Abstract
The adoptive transfer of regulatory Foxp3+ T (Treg) cells has been shown in various animal models to prevent inflammatory immune and autoimmune diseases. Translation into therapeutic applications, however, is hindered by the lack of suitable techniques and markers. CD25, commonly used to isolate Treg cells from mice, has only limited value in humans as it is also present on proinflammatory CD4+ effector cells. Here we show that clean populations of human Foxp3+ Treg cells can be obtained with antibodies directed against CD49d. The marker is present on proinflammatory peripheral blood mononuclear cells but is absent on immune-suppressive Treg cells. Depletion with α-CD49d removes contaminating interferon-γ (IFN-γ)– and interleukin-17 (IL-17)–secreting cells from Treg preparations of CD4+CD25high cells. More importantly, in combination with α-CD127 it allows the isolation of “untouched” Foxp3+ Treg (ie, cells that have not been targeted by an antibody during purification). The removal of CD49d+/CD127+ cells leaves a population of Foxp3+ Treg virtually free of contaminating CD25+ effector cells. The cells can be expanded in vitro and are effective suppressors both in vitro and in vivo. Thus, CD49d provides access to highly pure populations of untouched Foxp3+ Treg cells conferring maximal safety for future clinical applications.
Introduction
Regulatory T (Treg) cells are a key population for the maintenance of peripheral tolerance.1,2 They are suppressor cells that neutralize other immune cells by various bystander mechanisms. Their characteristic marker is the transcription factor Foxp3, which largely controls phenotype and function of Treg cells. Mutations in the gene can result in “immune dysregulation, polyendocrinopathy, enteropathy X-linked syndrome” (IPEX), a severe and rapidly fatal autoimmune disorder.3 Although the incidence of IPEX is low, more subtle deficits in Treg function appear to be a common cause of human autoimmune diseases. Treg defects have been discovered in patients with multiple sclerosis (MS), type 1 diabetes, psoriasis, myasthenia gravis (MG), and other autoimmune diseases.4 Similar links may also exist for atopy and allergic diseases.5
In many animal models the manifestation of inflammatory (auto-) immune diseases can be prevented by an adoptive transfer of Treg cells. This approach has been successfully applied in the treatment of experimental autoimmune encephalomyelitis (EAE),3 experimental type 1 diabetes,6 colitis,2 and several forms of allergy,5 where, depending on the model, either autologous “naive” Treg cells or in vitro expanded Treg cells were used.7 Similar results were also obtained in the field of transplantation3,8 : both graft rejection9 and graft-versus-host disease (GVHD)3 could be prevented or at least delayed. Protection was achieved here not only for allogeneic but also for more severe xenogeneic immune reactions.10
In most of these animal experiments Treg cells were isolated via targeting of CD25, the α-chain of the interleukin-2 (IL-2) receptor. However, in clinical practice CD25 is used mostly as a marker of proinflammatory rather than anti-inflammatory cells. α-CD25 (Daclizumab) is administered routinely as a potent immune suppressant. It is approved for the prevention of kidney graft rejections11 and also shows some effect in the treatment of MS.12 In addition, it has been shown that GVHD could be prevented when the CD25-expressing cells were eliminated from donor blood.13
Thus, in humans CD25 is a much less specific marker for immune-suppressive Foxp3+ Treg cells than in mice. Although human Treg cells stain brightly for CD25, a large fraction of nonregulatory cells expresses lower amounts of the marker, which includes the majority of CD4+ effector and memory T cells.14 Even highly pure populations of CD25highCD4+ T cells contain a substantial fraction of cytokine producing proinflammatory effector cells.15 At least some of these contaminations cannot be distinguished by intracellular Foxp3 staining, because activated human CD4+ effector T cells are known to express the transcription factor transiently.16 The risk of adverse reactions by contaminating proinflammatory effector cells has largely prevented the translation of Treg-based tolerogenic cellular therapies to humans. Only a single phase 1 trial using CD25-based Treg cell preparations has been launched so far.17
In this study we introduce CD49d, a new marker that may finally solve the problem. As α-chain of the integrin VLA-4 (α4β1), CD49d has already drawn considerable interest as therapeutic target for MS and Crohn disease.18 Here, we show that CD49d is present on the majority of proinflammatory effector cells but absent from immune-suppressive Foxp3+ Treg cells. The depletion of CD49d+ cells removes virtually all cytokine-producing CD4+ cells, which contaminate CD25-based Treg preparations, including transiently Foxp3-expressing effector cells. Moreover, in combination with antibodies against the α-chain of the IL-7 receptor (CD127), CD49d provides access to highly pure populations of Foxp3+ Treg cells, avoiding the use of CD25. Notably, because CD49d and CD127 are inversely correlated with Foxp3 expression, it allows obtaining the Treg cells in an “untouched” state, which ensures full functionality paired with maximal safety for human therapies.
Methods
Antibodies and reagents
Antibodies specific for CD4 were obtained from BD Biosciences (San Jose, CA; RPA-T4) or Coulter (T4). α-CD25 (MA251 and 2A3), α-CD49d (9F10), α-CD127 (hIL-7R-M21), and α-interferon (IFN)-γ (B27) were purchased from BD Biosciences. α-CD49d for cell separations was obtained from ImmunoTools (BU49) or Miltenyi Biotec (Bergisch Gladbach, Germany; MZ18-24A9). α-CD3 (UCHT-1) was produced at the Max-Delbrück-Center (MDC). α-Foxp3 (PCH101 and 236A/E7) and α–IL-17 (eBio64CAP17) were purchased from eBioscience (San Diego, CA), and intracellular staining was carried out according to the manufacturer's recommendation. 5-Carboxyfluorescein diacetate (CFDA) was obtained from Molecular Probes (Eugene, OR) and 3H-thymidine from GE Healthcare (Little Chalfont, United Kingdom).
Flow cytometry and cell preparation
Human peripheral blood mononuclear cells (PBMCs) were obtained from healthy volunteers. Mononuclear cells were isolated by Ficoll gradient centrifugation (GE Healthcare). Flourescence-activated cell sorting (FACS) analysis was carried out on a FACSCalibur, FACSCanto, or LSR II instrument (BD Biosciences). FACS sorting was carried out with the FACSAria instrument (BD Biosciences) or the MoFlo sorter (Dako). The sorting gates for CD25+ cells were defined by the staining of the isotype control; sorting gates separating the CD25highCD4+ cells from CD25lowCD4+ cells were based on the slightly lower CD4 expression of CD25highCD4+ cells. Data were analyzed using FACSDiva, CellQuest (both BD Biosciences), or FlowJo software (TreeStar, Ashland, OR). Approval for use of human PBMCs was obtained from the MDC (Berlin, Germany) and the IRCCS Santa Lucia (Rome, Italy) institutional review boards for these studies. Informed consent was obtained in accordance with the Declaration of Helsinki.
Cytokine detection
Cells were stimulated for 4 to 6 hours with phorbol 12-myristate 13-acetate (PMA) and ionomycin in the presence of brefeldin A for the final 3 to 4 hours. The cytokine secretion was determined by intracellular staining with α-IL-17 and α-IFN-γ using magnetic-activated cell sorting (MACS)– or FACS-sorted CD4+ T-cell subsets. Cells were either used freshly or maintained overnight in RPMI supplemented with 50 U/mL IL-2 before stimulation.
Treg isolation
For FACS, untouched CD4+ T cells were obtained from human PBMCs using the human CD4+ T-cell isolation kit II (Miltenyi Biotec). Cells were stained with α-CD49d–fluorescein isothiocyanate (FITC) and α-CD127-PE for 10 minutes at 4° to 8°C. After washing with MACS buffer, cells were resuspended in MACS buffer and sorted on the cell sorter using a sorting gate for CD49d−CD127− cells. Dead cells were excluded by propidium iodide (Sigma-Aldrich, St Louis, MO). FACS sorting was carried out with the FACSAria instrument (BD Bioscience) or the MoFlo sorter (Dako). MACS sorting: magnetic cell sorting was carried out using the MACS system (Miltenyi Biotec). Human PBMC were incubated with FITC-labeled α-CD49d, biotin-labeled α-CD127 for 10 minutes at 4° to 8°C. After washing with MACS buffer, cells were incubated with 15 μL α-FITC- and 20 μL α-biotin magnetic-beads per 107 cells for 15 minutes at 4° to 8°C. In most experiments the 2 antibodies were used in combination with the hCD4-isolation kit II (Miltenyi Biotec) to increase the purity. CD4+ cells were either isolated before the CD49d/CD127 depletion or the depletion was carried out in a single step. In this case, PBMC were incubated with FITC-labeled α-CD49d, biotin-labeled α-CD127 and the biotin-labeled antibody mix of the human CD4+ T-cell isolation kit II for 10 minutes at 4° to 8°C. After washing with MACS buffer, cells were incubated with 10 μL α-FITC- and 25 μL α-biotin magnetic-beads per 107 cells for 15 minutes at 4° to 8°C. After washing with MACS buffer, cells were separated using a LD column. Treg isolations using the CD4+/CD25+/CD127dim/− isolation kit (Miltenyi Biotec) were carried out according to the manufacturer's recommendation applying 1 depletion step followed by 2 consecutive positive sorting steps.
CFDA-based proliferation assay
CD4+ effector T cells were labeled with 0.5 μM CFDA (Molecular Probes) as described before.19 CD4+ effector T cells (25 000 cells/well) were incubated with irradiated CD4-depleted PBMCs (50 000 cells/well; 3000 rad) and 10 μg/mL anti-CD3 (UCHT-1) or alone with the Treg Suppression Inspector reagent, as suggested by the manufacturer (Miltenyi Biotec) for 3 to 5 days in 96-well V-bottom plates (Costar, Corning, NY). For T-cell suppression, Treg cells were added at the indicated ratio. Proliferation of CD4+ T cells was analyzed by FACS.
Long-term culture of Treg cells in vitro
Cells were cultivated in 96-well round-bottomed plates in RPMI/10% FCS (Invitrogen). Treg or CD4+ T cells (105) were stimulated with α-CD3/28 T-cell expander beads (Invitrogen) at a ratio of 4 beads/cell, in the presence of 500 U/mL recombinant IL-2 in 200 μL medium. Every 4 to 5 days, one-half of the medium was replaced by fresh RPMI/10% FCS supplemented with 200 U/mL IL-2.
Mixed lymphocyte reaction
Human PBMC (100 000 cells/well) were incubated with irradiated (3000 rad) allogeneic PBMC (100 000 cells/well) in RPMI/10% FCS (Invitrogen, Carlsbad, CA) for 5 days. For suppression of proliferation, isolated autologous Treg cells were added at the indicated ratio. Proliferation was monitored by 3H-thymidine incorporation (0.037 MBq/well) for an additional 10 to 15 hours of culture and determined using a β-plate reader (Wallac/PerkinElmer Life Sciences, Waltham, MA).
Xenogeneic GVHD
An acute form of GVHD was induced in Rag2−/− γc−/− mice (Taconic Farms, Hudson, NY) as described before.10 Briefly, PBMCs were depleted with α-CD25 microbeads (Miltenyi Biotec) using a LD column (Miltenyi Biotec). Treg cells were obtained from PBMCs of healthy donors by single-step depletion (CD49d/CD127 and CD4+ T-cell isolation kit II). One day before transfer, mice received 0.2 mL clodronate-containing liposomes intravenously. Four hours before the transfer of cells, mice were irradiated (350 rad). CD25-depleted PBMCs (30 × 106) were injected intravenously, either alone or as mixture with 0.5 × 106 Treg cells in PBS/0.1% human serum albumin. Engraftment of human PBMCs was confirmed by FACS analysis of murine blood with α-human CD4 (BD) and α-human pan–TCR- specific antibodies (Miltenyi Biotec), obtained by punctuation of the tail vein at day 3. The weight and clinical symptoms of the mice were determined over the entire period of the experiment; clinical signs of the disease were scored according to general appearence (ruffled fur, hunched posture) and mobility (impaired movement). All animal experiments were approved by the Landesamt für Arbeitsschutz, Gesundheitsschutz und Technische Sicherheit (Berlin, Germany).
Results
CD49d is absent on Foxp3+ CD4+ T cells
Several markers have been described so far to correlate with Foxp3 expression. In all these cases the cosegregation with human Foxp3 is incomplete. The most prominent example is CD25, which is expressed in high amounts on Treg cells (CD25high) but in lower amounts also on CD4+ effector and memory T cells (CD25low)14 (Figure 1A). For CD25high cells the counterstaining with Foxp3 indicates a near linear correlation with CD25 (Figure 1B left panel). A linear association was also reported for CD12720,21 (Figure 1B middle panel). In contrast to CD25, the correlation is inversed as cells with highest level of Foxp3 have the lowest expression level of CD127. The incomplete segregation is evident here in the fact that a considerable fraction of CD127−CD4+ cells are also Foxp3−. In the example shown in Figure 1B, it comprises approximately two-thirds of the CD127− cells.
CD49d removes Th1- and Th17-like cells from CD25high Treg preparations. (A) Double staining of human PBMC for CD4 and CD25. Percentage of CD4+ cells of total PBMCs is indicated. (B) Inverse correlation of CD49d with Foxp3 expression. Costaining of Foxp3 with CD25 (left panel), CD127 (middle panel), and CD49d (right panel) is shown for CD4+ cells gated according to Figure 1A. Numbers indicate percentage of cells in each quadrant. (C) Cytokine-secreting CD4+ cells express CD49d. CD4+ T cells were stimulated in vitro with PMA/ionomycin and analyzed by FACS. Staining is shown for CD49d versus IL-17 or IFN-γ. Numbers represent percentages of cells per quadrant. (D) Segregation of IFN-γ and IL-17 secretion with CD49d expression within the Foxp3+ subset. CD4+ cells were activated as described. After activation the cells were stained with α-Foxp3, α-CD49d, α–IFN-γ, and α–IL-17. Cells were gated on Foxp3. Density plots show the cytokine staining versus CD49d. Numbers refer to the percentage of cells in the quadrant. Average percentages of cytokine producers within the subsets (n = 6) were IL-17+Foxp3+CD49d+ (13% ± 6.8%), IL-17+Foxp3+CD49d− (2.6% ± 0.6%); IFN-γ+Foxp3+CD49d+ (13.5% ± 3.9%), IFN-γ+Foxp3+CD49d− (2% ± 1.2%). (E) Separation of cytokine-secreting effector cells from CD4+CD25high Treg cells. PBMC were stained with α-CD4, α-CD25, and α-CD49d and sorted by FACS into the 3 CD4+ subsets CD25low, CD49d+CD25high, and CD49d−CD25high. The sorted cell subsets were activated with PMA/ionomycin and stained intracellular for IFN-γ and IL-17. Numbers represent the percentage of cells in the indicated quadrant.
CD49d removes Th1- and Th17-like cells from CD25high Treg preparations. (A) Double staining of human PBMC for CD4 and CD25. Percentage of CD4+ cells of total PBMCs is indicated. (B) Inverse correlation of CD49d with Foxp3 expression. Costaining of Foxp3 with CD25 (left panel), CD127 (middle panel), and CD49d (right panel) is shown for CD4+ cells gated according to Figure 1A. Numbers indicate percentage of cells in each quadrant. (C) Cytokine-secreting CD4+ cells express CD49d. CD4+ T cells were stimulated in vitro with PMA/ionomycin and analyzed by FACS. Staining is shown for CD49d versus IL-17 or IFN-γ. Numbers represent percentages of cells per quadrant. (D) Segregation of IFN-γ and IL-17 secretion with CD49d expression within the Foxp3+ subset. CD4+ cells were activated as described. After activation the cells were stained with α-Foxp3, α-CD49d, α–IFN-γ, and α–IL-17. Cells were gated on Foxp3. Density plots show the cytokine staining versus CD49d. Numbers refer to the percentage of cells in the quadrant. Average percentages of cytokine producers within the subsets (n = 6) were IL-17+Foxp3+CD49d+ (13% ± 6.8%), IL-17+Foxp3+CD49d− (2.6% ± 0.6%); IFN-γ+Foxp3+CD49d+ (13.5% ± 3.9%), IFN-γ+Foxp3+CD49d− (2% ± 1.2%). (E) Separation of cytokine-secreting effector cells from CD4+CD25high Treg cells. PBMC were stained with α-CD4, α-CD25, and α-CD49d and sorted by FACS into the 3 CD4+ subsets CD25low, CD49d+CD25high, and CD49d−CD25high. The sorted cell subsets were activated with PMA/ionomycin and stained intracellular for IFN-γ and IL-17. Numbers represent the percentage of cells in the indicated quadrant.
We have now identified with CD49d a second surface marker absent on Foxp3+ Treg cells. CD4+ cells staining bright for Foxp3 are negative for CD49d (Figure 1B right panel). Cosegregation is incomplete here, too. More than one-third of the Foxp3−CD4+ cells lack expression of CD49d, and a minor fraction of cells expressing intermediate levels of Foxp3+ stain positive for the marker. On average, approximately 70% (72.1% ± 4.9%; n = 10) of the Foxp3+ cells are CD49d−. Importantly, however, in parallel studies we could establish that the marker discriminates Treg cells from cytokine-secreting CD4+ effector T cells, as essentially all CD4+ T cells releasing proinflammatory cytokines coexpress CD49d (Figure 1C and manuscript in preparation). This includes also some of the Foxp3+ Th1- and Th17-like effector cells (Figure 1D). Although Th17-like cells can apparently be generated directly from Foxp3+ Treg cells,22 the former are likely to represent CD25low cells transiently expressing Foxp3.16
CD49d separates contaminating Th1- and Th17-like cells from CD25high Treg populations
Human Treg preparations based solely on the isolation of CD25highCD4+ cells are usually contaminated with substantial amounts of CD25low effector T cells.15,23 The extent of this contamination in CD25-based Treg preparations depends on the stringency of the CD25 sorting gate. The additional use of CD49d, however, should allow the identification and removal of potentially dangerous Th1- and Th17-like cells from the CD25highCD4+ subset. CD25high cells were therefore separated into CD49d+ and CD49d− subsets by FACS sorting (Figure 1E). FACS analysis 5 hours after activation with PMA/ionomycin indeed confirmed that most of the cytokine-secreting CD25high cells were found among the CD49d+ cells. The frequency of IFN-γ– and IL-17–secreting cells was almost comparable with that of CD4+CD25low cells, which represent “true” effector and memory T cells. In contrast, the CD49d−CD25high subset was almost free of IFN-γ– or IL-17–secreting cells. Thus, the use of CD49d allows the removal of cytokine-secreting effector cells from CD25high Treg preparations.
Absence of CD49d and CD127 defines functional Foxp3+ Treg cells free of contaminating effector cells
As CD25 targets both effector and Treg cells, it still remains a risky marker for tolerogenic T-cell therapies. To avoid the use of CD25 we therefore tested whether antibodies against CD49d and CD127 alone are sufficient to identify Foxp3+ regulatory T cells within the CD4+ T-cell population. Although the segregation with Foxp3 for both markers was incomplete (Figure 1B), their combined use may provide access to clean populations of Foxp3+ Treg cells. To test this hypothesis CD4+ T cells were therefore costained with antibodies for CD49d and CD127.
FACS analysis revealed that CD4+ cells can be divided into 3 major populations: CD127+, CD49d−CD127− and CD49d+CD127− cells (Figure 2A). In line with previous publications20,21 Treg cells were only found within the CD127− subset. Importantly, however, CD49d separated the CD127− subset into 2 nearly equally large subpopulations, with the Treg cells accumulating within the CD49d−CD127− subset. Almost 80% of the CD49d−CD127− cells were CD25+ cells staining brightly for Foxp3. In contrast, only less than 20% of the CD49d+CD127− cells were CD25+Foxp3+. Moreover, their Foxp3 expression level was significantly lower compared with CD49d−CD127− cells (MFI Foxp3, 1577 vs 2311), reaching on average only approximately 70% (73.4% ± 6.7%; n = 6) of the Foxp3 MFI of CD49d−CD127− Treg. This correlates with a phenotype of activated effector cells, known to express lower amounts of Foxp3 transiently.16
Absence of CD49d and CD127 defines Foxp3+ Treg cells free of contaminating effector cells. (A) Foxp3 expression of CD127+, CD49d−CD127−, and CD49d+CD127− subsets of CD4+ T cells. Human PBMC were stained for CD4, CD25, CD127, CD49d, and Foxp3 and gated for CD4+ T cells. (Top panel) Costaining of CD49d and CD127. Gates and percentages of the 3 major populations are indicated. (Bottom panels) Costaining of CD25 and Foxp3 is shown for CD127+ (left), CD49d−CD127− (middle), and CD49d+CD127− cells (right). Numbers represent the percentages of CD25+Foxp3+ Treg cells in the indicated gate. Mean fluorescence intensity (MFI) of Foxp3 refers to the cells of the respective gate. One of 6 independent experiments is shown. (B) Release of IFN-γ and IL-17. Purified CD4+ T cells were stimulated in vitro with PMA/ionomycin to measure the cytokine production. The FACS analysis was carried out as in panel A, except that the cells were stained intracellularly with α–IFN-γ and α–IL-17.
Absence of CD49d and CD127 defines Foxp3+ Treg cells free of contaminating effector cells. (A) Foxp3 expression of CD127+, CD49d−CD127−, and CD49d+CD127− subsets of CD4+ T cells. Human PBMC were stained for CD4, CD25, CD127, CD49d, and Foxp3 and gated for CD4+ T cells. (Top panel) Costaining of CD49d and CD127. Gates and percentages of the 3 major populations are indicated. (Bottom panels) Costaining of CD25 and Foxp3 is shown for CD127+ (left), CD49d−CD127− (middle), and CD49d+CD127− cells (right). Numbers represent the percentages of CD25+Foxp3+ Treg cells in the indicated gate. Mean fluorescence intensity (MFI) of Foxp3 refers to the cells of the respective gate. One of 6 independent experiments is shown. (B) Release of IFN-γ and IL-17. Purified CD4+ T cells were stimulated in vitro with PMA/ionomycin to measure the cytokine production. The FACS analysis was carried out as in panel A, except that the cells were stained intracellularly with α–IFN-γ and α–IL-17.
FACS analysis of cells stimulated in vitro confirmed that a high proportion of the CD49d+CD127− cells are able to secrete proinflammatory cytokines (Figure 2B). Approximately 20% (22.8% ± 2.7%; n = 4) of the CD49d+CD127− cells released IFN-γ and IL-17 compared with less than 3% (2.2% ± 1.2%; n = 4) of the CD49d−CD127− cells. Thus, here too the vast majority of cytokine-secreting cells were found only within the CD49d+ subset.
The experiments above indicated that the use of the 2 markers provides access to clean populations of Treg cells as the double-negative subset consisted almost entirely of CD25+Foxp3+ cells. To confirm that the CD49d−CD127− subset indeed exhibits suppressive capacity, CD49d+CD127− and CD49d−CD127− cells were isolated by FACS sorting (Figure 3A) and tested in an in vitro suppression assay (Figure 3B). In line with the low number of Foxp3+ cells contained in the fraction, only a marginal inhibition was observed by CD49d+CD127− cells. In contrast, CD49d−CD127− cells effectively prevented the expansion of activated CD4+ effector T cells. Thus, absence of CD49d and CD127 defines functional Foxp3+ Treg cell virtually free of contaminating nonregulatory cells without the need of using any CD25-specific antibodies.
Suppressive capacity segregates with the CD49d−CD127− CD4+ T-cell subset. (A) CD49d/CD127 FACS sorting. CD4+ cells isolated from PBMC with a commercial MACS-depletion kit were stained with α-CD49d and α-CD127 and sorted by FACS into the CD49d−CD127− and the CD49d+CD127− subset (top panels). The Foxp3 versus CD25 staining is shown in the bottom panels. Numbers represent percentages of cells per quadrant. (B) In vitro suppression assay. CD49d−CD127− and CD49d+CD127− cells were incubated with CD4+ cells. Proliferation of CD4+ cells was induced by stimulation; suppressor cells were added at a ratio of 1:1. Inhibition of the proliferative response of CD4+ cells was determined in a FACS-based suppression assay as shown in Figure 4B. Suppression is expressed as “% inhibition” based on the fraction of dividing cells in reference to noninhibited stimulated CD4+ cells. Summarized data of 4 independent experiments are shown (% inhibition CD49d−CD127− cells, 76.9% ± 12.8%; CD49d+CD127− cells, 27.8% ± 7%).
Suppressive capacity segregates with the CD49d−CD127− CD4+ T-cell subset. (A) CD49d/CD127 FACS sorting. CD4+ cells isolated from PBMC with a commercial MACS-depletion kit were stained with α-CD49d and α-CD127 and sorted by FACS into the CD49d−CD127− and the CD49d+CD127− subset (top panels). The Foxp3 versus CD25 staining is shown in the bottom panels. Numbers represent percentages of cells per quadrant. (B) In vitro suppression assay. CD49d−CD127− and CD49d+CD127− cells were incubated with CD4+ cells. Proliferation of CD4+ cells was induced by stimulation; suppressor cells were added at a ratio of 1:1. Inhibition of the proliferative response of CD4+ cells was determined in a FACS-based suppression assay as shown in Figure 4B. Suppression is expressed as “% inhibition” based on the fraction of dividing cells in reference to noninhibited stimulated CD4+ cells. Summarized data of 4 independent experiments are shown (% inhibition CD49d−CD127− cells, 76.9% ± 12.8%; CD49d+CD127− cells, 27.8% ± 7%).
CD49d provides access to untouched Treg cells
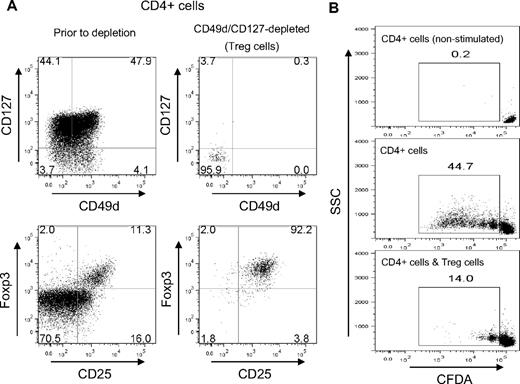
The above results suggested that the depletion of CD49d+CD127+ cells should produce a clean population of untouched Treg cells. To validate applicability of this approach, CD4+ T cells from human PBMC were incubated with α-CD49d and α-CD127 and depleted with conventional magnetic bead–labeled antibodies (Figure 4). The postsorting analysis revealed that the depletion produced CD49d−CD127− cells with a purity greater than 95% (Figure 4A top panels). Staining of CD25 and Foxp3 confirmed that in fact more than 92% of these cells were CD25+ Foxp3+ cells (Figure 4A bottom panels). An additional 2% were CD25-Foxp3+, a subset of Treg cells also reported to be immune-suppressive,24 adding to a total of approximately 94% Foxp3+ Treg cells.
Isolation of untouched Treg cells from CD4+ T cells by CD49d/CD127 depletion. CD4+ cells of human PBMCs were depleted of CD127/CD49d-expressing cells by MACS. (A) FACS analysis before and after depletion. FACS plots are shown for the CD4+ cell population before the depletion (left panels) and for the cells remaining after CD49d/CD127 depletion (right panels). Data are shown for the staining CD49d versus CD127 (top panels) and CD25 versus Foxp3 (bottom panels). Numbers refers to the percentage of cells in each quadrant. (B) Suppressive capacity. CD49d−CD127− cells isolated by MACS depletion were used as suppressor cells (Treg) in a FACS-based in vitro assay to inhibit the proliferation of CFDA-labeled CD4+ cells (ie, CD49d+CD127+ cells removed from the Treg population by the depletion). The panels indicate the CFDA staining without any stimulation (top panel), after stimulation (middle panel) or after stimulation in the presence of CD49d−CD127− Treg cells at a ratio of 1:1 (bottom panel). Numbers represent the percentage of dividing CD4+ cells. Data are representative of 8 independent experiments with blood from healthy donors (average inhibition, 78% ± 11%).
Isolation of untouched Treg cells from CD4+ T cells by CD49d/CD127 depletion. CD4+ cells of human PBMCs were depleted of CD127/CD49d-expressing cells by MACS. (A) FACS analysis before and after depletion. FACS plots are shown for the CD4+ cell population before the depletion (left panels) and for the cells remaining after CD49d/CD127 depletion (right panels). Data are shown for the staining CD49d versus CD127 (top panels) and CD25 versus Foxp3 (bottom panels). Numbers refers to the percentage of cells in each quadrant. (B) Suppressive capacity. CD49d−CD127− cells isolated by MACS depletion were used as suppressor cells (Treg) in a FACS-based in vitro assay to inhibit the proliferation of CFDA-labeled CD4+ cells (ie, CD49d+CD127+ cells removed from the Treg population by the depletion). The panels indicate the CFDA staining without any stimulation (top panel), after stimulation (middle panel) or after stimulation in the presence of CD49d−CD127− Treg cells at a ratio of 1:1 (bottom panel). Numbers represent the percentage of dividing CD4+ cells. Data are representative of 8 independent experiments with blood from healthy donors (average inhibition, 78% ± 11%).
To confirm functionality, isolated Treg cells were tested in a FACS-based in vitro suppression assay (Figure 4B). CD4+ effector T cells were used as responder cells and labeled with CFDA to monitor proliferation. Whereas without any stimulation the CD4+ cells did not divide (Figure 4B top panel), stimulation with α-CD3 triggered a typical proliferative response of the labeled cells (Figure 4B middle panel). The addition of CD49d/CD127-purified Treg cells, however, inhibited the proliferation almost completely, with most cells performing only a single replication cycle (Figure 4B bottom panel). Thus, the use of the 2 markers alone seems to be sufficient for the isolation of Foxp3+ Treg cells from CD4+ T cells.
Isolation of Treg cells from total PBMC by CD49d/CD127 depletion
The selectivity of CD49d for the isolation of Foxp3+ Treg cells is particularly striking when using total PBMC instead of prepurified CD4+ cells (Figure 5). More than 80% of the PBMC express CD49d, which leaves only a very small fraction of CD49d−CD127− cells (∼2%-4%). As most of these cells are in fact Treg cells, the MACS-depletion with α-CD49d and α-CD127 alone was already sufficient to obtain untouched Foxp3+ cells with a purity of approximately 75% (Figure 5A). The purity could be further increased when the CD49d/CD127-antibodies were used together with antibodies of a commercial CD4+ T-cell isolation kit (Figure 5B). The postsorting analysis revealed here that approximately 90% of the isolated CD4+ T cells were Foxp3+ Treg. Thus, due to the expression profile of CD49d, untouched Treg cells can be obtained directly from PBMCs in a single depletion step.
Isolation of Treg cells from total PBMC by single-step CD49d/CD127 depletion. Untouched Treg cells were isolated by CD49d/CD127 MACS depletion of total PBMC. (A) Depletion with α-CD49d and α-CD127 only. Depletion of human PBMC was carried out with α-CD49d and α-CD127 as described in Figure 4 except that total PBMC instead of purified CD4+ cells were used. Left panels show PBMC before depletion, right panel after depletion (CD49d−CD127− PBMC). (B) CD49d/CD127 depletion combined with a CD4+ T-cell isolation kit. To increase the purity, the antibody mix of a commercial CD4+ T-cell isolation kit was added to the CD49d/CD127 depletion. The left panel shows PBMC before depletion, the right panel after depletion (CD49d−CD127−CD4+ cells). Numbers indicate the percentage of cells in each quadrant. (C) Statistical analysis of Treg enrichment. Treg cells were isolated in 25 independent experiments from PBMCs of healthy donors. CD49d/CD127 depletions were carried out as shown in Figure 4 (10 experiments) or panel B (15 experiments). The fractions of CD25+ effector T cells (Teff) and CD25+ Treg cells were determined by FACS with α-CD127 and α-CD25 (left panels). Analysis gates for CD25+ Teff (CD25+ CD127+) and CD25+ Treg cells (CD25+CD127−) are indicated. The percentages of CD25+ Treg (●) and of CD25+ Teff cells (○) of the total population of CD4+ cells before and after the CD49d/CD127 depletion are indicated in the dot plot (middle panel). Average percentages before depletion were 9.0% plus or minus 2.5% for CD25+ Treg and 32.1% plus or minus 9.8% for CD25+ Teff; after depletion they were 86.1% plus or minus 5.1% for CD25+ Treg and 0.9% plus or minus 1.2% for CD25+ Teff. (D) Percentage of Foxp3+ cells after depletion. Twelve Treg preparations were analyzed with α-CD25 and α-Foxp3. The dot plot indicates the corresponding values of CD25+Foxp3+ T cells and of total Foxp3+ T cells, including CD25−Foxp3+ T cells. Average values are indicated.
Isolation of Treg cells from total PBMC by single-step CD49d/CD127 depletion. Untouched Treg cells were isolated by CD49d/CD127 MACS depletion of total PBMC. (A) Depletion with α-CD49d and α-CD127 only. Depletion of human PBMC was carried out with α-CD49d and α-CD127 as described in Figure 4 except that total PBMC instead of purified CD4+ cells were used. Left panels show PBMC before depletion, right panel after depletion (CD49d−CD127− PBMC). (B) CD49d/CD127 depletion combined with a CD4+ T-cell isolation kit. To increase the purity, the antibody mix of a commercial CD4+ T-cell isolation kit was added to the CD49d/CD127 depletion. The left panel shows PBMC before depletion, the right panel after depletion (CD49d−CD127−CD4+ cells). Numbers indicate the percentage of cells in each quadrant. (C) Statistical analysis of Treg enrichment. Treg cells were isolated in 25 independent experiments from PBMCs of healthy donors. CD49d/CD127 depletions were carried out as shown in Figure 4 (10 experiments) or panel B (15 experiments). The fractions of CD25+ effector T cells (Teff) and CD25+ Treg cells were determined by FACS with α-CD127 and α-CD25 (left panels). Analysis gates for CD25+ Teff (CD25+ CD127+) and CD25+ Treg cells (CD25+CD127−) are indicated. The percentages of CD25+ Treg (●) and of CD25+ Teff cells (○) of the total population of CD4+ cells before and after the CD49d/CD127 depletion are indicated in the dot plot (middle panel). Average percentages before depletion were 9.0% plus or minus 2.5% for CD25+ Treg and 32.1% plus or minus 9.8% for CD25+ Teff; after depletion they were 86.1% plus or minus 5.1% for CD25+ Treg and 0.9% plus or minus 1.2% for CD25+ Teff. (D) Percentage of Foxp3+ cells after depletion. Twelve Treg preparations were analyzed with α-CD25 and α-Foxp3. The dot plot indicates the corresponding values of CD25+Foxp3+ T cells and of total Foxp3+ T cells, including CD25−Foxp3+ T cells. Average values are indicated.
In 25 independent experiments the average purity of Treg cells was 86.1% plus or minus 5.1% when using the CD25+CD127− phenotype as Treg marker20,21 (Figure 5C). Because the isolation procedure also provides access to some CD25− Treg cells, the average purity increased to 91.7% plus or minus 3.0% Treg cells, if Foxp3 is used as Treg marker (Figure 5D). This excludes, however, Foxp3-expressing cytokine-secreting effector cells as they are mainly CD49d+ (Figure 1D). Importantly, the depletion almost completely removed CD25+ effector and memory T cells from the Treg fraction. While the population of CD4+ T cells before depletion contained 32.1% plus or minus 9.8% CD25+CD127+ cells, their number dropped to below 1% (0.9% ± 1.2%) after CD49d/CD127 depletion (Figure 5C). The average yield was with 0.24% plus or minus 0.14% (n = 25) of total PBMCs, in the range of commercially available isolation kits. In a direct comparison, this was only slightly below the yield obtained with the CD4+/CD25+/CD127dim/− regulatory T-cell isolation kit (Miltenyi Biotec; Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Long-term culture of untouched Treg cells
Treg cells have a hypoproliferative phenotype compared with nonregulatory CD4+ T cells.2 Residual CD4+ effector T cells contaminating the Treg preparation can therefore easily overgrow Treg cultures of low purity.25 To determine whether Treg cells isolated by CD49d/CD127 depletion can be expanded in vitro, they were cultivated for a period of more than 30 days. Expansion was triggered with α-CD3/CD28-coated beads in the presence of IL-2 and compared with a culture of total CD4+ T cells (Figure 6). In line with previous publications,25,26 the Treg cell culture expanded less efficiently than the culture of total CD4+ cells (Figure 6A). Importantly, however, even after more than 1 month most of the cultured cells stably expressed Foxp3 (Figure 6B). Only in 2 of 8 cultures a significant decrease in the purity was observed. In all other long-term cultures either slight or no reductions in the frequency of Foxp3+ cells were observed (Figure 6C). Foxp3 expression was confirmed by staining with PCH101 (Figure 6B) as well as with 236A/E7 (data not shown), which in contrast to PCH101 has not been implicated with artificial staining of activated T cells.27
Long-term in vitro culture of CD49d−CD127− Treg cells. Untouched Treg cells were obtained by CD49d/CD127 MACS depletion as described in Figure 4. (A) Expansion of Treg cells. Total CD4+ T cells (□) and CD49d−CD127− Treg cells (■) were expanded in vitro by stimulation with α-CD3/CD28 beads in the presence of IL-2. Cells were counted on days 21 and 33; absolute cell numbers are shown. (B) FACS analysis. Dot plots show the expression of CD25 and Foxp3 for the cultures on days 0 (top row) and 33 (bottom row). Numbers represent percentages of cells per quadrant. (C) Summarized purity of Foxp3+ cells of 8 independent experiments after the indicated cultivation period. The linked data points refer to purity of the culture at the beginning of cultivation and on days 33 to 36 based on Foxp3 expression (day 0, 91.01% ± 3.27%; days 33-36, 74.28% ± 12.74%).
Long-term in vitro culture of CD49d−CD127− Treg cells. Untouched Treg cells were obtained by CD49d/CD127 MACS depletion as described in Figure 4. (A) Expansion of Treg cells. Total CD4+ T cells (□) and CD49d−CD127− Treg cells (■) were expanded in vitro by stimulation with α-CD3/CD28 beads in the presence of IL-2. Cells were counted on days 21 and 33; absolute cell numbers are shown. (B) FACS analysis. Dot plots show the expression of CD25 and Foxp3 for the cultures on days 0 (top row) and 33 (bottom row). Numbers represent percentages of cells per quadrant. (C) Summarized purity of Foxp3+ cells of 8 independent experiments after the indicated cultivation period. The linked data points refer to purity of the culture at the beginning of cultivation and on days 33 to 36 based on Foxp3 expression (day 0, 91.01% ± 3.27%; days 33-36, 74.28% ± 12.74%).
Thus, untouched Treg, isolated by CD49d/CD127 depletion can be expanded and long term cultured in vitro. The high purity of the CD49d−CD127− cells and the removal of residual CD25+ effector T cells by CD49d seems to allow the expansion of Treg cells without a significant outgrowth of effector cells.
Inhibition of allogeneic and xenogeneic reactions in vitro and in vivo
To determine whether untouched Treg cells are able to control alloreactive responses, their inhibitory capacity was tested in mixed lymphocyte reactions (MLRs). MLR is the in vitro correlate of GVHD. It is based on the allogeneic activation of T cells by foreign major histocompatibility complex molecules, evident after mixing PBMCs of 2 different donors. In this experiment CD49d/CD127-purified Treg were added to autologous PBMCs and mixed with irradiated PBMCs of haplotype-mismatched donors (Figure 7A). In line with published results15,25 also the untouched Treg population proved to be a potent suppressor of the allogeneic response. They strongly inhibited the proliferation of the autologous PBMC.
Inhibition of allogeneic and xenogeneic reactions in vitro and in vivo. Untouched Treg cells were isolated from human PBMCs as shown in Figure 5B. (A) Inhibition of mixed lymphocyte reaction (MLR). PBMCs (105) were incubated with the same number of radiated PBMCs of a haplotype-mismatched second donor (allogen). The reaction was inhibited by adding 2.5 × 104 autologous CD49d−CD127− Treg cells (allogen and Treg). Proliferation was determined by 3H-thymidine incorporation and is expressed as counts per minute (cpm). The figure shows 6 independent experiments carried out in duplicate (average inhibition 72.5% ± 16.8%). (C) Prevention of acute GVHD. Acute xeno-GVHD was induced by the adoptive transfer of 30 × 106 CD25-depleted human PBMC (CD25− PBMC) into Rag2−/−γc−/− mice. Progression of the disease was recorded by monitoring for clinical signs of GVHD, expressed as percent free of GVHD (top panel), and by determining the average relative weight in reference to the start of the experiment, expressed as percent weight difference (bottom panel). One group received only CD25− PBMCs (●), a second group received CD25− PBMCs together with 0.5 × 106 untouched autologous Treg cells in a cotransfer (○). Groups of 6 mice were used.
Inhibition of allogeneic and xenogeneic reactions in vitro and in vivo. Untouched Treg cells were isolated from human PBMCs as shown in Figure 5B. (A) Inhibition of mixed lymphocyte reaction (MLR). PBMCs (105) were incubated with the same number of radiated PBMCs of a haplotype-mismatched second donor (allogen). The reaction was inhibited by adding 2.5 × 104 autologous CD49d−CD127− Treg cells (allogen and Treg). Proliferation was determined by 3H-thymidine incorporation and is expressed as counts per minute (cpm). The figure shows 6 independent experiments carried out in duplicate (average inhibition 72.5% ± 16.8%). (C) Prevention of acute GVHD. Acute xeno-GVHD was induced by the adoptive transfer of 30 × 106 CD25-depleted human PBMC (CD25− PBMC) into Rag2−/−γc−/− mice. Progression of the disease was recorded by monitoring for clinical signs of GVHD, expressed as percent free of GVHD (top panel), and by determining the average relative weight in reference to the start of the experiment, expressed as percent weight difference (bottom panel). One group received only CD25− PBMCs (●), a second group received CD25− PBMCs together with 0.5 × 106 untouched autologous Treg cells in a cotransfer (○). Groups of 6 mice were used.
To further document the functionality of “untouched” Treg cells in vivo, an acute GVHD model was used based on the transfer of CD25-depleted human PBMCs into Rag2−/−γc−/− mice. This model allows to trigger an acute aggressive form of the disease, which is apparently mediated by a massive cytokine storm.10 Although this model may not be as informative for the human GVHD, it represents one of the few models that allow determination of human Treg efficacy in vivo, as it can be controlled by the addition of human CD25+ Treg cells.10 All of the mice that received CD25-depleted PBMC exhibited a more or less pronounced weight loss within the first days of the experiment caused by the engraftment of xenogeneic PBMCs (Figure 7B). Two-thirds of the mice developed clinical symptoms (top panel), and half of them died during the experiment due to the acute-aggressive form of GVHD. The addition of CD49d/CD127-purified Treg cells completely prevented x-GVHD. None of the mice showed signs of weight loss (bottom panel) and all mice treated with Treg cells remained without symptoms for the entire course of the experiment (top panel). Thus, untouched Treg cells isolated by CD49d/CD127 depletion are potent suppressor cells capable of controlling proinflammatory immune responses in vitro and in vivo.
Discussion
In this study we have identified CD49d as a marker that discriminates between proinflammatory effector cells and immune-suppressive Treg cells. It is present on more than 80% of human PBMCs, including nonregulatory CD4+CD25low T cells and cytokine-secreting CD4+ effector cells, and also on Foxp3+ Th1- and Th17-like cells. Because the marker is absent from immune-suppressive Treg cells, it is possible to remove potentially dangerous effector cell contaminations from conventional CD25-based Treg preparations by a simple CD49d depletion. Moreover, together with CD127, CD49d provides access to highly pure populations of Treg cells that have not been tagged by an antibody during purification. The latter can be obtained in a single depletion step directly from PBMCs, where the combination of α-CD49d and α-CD127 removes the bulk of nonregulatory cells. These untouched Treg cells show efficient suppressive capacity in vitro in autologous as well as in allogeneic settings and were able to contain acute xenogeneic GVHD reactions in vivo when used in adoptive transfer models based on Rag2−/−γc−/− mice.
CD49d has gained considerable attention as a therapeutic target: CD49d-blocking antibodies, such as natalizumab, are highly effective for the treatment of Crohn disease and MS.18 As α-chain of the integrin VLA-4, it functions as costimulatory molecule28 and represents a key adhesion molecule for transmigration into inflamed tissues.29 Considering the lopsided distribution of CD49d on effector cells, the integrin seems to represent an important element in adjusting the effector/suppressor balance in inflamed tissues, which may explain the exceptional efficacy of VLA-4 blocking antibodies (M.K., G.B., L.B., O.R., K.F., manuscript in preparation). Despite the clinical relevance of the marker, the striking difference in expression has been largely overlooked. In previous studies elevated numbers of CD49d+ cells among the Treg population were reported. This discrepancy has presumably technical causes. One of these studies was based only on FACS staining of frozen cells.30 Another older study used Treg-cell populations isolated by MACS using anti-CD25.31 As shown by others,23 this procedure is prone to contaminations by CD25low effector cells, which may have added to the elevated percentage of CD49d+ cells.
Central to this study, however, is the role CD49d could play in the isolation of Treg cells for tolerogenic cell transfers. So far, safety concerns have prevented the successful translation into human applications, given the potentially fatal adverse reactions caused by effector cell contaminations. Up to now, only a single trial had been launched. It aimed at the prevention of GVHD after bone marrow transplantation and used Treg cell preparations containing approximately 40% nonregulatory CD4+CD25+ cells.23 As shown here, IFN-γ– and IL-17–secreting cells can be effectively removed from CD25highCD4+ Treg preparations by α-CD49d. Hence, employment of this marker may dramatically improve the safety of adoptive transfers of CD25-based Treg preparations.
Even more relevant for future Treg therapy may be the combination of CD49d with CD127. CD127 has already been used together with CD25,20,21 but with CD49d it provides access to untouched human Treg cells. The regulations for reagents used in human therapy are very strict, particularly for the cells, materials, and compounds injected into patients. Treg cells produced by positive sorting carry a foreign antibody on their surface, which may bind complement or induce functional changes in the tagged cells. As α-chain of the IL-2 receptor, CD25 is absolutely vital for the survival and function of Treg cells. Antibodies blocking this receptor can therefore inhibit expansion and suppressor function,32-35 whereas cross-linking of the IL-2 receptor by antibody-coupled beads may lead to a premature activation.36,37 Another issue is the cellular distribution of the marker. As mentioned before, in humans CD25 is expressed not only by Treg cells but also by activated effector and memory CD4+ T cells. Particularly because those nonregulatory CD25+ cells are one of the driving forces for destructive immune responses,11 the use of this marker for Treg isolations should generally be avoided. Compared with CD25-based methods, the CD49d/CD127 depletion appears to be much safer. It yields highly pure Foxp3+ Treg cells, untouched by antibodies and virtually free of contaminating CD25+ effector cells. The depletion of PBMC can be carried out in a single depletion step. It is therefore fast and cheap and—as a MACS-based method—compatible at least in principle with GMP regulations.
Although CD49d/CD127-depleted cells can be used directly for transfers, their untouched state and high purity also allow an expansion in vitro. A previous study suggested that only “naive-like” CD45RA+ Treg cells can be efficiently expanded, whereas cultures of CD45RO+ Treg cells tend to be unstable and quickly consist mostly of cytokine-producing effector cells.38 As CD45RO+ effector cells, in contrast to CD45RA+ cells, express low amounts of CD25, the observed instability may in part be explained by effector cell contaminations of “conventional” CD25-based CD45RO+ Treg preparations. Due to a bias in the CD127 expression CD49d/CD127-depletion enriches for CD45RO+ Treg cells but the percentage of CD45RA+ Treg cells in CD49d/CD127-depleted cells may be sufficient to account for the expansion (Figure S2). More studies are needed to fully address this question. In any case, the approach can be used effectively to obtain a starting population suitable for in vitro expansion. This may be used particularly in settings in which blood is limited or where antigen-specific expansion of the Treg cells is required.
Taken together, CD49d represents a marker that separates contaminating effector cells from immune-suppressive Foxp3+ Treg cells. Depletion with α-CD49d removes potentially dangerous Th1- and Th17-like cells from CD25highCD4+ Treg preparations and in combination with α-CD127 it provides access to highly pure populations of untouched Foxp3+ cells. The CD49d/CD127 approach is, therefore, a simple and cost-effective way to obtain human Foxp3+ cells for the treatment of autoimmune diseases,3 allergies,5 allogeneic transplant rejection,9 and GVHD.3 Clinical trials are planned or are already underway to prevent GVHD during allogeneic hematopoetic stem cell transplantation (HSCT).23 Other applications for tolerogenic Treg transfers are likely to follow.39 Therefore, the findings of this study may open the door to the routine use of Treg cells in human therapies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank H. P. Rahn for FACS sorting, C. Schnöller and S. Hartmann for providing chlodronate liposomes, and S. Giering for secretarial help.
M.K. was supported by the German Federal Ministry of Education and Research (BMBF) project 01GU5130300, M.S. by the Program to Support Research, Innovation, and Technology (PROFIT; Berlin, Germany) project 10134758, K.F. and O.R. are supported by the Deutsche Forschungsgemeinschaft (DFG) grant SFB650. G.B. and L.B. are partially supported by Italian Federation for Multiple Sclerosis (FISM) and by the Italian Ministry of Health (Progetto Finalizzato, Strategico, Articolo 56).
Authorship
Contribution: M.K. designed and performed the research, analyzed the data, and wrote the paper; M.S., D.D.M., and L.B. performed research and analyzed data; G.B. performed research, analyzed data, and wrote the paper; and O.R. and K.F. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Olaf Rötzschke, Singapore Immunology Network (SIgN), 8A Biomedical Grove, IMMUNOS, Singapore 138648; e-mail: olaf_rotzschke@immunol.a-star.edu.sg; or Kirsten Falk, Max-Delbrück-Center for Molecular Medicine (MDC), Robert-Rossle-Str 10, 13125 Berlin, Germany; e-mail: falk@mdc-berlin.de.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal