Abstract
Because of their potent immunoregulatory capacity, dendritic cells (DCs) have been exploited as therapeutic tools to boost immune responses against tumors or pathogens, or dampen autoimmune or allergic responses. Murine bone marrow–derived DCs (BM-DCs) are the closest known equivalent of the blood monocyte-derived DCs that have been used for human therapy. Current imaging methods have proven unable to properly address the migration of injected DCs to small and deep tissues in mice and humans. This study presents the first extensive analysis of BM-DC homing to lymph nodes (and other selected tissues) after intravenous and intraperitoneal inoculation. After intravenous delivery, DCs accumulated in the spleen, and preferentially in the pancreatic and lung-draining lymph nodes. In contrast, DCs injected intraperitoneally were found predominantly in peritoneal lymph nodes (pancreatic in particular), and in omentum-associated lymphoid tissue. This uneven distribution of BM-DCs, independent of the mouse strain and also observed within pancreatic lymph nodes, resulted in the uneven induction of immune response in different lymphoid tissues. These data have important implications for the design of systemic cellular therapy with DCs, and in particular underlie a previously unsuspected potential for specific treatment of diseases such as autoimmune diabetes and pancreatic cancer.
Introduction
Dendritic cells (DCs) exert a central role in the immune system as antigen-presenting cells that can induce and control immune responses. They have been implicated in the pathogenesis of, and in the protection against, infection, allergy, cancer, and autoimmunity.1 DCs may be generated in large numbers in vitro from progenitor cells, and modified to either boost an immune response against tumors or microbes, or dampen an autoimmune or allergic response, in many cases in an antigen-specific manner. Therefore, immunotherapy with DCs represents a new generation of potent personalized medicine to fight various cancers,2-4 chronic infectious diseases,4-6 and possibly autoimmune diseases.7,8 The feasibility of such an approach has been established through many clinical studies (http://www.clinicaltrials.gov).
One important aspect of DC immunotherapy that greatly impacts efficacy is the method of delivery of the DCs to the patient. To wield their effect, DCs must directly interact with or localize in close proximity to the cells that they regulate, which are primarily, but not limited to, T lymphocytes. Thus, DCs would be required to target secondary lymphoid tissues to influence T-cell priming and/or the tumor or inflamed lesions to control T-cell effector function. Secondary lymphoid tissues typically include the spleen and lymph nodes, most importantly the lymph nodes that drain the site of tumor growth, infection, or autoimmune destruction. Efficient homing of DCs to the most appropriate sites constitutes a crucial aspect of DC immunotherapy.9,10 Intradermal, subcutaneous, intranodal, or intralymphatic delivery of DCs has been used to specifically target local lymph nodes, for instance in the treatment of metastatic tumors, which typically metastasize into draining lymph nodes. These local routes of treatment have been extensively studied in DC homing studies.11-16 Likewise, the biodistribution of DCs following systemic intravenous administration has received much attention, but remains poorly characterized in regard to the different lymph nodes to which DCs traffic. In addition, other routes such as intraperitoneal injection have been less well studied. Imaging methods applied to mice and humans have consistently described homing of DCs to the spleen, lungs, and liver, all well-vascularized organs, after intravenous injection.11,12,16-22 Probably due to limited sensitivity, resolution, or time duration of the methods used, these in vivo imaging studies failed to demonstrate lymph node homing, unless ex vivo biodistribution was performed, but this has not been well characterized.17,18
Prompted by our initial observation that bone marrow–derived DCs (BM-DCs), modified by lentivirus to express the Firefly luciferase (Luc) reporter gene and injected intravenously into nonobese diabetic (NOD) mice, home preferentially to the pancreatic lymph nodes (PLNs),23 we set out to conduct a more detailed analysis of the biodistribution of BM-DCs from different Luc-transgenic mice following intravenous and intraperitoneal injection. In this report, we show that systemic administration of DCs does not result in uniform homing among lymph nodes, a feature that was previously overlooked. Our study highlights new drainage patterns for DCs, with important implications on the relevance of combining certain DCs and routes of administration to treat specific diseases.
Methods
Mice
Female NOD, NOD.B10 (NOD.B10Sn-H2b), NOD.CD45.2 (NOD.C-(Ptprc-DIMit262)Wehi), NOD.BDC2.5, FVB, and BALB/c mice were purchased from The Jackson Laboratory and bred in our animal facility. L2G85 (FVB) mice,24 expressing Firefly luciferase (Luc), were backcrossed into NOD and BALB/c mice and used at stage N5-N10 (L2G85.NOD) or N2-N3 (L2G85.BALB/c). L2G85.NOD.B10 (Luc+ I-Ab+ I-Ag7−) mice were obtained by backcrossing L2G85.NOD twice into NOD.B10 and used at stage N7. All bone marrow donors were 8 to 10 weeks old, whereas recipient mice were used at 10 to 12 weeks of age. All animal studies were conducted in compliance with National Institutes of Health (NIH)–approved guidelines from the Department of Laboratory Animal Medicine at Stanford and were approved by the Stanford University IACUC.
Dendritic cell culture and inoculation
Bone marrow was harvested from the femurs, tibias, and pelves of female L2G85 donor mice. The bone marrow cells were depleted of T cells, B cells, and granulocytes by AutoMACS (Miltenyi) using biotinylated anti-CD3, anti-B220 and anti–Gr-1 (eBioscience), and antibiotin microbeads (Miltenyi). Remaining cells were then cultured in complete RPMI, containing 10% FCS and 10 ng/mL recombinant mouse granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-4 (Peprotech). Medium was changed on day 2 or 3. Cells were split on day 4 or 5, and harvested on day 6 or 7, the purity (typically 75%-95% CD11c+ CD11b+) and phenotype were analyzed by fluorescence-activated cell sorting (FACS), and the luciferase activity was measured by luminometer (Turner Biosystems). Finally, Luc+ DCs were injected either intravenously (lateral tail vein) or intraperitoneally into recipient mice (5 × 106 cells per mouse).
Bioluminescence imaging and biodistribution
Recipient mice were imaged at different times, ranging from 4 hours to 12 days after Luc+ DC injection. The mice were first injected intraperitoneally with d-luciferin (3 mg/mouse), anesthetized for 10 minutes with isoflurane, and imaged for 5 minutes under anesthesia using the IVIS-100 system (Caliper).
For ex vivo biodistribution analysis, a minimum of 3 mice were killed in each group at each time point. Tissues (Table 1; Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article) were harvested, weighed, homogenized in PBS (liver and pancreas in PBS containing protease inhibitor cocktail III; Calbiochem), and frozen in dry ice. For analysis, the tissue samples were subjected to 3 freeze-thaw cycles, and centrifuged for 10 minutes. Total supernatant was collected and the luciferase activity (relative light units, RLU) in 10 μL was assessed using LAR2 reagent (Promega) on luminometer (total and relative activity refer to total RLU in whole supernatant and total RLU/mg tissue, respectively). For ex vivo imaging, mice were injected intraperitoneally with d-luciferin and killed 10 minutes later. Tissues were dissected, arranged on black paper, covered with d-luciferin (3 mg/mL), and imaged for 1 to 5 minutes using the IVIS-200 system (Caliper). In vivo and ex vivo imaging data were analyzed using the Living Image 2.50 software (Caliper).
List of tissues analyzed in this study, with symbol, description and location
| Symbol . | Tissue . | Description, location . |
|---|---|---|
| PLN | Pancreatic lymph nodes | Typically 2 nodes between the pancreas and the caudate lobe of the liver (right node also known as pancreaticoduodenal [PDLN], and left node as gastric [GLN]), used pooled unless otherwise stated |
| MLN | Mesenteric lymph nodes | A string of nodes in the mesentery, mostly connected to the small intestine (jejunal and colic lymph nodes) |
| ILN | Inguinal lymph nodes | Under the skin, in the groin area (pooled from both sides) (subiliac lymph nodes) |
| LLN | Lumbar lymph nodes | Periaortic, below the colon (pool of lumbar and sacral nodes) (lumbar aortic, medial iliac, and caudal mesenteric lymph nodes) |
| CLN | Cervical lymph nodes | Typically 4 nodes close to the salivary glands (mandibular and superficial parotid lymph nodes) |
| TLN | Paratracheal mediastinal lymph node | Typically one node, right side of the trachea, under the thymus and heart (tracheobronchol lymph node) |
| KLN | Popliteal lymph nodes | Behind knees (pooled from both sides) |
| RLN | Renal lymph nodes | Under the kidney (pooled from both sides) |
| SPL | Spleen | Whole tissue |
| THY | Thymus | Whole tissue |
| POT | Pancreatic and omental tissue | Pancreas with associated omental tissue |
| LIV | Liver | Left or caudate lobe as indicated. The absolute signal measured thus refer to that lobe (approximately 27% and 8% of whole liver mass, respectively), not to the whole tissue |
| LGS | Lungs | All lobes pooled |
| KID | Kidneys | Both kidneys pooled |
| INT | Intestine | A 2-inch section of ileum, emptied of its content |
| Others | Other tissues analyzed at least once | Axillary and brachial lymph nodes, heart, brain, femur (bone marrow) |
| Symbol . | Tissue . | Description, location . |
|---|---|---|
| PLN | Pancreatic lymph nodes | Typically 2 nodes between the pancreas and the caudate lobe of the liver (right node also known as pancreaticoduodenal [PDLN], and left node as gastric [GLN]), used pooled unless otherwise stated |
| MLN | Mesenteric lymph nodes | A string of nodes in the mesentery, mostly connected to the small intestine (jejunal and colic lymph nodes) |
| ILN | Inguinal lymph nodes | Under the skin, in the groin area (pooled from both sides) (subiliac lymph nodes) |
| LLN | Lumbar lymph nodes | Periaortic, below the colon (pool of lumbar and sacral nodes) (lumbar aortic, medial iliac, and caudal mesenteric lymph nodes) |
| CLN | Cervical lymph nodes | Typically 4 nodes close to the salivary glands (mandibular and superficial parotid lymph nodes) |
| TLN | Paratracheal mediastinal lymph node | Typically one node, right side of the trachea, under the thymus and heart (tracheobronchol lymph node) |
| KLN | Popliteal lymph nodes | Behind knees (pooled from both sides) |
| RLN | Renal lymph nodes | Under the kidney (pooled from both sides) |
| SPL | Spleen | Whole tissue |
| THY | Thymus | Whole tissue |
| POT | Pancreatic and omental tissue | Pancreas with associated omental tissue |
| LIV | Liver | Left or caudate lobe as indicated. The absolute signal measured thus refer to that lobe (approximately 27% and 8% of whole liver mass, respectively), not to the whole tissue |
| LGS | Lungs | All lobes pooled |
| KID | Kidneys | Both kidneys pooled |
| INT | Intestine | A 2-inch section of ileum, emptied of its content |
| Others | Other tissues analyzed at least once | Axillary and brachial lymph nodes, heart, brain, femur (bone marrow) |
Alternative names from Van den Broeck et al's nomenclatures25 are shown in italics.
Flow cytometry and immunofluorescence microscopy
DCs were generated from NOD and NOD.CD45.2 mice as described in “Dendritic cell culture and inoculation.” After harvest, NOD DCs were resuspended at 107/mL in cold PBS, containing 5 μM CFDA (Molecular Probes), incubated for 10 minutes at 37°C, and washed twice with complete medium. CFSE+ or CD45.2+ DCs were injected intravenously or intraperitoneally into recipient mice (5 × 106 cells per mouse). Tissues were collected 1 to 3 days later. For flow cytometry, spleen, lymph nodes, and omental tissue were disrupted between glass slides, and the resulting single-cell suspension was blocked with FcR Block (eBioscience) and mouse serum, and then stained with anti-CD11c and anti-CD45.2 antibodies (BD Biosciences, eBioscience). Samples were analyzed on LSR flow cytometer (BD Biosciences). T-cell responses (total cell number, CD4+ T-cell numbers, and CD69 and CD25 expression on CD4+ T cells) were also assessed in spleen and lymph node samples. Pancreas-associated omental tissue from CFSE+ DC-injected mice was placed between slide and cover glass and green cells were observed by epifluorescence microscopy (Eclipse TE300; Nikon Instruments Inc, Melville, NY).
Intrasplenic DC inoculation
Luc+ DCs were obtained from L2G85.NOD mice as described in “Dendritic cell culture and inoculation.” Recipient NOD mice were kept anesthetized with 3% isoflurane over a heated board during the whole surgical procedure, which was performed under aseptic conditions. Skin and peritoneum were incised and one end of the spleen was carefully pulled and exposed. Approximately 105 Luc+ DCs in 25 μL PBS were injected into the spleen. Leakage was prevented by applying pressure on the injection site with a cotton swab before the spleen was put back in place. Peritoneum and skin were separately stitched. Mice were monitored until full recovery and imaged up to 48 hours later.
In vivo stimulation of diabetogenic T cells
Spleen and pooled lymph nodes from 8-week-old female BDC2.5 mice were depleted by AutoMACS using a CD4 T-cell isolation kit (Miltenyi) supplemented with anti–CD25-biotin antibodies (eBioscience). Resulting CD4+ CD25− T cells were labeled with CFSE as described in “Flow cytometry and immunofluorescence microscopy.” Recipient mice (8-week-old females) were injected intravenously or intraperitoneally with 8 × 106 CFSE+ T cells. Lymph nodes (pancreaticoduodenal, gastric, renal, mesenteric, and cervical) were collected 20 hours and 3 days later. CD4+ CFSE+ T cells were analyzed by FACS for proliferation and CD69 expression.
Results
Spatial distribution and kinetics of BM-DC homing in NOD and FVB mice
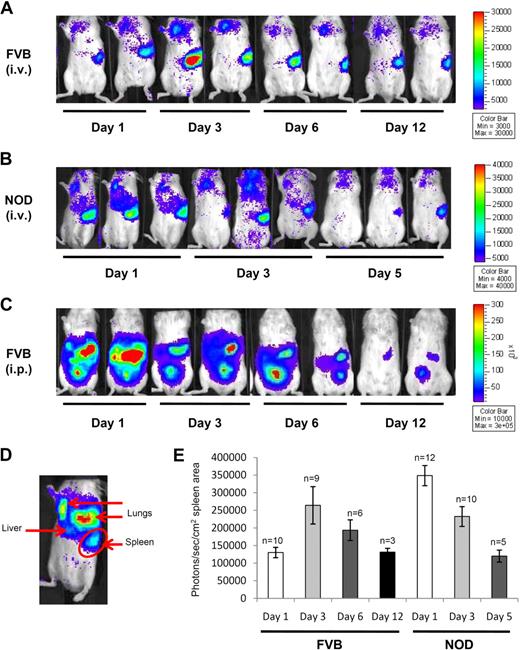
Bioluminescence imaging is a noninvasive optical approach for tracking the localization of Luc-expressing cells in living animals. In contrast to our previously published study, where DCs were transduced by lentivirus to express the Luc gene,23 we obtained the L2G85 Luc-transgenic mouse on the FVB background,24 which we backcrossed onto the NOD, NOD.B10, and BALB/c backgrounds. We first performed a kinetic analysis of DC homing in FVB and NOD mice, starting from 1 day after intravenous or intraperitoneal inoculation until the bioluminescent signal became undetectable. During that time frame, the clearest signal came from the spleen after intravenous injection, with a detectable signal in the lungs and liver (Figure 1A,B). Light emitted upon Luc activity can be quantified within a delimited region (such as the one drawn in Figure 1D). Figure 1E shows how the luciferase activity changes over time in the spleen, demonstrating that FVB DCs have slower homing kinetics than NOD DCs. After intraperitoneal injection in FVB (Figure 1C) and NOD mice (data not shown), the Luc signal remained confined to the peritoneum over time, with bright spots corresponding to tissues not easily identified in vivo. It was generally observed that in vivo signals disappeared faster in NOD mice (within a week), compared with FVB mice (up to 12 days) after injection.
In vivo imaging of BM-DCs. In vivo imaging of Luc+ DC homing over time in FVB mice (A) and NOD mice (B) after intravenous injection, and in FVB mice after intraperitoneal injection (C). Scale represents the number of photons per second/per cm2. (D) Annotation on the source of lights from Luc+ DCs after intravenous injection. (E) Quantitation of the signal emitted from Luc+ DCs in the spleen area (as delimited in panel D; mean ± SE, n value shown above each bar) after intravenous injection in FVB and NOD mice. Mice were imaged for 5 minutes under anesthesia.
In vivo imaging of BM-DCs. In vivo imaging of Luc+ DC homing over time in FVB mice (A) and NOD mice (B) after intravenous injection, and in FVB mice after intraperitoneal injection (C). Scale represents the number of photons per second/per cm2. (D) Annotation on the source of lights from Luc+ DCs after intravenous injection. (E) Quantitation of the signal emitted from Luc+ DCs in the spleen area (as delimited in panel D; mean ± SE, n value shown above each bar) after intravenous injection in FVB and NOD mice. Mice were imaged for 5 minutes under anesthesia.
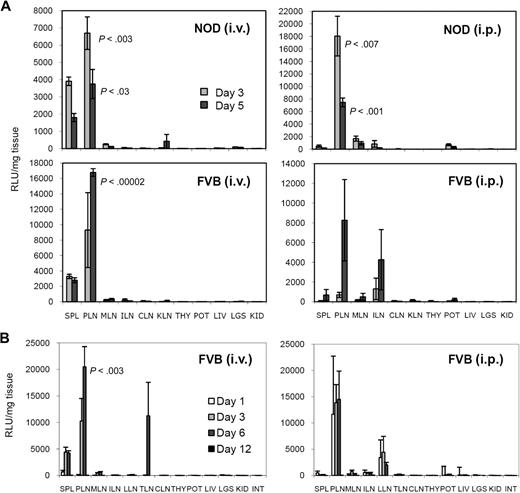
To assess homing of DCs in deeper and smaller tissues that are much more difficult to image in living mice, we performed a biodistribution analysis by killing 3 to 4 mice at each time point to evaluate the amount of Luc activity present in various tissues (including most lymph nodes), which are listed and described in Table 1. To better highlight the relative homing specificity, we normalized the total amount of Luc activity by the weight of the individual tissue. Figure 2A and B shows normalized data from 2 independent experiments, representative of several experiments conducted in the NOD and FVB mouse strains (and others). The total Luc activity (not normalized) in these tissues is depicted in Figure S2A and B. First, DC homing to lymph nodes was clearly not evenly distributed. Through the intravenous route, DCs migrated almost exclusively to the PLNs and tracheal lymph node (TLN, or lung-draining paratracheal mediastinal lymph node), whereas more lymph nodes were targeted using the intraperitoneal route, including PLNs, mesenteric lymph nodes (MLNs), and lumbar lymph nodes (LLNs). Homing to inguinal lymph nodes (ILNs) and renal lymph nodes (RLNs) was not consistently observed. Interestingly, after intraperitoneal inoculation, homing to the PLNs also predominated, whereas homing to the spleen was not as pronounced or consistent as with the intravenous route. In agreement with the kinetic differences seen in imaging, we observed that the signal in lymph nodes (and other tissues) decreased in NOD mice after day 1, whereas it was still increasing in FVB mice between day 3 and days 5 to 6, before decreasing. Second, DCs rapidly cleared from the lungs and liver after intravenous injection, detectable on day 1, but not thereafter. Finally, the last tissue that was found to hold high levels of DCs appeared to be the pancreas after intraperitoneal injection. No detectable signal was measured in the thymus, kidneys, and intestine at any time point, by any route. In some experiments, we also examined the axillary/brachial lymph nodes, brain, heart, and femoral bone marrow, where injected DCs were not detected (data not shown). Similar observations were made in BALB/c mice (Figure S2C) and NOD.B10 mice (data not shown).
Ex vivo biodistribution analysis of BM-DC homing. (A) Relative biodistribution (relative light units normalized per milligram of tissue) of Luc+ DCs in NOD and FVB mice on day 3 and day 5 after intravenous or intraperitoneal injection. (B) Relative biodistribution of Luc+ DCs in FVB mice on days 1, 3, 6, and 12 after intravenous or intraperitoneal injection. See Table 1 for tissue legend (left lobe of the liver was used). All data show the mean from 3 mice ± SE.
Ex vivo biodistribution analysis of BM-DC homing. (A) Relative biodistribution (relative light units normalized per milligram of tissue) of Luc+ DCs in NOD and FVB mice on day 3 and day 5 after intravenous or intraperitoneal injection. (B) Relative biodistribution of Luc+ DCs in FVB mice on days 1, 3, 6, and 12 after intravenous or intraperitoneal injection. See Table 1 for tissue legend (left lobe of the liver was used). All data show the mean from 3 mice ± SE.
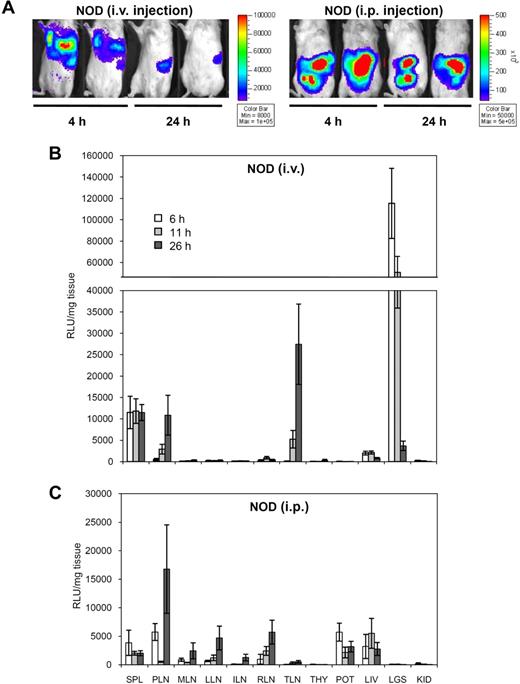
Next, we conducted a similar analysis at earlier time points to determine how fast injected DCs reached their target tissues. Representative in vivo imaging data from intravenously injected NOD mice showed rapid clearing of DCs from the lungs and liver between 4 and 24 hours, while the signal in the spleen remained stable (Figure 3A). These signals emitted from live animals were quantified (Figure S3A). In contrast, we did not see changes in the distribution of injected DCs in the peritoneal cavity within the first day after intraperitoneal injection (Figure 3A). The ex vivo biodistribution analysis at early time points is shown in Figure 3B and C (normalized signal) and in Figure S3B and C (total signal). The signal in the lungs was specific to the intravenous route and dropped 10- to 20-fold within the first 24 hours. A signal in the liver was found after both intravenous and intraperitoneal administration of DCs and did not change substantially within the first 24 hours. Injected DCs were detected in the spleen by 4 hours, and the signal remained stable until at least 24 hours. In contrast, after intravenous injection, DCs started to accumulate in the PLNs and TLNs between 6 and 11 hours, with levels culminating by 26 hours. After intraperitoneal injection, DCs appeared in the peritoneal lymph nodes with similar kinetics.
In vivo imaging and ex vivo biodistribution analysis of early BM-DC homing. (A) In vivo imaging of Luc+ DC homing in NOD mice 4 and 24 hours after intravenous or intraperitoneal injection. (B,C) Relative biodistribution of Luc+ DCs in NOD mice at 6, 11, and 26 hours after intravenous (B) or intraperitoneal (C) injection. See Table 1 for tissue legend (caudate lobe of the liver was used). Data show the mean from 3 mice ± SE.
In vivo imaging and ex vivo biodistribution analysis of early BM-DC homing. (A) In vivo imaging of Luc+ DC homing in NOD mice 4 and 24 hours after intravenous or intraperitoneal injection. (B,C) Relative biodistribution of Luc+ DCs in NOD mice at 6, 11, and 26 hours after intravenous (B) or intraperitoneal (C) injection. See Table 1 for tissue legend (caudate lobe of the liver was used). Data show the mean from 3 mice ± SE.
Preferential homing of BM-DCs to PLNs
Although preferential DC homing to the lung-draining TLNs after intravenous injection makes sense given the great number of DCs transiting through the lungs via this route, the reason for the preferential homing to the PLNs was unclear. By analogy with the lung/TLN system, we asked if well-vascularized tissues close to the PLNs could be the source of these DCs. To determine whether DCs can migrate from the spleen to the PLNs, we injected Luc+ DCs directly into the spleen. We imaged the mice several hours after injection, as well as 24 and 48 hours later (Figure S4). The Luc signal remained confined to the spleen at all time points. To ensure that no leakage of injected DCs from the spleen into the peritoneum occurred, which could have resulted in PLN homing via an indirect route, we killed some of the mice several hours after intrasplenic DC injection and verified the absence of signal outside the spleen (Figure S4). Other mice were killed 24 and 48 hours later for ex vivo biodistribution analysis of Luc+ DCs in the spleen, PLNs, and other proximal tissues (Figure 4). After intrasplenic injection, Luc activity was detected only in the spleen, suggesting that DCs did not traffic from the spleen to the PLNs. Injection into the liver, another possible source of PLN-homing DCs, could not be successfully achieved because of its greater depth and tissue friability.
Relative biodistribution of Luc+ DCs in NOD mice 24 and 48 hours after intrasplenic injection. See Table 1 for tissue legend (LIV-L and LIV-C are the left and caudate lobes of the liver, respectively; PANC indicates pancreas; KID-L, left kidney). All data show the mean from 3 mice ± SE.
Relative biodistribution of Luc+ DCs in NOD mice 24 and 48 hours after intrasplenic injection. See Table 1 for tissue legend (LIV-L and LIV-C are the left and caudate lobes of the liver, respectively; PANC indicates pancreas; KID-L, left kidney). All data show the mean from 3 mice ± SE.
The preferential DC homing to and retention in the PLNs was not associated with local inflammation, because it was seen in nondiseased strains (NOD.B10, FVB, BALB/c) in addition to prediabetic NOD mice. We inquired whether the PLNs could constitutively overexpress certain genes involved in chemoattraction or adhesion that could facilitate entry or retention of circulating DCs. By microarray analysis, we compared gene expression between the PLNs (efficient DC homing after intravenous or intraperitoneal injection) and the MLNs/ILNs (absent or poorer DC homing after intravenous or intraperitoneal injection, respectively) across the aforementioned strains of mice (7 conditions). Table S1 recapitulates the changes in expression level of most genes involved in cell trafficking, including chemokines and their receptors, integrins, selectins, cadherins, and other adhesion molecules. There was no gene whose expression was increased in the PLNs in all 7 conditions tested. A few genes were overexpressed more than 2-fold in 5 to 6 conditions (Cklfsf4, Itga8, Ccr3, Cdh26), as indicated by particular probes. However, other probes against the same genes showed no difference in expression, possibly reflecting differences in splice variants instead. Complete raw data are available on http://fathmanlab.stanford.edu/therapy.html.26 The microarray data have also been deposited into the GEO public database under accession number GSE15784, subseries GSE15748.27
Differential homing of DCs to lymphoid tissues correlates with magnitude of immune responses
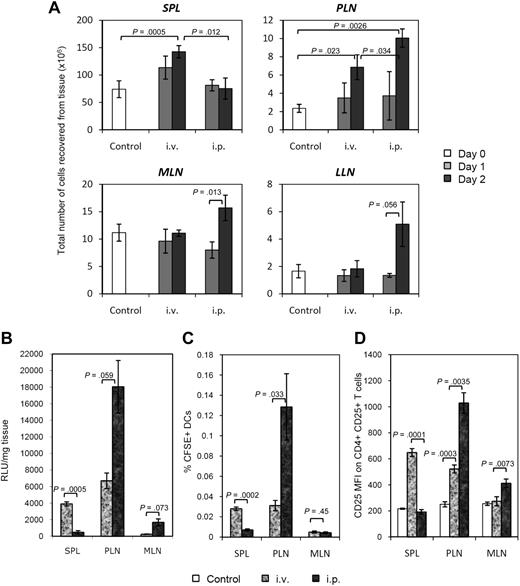
Previous studies have reported that injected BM-DCs can localize to the T-cell areas of spleen and lymph nodes.17,19,28,29 Using CD45.2+ DCs from congenic NOD.CD45.2 mice (host DCs in the NOD mouse are CD45.1+), we could also detect transferred DCs in the T-cell zones of spleen and lymph nodes by immunohistochemistry (data not shown). Most importantly, we observed the development of an immune response in targeted lymphoid tissues, as evidenced by increase in total cell number (Figure 5A), which could not have been contributed by DCs alone. This immune response was directed against bovine antigens that the DCs acquired in the FBS-supplemented in vitro culture (Figure S5A,B). A significant increase in T-cell numbers was measured only in tissues where DCs migrated most efficiently. For example, the number of splenocytes significantly rose after intravenousinjection (1.5- to 2-fold within 2 days), but not after intraperitoneal injection (Figure 5A).
Differential immune responses as a result of differential BM-DC homing. (A) Increase in cell number in DC-targeted lymphoid tissues (spleen, PLNs, MLNs, LLNs) on day 1 and day 2 after intravenous or intraperitoneal injection. Data show the mean from 3 mice ± standard deviation (SD) (except uninjected control group, n = 5). (B) Relative biodistribution of Luc+ DCs in spleen, PLNs, and MLNs 3 days after intravenous or intraperitoneal injection in NOD mice. All data show the mean from 3 mice ± SE. (C) Percentage of CFSE+ DCs in spleen, PLNs, and MLNs 2 days after intravenous or intraperitoneal injection in NOD mice. Data show the mean from 3 mice ± SD. (D) Mean fluorescence intensity of CD25 expression on CD4+ CD25+ T cells in spleen, PLNs, and MLNs 2 days after intravenous or intraperitoneal injection of CFSE+ DCs. Data show the mean from 3 mice ± SD (except uninjected control group, n = 5). t test was used for all statistical analyses.
Differential immune responses as a result of differential BM-DC homing. (A) Increase in cell number in DC-targeted lymphoid tissues (spleen, PLNs, MLNs, LLNs) on day 1 and day 2 after intravenous or intraperitoneal injection. Data show the mean from 3 mice ± standard deviation (SD) (except uninjected control group, n = 5). (B) Relative biodistribution of Luc+ DCs in spleen, PLNs, and MLNs 3 days after intravenous or intraperitoneal injection in NOD mice. All data show the mean from 3 mice ± SE. (C) Percentage of CFSE+ DCs in spleen, PLNs, and MLNs 2 days after intravenous or intraperitoneal injection in NOD mice. Data show the mean from 3 mice ± SD. (D) Mean fluorescence intensity of CD25 expression on CD4+ CD25+ T cells in spleen, PLNs, and MLNs 2 days after intravenous or intraperitoneal injection of CFSE+ DCs. Data show the mean from 3 mice ± SD (except uninjected control group, n = 5). t test was used for all statistical analyses.
Significant activation in the PLNs was seen after both intravenous and intraperitoneal injections, whereas significant activation in the MLNs and LLNs was observed only after intraperitoneal injection of DCs. In addition, the number of CD4+ T cells (and their expression of CD69) and the percentage of activated CD4+ CD25+ T cells (and their expression of CD25; Figure S5C) increased proportionally to the number of migrating DCs. Data presented in Figure 5B through D demonstrate the remarkable correlation between the signal emitted by Luc+ DCs, the number of CFSE+ DCs present, and the expression of CD25 on CD4+ T cells, respectively, in the spleen, PLNs, and MLNs after intravenous or intraperitoneal injection of DCs. Flow cytometric analysis of CFSE+ DCs in those tissues allowed an estimation of the number of migrating DCs 2 days after administration of 5 × 106 Luc+ DCs: approximately 50 000 in the spleen, 1000 to 3000 in the PLNs, and approximately 500 in the MLNs (percentage measured near background) after intravenous injection; fewer than 5000 cells in the spleen, 5000 to 20 000 in the PLNs, and 500 to 1000 in the MLNs following intraperitoneal injection.
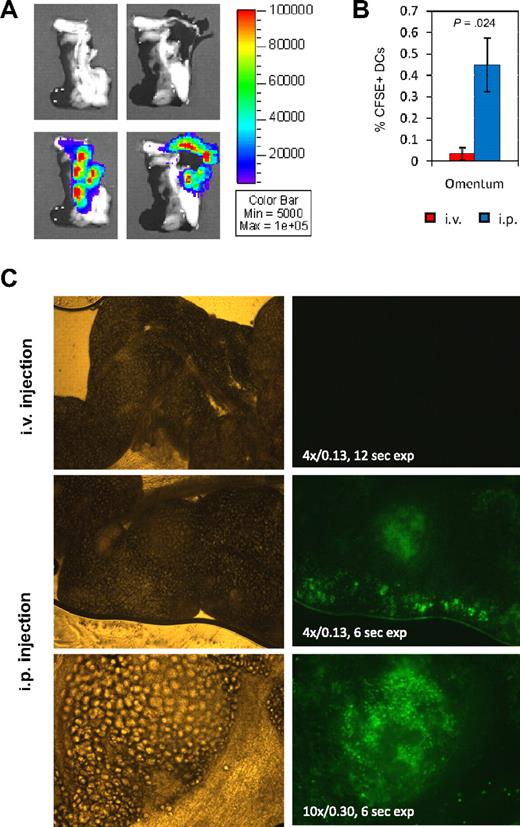
The omentum absorbs BM-DCs from the peritoneum
All the signals seen in the peritoneal cavity by in vivo imaging of mice after intraperitoneal inoculation of Luc+ DCs could not be explained simply by homing to specific lymph nodes, such as the PLNs, MLNs, and LLNs. In addition, CD45.2+ DCs could not be detected anywhere within the pancreas by immunohistochemistry despite seeing a signal by ex vivo biodistribution analysis. However, on the same sections, we found cells staining positive for the CD45.2 marker in residual tissue outside of the pancreas (Figure S6A). To examine this issue, the pancreas was imaged ex vivo 2 to 3 days after intraperitoneal injection of Luc+ DCs, allowing us to determine that the signal was in fact coming exclusively from omental tissue (Figure 6A). Part of this pancreas-associated omental tissue was found to be also connected to the spleen. CFSE+ DCs were observed in significant amounts in the omentum after intraperitoneal injection, but not after intravenous injection (Figure 6B,C). CFSE+ DCs within the omental tissue could be visualized through omental fat cells by epifluorescence microscopy; large numbers of them were observed in the periphery as well as in organized lymphoid structures known as “milky spots” (Figure 6C). Because it is attached to other tissues such as pancreas and spleen, omental tissue may contaminate them in ex vivo biodistribution assays, which may result in misleading signals. For instance, Luc signal was measured in the spleen after intraperitoneal injection of Luc+ DCs, although there was no evidence of T-cell activation (Figure 5D). Ex vivo tissue imaging indeed showed that such signal is either not detected (Figure 7A) or detected essentially in the zone of contact with the pancreas, which corresponds to omental tissue (Figure S6B). Overall, these observations suggest that intraperitoneally injected BM-DCs ultimately reside in the omentum-associated lymphoid tissue and peritoneal lymph nodes.
Homing of intraperitoneally injected DCs to omentum. (A) Ex vivo imaging of pancreas from a NOD mouse 3 days after intraperitoneal injection of Luc+ DCs. Omental tissue was detached and turned aside between left and right photos to show that signal comes exclusively from omental and not pancreatic tissue. The tissue was imaged for 5 minutes. Representative data from n = 6 mice equally treated. (B) Percentage of CFSE+ cells in pancreas-associated omentum 2 days after intravenous or intraperitoneal injection of CFSE+ DCs. t test was used for statistical analysis. (C) Visualization of CFSE+ DCs in omentum by fluorescence microscopy 2 days after intravenous or intraperitoneal injection. Brightfield and fluorescence pictures are shown on the left and right side, respectively. Numeric aperture and time of exposure are indicated on the fluorescence picture.
Homing of intraperitoneally injected DCs to omentum. (A) Ex vivo imaging of pancreas from a NOD mouse 3 days after intraperitoneal injection of Luc+ DCs. Omental tissue was detached and turned aside between left and right photos to show that signal comes exclusively from omental and not pancreatic tissue. The tissue was imaged for 5 minutes. Representative data from n = 6 mice equally treated. (B) Percentage of CFSE+ cells in pancreas-associated omentum 2 days after intravenous or intraperitoneal injection of CFSE+ DCs. t test was used for statistical analysis. (C) Visualization of CFSE+ DCs in omentum by fluorescence microscopy 2 days after intravenous or intraperitoneal injection. Brightfield and fluorescence pictures are shown on the left and right side, respectively. Numeric aperture and time of exposure are indicated on the fluorescence picture.
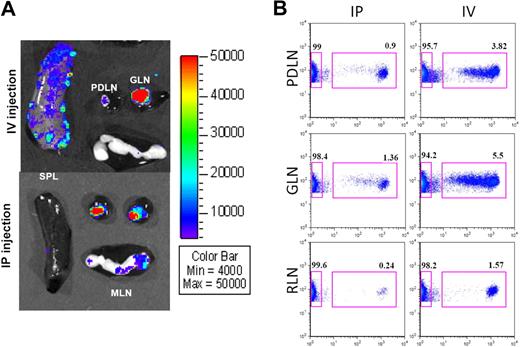
Differential homing of BM-DCs within PLNs. (A) Ex vivo imaging of spleen, PLNs (PDLN vs GLN), and MLNs from NOD mice 2 to 3 days after intravenous or intraperitoneal injection of Luc+ DCs. Tissues were imaged for 5 minutes. Representative data from more than 10 mice. (B) Proliferation of BDC2.5 T cells, by CFSE dilution (x-axis), in PDLN, GLN, and RLNs, 3 days after intraperitoneal or intravenous injection. Data shown were gated on CD4 (y-axis). Data from CLNs and MLNs (not shown) were similar to RLNs.
Differential homing of BM-DCs within PLNs. (A) Ex vivo imaging of spleen, PLNs (PDLN vs GLN), and MLNs from NOD mice 2 to 3 days after intravenous or intraperitoneal injection of Luc+ DCs. Tissues were imaged for 5 minutes. Representative data from more than 10 mice. (B) Proliferation of BDC2.5 T cells, by CFSE dilution (x-axis), in PDLN, GLN, and RLNs, 3 days after intraperitoneal or intravenous injection. Data shown were gated on CD4 (y-axis). Data from CLNs and MLNs (not shown) were similar to RLNs.
Differential homing of BM-DCs within the PLNs
The PLNs in the mouse strains studied typically have 2 anatomically distinct nodes, situated next to each other, which have been distinguished based on topography by another nomenclature25 as pancreaticoduodenal on the right side (PDLN) and gastric on the left side (GLN; Figure S1). Occasionally, each of these lymph nodes may exhibit 2 lobes, or more rarely, 2 distinct nodes. While performing ex vivo imaging of lymphoid tissues, we noticed that intravenously injected Luc+ DCs preferentially homed to the GLN (Figure 7A). Light emitted by the 2 nodes was quantified and summarized in Table 2. After intravenous injection, Luc+ DCs were infrequently found in similar amount in both nodes, but most of the time, they preferentially resided in the GLN (P = .026). In contrast, Luc+ DCs were found equally or indiscriminately distributed between the 2 nodes after intraperitoneal injection (P = .6). By microarray analysis, we found no meaningful difference in gene expression between these 2 lymph nodes (Table S2; microarray data deposited into the GEO public database under accession number GSE15784, subseries GSE15753). Given the differential homing of intravenously injected DCs to different nodes of PLNs, we asked whether such a disparity exists regarding the drainage of DCs from the pancreas (islets) to the PLNs. This could have been previously unnoticed given that published studies were conducted on pooled PLNs. During the pathogenesis of autoimmune diabetes, DCs migrate from the inflamed islets to the PLNs and present islet antigens to T cells. CD4+ T cells from BDC2.5 mice are specific for such an islet antigen; thus we tested whether they were differentially stimulated in PDLN and GLN. The BDC2.5 CD4+ T cells were purified (with depletion of CD25+ T cells for more efficient in vivo activation), labeled with CFSE, and injected either intravenously or intraperitoneal into NOD mice (Figure S7A). Within a day, these T cells had homed efficiently and equally to all tested lymph nodes after intravenous injection, whereas they preferentially homed to the PLNs after intraperitoneal injection (Figure S7B). After 3 days, proliferation of BDC2.5 T cells was clearly seen in both PDLN and GLN, regardless of the route of injection, whereas it was absent in other tested lymph nodes (Figure 7B). Similarly, up-regulation of CD69 on CFSE+ T cells was evident in both PDLN and GLN as early as 20 hours and after 3 days, but not in other tested lymph nodes (Figure S7C,D). In conclusion, DCs from the peritoneum and the pancreas drain into both PDLN and GLN, whereas DCs from the blood preferentially drain into the GLN.
Differential biodistribution of injected DCs within pancreatic lymph nodes
| . | PDLN (right PLN) . | GLN (left PLN) . |
|---|---|---|
| Luc+ DCs injected iv, n = 10, P = .026 | 4159 | 6421 |
| 2608 | 20 912 | |
| 1930 | 68 992 | |
| 10 734 | 42 320 | |
| 12 290 | 20 159 | |
| 3221 | 75 483 | |
| 720 | 24 200 | |
| 1056 | 1560 | |
| 3980 | 3793 | |
| 789 | 2410 | |
| Luc+ DCs injected ip, n = 5, P = .6 | 29 807 | 20 505 |
| 9414 | 209 820 | |
| 35 204 | 27 404 | |
| 72 334 | 5801 | |
| 21 093 | 33 595 |
| . | PDLN (right PLN) . | GLN (left PLN) . |
|---|---|---|
| Luc+ DCs injected iv, n = 10, P = .026 | 4159 | 6421 |
| 2608 | 20 912 | |
| 1930 | 68 992 | |
| 10 734 | 42 320 | |
| 12 290 | 20 159 | |
| 3221 | 75 483 | |
| 720 | 24 200 | |
| 1056 | 1560 | |
| 3980 | 3793 | |
| 789 | 2410 | |
| Luc+ DCs injected ip, n = 5, P = .6 | 29 807 | 20 505 |
| 9414 | 209 820 | |
| 35 204 | 27 404 | |
| 72 334 | 5801 | |
| 21 093 | 33 595 |
Luminescence measured (photons/sec per cm2) using ex vivo imaging of PDLN and GLN from NOD mice, 2 days after intravenous or intraperitoneal injection of Luc+ DCs, showing preferential homing to GLN after intravenous injection (P = .026, n = 10, paired t test), but not after intraperitoneal injection (P = .6, n = 5, paired t test). Data pooled from 2 independent experiments.
iv indicates intravenously; and ip, intraperitoneally.
Discussion
Efficacy of DC therapy requires that the DCs migrate in sufficient numbers to appropriate tissues to induce or modulate an immune response. Relevant antigens are best presented in lymph nodes draining the site of infection or a tumor. In the latter case, these lymph nodes are also privileged sites for metastasis. In addition, autoimmune responses can be initiated in the lymph nodes draining the targeted tissue (PLNs in autoimmune diabetes, for example). Therefore, it is crucial to define the homing capabilities of potentially therapeutic DCs, which seem to depend greatly on their route of delivery. Although other subsets of DCs have been tested, the most commonly used DCs for immunotherapy in animals and humans are the myeloid CD11c+ CD11b+ cells, generated from CD34+ bone marrow progenitors in mice or from CD14+ monocytes in humans. While studying the therapeutic efficacy of IL-4–producing DCs in the NOD mouse model of autoimmune diabetes, we serendipitously observed that such DCs predominantly migrated to the PLNs after systemic delivery using bioluminescence imaging methods.23 Thus, we performed an exhaustive evaluation of lymph nodes and other tissues to which BM-DCs can migrate following intravenous or intraperitoneal administration, and attempted to characterize the mechanism(s) underlying the preferential homing to the PLNs.
Our observations on the DC homing to large tissues (spleen, lungs, liver) after intravenous injection confirm previous studies done in mice17-21,30 and humans11,12,16,22 in vivo using scintigraphy, positron emission tomography, or magnetic resonance imaging, or in vitro using scintigraphy or bioluminescence. However, such imaging methods quickly reach their limit when it comes to detecting DCs in smaller tissues such as lymph nodes. It is possible to image DCs in some lymph nodes after subcutaneous, footpad, or intradermal injections, because they cannot easily disperse and the nodes are more superficial.14-16,20,29 In contrast, in vivo detection of DCs in lymph nodes following systematic delivery has not been reported, mostly due to the lack of sensitivity or resolution of current imaging methods. Because a low number of DCs (as low as a few hundreds) can have dramatic effects in lymph nodes, biodistribution analyses in animals remains necessary to document DC homing to those tissues. Some of such ex vivo studies in the mouse included pooled lymph nodes18 or a limited set of individual lymph nodes,17,30 none of which included the PLNs. Nevertheless, our studies highlighted the PLNs as a central destination for BM-DCs both after intravenous and intraperitoneal administration.
Our data demonstrated that the PLNs are the only major lymph nodes, besides the lung-draining TLN, that are targeted by BM-DCs following intravenous inoculation. We tested whether this peculiarity could be explained by higher expression levels of certain chemokines or adhesion genes, in the PLNs compared with other lymph nodes, that would result in greater attraction and retention of BM-DCs. Due to the large number of potential genes involved, microarray analysis was performed, leading us to the conclusion that there is no consistent difference in expression of these particular genes between the PLNs and other lymph nodes. Next, we attempted to determine whether there is a particular drainage pattern leading the DCs to converge into the PLNs. There are only 3 major tissues clearly identified in both mice and humans where DCs traffic after intravenous injection: lungs, liver, and spleen. A fraction of DCs from the lungs may migrate to the draining TLN, a process involving CCR7, CCR8, and CD38.31 Thus, this known drainage system can explain the accumulation of DCs in the TLN seen in our biodistribution studies. However, drainage to the PLNs, other than from the pancreas and the peritoneum,32 is not well defined in the mouse. We demonstrate here that DCs injected into the spleen did not traffic to the PLNs, leaving the possibility that the latter may act as liver-draining nodes. A group performed ink injections into the spleen and liver of BALB/c mice and reported the absence of splenic and hepatic lymph nodes that were described in humans and domestic mammals as being part of the celiac center, together with PDLN and GLN.25 The same group also proposed that hepatic and pancreaticoduodenal lymph nodes in the mouse may be the same tissue.25 In our hands, the GLN had relatively more intravenously injected DCs than the PDLN, suggesting that the role of hepatic lymph node surrogate in mouse may be played by the GLN instead.
In addition, although DCs can migrate from the peritoneal cavity to all the lymph nodes of that cavity (PLNs, MLNs, LLNs, RLNs), homing to the PLNs remains predominant via this route as well. Turley et al32 reported that antigens and splenocytes from the peritoneum preferentially drain to the PLNs. Here, we show that DCs likewise exhibit a preferential homing to the PLNs, further suggestive of a drainage pattern rather than a specific attraction. In contrast to intravenous injection, DCs from the peritoneum traffic indiscriminately to either left or right PLNs. We have also shown that the omental tissue harbors a great number of injected DCs within 4 to 6 hours after intraperitoneal injection, before such DCs appear in the lymph nodes. The omentum may act like a fishing net for the DCs in the peritoneum, which first reach the milky spots lymphoid structures of the omentum and then drain to the nearest lymph nodes. It is possible that a greater surface of omentum is associated with the PLNs, which could explain why more DCs migrate to these nodes. Some drainage patterns have been defined in the mouse using ink injected subcutaneously or in the footpad,33 but the drainage from tissues and peritoneum is still ill-defined. Our study better illustrates the trafficking of DCs from bloodstream to tissues to lymph nodes, and from peritoneum to lymph nodes. Finally, we did not observe significant migration of BM-DCs to tissues other than the aforementioned ones. Homing of BM-DCs to the thymus and bone marrow after intravenous injection was not detected. In contrast, splenic DCs (a subset distinct from BM-DCs) were shown, after intravenous injection, to be able to migrate to the bone marrow34 and the thymus,35 in a VCAM-1– and P-selectin–dependent fashion.
When BM-DCs efficiently home to lymphoid tissues (T-cell zones in particular), they induce activation and proliferation of T cells to an extent proportional to the number of injected DCs present. Assessing DC function is an important validation of DC homing. For example, Sheasley-O'Neill et al30 described differential integrin expression on CD8+ T cells after intravenous versus intraperitoneal injection of mature DCs. When DCs were injected intraperitoneally, they could activate T cells in the MLNs and instruct them to redistribute to both parathymic and paratracheal mediastinal lymph nodes,30 (to which BM-DCs do not home). In contrast, when DCs were injected intravenously, they could activate T cells in the paratracheal, but not in parathymic mediastinal nodes,30 which is consistent with our DC homing data. Detection of DCs in significant amounts in a lymphoid tissue, in the absence of measurable function, may signify that the DCs do not colocalize with the T cells in that tissue. This is best exemplified by residual omental tissue attached to the spleen that can contain large number of intraperitoneally injected DCs, leading to apparent DC homing to the spleen with no significant immune response.
This study demonstrates that the PLNs are at a central intersection between different tissue drainage systems, and a major destination for therapeutic DCs that are administered intravenously or intraperitoneally. The 2 nodes of the PLNs are ultimate destination for peritoneum- or pancreas-derived DCs, whereas blood-borne (and possibly liver-derived) DCs preferentially migrate to one of them (GLN). Implications for therapy include treatment of autoimmune diabetes with immunoregulatory DCs, via the aforementioned routes, to counteract the priming function of islet-derived DCs. Overall, this study brings about new models for using DCs in a more specific treatment of autoimmune diabetes, and possibly pancreatic cancer.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Ray Sobel for help with immunohistochemistry; Yuan Cao for L2G85 (FVB) mice; Olivier Gheysens for help with the intrasplenic injections; Demi Dang for help with processing microarrays; and Gudrun Debes, Eugene Butcher, and Michael Bachmann for useful discussions.
This work was supported by grants from the National Institutes of Health (AI36535-13, DK078123-03; C.G.F.), the Juvenile Diabetes Research Foundation (1-2005-1107, 3-2005-1019; C.G.F., R.J.C.), and the National Cancer Institute (Small Animal Imaging Resource Program R24CA92862, Imaging Facility).
National Institutes of Health
Authorship
Contribution: R.J.C., S.S.Y., and C.G.F. designed the research; R.J.C., S.S.Y., P.C., and J.C. performed experiments; C.H.C. and S.S.G. contributed vital reagents or analytical tools; R.J.C. and S.S.Y. analyzed and interpreted data; and R.J.C. and C.G.F. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: C. Garrison Fathman, Department of Medicine, Division of Immunology & Rheumatology, Stanford University School of Medicine, CCSR 2225, 300 Pasteur Dr, Stanford, CA 94305; e-mail: cfathman@stanford.edu.
References
Supplemental data
Data were processed and normalized using the GeneSpring GX7 software. Legend of conditions: B = BALB/c; F = FVB, N = NOD; NB = NOD.B10; pm = PLN vs MLN; pi = PLN vs ILN. Raw data (file “PLNvsMLN-ILN.xls”) are available on the Web site http://fathmanlab.stanford.edu/therapy.html, under “Comparison of gene expression between PLN and MLN/ILN.”
Data were processed and normalized using Feature Extraction and GeneSpring GX10 software. All the genes listed were increased over 1.5 fold in GLN in all four tested mice (ratio GLN / PDLN shown). Only Nos1 was increased over 2 fold in GLN in all four tested mice. Raw and filtered data (file “GLNvsPDLN.xls”) are available on the Web site http://fathmanlab.stanford.edu/therapy.html, under “Comparison of gene expression between GLN and PDLN.”