Abstract
Adhesion of acute myeloid leukemia (AML) blasts in the bone marrow microenvironment confers protection from chemotherapy-induced apoptosis. One mechanism for retention of blasts within the bone marrow is adhesion via very late antigen-4 (VLA-4), the α4β1 integrin heterodimer that binds to its main ligands, fibronectin, and vascular cell adhesion molecule-1 (VCAM-1). To examine the relationship of functional expression of VLA-4 to prognosis in AML, we studied marrow samples from 175 adult AML patients who underwent induction chemotherapy with anthracycline and cytarabine on Southwest Oncology Group trials. The studies included flow cytometry and functional in vitro assays for ligand binding and maximal β1 activation. VLA-4 expression varied widely, with mean expression 60.6% for α4, and was not significantly associated with response to chemotherapy, relapse-free, or overall survival (OS). However, increased binding of soluble VCAM-1 via VLA-4 was significantly associated with longer OS, corrected for age (P = .033). Estimated 5-year OS was 31% (95% confidence interval, 14%-48%) in 30 patients with soluble VCAM-1 binding greater than or equal to 40%, compared with 10% (confidence interval, 3%-17%) in 72 patients with lower binding. Adhesion and migratory properties of AML blasts thus appear to influence chemosensitivity and therefore may be therapeutic targets.
Introduction
The majority of adult patients with de novo acute myeloid leukemia (AML) will achieve an initial complete remission, but with chemotherapy alone, most will relapse. Moreover, there are poor prognostic groups, such as treatment-associated leukemia or transformed leukemia, or those with poor prognosis cytogenetics, who are less probable to achieve complete remission with induction treatment and for whom the overall survival (OS) is less than a year.1
Normal stem/progenitor cell retention in favorable anatomic location or niches in the bone marrow is critical for their survival and further development. The receptor CXCR-4 is thought to play a critical role in retention of human cells to the bone marrow after transplantation through interaction with its ligand, stromal-derived factor-1 (SDF-1),2 which is highly expressed in niches within the bone marrow. In addition to CXCR-4/SDF-1, this retention is facilitated by several other cooperative pathways, such as that of very late antigen-4 (VLA-4) integrin and its major ligands within the bone marrow, vascular cell adhesion molecule-1 (VCAM-1), and fibronectin.
Adhesive properties of leukemic cells are probably responsible for the complication of leukostasis in AML as well as leukemic meningitis, leukemia cutis, extramedullary leukemia, and formation of chloromas. Several adhesion mechanisms, including the VLA-4/VCAM-1 pathway, have been implicated in the attachment of leukemic blasts to the vessel wall.3 High levels of expression of VLA-4 are seen in all French-American-British classes of AML, M0-M5, with averages ranging from 72% to 95%, although there is a wide range (6%-98%).3 VLA-4 is involved in the migration of CD34+ cells and AML cells beneath marrow stromal cells.4 In addition, blocking of CXCR-4 by AMD 3100 results in mobilization of AML blasts into the circulation.5 Moreover, administration of an activating antibody to CD44, the hyaluronic acid receptor, which induces differentiation in vitro, blocks engraftment of AML cells in NOD-scid mice.6 Furthermore, administration of this antibody to CD44 can selectively eliminate the engrafted leukemia cells but has no effect on engrafted normal hematopoietic cells derived from cord blood or human bone marrow.6 Thus, multiple adhesion mechanisms, many of which are shared by normal cells, are probably critical for the movement to and retention of acute leukemia cells within a specific bone marrow microenvironment.
Adhesion of cells has been demonstrated to confer resistance to several chemotherapy agents, including cytarabine and etoposide. Growth of AML cells on HS-5 stroma reduces daunorubicin-or cytarabine-induced apoptosis.7 Adhesion of U937 to fibronectin via β1 integrins inhibits mitoxantrone- and etoposide-induced apoptosis8 ; similarly, adhesion of U937 or HL60 leukemia cell lines to fibronectin inhibits daunorubicin- or cytarabine-induced apoptosis.9
Several potential mechanisms have been proposed for the ability of integrin-mediated signaling to protect from chemotherapy toxicity, including activation of the phosphatidylinositol 3-kinase (PI3K)/Akt/bcl-2 pathway9 and an interaction between Wnt and adhesion-dependent signaling pathways.10 Furthermore, a function blocking antibody to VLA-4 restored chemotherapy sensitivity to cytarabine in a murine xenograft leukemia model of minimal residual disease.9 These investigators also reported that 5-year survival was 100% for patients whose leukemia cells exhibited low expression of VLA-4 (< 34.5%), compared with 44% for patients whose leukemia cells exhibited high expression of VLA-4 (≥ 34.5%),9 although the sample size included only 10 and 15 patients in each group, respectively.
To further explore the role of VLA-4 in survival in AML, we examined the expression and function of VLA-4 by flow cytometry, functional assays, and real-time quantitative reverse-transcribed polymerase chain reaction (RT-PCR) in 175 bone marrow samples obtained from patients with previously untreated AML, who subsequently received treatment on Southwest Oncology Group (SWOG) protocols with an anthracycline and cytarabine. The results of expression and function of VLA-4 were then correlated with response to induction chemotherapy, relapse-free survival (RFS), and OS.
Methods
Human subjects
This study was conducted with approval of the Fred Hutchinson Cancer Research Center Institutional Review Board. Informed consent was obtained in accordance with the Declaration of Helsinki.
Sample selection
We selected 175 cryopreserved pretreatment marrow specimens from patients with previously untreated AML who were eligible for and received anthracycline- and cytarabine-containing induction chemotherapy regimens on the following SWOG clinical trials: SWOG-8600, SWOG-9031, SWOG-9126, S9333, and S9500. Treatment regimens and clinical results of these trials have been reported separately.11-15 Eligible specimens had at least 3 vials of cryopreserved cells stored in the SWOG repository and probably had more than or equal to 15% blasts in the sample based on the repository's count, if available, or on the patient's clinical trial pretreatment data. All selected specimens had at least 27 million cells/vial, but this was not set as a minimal requirement. The selection included all eligible specimens from patients known to have prior myelodysplastic syndrome or treatment-related secondary AML. The remaining patients were randomly selected with stratification to ensure approximately equal numbers older and younger than age 56. The sample size of 175 was designed to ensure that a 2-sided test at the α = 5% critical level would have sufficient statistical power (88%) to detect an absolute difference of 25 percentage points in the probability of an event such as complete response (CR) or 1-year OS between groups defined by the presence (in 40%-60% of cases) of VLA-4 characteristics, assuming a 50% event rate for all patients combined. Characteristics of the 175 included patients are compared with those of the 1240 excluded patients on the same trials and treatment arms in Table 1 and Table S4 (available on the Blood website; see the Supplemental Materials link at the top of the online article). The included patients have relatively high white blood cell (WBC) count (median 42.9 vs 12.6 × 103/μL) and marrow blast percentages (median 76% vs 66%) and were more probable to have participated in the more recent studies.14,15 These differences reflect the greater availability of specimens from such patients in the repository.
Characteristics of 1415 adult patients with previously untreated AML by inclusion in the present study
| . | Included patients (N = 175) . | Excluded patients (N = 1240) . | ||
|---|---|---|---|---|
| No. . | % . | No. . | % . | |
| Sex | ||||
| Female | 77 | 44 | 567 | 46 |
| Male | 98 | 56 | 673 | 54 |
| Race/ethnicity | ||||
| Asian, non-Hispanic | 8 | 5 | 19 | 2 |
| Black, non-Hispanic | 18 | 10 | 118 | 10 |
| Native American | 0 | 0 | 5 | 0.4 |
| Pacific Islander | 2 | 1 | 2 | 0.2 |
| White, Hispanic | 5 | 3 | 24 | 2 |
| White, non-Hispanic | 141 | 81 | 1048 | 85 |
| Unknown | 0 | 0 | 24 | 2 |
| FAB class (local diagnosis) | ||||
| M1 | 39 | 22 | 270 | 22 |
| M2 | 53 | 30 | 357 | 29 |
| M3 | 6 | 3 | 99 | 8 |
| M4 | 49 | 28 | 279 | 23 |
| M5 | 15 | 9 | 95 | 8 |
| M6 | 0 | 0 | 41 | 3 |
| M7 | 1 | 1 | 11 | 1 |
| M0 | 7 | 4 | 20 | 2 |
| Other | 4 | 2 | 46 | 4 |
| AML, NOS | 1 | 1 | 22 | 2 |
| AML onset | ||||
| De novo | 59 | 34 | 243 | 20 |
| Secondary | 29 | 17 | 117 | 9 |
| Unknown | 87 | 50 | 880 | 71 |
| Study and treatment arm | ||||
| S8600 AD | 30 | 17 | 555 | 45 |
| S8600 hAD | 16 | 9 | 262 | 21 |
| S9031 AD | 21 | 12 | 97 | 8 |
| S9031 AD+G-CSF | 19 | 11 | 97 | 8 |
| S9126 AD | 3 | 2 | 22 | 2 |
| S9126 AD+CyA | 2 | 1 | 25 | 2 |
| S9333 AD | 43 | 25 | 119 | 10 |
| S9500 3+7+3 | 41 | 23 | 63 | 5 |
| Age, y | Median: 56 | Range: 18-84 | Median: 53 | Range: 15-88 |
| Marrow blasts, % | Median: 76 | Range: 15-99 | Median: 66 | Range: 0-99 |
| WBCs, 1000/μL | Median: 42.9 | Range: 0.8-274 | Median: 12.6 | Range: 0.4-416 |
| Peripheral blasts, % | Median: 44 | Range: 0-94 | Median: 30 | Range: 0-99 |
| . | Included patients (N = 175) . | Excluded patients (N = 1240) . | ||
|---|---|---|---|---|
| No. . | % . | No. . | % . | |
| Sex | ||||
| Female | 77 | 44 | 567 | 46 |
| Male | 98 | 56 | 673 | 54 |
| Race/ethnicity | ||||
| Asian, non-Hispanic | 8 | 5 | 19 | 2 |
| Black, non-Hispanic | 18 | 10 | 118 | 10 |
| Native American | 0 | 0 | 5 | 0.4 |
| Pacific Islander | 2 | 1 | 2 | 0.2 |
| White, Hispanic | 5 | 3 | 24 | 2 |
| White, non-Hispanic | 141 | 81 | 1048 | 85 |
| Unknown | 0 | 0 | 24 | 2 |
| FAB class (local diagnosis) | ||||
| M1 | 39 | 22 | 270 | 22 |
| M2 | 53 | 30 | 357 | 29 |
| M3 | 6 | 3 | 99 | 8 |
| M4 | 49 | 28 | 279 | 23 |
| M5 | 15 | 9 | 95 | 8 |
| M6 | 0 | 0 | 41 | 3 |
| M7 | 1 | 1 | 11 | 1 |
| M0 | 7 | 4 | 20 | 2 |
| Other | 4 | 2 | 46 | 4 |
| AML, NOS | 1 | 1 | 22 | 2 |
| AML onset | ||||
| De novo | 59 | 34 | 243 | 20 |
| Secondary | 29 | 17 | 117 | 9 |
| Unknown | 87 | 50 | 880 | 71 |
| Study and treatment arm | ||||
| S8600 AD | 30 | 17 | 555 | 45 |
| S8600 hAD | 16 | 9 | 262 | 21 |
| S9031 AD | 21 | 12 | 97 | 8 |
| S9031 AD+G-CSF | 19 | 11 | 97 | 8 |
| S9126 AD | 3 | 2 | 22 | 2 |
| S9126 AD+CyA | 2 | 1 | 25 | 2 |
| S9333 AD | 43 | 25 | 119 | 10 |
| S9500 3+7+3 | 41 | 23 | 63 | 5 |
| Age, y | Median: 56 | Range: 18-84 | Median: 53 | Range: 15-88 |
| Marrow blasts, % | Median: 76 | Range: 15-99 | Median: 66 | Range: 0-99 |
| WBCs, 1000/μL | Median: 42.9 | Range: 0.8-274 | Median: 12.6 | Range: 0.4-416 |
| Peripheral blasts, % | Median: 44 | Range: 0-94 | Median: 30 | Range: 0-99 |
FAB indicates French-American-British; NOS, not otherwise specified; AD, standard-dose cytarabine and daunomycin; hAD, high-dose cytarabine and daunomycin; G-CSF, granulocyte colony-stimulating factor; Cy-A, cyclosporin A; 3+7+3, AD followed by 3 days of high-dose cytarabine; and WBCs, white blood cells.
Thawing of bone marrow samples
Cryopreserved bone marrow samples received from the SWOG repository were kept frozen in liquid nitrogen until use. Samples were rapidly thawed by addition to prewarmed thaw media (2.5% bovine serum albumin [BSA] and 5% Dextran 40 from Sigma-Aldrich, St Louis, MO, with 50 U/mL DNAse I [Boehringer Ingleheim, Ridgefield, CT] in phosphate-buffered saline, sterile filtered). The cells were incubated in Iscove modified Dulbecco medium (IMDM), 15% heat-inactivated fetal bovine serum, 15% horse serum, 1% Pen-strep (IMDM, fetal bovine serum, and penicillin-streptomycin [Pen-strep] from Invitrogen, Carlsbad, CA); 49.5 μM β-mercaptoethanol and 49.4 μM monothioglycerol (Sigma-Aldrich); and interleukin-3 (IL-3; 100 ng/mL) and stem cell factor (SCF, 10 ng/mL) from R&D Systems (Minneapolis, MN) for 16 hours. Mononuclear cells were then isolated by density depletion and viable cells enumerated by trypan blue exclusion.
Effect of freeze, thaw, and overnight incubation on VLA-4 expression
The leukemia cell lines, HL60, MEG-01, and K562 (ATCC, Manassas, VA), and 2 patient AML samples were used to compare the effect of freeze/thaw and 16-hour incubation with low-dose SCF (10 ng/mL), SCF with IL-3 (100 ng/mL each), and thrombopoietin with Flt3/flk2 ligand (100 ng/mL each; cytokines from R&D Systems). VLA-4 expression was analyzed by flow cytometry.
Flow cytometric analysis
Flow cytometric analysis for VLA-4 expression by the leukemia blast population was performed on each SWOG sample. After the samples were thawed, they were labeled with 1:50 dilutions of the following antibodies (0.2-0.5 million cells/tube): phycoerythrin-conjugated antibodies CD49d (α4 clone 9F10) and CD29 (β1 clone MAR4), allophycocyanin-conjugated CD117 (c-kit, clone 104D2), fluorescein isothiocyanate (FITC)–conjugated CD45 (clone T2933), and CD34 (clone 8G12), and CD106 (VCAM-1 clone 429) with FITC-conjugated goat anti–rat secondary antibody (all antibodies from BD PharMingen, San Diego, CA, with the exception of CD45 from Dako North America, Carpinteria, CA). Cells were also stained with 5 μL 7-amino-actinomycin D (BD PharMingen) to select for viable cells. Cells were fixed with paraformaldehyde and then analyzed on the BD LSR (BD Biosciences, San Jose, CA). Sequential gates were applied; initially, the blast window defined by forward scatter/side scatter, then CD45/side scatter,16,17 then viable cells by exclusion of 7-amino-actinomycin D (Figure S1). The blast cells were then analyzed for CD49d (α4), CD29 (β1), CD34, CD117 (c-kit) percentage expression and mean fluorescence intensity (MFI). Twenty-nine samples were also examined for VCAM-1 (CD106). Analysis was performed using CellQuest software. The product of percentage expression and MFI (called “combined expression”) was also calculated as a measure of expression for each marker.
Functional adhesion assay to fibronectin
For 20 patient samples, CD34+ blast cells were isolated using the VarioMACS (Miltenyi Biotec, Auburn, CA). Lab Tek 8-well chamber slides were coated with 8 μg/mL per cm2 recombinant fibronectin fragment, CH-296 (Retronectin; Takara, Kyoto, Japan). Nonspecific adhesion was assessed in control wells coated with 2% heat-inactivated BSA; 105 patient CD34+ AML cells were incubated in serum-free media for 2 hours. The wells were washed, fixed with 1% paraformaldehyde, and counterstained with 4′,6 diamidino-2-phenylindole in Vectashield (Vector Laboratories, Burlingame, CA) and sealed. Ten fields per well were examined by fluorescence microscopy, and bound cells were enumerated.
Binding of fluorescently labeled soluble VCAM-1
After study of the first 73 patient samples for VLA-4 expression, we introduced a flow cytometry assay to examine functional binding of soluble VCAM-1 (sVCAM-1) for the remaining 102 patients. sVCAM-1 (R&D Systems) was fluorescently labeled with 5-(and 6)-carboxyfluorescein ester (Invitrogen) and the concentration determined by A280 (for 1 mg/mL, A280 = 0.84). A total of 2 × 105 AML cells/patient sample were incubated with 1 μM fluorescently labeled sVCAM-1, washed, and fixed. Nonspecific binding was determined as described.18 Percentage binding and MFI for specific and nonspecific binding were obtained.
Expression of activation-dependent epitope of β1
The expression of functionally activated β1 in 24 selected patient samples was assessed with 9EG7 antibody (BD PharMingen) before and after treatment with activating antibody 8A2.19 Then cells were labeled with 9EG7 antibody recognizing the activated epitope. Cells were then analyzed by flow cytometry.
Real-time RT-PCR for VLA-4
CD34+ blasts from 23 randomly selected patient samples were isolated using the VarioMACS. RNA was isolated using the RNeasy Kit and the RNAse-Free DNase set (QIAGEN, Valencia, CA). Reverse transcription was performed using the Reverse Transcriptase iSCRIPT cDNA Synthesis Kit (Bio-Rad, Hercules, CA) and real-time polymerase chain reaction (PCR) performed using α4 integrin–specific primers and QuantiTect SYBR Green PCR Kit (QIAGEN). Primer sequences were: (forward) 5′-GACGTGATTACAGGAAGCATACAGG-3′ and (reverse) 5′-GCAGTACAATAGCCTCTTATCAGTC-3′. For quantification of RNA molecules per microgram, a standard curve was prepared using log dilutions of plasmid DNA from cloned VLA-4 PCR product. The reactions were run on a LightCycler (Roche Diagnostics, Indianapolis, IN) programmed as follows: 95°C for 15 minutes, then 45 cycles of 95°C for 20 seconds, 59°C for 20 seconds, and 72°C for 80 seconds.
Chemotherapy resistance assays
We examined the ability of fresh and previously frozen primary AML cells to exhibit chemotherapy resistance afforded by plating on Retronectin or immobilized VCAM-1. These patient cell samples were obtained on the same Institutional Review Board–approved protocol but were not from the SWOG repository. Cells were preincubated with no azide, low endotoxin α4-blocking antibody, or isotype control antibody (BD PharMingen), or soluble VCAM-1 (R&D Systems) for 1 hour. Plates were then coated with 8 μg/mL per cm2 recombinant fibronectin fragment Retronectin (CH-296; Takara), 33 μg/mL per cm2 VCAM-1 (R&D Systems), or 2% heat-inactivated BSA. A total of 3 to 4 × 105 cells pretreated with either antibody to α4, isotype control antibody, sVCAM-1, or no antibody were then plated into coated plates. One hour after cell plating, 4 μM cytarabine (AraC) or no drug was added, and the cells were incubated for 24 hours. For cases for which there was no cytotoxicity with Ara-C alone, a combination of 5 μM daunorubicin and 4 μM Ara-C was used. Viability was then determined by trypan blue exclusion or propidium iodide staining (BD PharMingen), and apoptosis was assessed by flow cytometry for FITC-annexin V (BD PharMingen).
Statistical analysis
The following clinical information was available for analysis: WBC count at diagnosis, age, French-American-British classification, flow cytometry phenotype, cytogenetics, response to induction therapy (CR and resistant disease), time to CR, RFS (measured from CR until relapse or death from any cause, censored at last contact for patients last known to be alive without report of relapse), and OS (measured from study entry until death from any cause, censored at last contact for patients last known to be alive). Karyotypes were classified as favorable, intermediate, or unfavorable according to previously published criteria (Table S1).20 Data regarding FLT3–internal tandem duplication (ITD) (present vs absent, and ITD length) were available for 77 patients from a previous study (Table S2).21 We examined VLA-4 expression by flow cytometry for all 175 patients, and for subgroups examined functional adhesion to fibronectin, sVCAM-1 binding, and expression by quantitative real-time RT-PCR. Nonparametric methods were used to compare expression levels and MFIs between groups (Wilcoxon rank sum test) and to estimate correlation coefficients (Spearman R). Distributions of RFS and OS were estimated by the method of Kaplan and Meier.22 Multivariate analyses, based on logistic regression models (CR, resistant disease) or proportional hazards regression models (OS, RFS), were performed to examine the prognostic significance of VLA-4 and sVCAM-1 expression and function in the presence of other prognostic factors. Statistical significance was characterized by 2-sided P values. Analyses were based on data available April 5, 2007.
Results
Effect of thawing and cytokines on VLA-4 expression
No significant differences were observed in the percentage expression of α4, β1, CD34, CD117, and CD106, and there was no difference in sVCAM-1 binding between fresh and frozen samples (data not shown). There were no observed differences in expression of VLA-4 for HL60 or for primary patient leukemia cells for cytokine combinations of low-dose SCF alone (10 ng/mL), SCF with IL-3 (100 ng/mL each), and thrombopoietin with Flt3/flk2 ligand (100 ng/mL each; data not shown).
VLA-4 flow cytometric analysis
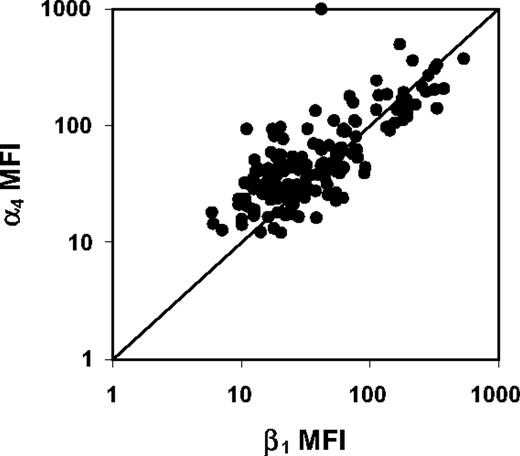
VLA-4 expression varied widely among the 175 patients, with a mean expression of 60.3% (range, 5.4%-99.7%) for α4 and 39.9% (range, 0.2%-99.2%) for β1 (Table 2). The average MFI was 74.9 (range, 12.2-999.1) for α4 and was 64.2 (range, 5.9-539.7) for β1. α4 expression and β1 percentage expression were significantly correlated (R = 0.65, P < .001). However, α4 expression exceeded β1 expression for most patients (139 of 175), probably related to difference in antibody affinity because the only known beta integrin association with α4 is β1. MFIs of α4 and β1 were also highly correlated (R = 0.71, P < .001; Figure 1). On visual inspection, the continuous distributions of VLA-4 expression did not cluster into obvious subgroups, in contrast to recently reported data on VLA-4 expression in chronic lymphocytic leukemia, where there were distinct subgroups of high and low expression that could be correlated with survival in combination with other prognostic factors.23
Measures of VLA-4 expression and function, and immunophenotype in 175 adult patients with previously untreated AML
| Measure of expression or function . | No. . | Median or % . | Range . |
|---|---|---|---|
| α4 | |||
| % expression | 175 | 68.7% | 5.4-99.7% |
| MFI | 175 | 43.4 | 12.2-999.1 |
| β1 | |||
| % expression | 175 | 34.8% | 0.2-99.2% |
| MFI | 175 | 31.6 | 5.9-539.7 |
| Activated expression before 8a2 | 24 | 7.6% | 1.8-30.2% |
| Activated expression after 8a2 | 24 | 10.3% | 3.8-39.2% |
| % of maximum activation | 24 | 69.5% | 6.0-100.0% |
| Soluble VCAM-1 binding | |||
| % binding | 102 | 20.5% | 2.0-96.8% |
| MFI | 102 | 53.2 | 10.4-520.1 |
| Fibronectin adhesion: adherent cell number number | 20 | 110 | 0-1922 |
| VLA-4 message: 104 RNA molecules/μg | 23 | 2.06 | 0.98-3.38 |
| CD34 | |||
| % expression | 175 | 8.0% | 0.2-90.4% |
| MFI | 175 | 33.0 | 7.2-94.7 |
| CD117 | |||
| % expression | 175 | 1.4% | 0.0-63.0% |
| MFI | 175 | 9.0 | 0.0-86.7 |
| Measure of expression or function . | No. . | Median or % . | Range . |
|---|---|---|---|
| α4 | |||
| % expression | 175 | 68.7% | 5.4-99.7% |
| MFI | 175 | 43.4 | 12.2-999.1 |
| β1 | |||
| % expression | 175 | 34.8% | 0.2-99.2% |
| MFI | 175 | 31.6 | 5.9-539.7 |
| Activated expression before 8a2 | 24 | 7.6% | 1.8-30.2% |
| Activated expression after 8a2 | 24 | 10.3% | 3.8-39.2% |
| % of maximum activation | 24 | 69.5% | 6.0-100.0% |
| Soluble VCAM-1 binding | |||
| % binding | 102 | 20.5% | 2.0-96.8% |
| MFI | 102 | 53.2 | 10.4-520.1 |
| Fibronectin adhesion: adherent cell number number | 20 | 110 | 0-1922 |
| VLA-4 message: 104 RNA molecules/μg | 23 | 2.06 | 0.98-3.38 |
| CD34 | |||
| % expression | 175 | 8.0% | 0.2-90.4% |
| MFI | 175 | 33.0 | 7.2-94.7 |
| CD117 | |||
| % expression | 175 | 1.4% | 0.0-63.0% |
| MFI | 175 | 9.0 | 0.0-86.7 |
Scatter plot of α4 and β1 MFI for 175 adult AML patients. Fluorescence intensities of α4 and β1 are highly correlated (Spearman R = 0.71, P < .001).
Scatter plot of α4 and β1 MFI for 175 adult AML patients. Fluorescence intensities of α4 and β1 are highly correlated (Spearman R = 0.71, P < .001).
Function of VLA-4: in vitro adhesion to recombinant fibronectin peptide and sVCAM-1 binding
Because VLA-4 expression does not ensure function, we wished to directly assay for functional VLA-4. We began with a standard in vitro adhesion assay, but this required isolation of the blast population, which could only be performed for the patients who exhibited high expression of CD34, with sufficient cell numbers for both the separation and subsequent assay. This in vitro adhesion assay assessed binding of VLA-4 to its 2 ligands, fibronectin and VCAM-1, coated on tissue-culture wells, for a subsample of 20 patients. Flow cytometric measures of α4, β1, and sVCAM-1 binding did not differ significantly for these 20 patients compared with the remaining 155 patients (Table S5). Adhesion of CD34+ blasts to recombinant fibronectin peptide CH-296 was significantly associated with α4 expression (R = 0.67, P = .001).
We then switched to the flow cytometry method for sVCAM-1 binding, which could be performed for all patient samples, because the function could be assessed on the gated blast population. Using this second functional assay for 102 patient bone marrow samples, the mean percentage binding and MFI of sVCAM-1 were 28.6% (range, 2.0%-96.8%) and 83.1 (range, 10.4-520.1), respectively. The percentage binding of sVCAM-1 increased with increasing α4 MFI (R = 0.35, P < .001), reflecting increased density of VLA-4 surface receptors. It is intuitive that there may be higher binding of the sVCAM-1 ligand for patients whose cells exhibited a higher density of VLA-4 receptors on the cell surface.
Activated β1 analysis
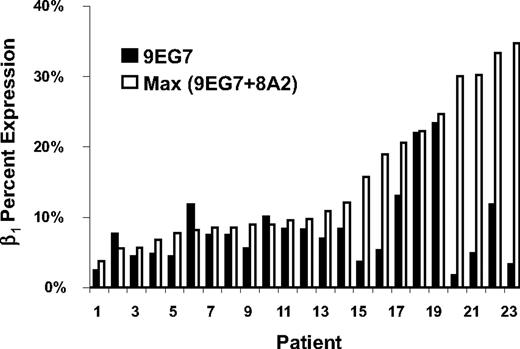
β1 integrin, which combines with α4 to form VLA-4, may be expressed with or without functional activation. The antibody 9EG7 recognizes the activated epitope of the β1 integrin. The anti-β1 antibody 8A2 induces maximal activation by stabilizing the high-affinity conformation,19 and analysis with 9EG7 after 8A2 will reflect the maximal potential for activation of β1. By this analysis, we were able to define whether the AML cells expressed activated β1 and whether there was additional potential for increasing the percentage of the activated form. Twenty-four randomly selected samples were thus examined before and after treatment with activating antibody 8A2. α4 and β1 MFI were higher in these 24 patients, compared with the remaining 151 patients, but the 2 groups did not differ significantly with respect to percentage expression of α4 or β1, or percentage binding of sVCAM-1 (Table S6). When β1 expression before 8A2 treatment was compared with expression after 8A2 treatment, the percentage of the maximum potential level of functional β1 expressed was determined (Figure 2). These 24 patients expressed a median 70.5% of their maximum potential level of functional β1 (range, 6.0%-100%). Thus, on average, the AML blasts from these patients expressed a high proportion of their maximal potential level of expression of functional β1, but there were patients whose cells could be further stimulated, and there was a broad range of expression of the activation-dependent epitope.
Maximum β1 level of activation as determined by 8A2 prestimulation of β1 (9EG7 epitope). The percentage expression of the activation epitope of β1 recognized by the 9EG7 antibody, before and after incubation with β1-activating antibody 8A2, ranged from 6% to 100% in a subset of 24 AML patients.
Maximum β1 level of activation as determined by 8A2 prestimulation of β1 (9EG7 epitope). The percentage expression of the activation epitope of β1 recognized by the 9EG7 antibody, before and after incubation with β1-activating antibody 8A2, ranged from 6% to 100% in a subset of 24 AML patients.
Quantitative real-time PCR for VLA-4
Because there have been several recent studies of RNA expression profiling in AML, we sought to determine whether there was a correlation between level of mRNA for α4 and percentage of surface expression of the protein receptor, The level of mRNA for VLA-4 was measured for 23 patients by quantitative real-time RT-PCR. A moderately significant relationship was found between high levels of α4 percentage expression by flow cytometry and the RNA message level of VLA-4 (Spearman R = 0.44, P = .038). Fourteen patients with α4 expression more than or equal to 40% by flow cytometry had a mean of 2.34 × 104 molecules/μg RNA, compared with 1.68 × 104 molecules/μg in 9 patients with lower α4 expression. A similar investigation by others also demonstrated that the mRNA expression of VLA-4 correlated with VLA-4 surface expression in normal or leukemic myeloid cells.24
Correlation of VLA-4 expression with patient characteristics and treatment outcomes
α4 MFI (R = 0.19, P = .013) and combined expression (R = 0.24, P = .002) increased significantly with increasing age, although α4 percentage expression did not (R = 0.09, P = .22). We also found that α4 combined expression was somewhat lower among women (P = .037). Among the 88 patients with known AML onset (de novo vs secondary), α4 percentage expression was somewhat higher in patients with secondary AML (P = .052).
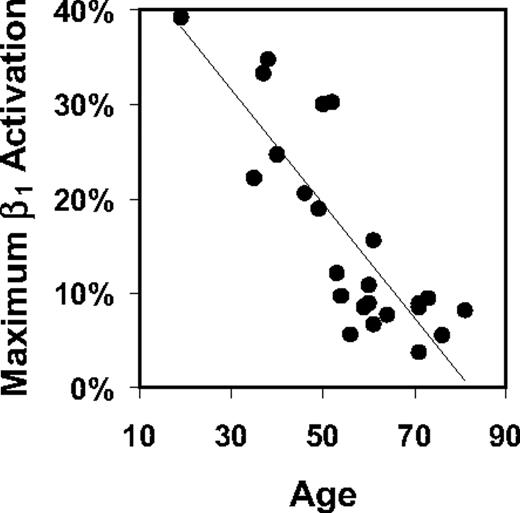
Maximal activated β1 expression, as determined by staining with 9EG7 antibody after pretreatment with 8A2, decreased sharply with increasing age (R = −0.83, P < .001) (Figure 3), whereas unstimulated β1 expression as a fraction of this maximum increased somewhat with age (R = 0.33, P = .12).
Maximum potential β1 activation vs age. The percentage expression of β1 after treatment with 8A2 activating antibody declined with increasing age in a subset of 24 AML patients (Spearman R = −0.83, P < .001).
Maximum potential β1 activation vs age. The percentage expression of β1 after treatment with 8A2 activating antibody declined with increasing age in a subset of 24 AML patients (Spearman R = −0.83, P < .001).
Among the 102 patients with sVCAM-1–binding data, sVCAM-1 MFI reflecting density of binding sites decreased with increasing age (R = −0.30, P = .002), although sVCAM-1 percentage binding did not (R = −0.11, P = .29). Among the 59 patients with sVCAM-1 binding data and known AML onset (primary or secondary), sVCAM-1 percentage binding, MFI, and combined expression were all somewhat lower in those with secondary AML (Wilcoxon P = .024, .13, and .045, respectively).
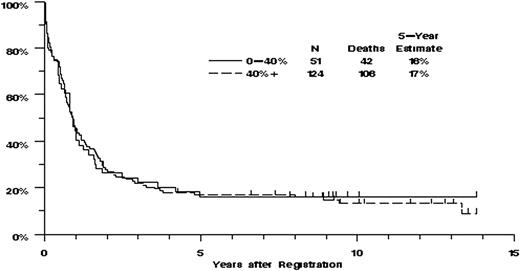
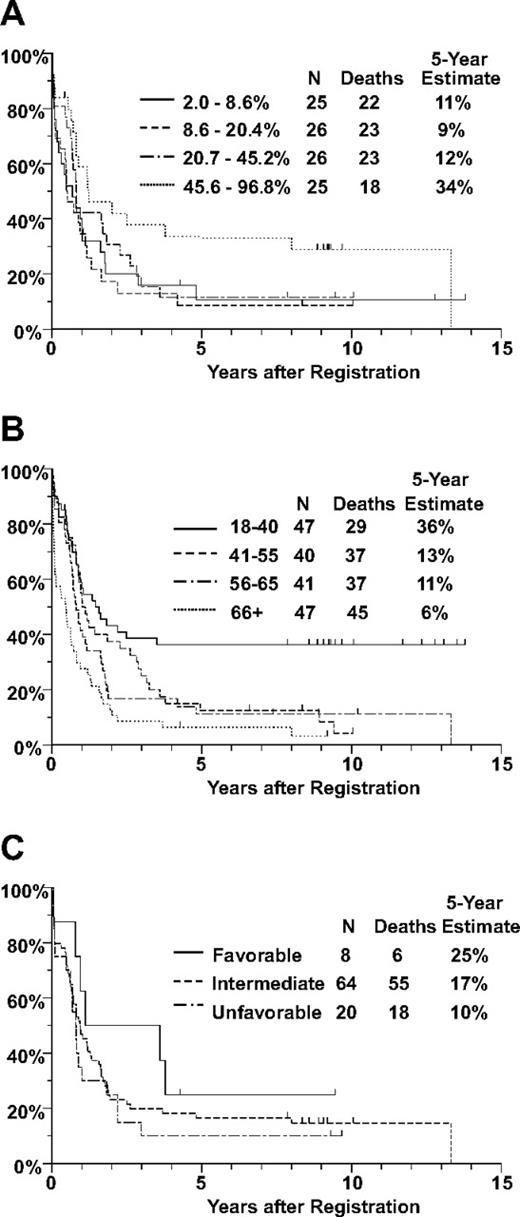
Eighty-four (48%) of the 175 patients achieved CR. There were no significant associations between CR rate and any of the measures of VLA-4 expression or function. The CR rate was somewhat higher for patients in the upper quartile of sVCAM-1 binding, compared with those with lower levels of sVCAM-1 binding (64% vs 44%), although the trend was not statistically significant (P = .18, adjusted for the effect of age on CR rate). Of the 175 patients, 148 have died and the remaining 27 were last known to be alive after 5 months to 13.8 years (median, 9.2 years). There were no clearly significant associations between OS and any of the measures of VLA-4 expression or function (Figure 4). However, OS increased with increasing percentage binding of sVCAM-1 (P = .023 for percentage expression, Figure 5). In multivariate analyses adjusting for the effect of age on OS, the effect of sVCAM-1 binding persisted (P = .033). This effect was largely the result of patients whose sVCAM-1 binding was in the highest quartile for whom the estimated mortality hazard ratio, relative to the lowest quartile of sVCAM-1 binding, was 0.57 (confidence interval, 0.30-1.07). The effect of sVCAM-1 binding on OS also persisted in multivariate analyses that adjusted for the effects of cytogenetic risk classification or FLT3-ITD status (Table S3).
OS by VLA-4 expression. Distributions of OS were estimated by the method of Kaplan and Meier. Tickmarks indicate censored values. The P values for the trends for survival were not significant: for percentage expression of α4, 0.62, and for MFI, 0.96. This figure represents the OS by α4 percentage expression with percentage positive dichotomized at 40% trial value. This value of 40% approximates the value of 34.5% chosen by Matsunaga et al as the breakpoint between α4 “positive” and “negative.”9
OS by VLA-4 expression. Distributions of OS were estimated by the method of Kaplan and Meier. Tickmarks indicate censored values. The P values for the trends for survival were not significant: for percentage expression of α4, 0.62, and for MFI, 0.96. This figure represents the OS by α4 percentage expression with percentage positive dichotomized at 40% trial value. This value of 40% approximates the value of 34.5% chosen by Matsunaga et al as the breakpoint between α4 “positive” and “negative.”9
OS of adult patients with previously untreated AML. Distributions of OS were estimated by the method of Kaplan and Meier. Tickmarks indicate censored values. (A) OS by quartile of sVCAM-1 binding, based on 103 patients with sVCAM-1–binding data. The P value was .023 for the trend (2-sided P value based on logistic regression). (B) OS by age, based on all 175 patients included in this study. (C) OS by cytogenetic risk group, based on 92 patients with evaluable cytogenetics. Note that the 5-year survival data for the highest quartile of sVCAM-1 binding are comparable with the 5-year survival of the youngest patients, with age younger than 40 years.
OS of adult patients with previously untreated AML. Distributions of OS were estimated by the method of Kaplan and Meier. Tickmarks indicate censored values. (A) OS by quartile of sVCAM-1 binding, based on 103 patients with sVCAM-1–binding data. The P value was .023 for the trend (2-sided P value based on logistic regression). (B) OS by age, based on all 175 patients included in this study. (C) OS by cytogenetic risk group, based on 92 patients with evaluable cytogenetics. Note that the 5-year survival data for the highest quartile of sVCAM-1 binding are comparable with the 5-year survival of the youngest patients, with age younger than 40 years.
In vitro studies overcoming adhesion-mediated chemotherapy resistance by anti VLA-4 antibody or sVCAM-1
Primary AML cells from 3 patients pretreated with IgG isotype or left untreated showed increased viability in the presence of chemotherapy when plated on retronectin or immobilized VCAM-1, compared with cells plated on BSA control. Viability improved by an average of 28% on retronectin and 24% on VCAM-1, compared with BSA control (data not shown). In addition, apoptotic activity assayed by annexin staining decreased by an average of 29.5% on retronectin, and 22.5% on VCAM-1, compared with BSA control (data not shown).
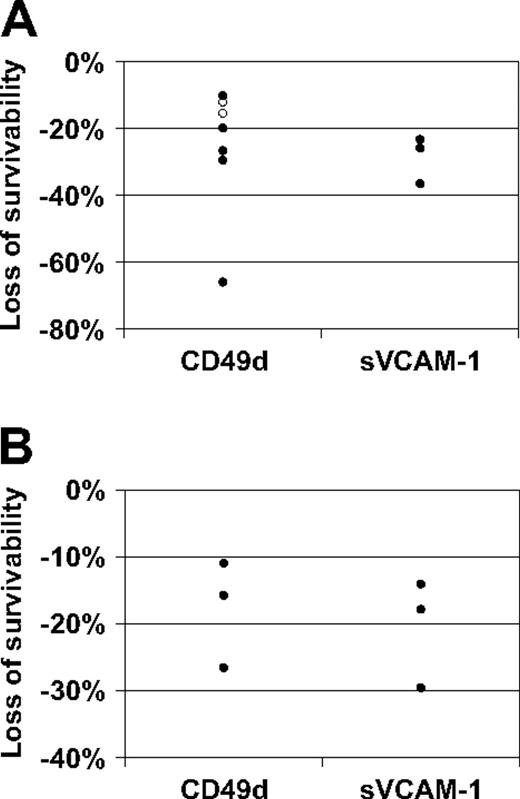
However, the “protection” afforded by the VLA-4 interaction diminished when cells were pretreated with either α4 blocking antibody or sVCAM-1. For cells plated on retronectin (Figure 6A), compared with the isotype control, survivability after exposure to Ara-C or Ara-C plus daunorubicin was an average of 26 plus or minus 7 (SE) percentage points lower for cells preincubated with α4-blocking antibody (one-sided P = .006 based on paired t test). One patient with more than 100% survivability in the cells (cells expanded in culture) preincubated with isotype control was a possible outlier; for the remaining 6 patients, survivability was an average of 19 plus or minus 3 percentage points lower for cells preincubated with CD49d (P = .001). Survivability was an average of 29 plus or minus 4 percentage points lower for cells preincubated with sVCAM-1 (P = .010). In addition, apoptotic activity of these cells that were disrupted by inhibition of adhesion, by annexin V staining, increased by an average of 29.5% on retronectin and 22.5% on sVCAM-1 (data not shown). For cells plated on immobilized VCAM-1 (Figure 6B), compared with the IgG isotype, survivability after exposure to Ara-C on VCAM-1 was an average of 18 plus or minus 5 percentage points lower for cells preincubated with CD49d (P = .031) and an average of 21 plus or minus 5 percentage points lower for cells preincubated with sVCAM-1 (P = .024).
Results of chemoresistance assays. Survivability is defined as the viability of cells exposed to cytotoxic agents, relative to the viability of unexposed cells, expressed as a percentage. Loss of survivability is the absolute decrease in survivability after exposure to α4-blocking agents. (A) Chemotherapy resistance assay results for cells plated on retronectin from patients with data for anti-VLA-4 antibody (N = 7) or sVCAM-1 (N = 3). Compared with the IgG isotype, survivability after exposure to Ara-C (N = 5, ●) or Ara-C plus daunorubicin (N = 2, ○) was an average of 26 plus or minus 7 (SE) percentage points lower for cells preincubated with α4 blocking antibody CD49d (one-sided P = .006 based on paired t test). A 66% decrease in survivability in cells preincubated with CD49d was observed for 1 patient; however this patient, who had more than 100% survivability in the cells preincubated with IgG isotype, was a possible outlier; for the remaining 6 patients, survivability was an average of 19 plus or minus 3 percentage points lower for cells preincubated with CD49d (P < .001). Survivability was an average of 29 plus or minus 4 percentage points lower for cells preincubated with sVCAM-1 (P = .010). (B) Chemotherapy resistance assay results for cells on immobilized VCAM-1 from the 3 patients with sVCAM-1 data in panel A. Compared with the IgG isotype, survivability after exposure to Ara-C on VCAM-1 was an average of 18 plus or minus 5 percentage points lower for cells preincubated with CD49d (P = .031) and an average of 21 plus or minus 5 percentage points lower for cells preincubated with sVCAM-1 (P = .024).
Results of chemoresistance assays. Survivability is defined as the viability of cells exposed to cytotoxic agents, relative to the viability of unexposed cells, expressed as a percentage. Loss of survivability is the absolute decrease in survivability after exposure to α4-blocking agents. (A) Chemotherapy resistance assay results for cells plated on retronectin from patients with data for anti-VLA-4 antibody (N = 7) or sVCAM-1 (N = 3). Compared with the IgG isotype, survivability after exposure to Ara-C (N = 5, ●) or Ara-C plus daunorubicin (N = 2, ○) was an average of 26 plus or minus 7 (SE) percentage points lower for cells preincubated with α4 blocking antibody CD49d (one-sided P = .006 based on paired t test). A 66% decrease in survivability in cells preincubated with CD49d was observed for 1 patient; however this patient, who had more than 100% survivability in the cells preincubated with IgG isotype, was a possible outlier; for the remaining 6 patients, survivability was an average of 19 plus or minus 3 percentage points lower for cells preincubated with CD49d (P < .001). Survivability was an average of 29 plus or minus 4 percentage points lower for cells preincubated with sVCAM-1 (P = .010). (B) Chemotherapy resistance assay results for cells on immobilized VCAM-1 from the 3 patients with sVCAM-1 data in panel A. Compared with the IgG isotype, survivability after exposure to Ara-C on VCAM-1 was an average of 18 plus or minus 5 percentage points lower for cells preincubated with CD49d (P = .031) and an average of 21 plus or minus 5 percentage points lower for cells preincubated with sVCAM-1 (P = .024).
Discussion
Both VLA-4 and CXCR-4 have been proposed as mechanisms for retention of AML blasts in the marrow. Functional inhibition of either of these receptors has been shown to dislodge AML cells and enhance chemotherapy-induced apoptosis in vitro and improve eradication of leukemia in vivo.9,25 To study the role of VLA-4 expression by AML blasts and its relevance in clinical outcome of AML, we analyzed VLA-4 expression and function in bone marrow samples from 175 adult AML patients who received anthracycline- and cytarabine-containing induction chemotherapy regimens.
This study's use of banked cryopreserved cells must be considered when interpreting these results. Patients for whom samples were available were not representative of all patients on the clinical trials from which they were selected and, in particular, tended to have higher WBC and blast counts than excluded patients. This limits the generalizability of these results but not their validity. In addition, the use of cryopreserved samples necessitated thawing of the cells before examination. AML cells undergo apoptosis in culture without media containing serum and cytokines24 ; therefore, AML cell viability was maintained by incubating cells in media supplemented with serum, SCF, and IL-3. With the short overnight incubation time and dose of cytokines used, we did not observe changes in VLA-4 expression, as demonstrated by comparing various cytokine concentrations and fresh versus frozen AML samples. This is consistent with our previous findings showing that a longer incubation time in cytokines was required to affect VLA-4 expression in HL60 cells.26
By means of a successive gating strategy, the flow cytometry analyses were restricted to viable AML blasts (Figure S1). β1 (the main partner of α4) expression was less than α4 expression in most samples, whereas the fluorescence intensities of expression for the 2 subunits were more nearly equal. This may be the result of the differences in the affinity of the antibodies used, α4 clone 9F10 and β1 clone MAR4. Other investigators have had similar results with these antibody clones, in that there was always higher α4 expression than β1 expression for the same cell populations.27 Other α integrin partners with β1 (eg, α1, α2, α3, α5, α6) are minimally expressed by myeloid cells, and the alternative partner of α4, β7, occurs mainly in lymphoid cells with tropism for the gastrointestinal tract.
For all integrins, expression does not imply function, and only activated integrins have binding capacity. To test the functional status of α4, binding to sVCAM-1 was measured, and the level of β1 activation was assayed by staining with activation-dependent anti-β1 antibody 9EG7 before or after treatment with the anti-β1–activating antibody 8A2. The level of endogenous β1 activation varied widely among the 24 samples examined (range, 6.0%-100%). β1 activation was modestly correlated with α4 percentage expression (Spearman R = 0.35, P = .10) and MFI (R = 0.47, P = .020), and consequently with combined α4 expression (Spearman R = 0.58, P = .003), suggesting that activation may be related to the total number of α4 receptors. However, the maximal functional activity of β1 declined significantly with increasing age, which is noteworthy because of the known poor response to chemotherapy in older patients.28 Furthermore, there was increased sVCAM-1 binding with increasing density of α4 surface expression, as reflected by MFI (P < .001). This correlation again underscores the importance of assaying the function of VLA-4, rather than simple expression.
A significant correlation between binding of sVCAM-1, reflecting functional activity of VLA-4, and OS in AML was documented, with the mortality rate reduced by 43% in the highest quartile of sVCAM-1–binding MFI, compared with the lowest quartile. In contrast to a previous report,9 we found no clearly significant associations of VLA-4 percentage expression with response to chemotherapy or with disease-free survival or OS (Figure 4). Notably, Matsunaga et al reported no deaths in 10 patients they classified as negative for α4 expression (≤ 18.6%), who had median follow-up more than 3 years,9 whereas in our study 13 of 23 patients with α4 expression less than or equal to 18.6% died within 12 months. Potential differences between this study and the previously reported study include heterogeneity of patient inclusion criteria, choice of chemotherapy regimens, and duration of treatment. In addition, the activation state of α4 integrin, which could have influenced the outcome in a small group of patients, was not assessed in the previous study.
Our studies clearly demonstrated that sVCAM-1 was as effective as anti–VLA-4 antibody in restoring sensitivity to chemotherapy in vitro for leukemia cells attached to immobilized VCAM-1 or retronectin. This observation allows the formulation of a putative scenario to explain the pronounced effect of sVCAM binding on survival. If, within the bone marrow microenvironment, sVCAM-1 is increased (released by leukemic cells or by chemotherapy induced cell lysis), the VLA-4–expressing leukemic cells bound to sVCAM-1 will be dislodged, enhancing the effect of chemotherapy. This movement would be similar to the migration of lymphocytes induced by sVCAM-1,29 although the leukemia cells would not necessarily need to leave the bone marrow to undergo apoptosis. Others have reported that plasma levels of sVCAM-1 are elevated in AML patients compared with healthy controls (P < .001).30 In that report, increased levels of either sE-selectin or sVCAM-1 were present in 32 of 40 leukemic patients (80%) and the highest levels were measured in M4-AML.29 We did not measure plasma sVCAM-1 levels in our study.
The exact mechanism by which blocking of adhesion leads to increased chemosensitivity is uncertain. Matsunaga et al demonstrated that binding of AML blasts to fibronectin via VLA-4 resulted in signaling through the PI3K/Akt pathway, leading to increased bcl2 and enhanced survival when the leukemia cells were adherent.9 Antibody blocking of VLA-4, PI3K inhibitors, or antisense oligonucleotides to bcl-2 all restored chemotherapy-induced apoptosis. It is also possible that there may be alternative downstream signaling pathways for VLA-4, analogous to the distinct pathways induced by the Flt3 tyrosine kinase domain mutations as opposed to the Flt3-ITDs.31 Moreover, there are countless other potentially contributing influences; for example, Flt3 ligand itself has been shown to enhance adhesion via VLA-4,32 and Flt3-ITDs may enhance the expression of CXCR-4, involved in marrow homing/retention through binding of SDF-1, and thought to convey poor prognosis in AML.33-35
These data suggest that sVCAM-1 binding by AML blasts may have value as a prognostic parameter for OS at diagnosis. It is evident that it is not simply adhesion receptor expression, but the functional interactions with stromal cells and other cells in the bone marrow microenvironment that may ultimately determine the susceptibility to chemotherapy, protection from apoptosis, or migratory behavior of leukemic cells. Disruption of the adhesion pathways on which leukemia cell survival is dependent might be of therapeutic benefit, and clinical investigations in this direction are underway. Prospective study of functional VLA-4 expression and response to treatment is warranted.
Presented as an abstract at the 47th Annual Meeting of the American Society of Hematology, Atlanta, GA, December 10, 2005.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thankfully acknowledge the Southwest Oncology Group Leukemia Repository for providing the patient bone marrow samples. We are grateful to Dr Erica Jonlin for assistance with the regulatory documents. We acknowledge Dr Xin Zhao for technical assistance and data analysis.
This work was supported by Southwest Oncology Group Development Funds (National Cancer Institute), a grant from the Leukemia and Lymphoma Society Translational Research Program (P.S.B.), and the Douglas Kroll Research Foundation.
National Institutes of Health
Authorship
Contribution: P.S.B. designed and performed research, analyzed data, and wrote the paper; K.J.K. designed research, analyzed data, and wrote the paper; A.N.W. performed research, analyzed data, and wrote the paper; S.C. performed research, contributed to study design, and analyzed data; J.M.H. contributed vital reagent and contributed to study design; C.L.W. contributed repository samples and methodology and contributed to study design; S.H.P. contributed to study design and patients for study; D.L.S. contributed data; and T.P. and F.R.A. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Pamela S. Becker, University of Washington, Box 358056, 815 Mercer Street, Seattle, WA 98109; e-mail: pbecker@u.washington.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal