Abstract
Previous work showed that administration of antigen-expressing apoptotic cells in vivo results in antigen-specific CD8+ T-cell responses independent of Toll-like receptor signaling. We report here that natural killer (NK) cells can serve a function directly upstream of this pathway and initiate robust adaptive immune responses via killing of antigen-expressing target cells. This pathway is highly sensitive, in that administration of as few as 104 target cells induced detectable antigen-specific CD8+ T-cell responses. Importantly, NK cell–mediated cytotoxicity of target cells could also induce robust antigen-specific CD4+ T-cell responses, which were critical for subsequent CD8+ T-cell priming and IgG responses. Unlike adaptive immune responses induced by gamma-irradiated cells, the NK-cell pathway required myeloid differentiating factor 88 (MyD88) and Toll/interleukin-1 receptor domain–containing adapter-inducinginterferon-β (Trif) signaling. NK cells have previously been shown to detect and kill pathogen-infected host cells, as well as neoplastic cells and tissue allografts. The present data provide further evidence that they also discharge a strong tie with their relatives in the adaptive immune system. We think that the recognition and killing of target cells by NK cells represents an important pathway for the generation of robust CD8+ T and humoral responses that may be exploited for vaccine development.
Introduction
Despite recent advances in the understanding of innate and adaptive immunity, the interactions between the 2 systems remain poorly understood. This is true both in cases of infectious diseases and in those of sterile inflammation (eg, allograft rejection, tumor immunosurveillance), as well as in the mechanistic underpinnings of commonly used adjuvants. All such immune activation involves stimulation of an innate inflammatory response, followed by antigen-specific adaptive immune responses.1,2
Previous reports revealed that in vivo administration of antigen-expressing dying cells can effectively trigger adaptive immune responses.3-5 Our laboratory recently showed such immune activation can occur independent of Toll/interleukin-1 receptor domain (TIR) signaling and required presentation of apoptotic cell–derived antigen by a distinct subset of dendritic cells (DCs).6 In this model, efficient CD8+ T-cell responses were induced when mice were immunized with antigen-expressing (act-mOVA; membrane-bound ovalbumin under an actin promoter) splenocytes rendered apoptotic either through gamma-irradiation, ultraviolet irradiation, or activation of the Fas pathway.6 The latter suggested that adaptive immune responses might be initiated directly by cytolytic effector cells of the innate immune system, including natural killer (NK) cells.
Injection of allogeneic cells in vivo results in a rapid recognition and rejection of these cells by NK cells with subsequent uptake by DCs.7 In addition, robust antitumor responses could be obtained when allogeneic tumor cells expressing costimulatory molecules were injected into recipients,8,9 indicating a role for NK cells in the onset of adaptive immune responses. In the present study, we tested the potential of NK cells to initiate adaptive immune responses via induction of apoptosis in target cells. NK cells are able to recognize expression of surface molecules that can be classified as constitutive self, stress-induced self, and non-self (ie, pathogen-derived molecules). For instance, NK cells recognize class I major histocompatibility complex (MHC) molecules directly and indirectly via surface receptors, leading to signals that inhibit rather than activate NK-cell cytolytic function.10-12 Using missing-self target cells expressing ovalbumin (transgenic act-mOVA.Kb−/− cells), we assessed the induction of T and B cell responses in mice in vivo. These NK-cell targets were rapidly depleted from the circulation, and induced robust, antigen-specific T- and B-cell responses via a pathway that is far more sensitive than that observed after injection of equivalent numbers of antigen-expressing irradiated cells. Unlike irradiated cells, this pathway required MyD88/Trif (myeloid differentiating factor 88/TIR domain-containing adapter-inducing interferon [IFN]–β) signaling for optimal CD8+ T-cell priming. These data provide further evidence for a functional role of NK cells as initiators of adaptive immune responses that may be relevant for infectious diseases, tumor surveillance, and transplantation, but may also be exploited for vaccine development.
Methods
Mice
All experiments were performed according to US National Institutes of Health (NIH, Bethesda, MD) guidelines. C57BL/6, MyD88/Trif double-deficient mice, act-mOVA.Kb+/+, act-mOVA.Kb−/−, act-mOVA.I-Ab−/−, IFN-α receptor (IFN-αR) knockout, BALB/c.act-mOVA, and C3HHeN.act-mOVA mice, and OT-I recombination-activating gene (RAG)−/− CD45.1 mice were housed in the vivarium of The Scripps Research Institute. All knockout mice were transferred to a C57BL/6 background by repeated backcrossing (at least 6 times). Act-mOVA transgenic mice were a kind gift from Dr Mark Jenkins (University of Minnesota Medical School, Minneapolis, MN). C57BL/6J-Prf1tm1Sdz, C57BL/6Jgld/gld, B6.129S7-Il1r1tm1Imx/J, and B6.129P2-Il18tm1Aki/J mice were obtained from The Jackson Laboratory. Class II MHC-deficient and IFN-γR–deficient mice were kindly provided by Dr Charles D. Surh (The Scripps Research Institute). All mice experiments were approved by the Institutional Animal Care and Use Committees of the Cincinnati Children's Hospital and The Scripps Research Institute.
Antibodies and reagents
Fluorescent-labeled antibodies against IFN-γ, NKp46, CD4, NK1.1, DX5, and CD8 were obtained from eBioscience. CFSE (5- and 6-carboxyfluorescein diacetate succinimidyl ester) was obtained from Molecular Probes. Ovalbumin-specific peptides OVA257-264; SIINFEKL) and OVA323-339; ISQAVHAAHAEINEAGR were purchased from A&A Labs.
Preparation and treatment of splenocytes
Spleens were homogenized using a cell strainer (70 μm) and washed in complete Iscove modified Dulbecco medium (IMDM) supplemented with 10% fetal calf serum, 2% penicillin/streptomycin (Invitrogen), and 50 μM β-mercaptoethanol (Sigma-Aldrich). Cells were left untreated or exposed to gamma-irradiation (1500 rad) as described elsewhere.6 After treatment, cells were resuspended in Hanks balanced salt solution (HBSS; HyClone) and immediately injected intravenously into recipient mice.
Measurement of CD4+ T and CD8+ T-cell activation
CD8+ T-cell responses were determined ex vivo by intracellular IFN-γ staining after a 5-hour stimulation with 10−7 M OVA257-264 peptide as described before.6
Cytolytic CD8+ T-cell effector function was determined by standard 51Cr-release assay. Briefly, splenocytes were restimulated for 5 days with 10−8 M OVA257-264 peptide in complete IMDM (see “Preparation and treatment of splenocytes”). Subsequently, cells were collected and 3-fold serial dilutions were cultured with 51Cr-labeled control or OVA257-264-pulsed EL-4 target cells. Supernatants were analyzed for 51Cr release using the ICN Isomedic gamma-radiation counter (VWR Scientific). Percentage of specific lysis was calculated based on measurement of 51Cr release after total lysis and spontaneous 51Cr release.
Antigen-specific CD4+ T-cell responses were determined by standard enzyme-linked immunospot (ELISPOT) assay upon overnight stimulation with I-Ab-restricted OVA323-339 peptide (10−7 M final concentration).13 Reactive spots were counted by computer-assisted image analysis (Zeiss KS ELISPOT reader).
In vivo depletion of NK and CD4+ T cells
Depletion of NK cells in vivo was performed by injecting mice intraperitoneally with 20 μL of anti-asialo GM1 antibody as recommended by the manufacturer (Wako Pure Chemical Industries) or using anti-NK1.1 treatment (200 μg/injection; clone PK136). In vivo depletion of CD4+ T cells was achieved by injecting 200 μg of anti-CD4 antibody intraperitoneally (clone GK1.5). All mice were treated twice, one day before and one day after immunization. NK cell depletion was determined 7 days after the final injection using flow cytometry.
OVA-specific total IgG, IgG1, and IgG2c isotype levels using ELISA
Serum levels of OVA-specific IgG isotypes were determined by ELISA as described before.14
Listeria monocytogenes challenge
Experimental challenges with L monocytogenes were performed as described elsewhere.15
Statistical data analysis
Data were analyzed using the GraphPad Prism4 software (GraphPad Software). The statistical significance of the differences among groups was determined from the mean and standard deviation by Student 2-tailed test or by analysis of variance (ANOVA) followed by Dunnett test for 3 or more groups. Data were considered significant when P values were less than .05.
Results
Administration of antigen-expressing NK-cell targets induces robust CD8+ T-cell responses
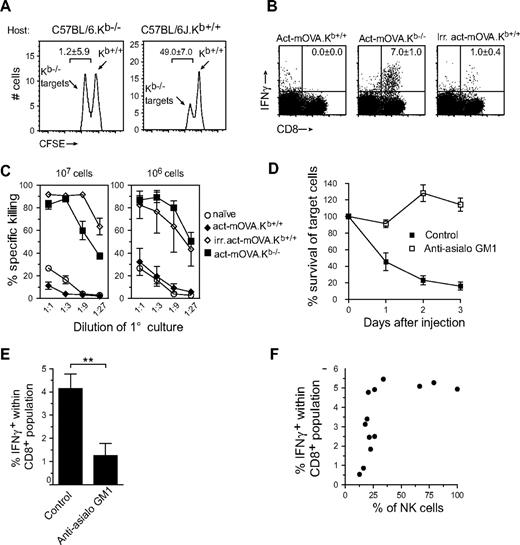
To test the potential of NK cells to initiate adaptive immune responses, we first established a model to measure NK cell–specific killing of target cells. It is well appreciated that NK-cell activation can be triggered by an absence of class I MHC expression on the surface of target cells. We thus isolated splenocytes from wild-type C57BL/6 or C57BL/6.Kb−/− mice and labeled them with different intensities of CFSE to track control and target populations in vivo. Recipient C57BL/6 and Kb-deficient mice were injected with 107 cells intravenously and, after 24 hours, a blood sample was collected and the presence of each population was determined by flow cytometry. In C57BL/6 recipient mice, only the Kb-deficient cells were removed from the circulation, while in Kb-deficient recipients both populations were retained (Figure 1A).
Administration of antigen-expressing NK-cell targets results in early clearance and robust CD8+ T-cell responses. (A) In vivo clearance of CFSE-labeled Kb-deficient splenocytes in wild-type but not Kb-deficient hosts. Twenty-four hours after transfer, blood samples were collected and analyzed for the presence of Kb-sufficient (high CFSE) and Kb-deficient splenocytes (low CFSE). Numbers in graph represent percentage killing ± SD (n = 3). (B,C) CD8+ T-cell responses measured 8 days after immunization with either 107 live Kb-sufficient or -deficient act-mOVA cells or with 107 irradiated act-mOVA.Kb+/+ cells. Responses were quantified by IFN-γ production after stimulation with OVA peptide ex vivo (B) or the ability to kill OVA-expressing target cells in vitro (C). (D) Survival of Kb-deficient splenocytes in wild-type and anti-asialo GM1-treated recipient mice (percentage is calculated from the ratio between Kb-deficient and Kb-sufficient cells in Kb-deficient recipients for each day). (E) Frequency of antigen-specific CD8+ T cells in wild-type or mice receiving anti-asialo GM1 treatment before and after immunization. (F) Association curve between NK-cell presence and antigen-specific CD8+ T-cell responses measured after mice received different doses of anti-asialo GM1 antibody 1 day before and 1 day after vaccination with 106 Kb−/− act-mOVA splenocytes.
Administration of antigen-expressing NK-cell targets results in early clearance and robust CD8+ T-cell responses. (A) In vivo clearance of CFSE-labeled Kb-deficient splenocytes in wild-type but not Kb-deficient hosts. Twenty-four hours after transfer, blood samples were collected and analyzed for the presence of Kb-sufficient (high CFSE) and Kb-deficient splenocytes (low CFSE). Numbers in graph represent percentage killing ± SD (n = 3). (B,C) CD8+ T-cell responses measured 8 days after immunization with either 107 live Kb-sufficient or -deficient act-mOVA cells or with 107 irradiated act-mOVA.Kb+/+ cells. Responses were quantified by IFN-γ production after stimulation with OVA peptide ex vivo (B) or the ability to kill OVA-expressing target cells in vitro (C). (D) Survival of Kb-deficient splenocytes in wild-type and anti-asialo GM1-treated recipient mice (percentage is calculated from the ratio between Kb-deficient and Kb-sufficient cells in Kb-deficient recipients for each day). (E) Frequency of antigen-specific CD8+ T cells in wild-type or mice receiving anti-asialo GM1 treatment before and after immunization. (F) Association curve between NK-cell presence and antigen-specific CD8+ T-cell responses measured after mice received different doses of anti-asialo GM1 antibody 1 day before and 1 day after vaccination with 106 Kb−/− act-mOVA splenocytes.
We next examined whether NK cell–mediated killing could induce CD8+ T-cell responses. C57BL/6J mice were injected intravenously with 107 live act-mOVA on either a Kb-sufficient (control group) or -deficient background. As a positive control, mice received 107 million gamma-irradiated act-mOVA.Kb+/+ or act-mOVA.Kb−/− cells intravenously, as described.6 After 8 days, splenocytes were isolated and restimulated ex vivo with OVA257-264 peptide, followed by measurement of intracellular IFN-γ production. Interestingly, immunization with live Kb-deficient act-mOVA cells caused a significantly stronger antigen-specific CD8+ T-cell response than observed with gamma-irradiated act-mOVA.Kb+/+ or act-mOVA.Kb−/− cells (Figure 1B and Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article), suggesting that NK cell–mediated killing amplifies CD8+ T-cell responses. As previously observed, no CD8+ T-cell response could be measured when mice were administered syngeneic live act-mOVA.Kb+/+ cells6 (Figure 1B,C). In all groups, priming of CD8+ T cells, as measured by IFN-γ production, correlated well with their ability to kill OVA257-264-loaded EL-4 target cells (Figure 1C).
To confirm the role of NK cells in killing “missing self” target cells we depleted NK cells in vivo using anti-asialo GM1 or anti-NK1.1 (clone PK136) antibodies. Although NK-cell depletion via this method was never absolute, anti-asialo GM1 antibodies proved far more effective in the removal of NK cells than did anti-NK1.1 (Figure S1B). Upon treatment with anti-asialo GM1 (and to a lesser extent PK136), recognition and removal of Kb-deficient act-mOVA cells was abrogated (Figure 1D) and a marked reduction in CD8+ T-cell responses was observed (Figure 1E and Figure S1C). To identify the number of NK cells necessary for optimal CD8+ T-cell responses, we treated mice with various doses of anti-asialo GM1 antibodies. Interestingly, the presence of approximately 25% of normal NK-cell levels (as measured by day 7 after treatment) appeared sufficient for obtaining effective CD8+ T-cell responses (Figure 1F). Although the removal of gamma-irradiated cells from the circulation appeared faster (within 24 hours), no apparent differences concerning tissue distribution were observed between injection of gamma-irradiated cells and Kb-deficient cells (Table S1).
The NK-cell adjuvant pathway is sensitive for induction of CD8+ T-cell responses
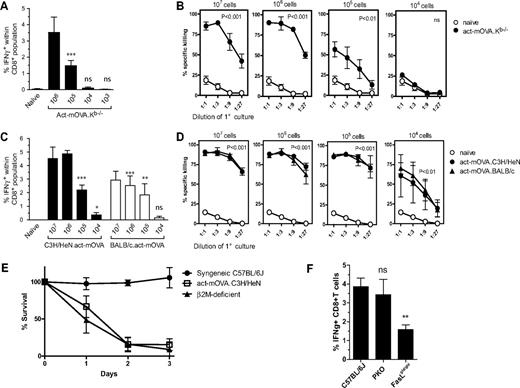
We next determined the minimal number of NK-cell targets capable of inducing antigen-specific CD8+ T-cell responses. We immunized mice with different doses of live act-mOVA.Kb−/− cells and 8 days later CD8+ T-cell responses were measured ex vivo. Strong CD8+ T-cell responses were observed when mice were injected with 107 or 106 NK-cell targets; immunization with 105 cells resulted in suboptimal CD8+ T-cell responses, whereas no significant antigen-specific CD8+ T-cell responses were observed with 104 cells (Figure 2A,B). In comparison, antigen-specific CD8+ T-cell responses induced by gamma-irradiated act-mOVA.Kb+/+ cells were undetectable when mice received less than 106 irradiated cells (Figure S1D), suggesting an enhanced adjuvanticity when live Kb-deficient target cells were injected.
CD8+ T-cell priming upon administration of Kb-deficient or allogeneic NK-cell targets is highly sensitive. CD8+ T-cell responses upon administration of various doses of Kb-deficient (A,B) or allogeneic (C-D) NK-cell targets. CD8+ T-cell responses were quantified 8 days after immunization by measuring IFN-γ production upon restimulation with OVA257-264 ex vivo (A) or the ability of CD8+ T cells to kill OVA-expressing target cells in vitro (B). (C,D) C57BL/6 mice were either immunized with act-mOVA targets on a mixed H-2b × H-2k (closed bars) or H-2b × H-2d (open bars) background (± SEM; n = 3). (E) Survival of allogeneic C3H/HeN-act-mOVA and β2m-deficient splenocytes in C57BL/6J recipient mice (percentage is calculated from the ratio between β2m-deficient and C57BL/6J cells in β2m-deficient recipients for each day). (F) CD8+ T-cell responses upon administration of 106 allogeneic C3H/HeN.act-mOVA target cells in C57BL/6J control, C57BL/6J-Prf1tm1Sdz, and C57BL/6Jgld/gld recipient mice (n = 6 for each group).
CD8+ T-cell priming upon administration of Kb-deficient or allogeneic NK-cell targets is highly sensitive. CD8+ T-cell responses upon administration of various doses of Kb-deficient (A,B) or allogeneic (C-D) NK-cell targets. CD8+ T-cell responses were quantified 8 days after immunization by measuring IFN-γ production upon restimulation with OVA257-264 ex vivo (A) or the ability of CD8+ T cells to kill OVA-expressing target cells in vitro (B). (C,D) C57BL/6 mice were either immunized with act-mOVA targets on a mixed H-2b × H-2k (closed bars) or H-2b × H-2d (open bars) background (± SEM; n = 3). (E) Survival of allogeneic C3H/HeN-act-mOVA and β2m-deficient splenocytes in C57BL/6J recipient mice (percentage is calculated from the ratio between β2m-deficient and C57BL/6J cells in β2m-deficient recipients for each day). (F) CD8+ T-cell responses upon administration of 106 allogeneic C3H/HeN.act-mOVA target cells in C57BL/6J control, C57BL/6J-Prf1tm1Sdz, and C57BL/6Jgld/gld recipient mice (n = 6 for each group).
Notably, the Kb-deficient model is based on expression of a single minor histocompatibility antigen and a single haplotype deficiency. It is conceivable that the NK-cell pathway is relevant for allogeneic responses, for which more pronounced antigen- and haplotype-specific genetic differences exist. We were thus interested in assessing the sensitivity observed for Kb deficiency–mimicked responses observed for allogeneic cells. We injected C57BL/6J mice with identical cell numbers of live C3H/HeN.act-mOVA or BALB/c.act-mOVA splenocytes and measured killing of allogeneic cells by NK cells and the subsequent OVA-specific CD8+ T response. Similar to Kb-deficient target cells, allogeneic C3H/HeN cells were removed within 2 to 3 days after injection (Figure 2E) and induced robust OVA-specific CD8+ T-cell responses (Figure 2C,D). Importantly, with 104 allogeneic target cells, still measurable CD8+ T-cell responses were observed (Figure 2C,D), showing that the pathway is highly sensitive and can be induced with relative small numbers of antigen-expressing NK-cell targets in vivo.
Activation of Fas receptor contributes to CD8+ T-cell priming via allogeneic target cells
Recent work suggests that the pathway triggering cell death influences the immunogenicity of cells.16 We thus sought to investigate whether cytolytic pathways could influence the immune responses induced by target cells. The main cytolytic pathways used by NK cells involve exocytosis of cytoplasmic granules containing perforin, and Fas ligand (FasL)–mediated activation of the Fas receptor (CD95) on target cells.17-19 Five-week-old FasL mutants (C57BL/6Jgld/gld) and perforin-deficient (C57BL/6J-Prf1tm1Sdz) mice were injected with allogeneic C3H/HeN.act-mOVA cells. Interestingly, disruption of the Fas/FasL, but not the perforin, pathway led to a significant reduction of the OVA257-264-specific CD8+ T-cell response (Figure 2F). As expected, given the redundant role of these cytolytic pathways, NK cell–specific killing of MHC class I–deficient target cells was normal in FasL-deficient mice and was moderately affected in perforin-deficient mice (Figure S2A). Importantly, no differences in CD8+ T-cell responses were observed after administration of gamma-irradiated act-mOVA cells, suggesting that the observed difference in CD8+ T-cell responses was not due to an intrinsic defect in the CD8+ T cells themselves (Figure S2B). Taken together, these data suggest that FasL influences CD8+ T-cell responses independent of its killing potential and instead may determine immunogenicity of target cells.
NK cell–mediated killing of OVA-expressing target cells results in significant CD4+ T- and B-cell responses
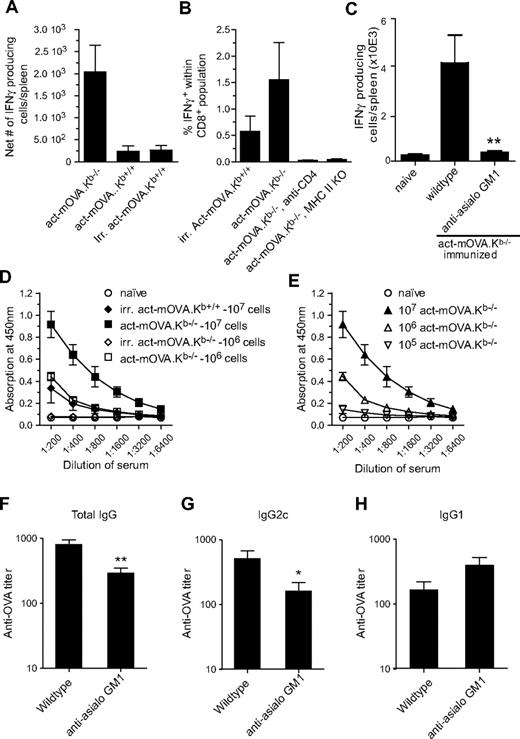
To test whether the amplified CD8+ T-cell responses induced by NK-cell targets correlated with the generation of an increased T helper response, we assessed the induction of antigen-specific CD4+ T-cell responses by IFN-γ–specific ELISPOT analysis. Immunization of mice with act-mOVA.Kb−/− cells resulted in significantly increased CD4+ T-cell responses compared with mice immunized with gamma-irradiated or live act-mOVA.Kb+/+ cells (Figure 3A). As induction of CD8+ T-cell responses by exogenous antigens has been reported to require CD4 help,20 we next examined whether CD4 help is essential for NK cell–mediated CD8+ T-cell responses. Using class II MHC-deficient and CD4-depleted mice, we observed a complete loss of antigen-specific CD8+ T cells in the absence of CD4 help (Figure 3B), suggesting a requirement for CD4 help for primary expansion and effector function of antigen-specific CD8+ T cells via this pathway. These data are consistent with previous observations in which CD4 help was essential for graft rejection.21 Further analysis of the OVA-specific CD4+ T cells showed that they produced IFN-γ, whereas interleukin (IL)–4 and IL-5 were undetectable. Importantly, NK cells were essential for the induction or polarization of CD4+ T-cell responses. Immunization of anti-asialo GM1–treated mice resulted in significantly less OVA-specific IFN-γ–producing CD4+ T cells (Figure 3C), whereas IL-4 and IL-5 remained undetectable (results not shown). These data confirm the importance of NK cells in the induction of antigen-specific CD4+ T-cell responses.
Administration of NK-cell targets induces strong CD4+ T- and B-cell responses. (A) OVA323-339-specific IFN-γ production as measured by ELISPOT by isolated splenocytes from mice immunized with either 106 live Kb-sufficient or -deficient act-mOVA cells or with 106 irradiated act-mOVA.Kb+/+ cells. Data are expressed as the average number of IFN-γ-producing cells per spleen (n = 4 per group). (B) Absence of CD4 help either through CD4 depletion via Ab treatment or using class II MHC-deficient recipient mice leads to complete abrogation of CD8+ T-cell responses as measured by antigen-specific intracellular IFN-γ production. (C) Anti-asialo GM1 treatment abrogates CD4+ T-cell response in mice immunized with 107 act-mOVA.Kb−/−-deficient target cells. (D,E) OVA-specific total IgG titers after immunization of mice with various doses of live Kb-sufficient or -deficient act-mOVA cells or irradiated act-mOVA.Kb+/+ cells. (F-H) Polarization of antibody responses as measured by OVA-specific IgG1 and IgG2c levels induced by 106 act-mOVA.Kb−/− in the presence or absence of NK cells. Sera are collected at day 14 after immunization. Data represent mean values ± SEM (n = 8). * P < .05; ** P < .01.
Administration of NK-cell targets induces strong CD4+ T- and B-cell responses. (A) OVA323-339-specific IFN-γ production as measured by ELISPOT by isolated splenocytes from mice immunized with either 106 live Kb-sufficient or -deficient act-mOVA cells or with 106 irradiated act-mOVA.Kb+/+ cells. Data are expressed as the average number of IFN-γ-producing cells per spleen (n = 4 per group). (B) Absence of CD4 help either through CD4 depletion via Ab treatment or using class II MHC-deficient recipient mice leads to complete abrogation of CD8+ T-cell responses as measured by antigen-specific intracellular IFN-γ production. (C) Anti-asialo GM1 treatment abrogates CD4+ T-cell response in mice immunized with 107 act-mOVA.Kb−/−-deficient target cells. (D,E) OVA-specific total IgG titers after immunization of mice with various doses of live Kb-sufficient or -deficient act-mOVA cells or irradiated act-mOVA.Kb+/+ cells. (F-H) Polarization of antibody responses as measured by OVA-specific IgG1 and IgG2c levels induced by 106 act-mOVA.Kb−/− in the presence or absence of NK cells. Sera are collected at day 14 after immunization. Data represent mean values ± SEM (n = 8). * P < .05; ** P < .01.
The increased CD4+ T-cell responses induced via this pathway suggested potential differences in humoral responses as well. Immunization of wild-type C57BL/6J recipients with either 107 or 106 (Figure 3D) Kb-deficient act-mOVA cells resulted in robust OVA-specific IgG responses that were absent in mice immunized with 107 gamma-irradiated act-mOVA splenocytes. Consistent with CD8+ T-cell responses, administration of 105 act-mOVA.Kb−/− cells still induced measurable anti-OVA IgG levels (Figure 3E), confirming the sensitivity of this pathway for induction of adaptive immune responses. To further investigate polarization of the immune response, we analyzed the presence of antigen-specific IgG1 and IgG2c isotype levels in wild-type and NK cell–depleted mice immunized with 106 act-mOVA.Kb−/− cells. In untreated wild-type mice, Ig isotype production was predominantly skewed toward IgG2c. However, after anti-asialo GM1 treatment, total IgG levels were reduced and class-switching skewed toward IgG1 (Figure 3G,H).
Taken together these data reveal that NK cell–mediated killing, besides priming CD8+ T cells, also initiates antigen-specific CD4+ T helper cell and antigen-specific humoral responses, predominantly polarized toward a Th1 response.
Type I and II IFNs determine CD8+ T-cell priming via MHC-deficient target cells
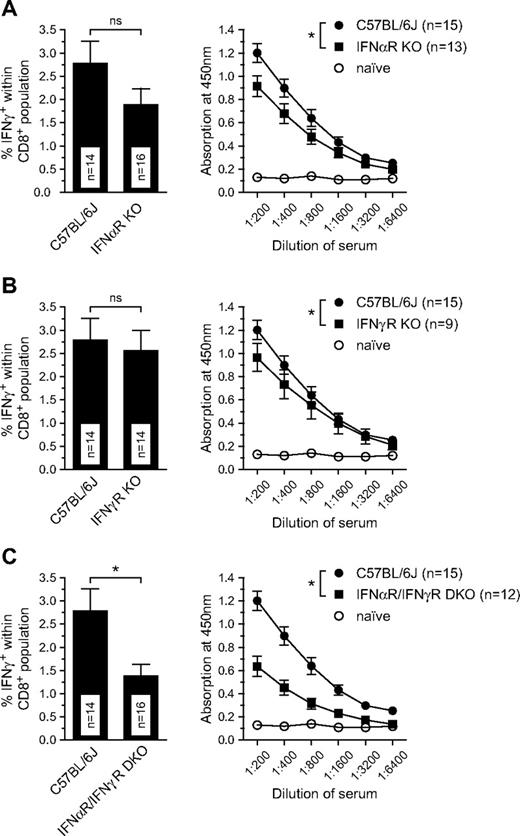
Several studies have demonstrated that NK cells and DCs directly influence each other through production of cytokines such as IFN-γ and/or type I IFN.18,22 In previous studies we found that CD8+ T-cell responses induced by irradiated act-mOVA cells were dependent on type I IFN production by a defined subset of DCs.6 Because activation of NK cells results in IFN-γ production, and both type I and type II IFNs are capable of inducing costimulatory molecule expression on DCs, we wanted to assess the role of these cytokines in NK cell–mediated adaptive immune responses. Although the CD8+ T-cell responses after transfer of NK-cell targets appear to be diminished particularly in IFN-α receptor knockout (IFN-αR−/−), a significant reduction was only observed in recipients lacking both type I and type II IFN receptors (Figure 4). A similar profile was observed for OVA-specific Ig responses (Figure 4), suggesting that both type I and type II IFNs are redundant in function and support the onset of adaptive immune responses via this pathway. No differences were observed for the killing of target cells administered in vivo in these mice (results not shown).
IFNs are key components of the NK cell–mediated CD8+ T-cell responses. (A-C) CD8+ T-cell responses as measured by IFN-γ production upon restimulation with OVA257-264 peptide ex vivo (day 8) and OVA-specific total IgG (measured day 14) in wild-type, IFN-αR, IFN-γR, and IFN-α/IFN-γR double-deficient mice. Values represent the mean ± SEM of pooled data from 3 to 4 independent experiments (*P ≤ .05).
IFNs are key components of the NK cell–mediated CD8+ T-cell responses. (A-C) CD8+ T-cell responses as measured by IFN-γ production upon restimulation with OVA257-264 peptide ex vivo (day 8) and OVA-specific total IgG (measured day 14) in wild-type, IFN-αR, IFN-γR, and IFN-α/IFN-γR double-deficient mice. Values represent the mean ± SEM of pooled data from 3 to 4 independent experiments (*P ≤ .05).
Involvement of the MyD88/Trif pathway in NK cell–mediated immune responses
Previous studies in our laboratory have revealed that CD8+ T-cell responses induced by OVA-expressing irradiated cells occur independent of MyD88/Trif signaling. We thus sought to determine whether MyD88/Trif signaling is dispensable for NK cell–mediated CD8+ T-cell responses as well. Both wild-type and MyD88/Trif double-deficient mice were administered 106 gamma-irradiated act-mOVA.Kb+/+ cells or 106 live act-mOVA.Kb−/− cells. After 8 days, splenocytes were isolated and assessed for antigen-specific CD8+ T-cell responses. Surprisingly, CD8+ T-cell responses after injection of NK-cell targets were reduced in MyD88/Trif double-deficient mice and comparable with irradiated act-mOVA cells (Figure 5A,B), suggesting a role for MyD88/Trif in the amplification of CD8+ T-cell responses in the NK-cell pathway. In addition, strongly abrogated B-cell responses were observed in MyD88/Trif double-deficient mice immunized with OVA-expressing NK-cell targets (Figure 5C). To asses whether MyD88/Trif double-deficient mice were defective in the killing of target cells, we performed an in vivo cytotoxicity assay with class I MHC-deficient target cells. Although slightly diminished killing was observed, most cells were killed after 1 to 2 days similar to wild-type C57BL/6 mice (Figure S3A).
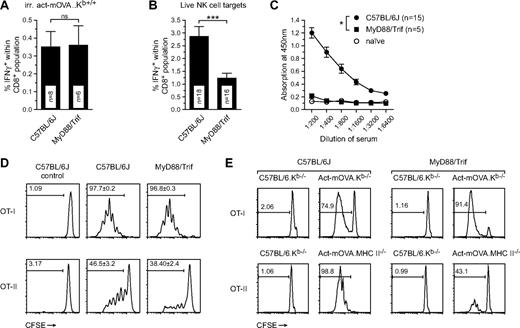
Involvement of MyD88/Trif signaling in the amplification of CD8+ T-cell responses induced by NK-cell targets. (A,B) Frequency of CD8+IFN-γ+ T cells as measured by restimulation ex vivo 8 days after immunization of wild-type and MyD88/Trif double-deficient mice with either 106 irradiated cells or 106 live act-mOVA.Kb−/− splenocytes (n ≥ 8 for each group). (C) B-cell responses in wild-type and MyD88/Trif double-deficient mice immunized with 106 live act-mOVA.Kb−/− cells as measured by OVA-specific total IgG levels in sera collected 14 days after immunization. (D,E) Activation of OT-I and OT-II cells in wild-type and MyD88/Trif double-deficient mice either in vivo (D) or in vitro (E). (D) Mice were injected with 106 live act-mOVA.Kb−/− cells followed by administration of CD8+ OT-I or CD4+ OT-II cells intravenously after 3 days. (E) Activation of OT-I and OT-II cells by Flt3L-treated bone marrow–derived DCs from either wild-type or MyD88/Trif double-deficient mice. Proliferation of OT-I and OT-II cells is measured by flow cytometry measuring the dilution of CFSE upon each mother cell division (n = 4). *P ≤ .05; ***P ≤ .001.
Involvement of MyD88/Trif signaling in the amplification of CD8+ T-cell responses induced by NK-cell targets. (A,B) Frequency of CD8+IFN-γ+ T cells as measured by restimulation ex vivo 8 days after immunization of wild-type and MyD88/Trif double-deficient mice with either 106 irradiated cells or 106 live act-mOVA.Kb−/− splenocytes (n ≥ 8 for each group). (C) B-cell responses in wild-type and MyD88/Trif double-deficient mice immunized with 106 live act-mOVA.Kb−/− cells as measured by OVA-specific total IgG levels in sera collected 14 days after immunization. (D,E) Activation of OT-I and OT-II cells in wild-type and MyD88/Trif double-deficient mice either in vivo (D) or in vitro (E). (D) Mice were injected with 106 live act-mOVA.Kb−/− cells followed by administration of CD8+ OT-I or CD4+ OT-II cells intravenously after 3 days. (E) Activation of OT-I and OT-II cells by Flt3L-treated bone marrow–derived DCs from either wild-type or MyD88/Trif double-deficient mice. Proliferation of OT-I and OT-II cells is measured by flow cytometry measuring the dilution of CFSE upon each mother cell division (n = 4). *P ≤ .05; ***P ≤ .001.
Both MyD88 and Trif are key adaptor molecules involved in the downstream signaling cascades of TLRs or the IL-1R family of cytokines that include IL-1α/β, IL-18, and IL-33. Previous work suggests that cell death can induce robust IL-1α/β production in vivo.23,24 Indeed, IL-1β secretion can be readily measured in vitro upon exposure of Flt3L-induced bone marrow–derived DCs to apoptotic cells, a process that also occurs in DCs derived from MyD88/Trif double-deficient mice (results not shown). Thus, IL-1 signaling through MyD88 may be responsible for the amplification of adaptive immune responses observed after injection of NK-cell targets. In addition, a recent report revealed a role for IL-18 in NK-cell priming25 and, as such, a lack of IL-18 signaling could affect target cell killing. Analyses of CD8+ T-cell responses in IL-1R– and IL-18–deficient mice showed only mild reductions in IL-1R-deficient and slight but insignificant increases of CD8+ T-cell responses in IL-18– and MyD88-deficient mice (Figure S3B-D). Overall, these effects did not recapitulate the strong reduction of adaptive immune responses observed in MyD88/Trif double-deficient mice. Furthermore, CD8+ T-cell responses were found to be normal in Trif-deficient mice (Figure S3D), ruling out the possibility that Trif alone mediates adjuvanticity for this pathway.
A recent report suggested an important role for TLR4—which activates both Myd88 and Trif signaling—as a sensor of high-mobility-group box 1 (HMGB1) protein secreted by dying tumor cells and subsequently mediating T-cell responses.26 However, in our system no differences were observed for CD8+ T-cell responses induced in wild-type and TLR4-deficient mice (Figure S3E), thereby rejecting this hypothesis.
The NK-cell pathway requires Myd88/Trif for CD4+ but not CD8+ T-cell responses
The lack of B-cell responses in MyD88/Trif double-deficient mice suggested a role for these adaptors in induction of CD4 B-cell helper activation. We therefore investigated whether transgenic CD8+ OT-I or CD4+ OT-II cells could be activated in a MyD88/Trif double-deficient background. Wild-type and MyD88/Trif double-deficient mice were injected intravenously with live act-mOVA.Kb−/− splenocytes and after 3 days mice received CFSE-labeled congenic CD8+ OT-I (CD45.1) or CD4+ OT-II (CD90.1) cells. After 3 days, OT-I/II proliferation was determined by flow cytometry measuring CFSE dilution upon each cell division. Whereas no differences were observed for activation of CD8+ OT-I T cells, marked reductions were observed for activation of CD4+ OT-II cells in MyD88/Trif double-deficient mice (Figure 5D). This suggested that cross-priming of CD8+ T cells in Myd88/Trif double-deficient mice was normal, whereas priming of CD4+ T cells was affected. We next assessed whether this observation could be linked to a suboptimal DC function in MyD88/Trif double-deficient mice. We treated bone marrow–derived cells with Flt3L and 8 days later isolated mPDCA1− cells as described before.6 Subsequently, DCs were exposed to either irradiated act-mOVA.Kb−/− or act-mOVA.MHC-II−/− cells. After 24 hours of incubation, CFSE-labeled CD8+ OT-I or CD4+ OT-II T cells were added to the wells and proliferation was measured after 3 days incubation using flow cytometry. In accordance with CD4+ T-cell activation in vivo, OT-II but not OT-I T-cell proliferation was abrogated in the presence of MyD88/Trif double-deficient DCs (Figure 5E). Further analysis revealed that BMDCs from MyD88/Trif double-deficient mice express reduced levels of class II MHC and costimulatory molecules such as CD86 (Figure S3F,G). Overall, these data suggest that DC maturation and activation of T cells, specifically via the class II MHC pathway, may be hindered in MyD88/Trif double-deficient mice.
Vaccination with NK target cells induces protective memory against a secondary pathogenic challenge
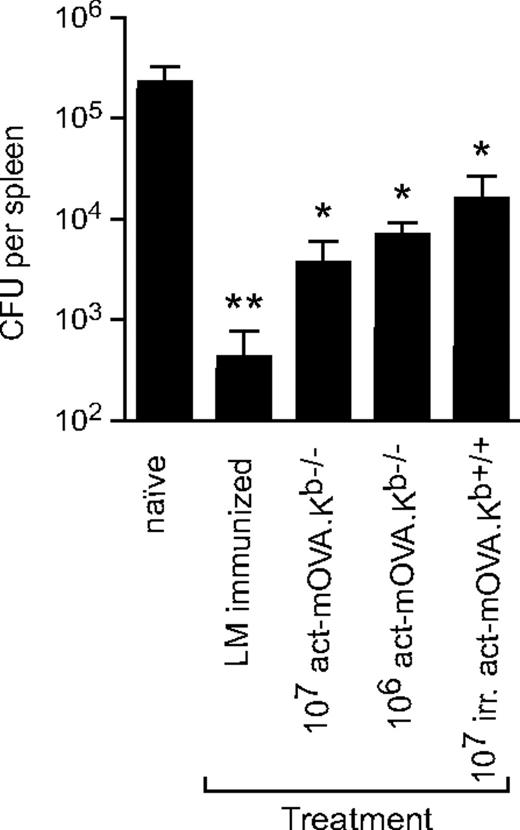
The generation of long-lived CD8+ T-cell memory cells is central to vaccine development,27-29 including vaccines intended to prevent infection with intracellular microbes. The intracellular bacterium L monocytogenes serves as an ideal model for assessing CD8+ T-cell memory due to the well-defined kinetics of bacterial growth and the absolute dependence on Listeria-specific CD8+ T cells for protective immunity. To assess whether the NK cell–mediated adjuvant pathway can be used to provide protection against a sublethal challenge with L monocytogenes, C57BL/6 mice were immunized with either 107 gamma-irradiated act-mOVA.Kb+/+ or live 107 act-mOVA.Kb−/− cells or with 103 CFU OVA-expressing L monocytogenes (LM-OVA). Thirty-five days after injection, mice were challenged with 105 CFU of LM-OVA. After another 3 days, spleens were isolated and LM-OVA colonies were quantified as described before.6 As expected, mice immunized with a low dose of LM-OVA revealed the strongest protection; however, immunization with act-mOVA.Kb−/− target cells resulted in a protection almost as robust as observed in the LM-OVA–immunized group (Figure 6). A similar protection was observed for mice immunized with 106 act-mOVA.Kb−/− cells, whereas immunization of mice with 107 irradiated act-mOVA cells induced a significant but less potent protection against L monocytogenes (Figure 6).
Vaccination with NK-cell targets results in effective protection against OVA-expressing L monocytogenes. C57BL/6 mice were immunized with either 107 irradiated cells or 107 or 106 live act-mOVA.Kb−/− splenocytes. As a positive control, mice were immunized with a low dose (103) of L monocytogenes. On day 35 after immunization, mice were challenged with 105 CFU of LM-OVA and splenic titers were determined after an additional 3 days (n = 8 for each group). *P ≤ .05; **P ≤ .01 as determined by ANOVA.
Vaccination with NK-cell targets results in effective protection against OVA-expressing L monocytogenes. C57BL/6 mice were immunized with either 107 irradiated cells or 107 or 106 live act-mOVA.Kb−/− splenocytes. As a positive control, mice were immunized with a low dose (103) of L monocytogenes. On day 35 after immunization, mice were challenged with 105 CFU of LM-OVA and splenic titers were determined after an additional 3 days (n = 8 for each group). *P ≤ .05; **P ≤ .01 as determined by ANOVA.
These data indicate that adaptive immune responses induced by NK cells yield long-lived memory responses that provide effective protection against pathogen rechallenges.
Discussion
NK cells have been particularly appreciated for their innate ability to recognize and kill tumor cells, grafted cells, or infected cells. In general, such conditions coincide with robust CD8+ T-cell responses, but the role of NK cells in the initiation of adaptive responses is less well understood. Here we show that injection of 104 to 105 antigen-expressing NK-cell targets induces significant antigen-specific T- and B-cell responses. Such numbers present only a small fraction of the cells that undergo apoptosis or cytolysis during a systemic viral infection. Consistent with the overall strategy of the innate immune system, which detects and contains infection at a very early stage, the NK cell–driven pathway would presumably be initiated soon after viral inoculation (at a stage when only a few thousand cells might be infected and converted to targets for NK-cell attack). Our data provide evidence for the remarkable sensitivity of this pathway and the role that NK cells play in the initiation of adaptive immune responses. Indeed, recent reports suggest an important role for NK cells in CD8+ T-cell responses during viral infections such as poxvirus and murices cytomegalovirus (MCMV).30,31 In the latter model, however, NK cells seemed mostly involved in restricting the overwhelming immune response observed after MCMV infection. Unlike the low levels of type I IFNs in our model,6 MCMV infections in Ly49H-deficient BALB/c mice result in high concentrations of IFN-α/β, which can cause an ablation of DCs and immunosuppression.32,33 Early NK-cell activation limits viral replication and type I IFN production to a level that promotes optimal CD8+ T-cell activation.31 Whether forced cross-presentation of viral antigens via this process contributes to improved CD8+ T-cell responses remains unclear. Note also that killing of target cells alone does not suffice for initiation of adaptive immune responses. Our data indicate that the FasL/Fas pathway influences T-cell responses independent of the killing of target cells. Previous work suggests that activation of Fas receptor coincides with the release of a variety of inflammatory mediators, including but not limited to IL-1, TNF, and (human) IL-8.24,34 The biologic relevance of these factors in the immunogenicity of cell death is still unclear.
CD8+ T-cell priming can occur via antigen presentation in class I MHC on DCs via direct presentation and via cross-presentation of exogenous soluble or particulate antigens. The latter pathway would be relevant for NK cell–mediated adaptive immune responses induced by class I MHC–deficient target cells. Indeed, previous work showed NK cell–mediated cell death can induce cross-presentation of antigens by CD8+ DCs, resulting in activation of transgenic CD8+ OT-I and CD4+ OT-II T cells in vitro.35 In our model, CD8+ T-cell responses upon injection of antigen-expressing Kb-deficient target cells are entirely dependent on cross-priming and would presumably resemble sterile inflammatory responses as they occur during allograft or tumor cell rejection. Importantly, both the initiation of adaptive immune responses and killing of Kb-deficient target cells are abrogated in the absence of NK cells, demonstrating the requirement for NK cells in this pathway. A recent report suggested that NK cell–mediated clearance of β2-microglobulin–deficient male cells in female recipient mice reduced H-Y-specific CD4+ and CD8+ T-cell activation compared with injection of wild-type H-Y cells.36 In this model, H-Y-specific antigen presentation was reduced after transfer of β2-microglobulin donor cells compared with wild-type cells 11 days after transfer, and the authors suggested that long-lived antigen is required for DC licensing and helper-dependent CD8+ T-cell activation. However, using Kb-deficient target cells, we find a rapid clearance and a robust helper-dependent CD8+ T-cell response 8 days after injection of target cells, implying that antigen persistence is not a determinant in our model.
Our data further indicate involvement of type I and type II IFNs in this pathway. Previously, we showed that type I IFN is an important mediator of adjuvanticity via TIR-dependent and -independent pathways,6,37 and our present data indicate that it also mediates adjuvanticity elicited by NK cell–mediated cell death. Type I IFN can be induced by cell death upon recognition of a specific subset of DCs and as such serves a function in the priming of CD8+ T cells by inducing maturation of DCs in a autocrine or paracrine manner.6
Previous work suggested that NK cells are intimately involved in DC function, providing positive feedback mechanisms beneficial to both cell types.22,38-43 For example, NK-cell activation induces release of IFN-γ, which in turn can cause maturation of DCs37 and polarize T-cell responses.44 In contrast, DC activation often results in production of type I IFNs and IL-18, which can prime NK cells and increase cytolytic effector function. In this context, NK cells are thought to primarily serve a function as DC “helper” cells in initiating T-cell responses.45 Because the activation of NK cells results in IFN-γ production, we hypothesized that this may present a mechanism by which the amplification of adaptive immune responses could be achieved. However, only modest reductions in T- or B-cell responses were observed in the absence of functional IFN-γ signaling, effectively refuting a major role of IFN-γ in the amplification of this pathway.
Instead, our data suggest that the amplification of immune responses upon administration of NK-cell targets is largely dependent on MyD88/Trif signaling. Interestingly, previous work showed that the T-cell responses induced by irradiated cells expressing antigen were equivalent in the absence of MyD88 and Trif signaling. Most, if not all, inflammatory mediators released upon exposure of DCs to cell death are induced independently of MyD88/Trif signaling6 (results not shown), and thus the recognition of cell death in our model is mediated predominantly via TLR-independent pathways. Our data indicate that in the absence of MyD88 and Trif signaling, DC development and maturation, particularly as pertaining to class II MHC–mediated responses, are affected. Besides a low class II MHC expression in MyD88/Trif double-deficient DCs, maturation and expression of costimulatory molecules remain muted but may still occur via activation of TLR-independent receptors such as NLRs or RLRs. Interestingly, the activation of CD8+ T cells seems less dependent on MyD88/Trif signaling. Hence, a clear divergence in the requirements for CD8+ and CD4+ T-cell activation seems to exist. Whether this can be linked to DC maturation alone or whether it also involves qualitative/quantitative differences in antigen presentation (ie, cross-presentation versus phagosomal loading of class II MHC peptides) remains to be determined.
Our data show that NK cells are capable of initiating robust adaptive immune responses. NK cells detect and kill pathogen-infected host cells, as well as neoplastic cells and tissue allografts. Such conditions entail sterile inflammatory responses and are often associated with strong cellular and humoral adaptive immune responses.46-48 Recognition of tumor cells can occur via down-regulation of class I MHC molecules, as well as expression of stress-associated ligands that engage NK-cell signaling.49,50 The coinciding inflammatory signals with cytotoxicity appear optimally geared toward the generation of adaptive immune responses. Importantly, the NK-cell pathway is able to activate different arms of the adaptive immune system inducing both T- and B-cell responses. Current vaccines are mostly designed to generate either humoral or cell-mediated immune responses, whereas an ideal vaccine would target both arms. Together with the induction of effective memory responses, we think that this pathway may be well exploitable for the development of novel approaches to vaccination, an aspect that thus far has been hardly explored.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
P.K. was supported by a long-term fellowship from the European Molecular Biology Organization. This research was funded by the International AIDS Vaccine Initiative and the NIH.
National Institutes of Health
Authorship
Contribution: P.K. conducted and designed experiments and performed data analyses; M.J.B., K.L., and K.W. provided experimental assistance; K.B. provided L monocytogenes reagents; B.B. and E.J. provided critical discussions; and K.H. was responsible for experimental design and data analyses and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kasper Hoebe, Division of Molecular Immunology, Cincinnati Children's Hospital Research Foundation, University of Cincinnati College of Medicine, Rm S5.421, 3333 Burnet Ave, Cincinnati, OH 45229; e-mail: kasper.hoebe@cchmc.org; or Philippe Krebs, Department of Genetics, The Scripps Research Institute, 10550 N Torrey Pines Rd, SP293, La Jolla, CA 92037; e-mail: pkrebs@scripps.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal