ″AML with multilineage dysplasia- with prior MDS″); and vacc, vaccination.
Protocol administration and toxicity
The median number of vaccinations was 11, with a range from 4 to 27, with 2 patients still on treatment. Four patients received fewer than 6 vaccinations due to disease progression (n = 3) or intercurrent soft tissue infection (n = 1). Toxicities included transient local erythema and induration, grade 1 or 2 fatigue, grade 1 fever, and grade 1 or 2 pruritus. Three patients (nos. 12, 15, and 18) developed transient erythema-nodosum–like lesions. Two of the patients with erythema-nodosum–like lesions (nos. 12 and 15) and one additional patient (no. 9) developed a persistent grade 2 cough within the first 2 months without radiologic abnormalities of the lungs. There was no renal toxicity in any patient.
T-cell response analysis
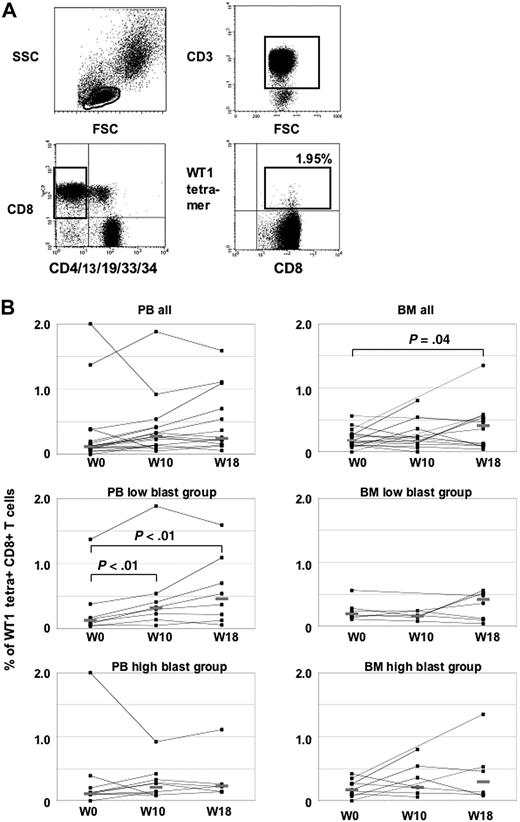
Eighteen patients with PB samples available at least at baseline and week 10 were evaluable for T-cell response assessment (Figure 1). Overall, the frequency of WT1 tetramer+ T cells increased from a median of 0.12% at baseline to 0.28% in week 10 and 0.25% in week 18, without statistical significance (Figure 1B). Compared with 12 healthy controls having a median of 0.16% WT1 tetramer+ CD3+CD8+ T cells, 4 patients with more than 0.3% (mean ± 2 standard deviations of healthy controls) were classified as having WT1 tetramer+ T cells at baseline. When arbitrarily defining induction of a T-cell response to WT1 by vaccination as a more than 2-fold increase compared with baseline, none of the 4 patients with WT1-T cells at baseline but 8 of the 14 patients without WT1-specific T cells at baseline mounted a T-cell response to the vaccine in week 10. In BM, a significant increase of the median frequency of WT1-tetramer+ T cells from 0.18% in week 0 to 0.41% in week 18 could be demonstrated (P = .04, Figure 1B).
Tetramer analyses in PB and BM. (A) Representative flow cytometry analysis of WT1 tetramers in patient no. 1. Lymphocytes were gated on CD3+ and CD8+ T cells, whereas CD4+, CD13+, CD19+, CD33+, and CD34+ cells were excluded to avoid nonspecific tetramer staining. WT1-specific T cells were calculated as CD8+ tetramer+ T cells. (B) WT1-tetramer CD3+CD8+T cells in PB and BM at baseline and during the course of vaccination. Patients with high and low blasts in BM at baseline are shown separately.
Tetramer analyses in PB and BM. (A) Representative flow cytometry analysis of WT1 tetramers in patient no. 1. Lymphocytes were gated on CD3+ and CD8+ T cells, whereas CD4+, CD13+, CD19+, CD33+, and CD34+ cells were excluded to avoid nonspecific tetramer staining. WT1-specific T cells were calculated as CD8+ tetramer+ T cells. (B) WT1-tetramer CD3+CD8+T cells in PB and BM at baseline and during the course of vaccination. Patients with high and low blasts in BM at baseline are shown separately.
To test the hypothesis whether the magnitude of leukemic blasts may limit a patient's ability to mount a T-cell response against the vaccine, patients with low (< 40%, n = 9) and high (> 50%, n = 9) blast counts in BM at baseline were compared in an explorative analysis. The groups were dichotomized at the median of 45% of BM blasts at baseline. Both groups were rather balanced for age, sex, and cohort 1 or 2, but showed a difference with respect to previous treatment, with 6 patients previously receiving chemotherapy in the low blast group and only 2 patients with chemotherapy in the high blast group. At baseline, a T-cell response to WT1 measured by tetramer assay could be detected in 2 patients of each group with frequencies in the same range. A significant increase of the median frequency of PB WT1-tetramer positive T cells from baseline (0.11%) to week 10 (0.30%, P < .01) and week 18 (0.46%, P < .01) could be demonstrated in the low blast group, but not in the high blast group (0.12% at baseline, 0.27% in week 10, 0.23% in week 18).
Next we studied the functionality of T-cell responses detected by tetramers in PB (Table 2). IFNγ- and/or TNFα-producing T cells in response to WT1 were detected in 11% of patients at baseline, in 39% of patients in week 10, and 50% in week 18. At baseline, 2 of 4 patients; in week 10, 6 of 11 patients; and in week 18, 5 of 10 patients with tetramer responses also showed a cytokine response. Conversely, only 1 patient with less than 0.25% WT1-tetramer+ T cells in week 18 showed a cytokine response (Table 2).
Bone marrow blast count and peripheral blood frequency of WT1 tetramer+ CD3CD8+ T cells and cytokine response to ex vivo stimulation with WT1 peptide
| Blast count in BM at baseline . | Patient no. . | Baseline . | Week 18 . | Week 18 . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tetramer . | IFNγ . | TNFα . | Tetramer . | IFNγ . | TNFα . | Tetramer . | IFNγ . | TNFα . | ||
| Low | ||||||||||
| 10 | 1 | 0.08 | 0 | 0 | 0.33 | 0 | 0 | 0.54 | 0.6 | 0.6 |
| 5 | 4 | 0.15 | 0 | 0 | 0.3 | 0 | 0 | 0.37 | 0.1 | 0.35 |
| 12.5 | 5 | 1.37* | 0 | 0 | 1.88* | 0 | 0 | 1.59* | 0.21 | 0.41 |
| 10 | 11 | 0.04 | 0 | 0 | 0.14 | 0 | 0 | 0.06 | na | na |
| 12 | 12 | 0.38* | 0.25 | 0 | 0.54* | 0 | 0.11 | 1.09 | 0 | 0.05 |
| 10 | 14 | 0.06 | 0 | 0 | 0.05 | 0 | 0 | 0.13 | 0 | 0 |
| 5 | 17 | 0.17 | 0 | 0 | 0.41 | 0 | 0 | 0.7 | 0.53 | 0 |
| 30 | 19 | 0.11 | 0 | 0 | 0.23 | 0.05 | 0 | 0.22 | 0 | 0 |
| High | ||||||||||
| 85 | 2 | 0.2 | 0 | 0 | 0.42 | 0 | 0.09 | na | na | na |
| 60 | 3 | 0.39* | 0.06 | 0 | 0.08 | 0 | 0 | na | na | na |
| 60 | 6 | 0 | 0 | 0 | 0.14 | 0 | 0 | na | na | na |
| 70 | 7 | 0.11 | 0 | 0 | 0.14 | 0 | 0 | 0.23 | 0 | 0 |
| na | 8 | 0.11 | 0 | 0 | 0.28 | 0.22 | 0.1 | 0.22 | 0 | 0 |
| 80 | 9 | 2.0* | 0 | 0 | 0.92* | 0.13 | 0.11 | 1.11* | 0 | 0 |
| 60 | 13 | 0.12 | 0 | 0 | 0.33 | 0 | 0 | 0.26 | 0 | 0 |
| 60 | 15 | 0.1 | 0 | 0 | 0.09 | 0 | 0.12 | 0.14 | 0.09 | 0 |
| 50 | 16 | 0.13 | 0 | 0 | 0.27 | 0 | 0.25 | 0.14 | 0 | 0 |
| 40 | 18 | 0.1 | 0 | 0 | 0.12 | 0 | 0 | na | na | na |
| Blast count in BM at baseline . | Patient no. . | Baseline . | Week 18 . | Week 18 . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tetramer . | IFNγ . | TNFα . | Tetramer . | IFNγ . | TNFα . | Tetramer . | IFNγ . | TNFα . | ||
| Low | ||||||||||
| 10 | 1 | 0.08 | 0 | 0 | 0.33 | 0 | 0 | 0.54 | 0.6 | 0.6 |
| 5 | 4 | 0.15 | 0 | 0 | 0.3 | 0 | 0 | 0.37 | 0.1 | 0.35 |
| 12.5 | 5 | 1.37* | 0 | 0 | 1.88* | 0 | 0 | 1.59* | 0.21 | 0.41 |
| 10 | 11 | 0.04 | 0 | 0 | 0.14 | 0 | 0 | 0.06 | na | na |
| 12 | 12 | 0.38* | 0.25 | 0 | 0.54* | 0 | 0.11 | 1.09 | 0 | 0.05 |
| 10 | 14 | 0.06 | 0 | 0 | 0.05 | 0 | 0 | 0.13 | 0 | 0 |
| 5 | 17 | 0.17 | 0 | 0 | 0.41 | 0 | 0 | 0.7 | 0.53 | 0 |
| 30 | 19 | 0.11 | 0 | 0 | 0.23 | 0.05 | 0 | 0.22 | 0 | 0 |
| High | ||||||||||
| 85 | 2 | 0.2 | 0 | 0 | 0.42 | 0 | 0.09 | na | na | na |
| 60 | 3 | 0.39* | 0.06 | 0 | 0.08 | 0 | 0 | na | na | na |
| 60 | 6 | 0 | 0 | 0 | 0.14 | 0 | 0 | na | na | na |
| 70 | 7 | 0.11 | 0 | 0 | 0.14 | 0 | 0 | 0.23 | 0 | 0 |
| na | 8 | 0.11 | 0 | 0 | 0.28 | 0.22 | 0.1 | 0.22 | 0 | 0 |
| 80 | 9 | 2.0* | 0 | 0 | 0.92* | 0.13 | 0.11 | 1.11* | 0 | 0 |
| 60 | 13 | 0.12 | 0 | 0 | 0.33 | 0 | 0 | 0.26 | 0 | 0 |
| 60 | 15 | 0.1 | 0 | 0 | 0.09 | 0 | 0.12 | 0.14 | 0.09 | 0 |
| 50 | 16 | 0.13 | 0 | 0 | 0.27 | 0 | 0.25 | 0.14 | 0 | 0 |
| 40 | 18 | 0.1 | 0 | 0 | 0.12 | 0 | 0 | na | na | na |
IFNγ and TNFα indicate intracellular cytokine response as detected by flow cytometry after stimulation with WT1 peptide in percent (%) of CD3+CD8+ T cells (a cytokine response was considered positive—printed in bold—if the percentage of WT1 peptide–specific cytokine-producing CD3+CD8+ T cells was at least 2-fold the percentage of an HIV control peptide; na, not available; Patient no., sequential number of inclusion into study; and tetramer indicates WT1-specific tetramer-positive T cells in percent (%) of CD3+CD8+ T cells, which were considered positive (indicated by an asterisk [*]), if the frequency of tetramer-positive CD3+CD8+ T cells at baseline exceeded 0.3%, which was the mean plus 2 standard deviations (0.16% + 0.14%) observed in 12 healthy control subjects: an increase of at least 2-fold in frequency as compared to baseline was considered as vaccine-induced response (indicated in bold).
Comparison of the tetramer and cytokine responses among patients from cohort 1 (4 × biweekly, then monthly) and cohort 2 (continual biweekly) did not suggest an advantage of continuing biweekly vaccinations in cohort 2 beyond the initial 4 vaccines (data not shown).
Clinical outcome
Clinical outcome data for all patients categorized by pretreatment disease status were summarized in Table 1. For primary analysis, clinical outcome was assessed according to the classical IWG criteria.23 According to the classical response criteria, disease stabilization with a median interval of 155 days (range, 101 to 571+ days) was observed in 10 AML patients, either with prior chemotherapy (n = 4) or untreated (n = 6). Since a heterogeneous group of patients was included in this trial, we report separately on the 3 major subgroups of patients.
Patients with AML and prior chemotherapy.
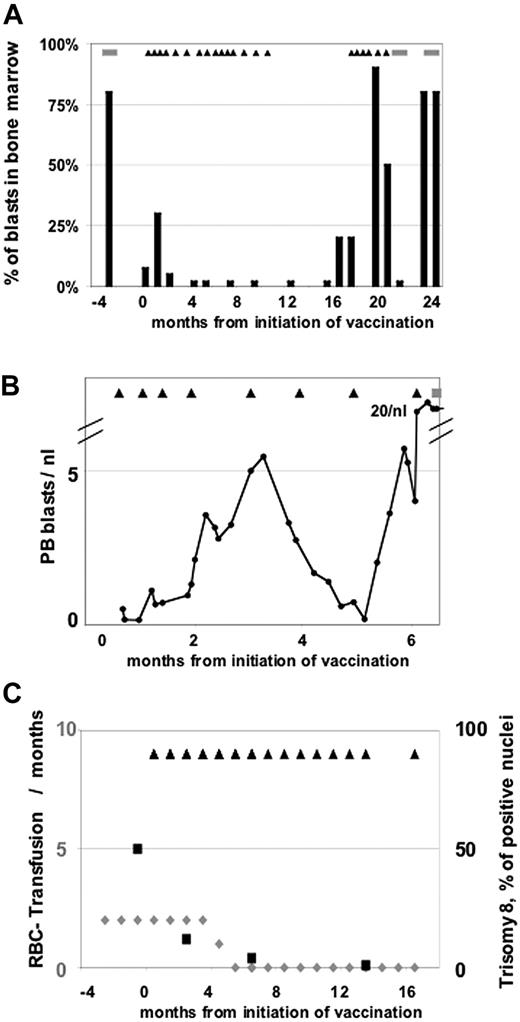
Nine of 17 AML patients had received previous chemotherapy and were in first PR (n = 3), second PR (n = 2), or third PR (n = 1), or had progressive disease (PD; n = 3) when vaccination was initiated. These patients had a median of 10% marrow blasts (range, 5%-60%) at baseline, received a median of 9 vaccinations (range, 4-27+ vaccinations), and had a median PFS of 50 days (range, 21-571+ days). The most remarkable case in this group is patient no. 17 with secondary AML from MDS, and with 5% marrow blasts at baseline and severe transfusion-dependent anemia after having received 3 cycles of chemotherapy. On protocol treatment the anemia subsided and a complete cytogenetic response was observed with reduction of trisomy 8 clones from 44% to undetectable levels by fluorescent in situ hybridization (FISH) on day 571 (Figure 2C). Three other patients (nos. 5, 11, and 19) had disease stabilization lasting for 201, 134, and 168 days, respectively.
Clinical observations. (A) Patient 1 with initial progression and subsequent mCR lasting for 12 months. ■ indicates the percentage of blasts in bone marrow; and ▴, the time points of vaccinations and the stripes periods of chemotherapy. (B) Patient 9 with initial progression and subsequent transient reduction of PB blasts. The amount of peripheral blasts per nanoliter is shown. ▴ indicates the time points of vaccinations; and the stripes, periods of chemotherapy. (C) Patient 17 with major erythroid response and a molecular response with complete clearance of the trisomy 8 disease marker.  (belonging to the left axis) indicates the number of RBC transfusions per months; ■ (belonging to the right axis) shows the percentage of positive nuclei for trisomy 8; and ▴, the time points of vaccinations.
(belonging to the left axis) indicates the number of RBC transfusions per months; ■ (belonging to the right axis) shows the percentage of positive nuclei for trisomy 8; and ▴, the time points of vaccinations.
Clinical observations. (A) Patient 1 with initial progression and subsequent mCR lasting for 12 months. ■ indicates the percentage of blasts in bone marrow; and ▴, the time points of vaccinations and the stripes periods of chemotherapy. (B) Patient 9 with initial progression and subsequent transient reduction of PB blasts. The amount of peripheral blasts per nanoliter is shown. ▴ indicates the time points of vaccinations; and the stripes, periods of chemotherapy. (C) Patient 17 with major erythroid response and a molecular response with complete clearance of the trisomy 8 disease marker.  (belonging to the left axis) indicates the number of RBC transfusions per months; ■ (belonging to the right axis) shows the percentage of positive nuclei for trisomy 8; and ▴, the time points of vaccinations.
(belonging to the left axis) indicates the number of RBC transfusions per months; ■ (belonging to the right axis) shows the percentage of positive nuclei for trisomy 8; and ▴, the time points of vaccinations.
Patients with AML and no prior chemotherapy.
Eight of the 17 AML patients had not received prior chemotherapy. This group of patients had progressive disease at baseline and a median of 60% marrow blasts (range, 40%-85%). The median number of vaccinations in this group was 11 (range, 4-23 vaccinations). In 6 of these 8 AML patients, disease stabilization for a median of 134 days (range, 101-506+ days) was observed. In 2 patients (nos. 15 and 18) disease stabilization was accompanied by a more than 50% blast reduction in BM. Another patient (no. 7) had a 50% reduction in red blood cell (RBC) transfusion requirements for 7 months.
Patients with myelodysplastic syndrome.
Both patients with high-risk MDS RAEB I and II (nos. 12 and 14) showed a major neutrophil response lasting for 10 and 2 months, respectively. The neutrophil response consisted of an increase in neutrophil levels in peripheral blood from 0.4/nL and 0.03/nL at baseline to 1.2/nL and 0.9/nL, respectively. This response was already observed within 4 weeks of treatment and continued until progression of the disease. In addition, patient no. 12 had a platelet response lasting for 3 months. In contrast to a previous trial,6 there was no evidence for hematotoxicity in our 2 MDS patients.
Since we had 4 patients with early progression, followed by subsequent responses, a second modified analysis was performed disregarding transient early disease progression in case of subsequent response. In addition to the responses described before, 4 of the 19 patients had a clinical response preceded by a period of transient early progression (until days 65 to 231). We therefore present modified response criteria, allowing for transient early disease progression if followed by subsequent response. According to these modified response criteria, 1 patient was classified as modified CR (mCR, patient no. 1), and 3 were classified as modified SD (mSD) in addition to the 10 SDs according to the conventional response criteria. Patient no. 1 had a remarkable clinical response. She had PR with 10% blasts after second-line chemotherapy when vaccination was initiated. Early information on this patient has been reported previously26 and was updated in Figure 2A. Initially, the disease progressed with an increase of marrow blasts from 10% to 30% and development of transfusion-dependent thrombocytopenia on day 28 of protocol treatment. According to conventional response criteria, the patient had to be classified as PD. As continuation of the vaccination was permitted in the protocol despite progression prior to vaccine no. 6, vaccination was continued and subsequently the marrow blasts declined to less than 5% on day 65. A formal mCR with full PB reconstitution was reached on day 99 and lasted 12 months. Vaccination was discontinued after 1 year. Five months after discontinuation of vaccination, a relapse with 20% marrow blasts was noted and vaccination was resumed with subsequent disease stabilization for 2 months, unfortunately followed by rapid progression. Of 2 other previously untreated patients with initial transient progression, in patient no. 9 disease stabilization occurred after initial rise of peripheral blasts and was characterized by a subsequent more than 50% blast reduction in PB (Figure 2B). Patient no. 16 showed an increase of marrow blasts from 50% at baseline to 75% on day 66, which was followed by a disease stabilization and reduction of marrow blasts to 40% on day 112. In addition, patient no. 12 with RAEB I at baseline had a major neutrophil response within 4 weeks of treatment lasting for 10 months and a platelet response for 3 months. However she demonstrated with an initial increase of marrow blasts to 40% on day 112, followed by a subsequent decline to 20% on day 231. The other patient with RAEB (no. 14) showed a major neutrophil response within 4 weeks of treatment lasting for 2 months.
Since the amount of blasts may limit not only the ability to mount a WT1-specific T-cell response but also the clinical effectiveness of the vaccine, the high (> 50%, n = 9) and low (< 40%, n = 10) blast group were also compared with respect to clinical outcome. The group of patients with low blasts showed in an exploratory analysis a significant better mPFS with a median of 201 days (range, 37-571+ days) in comparison with a median of only 136 days (range, 49-262 days) in the high blast group (P = .02).
WT1 mRNA levels
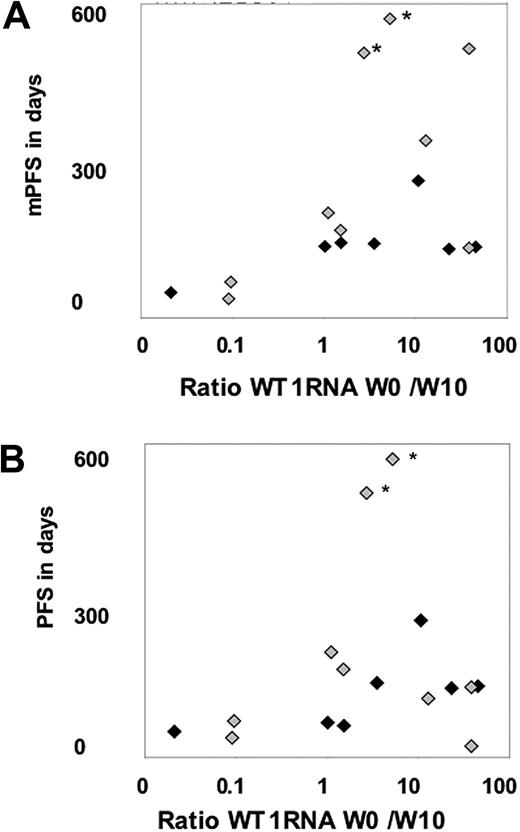
Peripheral blood and BM values at baseline and in week 10 were available in 17 patients and used to describe disease evolution and verify antigen expression. The ratio of baseline to week-10 WT1 mRNA levels was calculated and shown in Figure 3. The ratio increased at least 3-fold in 6 (38%) of 16 patients, indicating a molecular response, and remained stable over at least 10 weeks in another 7 patients (44%), whereas there was a decrease in 3 patients (18%) indicating early disease progression.
Comparison of WT1 mRNA response and clinical outcome. (A) Comparison of mPFS and the ratio of week-0 to week-10 WT1 mRNA levels in BM. (B) Comparison of PFS and the ratio of week-0 to week-10 WT1 mRNA levels in BM. ♦ indicates patients with high blasts in BM at baseline; and  , patients with low blasts in BM at baseline. *The 2 patients still on treatment with ongoing SD.
, patients with low blasts in BM at baseline. *The 2 patients still on treatment with ongoing SD.
Comparison of WT1 mRNA response and clinical outcome. (A) Comparison of mPFS and the ratio of week-0 to week-10 WT1 mRNA levels in BM. (B) Comparison of PFS and the ratio of week-0 to week-10 WT1 mRNA levels in BM. ♦ indicates patients with high blasts in BM at baseline; and  , patients with low blasts in BM at baseline. *The 2 patients still on treatment with ongoing SD.
, patients with low blasts in BM at baseline. *The 2 patients still on treatment with ongoing SD.
Comparison of clinical, molecular, and immunologic parameters
The comparison of mPFS and the WT1 mRNA ratio of baseline to week 10 revealed a significant association, when considering the number of patients below or above the median for both measurements (Figure 3, P < .01). In contrast there was no correlation between clinical outcome and T-cell response in PB or in BM, detected by either tetramers or cytokine response (not shown).
Discussion
The primary objective of this vaccination trial was the assessment of the immunologic efficacy. Because it was uncertain whether the presence of AML blasts would impair the hosts' ability to mount an immune response upon peptide vaccination, the goal was conservatively set at induction or augmentation of a tetramer response in at least 25% of patients in week 10. The vaccine adjuvants GM-CSF and KLH were chosen due to their immunologic efficacy in previous melanoma peptide vaccination trials.27 According to iSBTc recommendations,28 2 ex vivo assays were used to determine the T-cell response in PB and also in BM as “tumor compartment.” With both assays, the immunologic efficacy of the vaccination protocol was higher than 25% and with the tetramer assay approximately 50% in both compartments, which corresponds well to results with peptide vaccination and the same adjuvants in melanoma patients.27 Our findings are in accordance with reports of 2 vaccination trials in AML patients in CR by other authors.6,11 In the trial reported by Sugiyama and colleagues,6 12 AML patients in CR and 2 patients with MDS were vaccinated, resulting in a T-cell tetramer response in 9 of 13 patients, and a molecular response defined by a decrease of initially elevated WT1 mRNA in 5 of 14 patients, respectively. Similarly, Rezvani et al observed induction of WT1-specific T cells in 5 of 8 AML/MDS patients in CR in response to the HLA-A2.126-134 peptide applied in Montanide and GM-CSF, accompanied by WT1 mRNA decrease in 3 of 6 patients.11
Thus, in all 3 trials using different vaccination schemes, peptides, peptide concentrations, and adjuvants, evidence for immunologic and molecular efficacy of WT1 peptide vaccination was observed. However, in the 2 previous studies in AML patients in CR6,11 the percentage of patients with an induction of a T-cell response was 70% and 62.5%, whereas in our trial in patients with active disease the immunologic response rate was only 44%, suggesting an inverse correlation between blast count and induction of immunity.
In our study, comparison of T-cell markers and clinical outcome did not reveal a correlation between T-cell response and any outcome parameter, which may be related to the fact that 4 patients already had spontaneous WT1-tetramer+ T cells, which did not increase upon vaccination. Importantly, 2 of the 4 patients with prevaccinination WT1-tetramer T cells had no increase in T-cell frequency in PB despite disease stabilization. Furthermore, in 3 additional patients no T-cell response was detectable despite evidence for clinical activity. Thus the results of the monitoring assays used may underestimate the frequency of immunologic responses. It can only be hypothesized whether the difficulty in detecting T-cell responses in patients with evidence of clinical activity may be due to the nature of the immune response, which may not be visible in the assays used, or whether interaction between blasts and T cells may have interfered. Since blasts can have profound effects on lymphocyte number and function, we specifically evaluated differences between patients with low and high numbers of blasts in BM.29,30 The separate analysis of the 2 groups showed statistically significantly increased frequencies of PB WT1-specific tetramer responses in patients with low but not with high blasts in BM, suggesting a potential suppressive effect of high leukemic burden on the induction of WT1-specific T-cell responses. There was, however, a significant association between mPFS and decrease in BM WT1 transcripts, suggesting that WT1, although being the target antigen, maintained its role as disease marker.
Overall, the protocol treatment was well tolerated and associated only with transient local grade 1/2 toxicities typically seen with administration of GM-CSF and KLH. Pulmonary symptoms observed in 3 patients may possibly be related to induction of autoantibodies against GM-CSF.31 There was concern of potential hematologic or renal toxicity, because WT1 is expressed in CD34+ hematopoietic progenitor cells and in nephritis.32,33 A previous study had reported hematotoxicity of WT1 vaccination specifically in patients with MDS,34 but we and the group of Rezvani et al11 did not observe hematotoxicity in the 4 MDS patients treated in both studies. Actually, the major neutrophil responses we observed in both of our patients warrant specific trials in MDS patients. Furthermore, no renal toxicity was observed.
One remarkable observation in this study was the occurrence of a clinical response preceded by a period of transient early progression in 4 of 19 patients, including 1 mCR and 3 mSD. The phenomenon of disease stabilization after initial progression occurred in 2 AML patients with newly diagnosed AML with 50% and 80% marrow blasts at baseline, and in 1 patient with transformation of MDS into AML early after initiation of vaccination. All 3 of these patients were previously untreated, but did not have rapid disease kinetics. The fourth patient, whose clinical course was previously published, experienced progression of the AML with a blast doubling time of approximately 2 weeks shortly after reinduction chemotherapy, progressed further during the initial 2 vaccinations, and subsequently responded after receiving 4 vaccines. The observation of early progression despite a later response to the vaccine seems to be related to the fact that a certain time is required to mount an effective immune response and is in line with analysis of other cancer vaccine studies in which a delayed onset of activity has been frequently observed.25 This clearly points to the need for modified response criteria in therapeutic vaccine studies as, for example, suggested by the Cancer Vaccine Clinical Trial Working Group. Using such modified response criteria, 15 of 19 patients displayed evidence for clinical efficacy of treatment. Although there was only one CR and no formal PR, the fraction of patients with SD was remarkable, considering the disease setting. In addition, several patients with SD had evidence for hematologic or molecular improvement. Similarly to our results, decrease in WT1 mRNA upon vaccination with WT1 peptide was reported in AML patients in CR.6,11
Thus, further investigation of WT1-based immunotherapies in AML and also in the large variety of WT1-expressing carcinomas is warranted. However, when pursuing further clinical investigations, it will be of crucial importance to identify the mechanisms responsible for disease progression after initial efficacy.
Presented in part as abstract in an oral session at the 48th Annual Meeting of the American Society of Hematology, Orlando, FL, December 9, 2006, and at the 43rd Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 5, 2007.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by a grant from the from the José-Carreras Leukemia Foundation and from the Stiftung zur Bekaempfung der Leukaemie.
Authorship
Contribution: U.K., A.L., and C.S. designed and performed research, collected, analyzed, and interpreted data, and wrote the paper; A.B., A.M.A., and S.B. analyzed and interpreted data; I.W.B. and L.U. supplied patient material; W.-K.H. supplied patient material and discussed the paper; and E.T. designed research and discussed the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ulrich Keilholz, Department of Medicine III (Hematology and Medical Oncology), Charité, Hindenburgdamm 30, 12200 Berlin, Germany; e-mail: ulrich.keilholz@charite.de.
References
Author notes
*U.K. and A.L. share first authorship.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal