CX3CL1 (Fractalkine) is an atypical chemokine with a documented role in the development of numerous inflammatory diseases including atherosclerosis. In this issue of Blood, Landsman and colleagues demonstrate that interactions between CX3CL1 and its receptor CX3CR1 are involved in monocyte survival via antiapoptotic mechanisms and thus promote atherogenesis.
Chemokines (chemotactic cytokines) are a family of low molecular weight proteins which function to coordinate leukocyte trafficking in health and disease. Divided on the basis of structure into 4 families (C, CC, CXC, and CX3C), chemokines signal through G protein–coupled 7-transmembrane receptors to induce the directed migration of cells along a chemoattractant gradient.
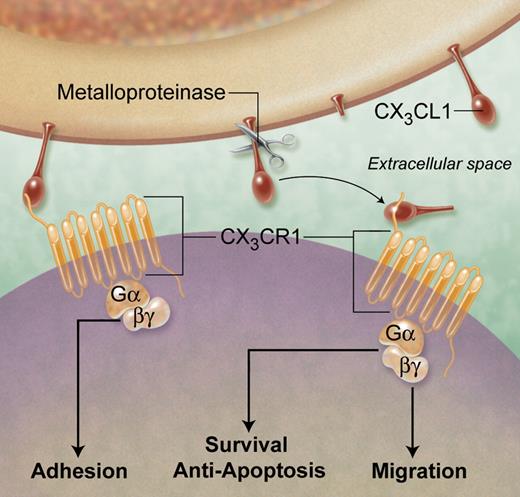
CX3CL1 is the only member of the CX3C family of chemokines and signals through a single Gαi-linked receptor: CX3CR1.1,2 Cell surface expression of CX3CR1 defines 2 circulating monocyte subsets: CX3CR1low cells correspond to the “inflammatory” Gr1hi (Ly6Chi) subset in mice and the CD14++ CD16− subset in humans.3 The murine Gr1low (Ly6Clow) subset expresses significantly higher levels of CX3CR1 and corresponds to the CD14+ CD16+ monocyte population in humans. CX3CL1 is synthesized as a membrane-bound molecule with the chemokine domain presented on a mucin-like stalk which mediates the direct capture of circulating leukocytes, as shown in the figure.2 Cleavage at the base of this stalk by metalloproteinases generates a soluble chemokine, which functions as a classical chemoattractant.4 It is becoming increasingly clear that CX3CL1, in common with other chemokines, has a wide range of functional roles that contribute to pathology.
Functions of CX3CL1 (Fractalkine) contributing to atherogenesis. Membrane-bound CX3CL1 enables the integrin-independent capture and firm adhesion of leukocytes via the Gαi coupled receptor CX3CR1. Under inflammatory conditions, cleavage of cell-surface CX3CL1 by metalloproteinases generates a soluble chemokine, which binds CX3CR1 on nearby cells and, via signaling intermediates, can induce cell chemotaxis and survival. Thus, CX3CL1 can have multiple roles in all stages of atherogenesis, including the recruitment, retention, and survival of monocytes in early atherosclerotic lesions as well as foam cell persistence in more advanced plaques. Professional illustration by A. Y. Chen.
Functions of CX3CL1 (Fractalkine) contributing to atherogenesis. Membrane-bound CX3CL1 enables the integrin-independent capture and firm adhesion of leukocytes via the Gαi coupled receptor CX3CR1. Under inflammatory conditions, cleavage of cell-surface CX3CL1 by metalloproteinases generates a soluble chemokine, which binds CX3CR1 on nearby cells and, via signaling intermediates, can induce cell chemotaxis and survival. Thus, CX3CL1 can have multiple roles in all stages of atherogenesis, including the recruitment, retention, and survival of monocytes in early atherosclerotic lesions as well as foam cell persistence in more advanced plaques. Professional illustration by A. Y. Chen.
Landsman et al demonstrate that under steady state conditions, mice lacking either CX3CL1 or CX3CR1 (Cx3cr1gfp/gfp) have significantly fewer circulating monocytes, specifically those of the Gr1low subset. Furthermore, cotransfer of Cx3cr1gfp/gfp and wild-type bone marrow into wild-type mice results in a selective reduction of circulating Cx3cr1gfp/gfp Gr1low monocytes. The forced transgenic expression of the antiapoptotic factor Bcl2 in monocytes rescues this phenotype, indicating that the CX3CL1-CX3CR1 interaction confers a survival signal under homeostatic conditions. In human monocytes, soluble CX3CL1 rescues cells from apoptosis induced by serum-deprivation or cytotoxic stress, though this is not confined to a particular subset.
In the apolipoprotein E (Apoe−/−) deficient mouse model of atherosclerosis, the absence of either CX3CL1 or CX3CR1 reduces total lesion area and plaque macrophage content.5,6 Landsman et al demonstrate that transfer of Cx3cr1gfp/gfp bone marrow into Apoe−/− recipients results in reduced atherosclerotic plaque burden (compared with transfer of Cx3cr1+/+ bone marrow), and these mice have reduced levels of circulating Gr1low monocytes. In addition, plaques from these animals contain increased levels of TUNEL+ apoptotic cells, consistent with a role for CX3CR1 in preventing apoptosis. However, enforced survival of monocytes and plaque foam cells in these mice restore atherosclerosis to levels seen in CX3CR1-proficient animals. These data suggest that plaque resident cells depend on the CX3CL1-CX3CR1 interaction for their survival, providing a plausible mechanism for the proatherogenic function of CX3CL1.
However, the role of CX3CL1 in controlling levels of circulating monocytes remains controversial. A recently published study failed to show an effect of CX3CL1 deficiency on levels of total blood monocytes or individual subsets in Apoe−/− mice.7 Instead, CCR2 deficiency reduced circulating monocyte numbers, and this was not further diminished in Ccr2−/− Cx3cl1−/− animals. A second study found significantly reduced levels of circulating Gr1low monocytes in Cx3cr1−/− mice in both homeostatic and inflammatory (Apoe−/−) conditions.8 However, CCL2 deficiency caused a more marked reduction in blood monocytes while Ccl2−/− Cx3cr1−/− Apoe−/− mice showed an additive reduction in total and individual monocyte subsets. Landsman et al provide a mechanistic explanation for the effect of CX3CL1 on circulating levels of specific monocyte subsets, but this may prove to be a more general function of chemokines.
Does this study have any implications for targeting chemokines in cardiovascular dis-ease? One key question is whether long-term inhibition of a single chemokine/receptor would be an effective therapeutic strategy. Combined inhibition of both CCL2 and CX3CL1 may be required to overcome redundancy in the chemokine network, but the resultant chronic monocyte suppression could have significant undesirable effects. Although targeting CX3CL1 in early atherosclerosis might reduce monocyte accumulation and survival in fatty streak lesions, macrophage survival may be crucial in late disease stages to ensure clearance of necrotic and potentially thrombogenic material. The study presented here adds to our appreciation of CX3CL1 as an unusual chemokine and reminds us that chemokines, originally discovered and named for their role in cell migration, have a plethora of functions that are only now being revealed.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal