Abstract
The protease thrombin is required for normal hemostasis and pathologic thrombogenesis. Since the mechanism of coagulation factor XI (FXI)–dependent thrombus growth remains unclear, we investigated the contribution of FXI to thrombus formation in a primate thrombosis model. Pretreatment of baboons with a novel anti–human FXI monoclonal antibody (aXIMab; 2 mg/kg) inhibited plasma FXI by at least 99% for 10 days, and suppressed thrombin-antithrombin (TAT) complex and β-thromboglobulin (βTG) formation measured immediately downstream from thrombi forming within collagen-coated vascular grafts. FXI inhibition with aXIMab limited platelet and fibrin deposition in 4-mm diameter grafts without an apparent increase in D-dimer release from thrombi, and prevented the occlusion of 2-mm diameter grafts without affecting template bleeding times. In comparison, pretreatment with aspirin (32 mg/kg) prolonged bleeding times but failed to prevent graft occlusion, supporting the concept that FXI blockade may offer therapeutic advantages over other antithrombotic agents in terms of bleeding complications. In whole blood, aXIMab prevented fibrin formation in a collagen-coated flow chamber, independent of factor XII and factor VII. These data suggest that endogenous FXI contributes to arterial thrombus propagation through a striking amplification of thrombin generation at the thrombus luminal surface.
Introduction
Blood coagulation during hemostasis is initiated by the tissue factor (TF)/factor VIIa complex (the extrinsic pathway) that activates factors IX and X, and ultimately produces thrombin at sites of vascular injury.1 In thrombosis, intravascular blood coagulation may also be initiated by the extrinsic pathway.2,3 However, impairment of the TF/factor VIIa pathway does not provide full protection from thrombosis, since symptomatic factor VII deficient subjects can develop concurrent thrombosis and severe bleeding.4 The functions of the contact proteins (factor XI, factor XII, prekallikrein, and high-molecular-weight kininogen) in hemostasis are less clear. The physiologic role of factor XI (FXI) has been difficult to determine because of the variable bleeding disorder associated with FXI deficiency,5 and because monospecific FXI inhibitors have not been widely available for experimental investigation. FXI activation is thought to proceed through thrombin- and/or factor XII–dependent mechanisms, and activated FXI (FXIa) contributes to sustained thrombin generation after initiation of blood clotting by activating factor IX. These activities ultimately promote coagulation, platelet activation, and preservation of fibrin clot integrity.6,7 Thrombin also increases the density of fibrin networks8 and indirectly inhibits fibrinolysis through activation of carboxypeptidase B (thrombin-activatable fibrinolysis inhibitor, TAFI).9 Thus, FXI may support thrombus propagation and clot stability by increasing thrombin generation.10,11
Compelling circumstantial evidence suggests a contributory role for FXI in the pathogenesis of thrombosis. An elevated plasma FXI level appears to be an independent risk factor for deep vein thrombosis (DVT),12 ischemic stroke,13 and myocardial infarction14 in humans. While one study did not detect a reduced incidence of myocardial infraction in patients with severe factor XI deficiency,15 the incidence of ischemic stroke appears to be significantly lower in FXI deficiency than in the general population.16 FXI deficiency reduces occlusive thrombus formation in mouse models,17,18 and pharmacologic inhibition of FXI is antithrombotic in rabbits19 and primates.20 Despite these findings, FXI appears to play a supportive role in normal hemostasis, and only a fraction of the individuals with severe factor XI deficiency exhibit a mild to moderate bleeding tendency upon injury.5,21 In contrast, hemophilia (factor VIII or IX deficiency) or factor deficiencies in the common pathway of coagulation (factors II, V, or X) are associated with severe bleeding or are incompatible with life.22,23 Taken together, these observations suggest that thrombosis and hemostasis, while linked in many respects, possess mechanistic differences that may allow development of more thrombosis-specific anticoagulant strategies such as targeting of FXI.
To investigate the mechanism by which FXI contributes to acute thrombus formation, baboon and flow chamber models were used. To block FXI activity, a potent monospecific neutralizing antibody was generated. A sensitive model was developed for locally measuring soluble markers of activated coagulation, platelets, and fibrinolysis at sites of experimental thrombus formation in baboons. Platelet and fibrin accumulation during arterial thrombogenesis and the occlusion of thrombogenic blood conduits were determined in the presence and absence of the antibody.
Methods
Experimental animals
Thirty-nine nonterminal studies were performed using 17 male baboons (Papio anubis, 9-11 kg). All studies were approved by the Institutional Animal Care and Use Committee of Oregon Health & Science University. Thrombosis experiments were conducted on non-anticoagulated, awake animals that had chronic exteriorized femoral arteriovenous shunts, as described elsewhere.24 Baseline shunt blood flow exceeded 250 mL/min in all study animals. Anxiety was managed with low-dose ketamine (< 2 mg/kg per hour). Platelet counts, red cell counts, and hematocrits were measured daily, before and after the experiments. Calculated blood loss did not exceed 4% of total blood volume on any experimental day.
Thrombosis model
Thrombus formation was initiated within chronic arteriovenous shunts in baboons by interposing a prosthetic vascular graft segment for up to 60 minutes, essentially as described.24 The hypothrombogenic graft (expanded-polytetrafluoroethylene; W. L. Gore & Associates, Flagstaff, AZ)20 was coated with collagen, which consistently triggers platelet-dependent thrombus formation. Graft segments 20 mm in length (with internal diameters [id] of either 2 or 4 mm) were filled with equine type I collagen (1 mg/mL; Nycomed Arzeneimittel, Munich, Germany) for 15 minutes, and then dried overnight under sterile airflow. The thrombogenic collagen-coated grafts were then incorporated between segments of silicon rubber tubing, deployed into the shunts (Figure 1), and exposed to blood flow. The flow rate through the graft was restricted to 100 mL/min (measured by the Transonics Systems flow meter [Ithaca, NY]) by clamping the proximal shunt segment, thereby producing initial mean wall-shear rates of 265 s−1 (4-mm id) or 2120 s−1 (2-mm id). The 4-mm id grafts remained patent without flow rate reduction for 60 minutes. In the 2-mm id grafts, flow rates progressively declined due to occlusive thrombus formation. The grafts were removed from the shunts either at 60 minutes (4-mm id grafts) or when the flow rate fell from 100 mL/min to 20 mL/min (2-mm id grafts), signaling imminent occlusion. The time from initiation of blood flow through the graft until the flow reached 20 mL/min was taken as the occlusion time.
Thrombogenic vascular graft and blood collection device. Thrombogenesis started and thrombi developed in a collagen-coated (4-mm internal diameter [id]) expanded-polytetrafluoroethylene vascular graft that was deployed for 60 minutes into a chronic high flow arteriovenous shunt in healthy baboons. Blood samples were drawn from the coagulation marker concentration boundary layer by a syringe pump 1 cm downstream from acutely developing thrombi. PPACK anticoagulant was infused 3 mm proximal to the sample port to prevent the sample port from occluding during the 1-hour study. Blood flow through the graft was maintained at a fixed rate of 100 mL/min for the entirety of each study by proximal clamping.
Thrombogenic vascular graft and blood collection device. Thrombogenesis started and thrombi developed in a collagen-coated (4-mm internal diameter [id]) expanded-polytetrafluoroethylene vascular graft that was deployed for 60 minutes into a chronic high flow arteriovenous shunt in healthy baboons. Blood samples were drawn from the coagulation marker concentration boundary layer by a syringe pump 1 cm downstream from acutely developing thrombi. PPACK anticoagulant was infused 3 mm proximal to the sample port to prevent the sample port from occluding during the 1-hour study. Blood flow through the graft was maintained at a fixed rate of 100 mL/min for the entirety of each study by proximal clamping.
Thrombus formation was assessed in real time during the experiments by quantitative gamma camera imaging of radiolabeled platelet accumulation within the graft segment, and by end-point determinations of radiolabeled fibrinogen/fibrin deposition, as described elsewhere.24 Measurements of platelet-associated radioactivity on the grafts were recorded at 5-minute (4-mm id) or 3-minute (2-mm id) intervals using a General Electric (Milwaukee, WI) 400T gamma scintillation camera interfaced with a NuQuest InteCam computer system (MEDX, Arlington Heights, IL). Embolic events were recorded as abrupt decreases in the number of platelets in the graft between subsequent imaging frames.
Blood sample collection
Systemic samples were collected proximal to the graft from the midstream of the shunt into 3.8% citrate (1:9, vol/vol) before graft deployment, and then at 30 and 60 minutes. One sample was processed for platelet poor plasma and used to assess FXI procoagulant activity levels, while a second sample was used for determination of systemic coagulation markers. For the assessment of thrombosis markers generated within the 4-mm id grafts, samples were also taken from the bloodstream adjacent to the vessel wall (intraluminal boundary layer) immediately distal to the thrombus. Blood was collected at a rate of 100 μL/min during 10-minute intervals through a 0.64-mm id port located 10 mm distal to the graft (Figure 1). To maintain patency of the sampling port, Phe-Pro-Arg-chloromethylketone (PPACK, 0.5 mg/mL, 1 mM), which inhibits thrombin and other coagulation proteases,25 was infused at a rate of 20 μL/min into a second 0.64-mm id port located 3 mm proximal to and in line with the collection port. Anticoagulant infusion and local blood sampling were regulated using syringe pumps (Harvard Apparatus, Holliston, MA).
Blood sample analysis
Blood cell counts were determined using a micro-60 automated cell counter (Horiba ABX Diagnostics, Montpellier, France). Blood samples were divided into aliquots and processed according to specific test requirements. Plasma was prepared from 1 aliquot and activated partial thromboplastin times (aPTTs) and prothrombin times (PTs) were measured on-site. Another aliquot was placed on ice for 10 minutes and centrifuged at 4°C for 10 minutes at 12 900g. For β-thromboglobulin (βTG) determinations, the samples were supplemented with 4 μg/mL prostaglandin E1, 4.3 mg/mL aspirin (ASA), and 50 μg/mL PPACK. The plasma was then frozen and stored at −80°C until assaying. Cross-reacting enzyme-linked immunosorbent assays (ELISAs) were used to determine D-dimer levels (IMUCLONE D-Dimer; American Diagnostica, Stamford, CT), the platelet activation marker βTG (Asserachrom; Diagnostica Stago, Asnières sur Seine, France), and thrombin-antithrombin complexes (TAT; Enzygnost-TAT; Siemens Healthcare Diagnostics, Berlin, Germany).
Derivation of neutralizing anti–factor XI antibody
Because previous studies showed that polyclonal antibodies to human FXI required very high doses to achieve near-complete inhibition of FXI in baboons,20 we generated a new reagent, a potent neutralizing anti–human FXI monoclonal antibody (aXIMab) that cross-reacts with baboon FXI. Hybridomas were derived from BalbC mice immunized with purified human FXI using standard procedures.26 Hybridomas were screened using solid phase ELISA against human FXI, and those that showed binding were subcloned twice by limiting dilution. The clone that produced the most potent neutralizing antibody, which inhibited the activation of FXI and/or the activity of FXIa, was selected based on prolongation of the clotting time of recalcified normal human plasma (NHP) and normal baboon plasma (NBP) by the cell culture supernatant. The cell line producing aXIMab (1A6.1.1) was grown in a CL1000 bioreactor according to the manufacturer's protocol (Integra Biosciences, Chur, Switzerland), and the antibody was purified from the media using cation exchange and protein A chromatography.
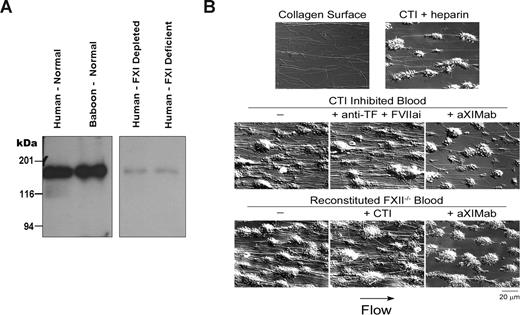
Human and baboon FXI in plasma was recognized by aXIMab as a single band at 160 kDa on Western blots (Figure 2A). The antibody specifically recognized the third apple (A3) domain of the FXI heavy chain, as assessed by immunoblotting of recombinant FXI/prekallikrein chimeras, as described elsewhere (data not shown).27 The IC50 and IC99 of aXIMab in vitro were 2.5 nM and 10 nM, respectively, in an aPTT-based clotting assay using FXI deficient human plasma (George King Bio-Medical, Overland Park, KS) with serial dilutions of NBP as standards.28 Purified aXIMab, tested within a concentration rage from 0 to 40 nM, prolonged the aPTT (HemosIL SynthASil; Instrumentation Laboratory, Lexington, MA) similarly in both NHP and NBP in a concentration-dependent manner without affecting the PT (Innovin; Siemens Healthcare Diagnostics).
Pharmacologic inhibition of FXI and platelet activity in vivo
In a pilot experiment, aXIMab (2 mg/kg) was administered to a single baboon, and blood samples were collected into citrate anticoagulant over 4 weeks to measure circulating FXI antigen (FXI:Ag) concentrations, FXI inhibitor, and FXI procoagulant activity. This dose of aXIMab was chosen with the intent to achieve sustained and near-complete inhibition of FXI, assuming an initial dilution of the antibody into 60 mL blood volume per kilogram of body weight after injection. The maximum achievable prolongation of the aPTT was approximately 2.5-fold. FXI:Ag was measured by ELISA using goat anti–human FXI polyclonal capture and detection (horseradish peroxidase conjugated [HRP]) antibodies (Affinity Biologicals, Ancaster, ON), which also recognized baboon FXI and its complex with aXIMab. A standard curve was constructed with serial dilutions of NBP, and FXI concentrations were determined as a percentage of NBP. Western blots for FXI were performed by size fractionation of 1 μL samples of plasma under nonreducing conditions on 7.5% polyacrylamide-SDS gels, followed by transfer to polyvinylidene difluoride membranes. Detection was with a goat anti–human FXI polyclonal antibody conjugated to HRP and chemiluminescence. In the same samples, the Bethesda assay29 was used to determine excess (noncomplexed) circulating FXI inhibitor (aXIMab) activity levels, and the FXI procoagulant activity was measured using a clotting assay.28 P-selectin (monkey sP-selectin ELISA; Bender MedSystem, Burlingame, CA) and fibrinogen (Clauss assay) levels were measured as global plasma markers for inflammation.
aXIMab was administered as a bolus (2 mg/kg intravenously) at least 24 hours before the thrombosis experiments. The anticoagulant effect was monitored daily, and thrombosis experiments were performed while the systemic FXI procoagulant activity was reduced by at least 99%. We previously showed that inhibition of FXI by polyclonal antibodies is safer and as effective as high-dose heparin in baboons.20 ASA has less pronounced effects on hemostasis than heparin, and is often used in the treatment of arterial-type platelet dependent thrombosis. The effect of ASA treatment was compared with aXIMab on the occlusion-prone 2-mm id grafts. ASA (32 mg/kg) was administered orally 2 to 4 hours before each thrombosis experiment, as described previously.30 Four weeks were allowed for washout of each aXIMab and ASA before performing new experiments in the same animal.
Hemostasis assessment
The effects of FXI inhibition and ASA on hemostasis in baboons were assessed using the standard template, skin bleeding time test (Surgicutt; International Technidyne, Piscataway, NJ). Experimentally, this test has been shown to be sensitive to the effects of therapeutic anticoagulants and antiplatelet agents in nonhuman primates.31,32 All bleeding time measurements were performed by the same expert technician.
Modeling of thrombosis marker mass transport and local sampling
A 3D computational fluid-dynamics model, similar to that presented by Xu and Wootton,33 was used to estimate the concentrations of thrombus-derived macromolecules in blood that flows over forming thrombi and transports the markers along the adjacent vessel wall distally. The model was based on the geometry shown in Figure 1, with typical values for blood density and viscosity.34 The model of local blood sampling was implemented using the finite element software ADINA (Watertown, MA). In addition, a 2D axisymmetric computational model, similar to that of Markou et al,35 was used to estimate thrombosis marker distribution within the flow field. Computational modeling predicted that molecules of interest (βTG, fibrin D-dimer, and TAT) released or generated at sites of thrombus formation would be concentrated (> 99%) within a very thin peripheral blood boundary layer (an approximately 0.1-mm concentration boundary layer) along the immediately distal vessel wall (data not shown). Thus, as used here, the local sampling method effectively sampled the entire near-wall concentration boundary layer region, immediately distal to the forming thrombus, for platelet and coagulation markers of interest.
Flow chamber coagulation studies
Glass capillary tubes coated with 100 μg/mL Horm collagen (Nycomed Arzeneimittel) were used as thrombus chambers. Whole human blood was collected into corn trypsin inhibitor (CTI, 40 μg/mL), discarding the first 1 mL to limit activation of coagulation. The blood was perfused through the thrombus chambers at a shear rate of 265 s−1 for 10 minutes. Before each experiment, blood was incubated with aXIMab (20 μg/mL), heparin (15 U/mL), a combination of an inhibitory anti-TF antibody (20 μg/mL; Genentech, South San Francisco, CA) and active-site–inhibited FVII (FVIIai, 1 μg/mL; NovoNordisk), and/or PBS vehicle (all concentrations final). In separate experiments, washed platelets and red blood cells (RBCs) were processed as described,36 then mixed with FXII-deficient human plasma (<1% FXII; Haematologic Technologies, Essex Junction, VT) to reach a hematocrit of 40% and platelet count of 300 × 103/μL. The reconstituted blood was incubated with aXIMab (20 μg/mL) or CTI (40 μg/mL), recalcified with 7.5 mM CaCl2 and 3.75 mM MgCl2, and then perfused immediately through collagen coated capillary tubes. Recalcified FXII-deficient human plasma without RBCs or platelets was also perfused over collagen. Images were obtained by differential interference contrast microscopy after 3 minutes of perfusion with modified Tyrode buffer to wash out unbound blood components.
Data analysis
Mean values plus or minus 1 standard error of the mean (SEM) are given. Occlusion data were compared using the log-rank test. The 2-tailed Student t test was used for all other single pair comparisons. A P value of .05 or less was considered significant.
Results
Inhibition of FXI by aXIMab ex vivo prevents fibrin formation independent of FXIIa
When CTI-anticoagulated, fresh human whole blood was perfused over collagen under arterial shear (265 s−1), platelets were deposited in large aggregates which became enveloped by forming fibrin strands (Figure 2B). In stark contrast, when CTI-anticoagulated blood was further anticoagulated with either heparin or aXIMab, fibrin deposition was greatly reduced. In these short experiments, there was no obvious reduction in platelet adhesion to collagen by the anticoagulants. The outcome was similar when using reconstituted FXII-deficient blood, with or without CTI. Fibrin was generated independent of FXII, and FXI inhibition with aXIMab interrupted fibrin thrombus formation. Inhibition of the extrinsic pathway with a combination of FVIIai and the anti-TF antibody, which nearly doubled the PT, had no effect on fibrin formation or platelet deposition. No fibrin was formed when recalcified FXII-deficient human plasma alone was perfused over collagen (data not shown), illustrating the importance of platelets and/or other blood cells in fibrin thrombus formation in this model. These data suggest that, under physiologically relevant shear flow, FXI and corpuscular blood components promote fibrin formation on collagen, independent of FXIIa or TF/FVIIa.
The anti-FXI monoclonal antibody binds human and baboon FXI, and inhibits fibrin formation in FXII-inhibited or deficient human blood under flow. (A) Binding of the anti-FXI monoclonal antibody (aXIMab) to the FXI dimer (160 kDa) in platelet free NHP and NBP was demonstrated by Western blotting, developed using a secondary anti–mouse IgG antibody. aXIMab binding was minimal in FXI-depleted plasma or plasma from a FXI-deficient patient. (B) aXIMab prevented visible fibrin formation in FXII-inhibited or deficient blood under flow. Human whole blood, anticoagulated with CTI (40 μg/mL) to inhibit FXIIa, or reconstituted FXII deficient human blood was perfused through collagen-coated capillary tubes (thrombus chambers) at a shear rate of 265 s−1 for 10 minutes. Before each experiment, blood was incubated with unfractionated heparin (15 U/mL), anti-TF antibody (20 μg/mL) plus FVIIai (1 μg/mL), aXIMab (20 μg/mL), CTI (40 μg/mL) for reconstituted blood where indicated, or PBS vehicle (−), as marked above each panel. Images were obtained via Kohler-illuminated Nomarski differential interference contrast microscopy with a Zeiss Axiovert 200M microscope using a Zeiss 63× oil-immersion 1.40 NA plan-apochromat lens (Zeiss, Göttingen, Germany). Images were captured using a Zeiss AxioCam with Slidebook 4.0 (Intelligent Imaging Innovations, Denver, CO) after 3 minutes of perfusion with modified Tyrodes buffer to wash the thrombus of unbound cells. All experiments were performed at 37°C. Each image is representative of 2 or 3 experiments.
The anti-FXI monoclonal antibody binds human and baboon FXI, and inhibits fibrin formation in FXII-inhibited or deficient human blood under flow. (A) Binding of the anti-FXI monoclonal antibody (aXIMab) to the FXI dimer (160 kDa) in platelet free NHP and NBP was demonstrated by Western blotting, developed using a secondary anti–mouse IgG antibody. aXIMab binding was minimal in FXI-depleted plasma or plasma from a FXI-deficient patient. (B) aXIMab prevented visible fibrin formation in FXII-inhibited or deficient blood under flow. Human whole blood, anticoagulated with CTI (40 μg/mL) to inhibit FXIIa, or reconstituted FXII deficient human blood was perfused through collagen-coated capillary tubes (thrombus chambers) at a shear rate of 265 s−1 for 10 minutes. Before each experiment, blood was incubated with unfractionated heparin (15 U/mL), anti-TF antibody (20 μg/mL) plus FVIIai (1 μg/mL), aXIMab (20 μg/mL), CTI (40 μg/mL) for reconstituted blood where indicated, or PBS vehicle (−), as marked above each panel. Images were obtained via Kohler-illuminated Nomarski differential interference contrast microscopy with a Zeiss Axiovert 200M microscope using a Zeiss 63× oil-immersion 1.40 NA plan-apochromat lens (Zeiss, Göttingen, Germany). Images were captured using a Zeiss AxioCam with Slidebook 4.0 (Intelligent Imaging Innovations, Denver, CO) after 3 minutes of perfusion with modified Tyrodes buffer to wash the thrombus of unbound cells. All experiments were performed at 37°C. Each image is representative of 2 or 3 experiments.
Inhibition of FXI with aXIMab, in vivo
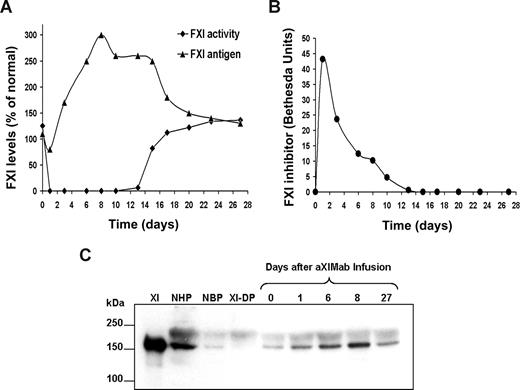
A baboon given aXIMab (2 mg/kg) was followed for 4 weeks after injection to assess FXI:Ag, anticoagulant activity, and antibody inhibitor levels. FXI:Ag levels (complexed and native) decreased from 110% to 40% of NBP levels by 60 minutes, but steadily increased thereafter, reaching 300% of control by day 8 after treatment (Figure 3A). FXI:Ag remained above 200% of control through day 15, after which there was a decrease to 130% by day 27. FXI activity decreased from 125% to less than 1% 1 hour after aXIMab administration, and remained inhibited by more than 99% for 10 days after administration. FXI activity gradually increased to 136% by day 27. Maximum circulating FXI inhibitor levels were observed 1 hour after aXIMab infusion (72 Bethesda units [BU]), which decreased to 0.6 BU by day 13, and were undetectable thereafter (Figure 3B). Consistent with the FXI:Ag ELISA data, the 160-kDa band representing the FXI homodimer on SDS–polyacrylamide gel electrophoresis (PAGE) and Western blots of plasma increased in intensity between days 0 and 8 (Figure 3C). These results indicate that the anticoagulant effect of aXIMab was due to its sustained presence in the circulation and its ability to block FXI activation and/or activity, and not to clearance of the zymogen from the circulation. Since aXIMab separates from FXI during SDS-PAGE, it remains unclear whether inhibition of FXI by aXIMab caused a rebound increase in FXI secretion or the FXI-aXIMab immune complexes were cleared slowly from the circulation. In the same samples, P-selectin and fibrinogen levels were determined as indirect measures of inflammation, with no remarkable changes from baseline (data not shown).
Sustained inhibition of circulating FXI procoagulant activity after administration of aXIMab. An intravenous injection of aXIMab (2 mg/kg) was given over 5 minutes to a single baboon. Plasma samples were collected into citrate anticoagulant and tested over 4 weeks for (A) FXI procoagulant activity, FXI antigen (FXI:Ag), and (B) inhibitor levels, with each time point being the mean of duplicate measurements. The FXI:Ag ELISA could be used to detect both free and complexed FXI. Because the Bethesda assay detected only free FXI inhibitor (aXIMab), FXI:Ag and FXI activity at low inhibitor levels did not correlate until all complexes were cleared from circulation. The 1-hour time points for all levels were omitted for clarity. (C) A Western blot of 1-μL NHP and NBP samples size-fractionated by nonreducing 7.5% SDS-PAGE. Detection was with a polyclonal antibody against human FXI. The 5 lanes on the right represent samples before (0) or 1, 6, 8, and 27 days after infusion of aXIMab. XI indicates 100 ng purified human FXI; NHP, normal human plasma; NBP, normal baboon plasma; and XI-DP, FXI-deficient human plasma.
Sustained inhibition of circulating FXI procoagulant activity after administration of aXIMab. An intravenous injection of aXIMab (2 mg/kg) was given over 5 minutes to a single baboon. Plasma samples were collected into citrate anticoagulant and tested over 4 weeks for (A) FXI procoagulant activity, FXI antigen (FXI:Ag), and (B) inhibitor levels, with each time point being the mean of duplicate measurements. The FXI:Ag ELISA could be used to detect both free and complexed FXI. Because the Bethesda assay detected only free FXI inhibitor (aXIMab), FXI:Ag and FXI activity at low inhibitor levels did not correlate until all complexes were cleared from circulation. The 1-hour time points for all levels were omitted for clarity. (C) A Western blot of 1-μL NHP and NBP samples size-fractionated by nonreducing 7.5% SDS-PAGE. Detection was with a polyclonal antibody against human FXI. The 5 lanes on the right represent samples before (0) or 1, 6, 8, and 27 days after infusion of aXIMab. XI indicates 100 ng purified human FXI; NHP, normal human plasma; NBP, normal baboon plasma; and XI-DP, FXI-deficient human plasma.
In thrombosis studies using aXIMab, FXI procoagulant activity was inhibited by 99.0% (98.8%-99.5%) 1 hour after injection and remained inhibited by 99% in all animals for at least 7 days. No observable adverse events were associated with the treatment. aPTT values were prolonged to 65.6 plus or minus 2.0 seconds after aXIMab administration compared with 30.5 plus or minus 0.7 seconds in control animals, while the PT values remained comparable with baseline (9.1 ± 0.1 seconds after aXIMab administration vs 9.0 ± 0.1 seconds in controls, n = 11 for each). Platelet aggregation in platelet rich plasma in response to adenosine diphosphate and collagen was not affected by aXIMab treatment (data not shown).
Protection of aXIMab-treated baboons from collagen-initiated thrombus development
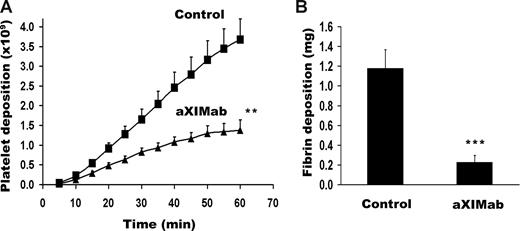
Administration of aXIMab reduced platelet and fibrin deposition in the 4-mm id collagen-coated vascular grafts. Significant differences in platelet accumulation between aXIMab- and vehicle-treated groups were seen as early as 10 minutes after graft exposure to blood flow (0.13 ± 0.03 × 109 vs 0.23 ± 0.03 × 109 platelets, respectively; P < .05). The differences remained statistically significant throughout the time course of thrombus propagation (Figure 4A). Graft platelet accumulation at 60 minutes was 63% lower in aXIMab-treated animals than in vehicle controls (1.38 ± 0.26 × 109 vs 3.68 ± 0.52 × 109 platelets, n = 6 and 8, respectively, P < .01; Figure 4A). Systemic platelet counts were similar in aXIMab (341 ± 27 × 103/μL) and control groups (337 ± 31 × 103/μL), and did not change significantly after thrombosis experiments. End-point fibrin deposition was 81% lower in aXIMab-treated animals than in controls (0.23 ± 0.07 mg and 1.18 ± 0.19 mg, respectively, P = .001; Figure 4B). Since aXIMab is monospecific for the A3 domain of FXI, these data verify that FXI plays an important role in thrombus propagation under arterial-type flow conditions in primates.
FXI inhibition reduces platelet and fibrin deposition on collagen-coated vascular grafts. Effects of FXI inhibition on (A) platelet and (B) fibrin deposition on collagen-coated (4-mm id) vascular grafts. The grafts were placed in vehicle-treated (n = 8) or aXIMab-treated (n = 6) animals (≥ 99% FXI inhibition). Blood was allowed to flow through the devices at 100 mL/min, producing an initial average wall shear rate of 265 s−1. Significance levels (**P < .01, ***P = .001) were calculated by the 2-tailed Student t test. Values are means plus or minus SEM.
FXI inhibition reduces platelet and fibrin deposition on collagen-coated vascular grafts. Effects of FXI inhibition on (A) platelet and (B) fibrin deposition on collagen-coated (4-mm id) vascular grafts. The grafts were placed in vehicle-treated (n = 8) or aXIMab-treated (n = 6) animals (≥ 99% FXI inhibition). Blood was allowed to flow through the devices at 100 mL/min, producing an initial average wall shear rate of 265 s−1. Significance levels (**P < .01, ***P = .001) were calculated by the 2-tailed Student t test. Values are means plus or minus SEM.
Reduced thrombin generation and platelet activation in aXIMab-treated baboons
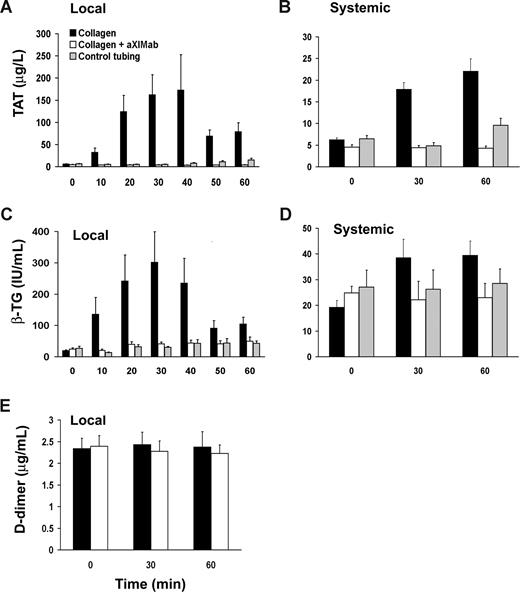
Because inhibition of FXI could reduce thrombus formation in vivo both by limiting thrombin-mediated platelet activation and fibrin formation and/or by increasing thrombolysis, levels of βTG, TAT, and D-dimer were measured. Systemic pretreatment βTG, TAT, and D-dimer levels were comparable in aXIMab- and vehicle-treated baboons (24.8 ± 3.8 vs 19.3 ± 2.6 IU/mL, 4.6 ± 0.6 vs 6.2 ± 0.4 μg/L, and 2.4 ± 0.2 vs 2.3 ± 0.2 μg/mL, respectively). We observed a robust increase by more than 20-fold in TAT release into the blood stream from the graft thrombus area, as measured in samples taken locally from the near-wall region, immediately downstream of thrombus formation in vehicle-treated baboons. Pretreatment of baboons with aXIMab prevented the increase in local TAT levels, indicating a profound reduction in thrombin generation in the absence of FXI activity (Figure 5A). TAT levels in plasma obtained by local sampling were lower in aXIMab-treated animals than in untreated controls, by up to 98% at 40 minutes, while systemic TAT levels were 81% lower at 60 minutes (4.3 ± 0.5 vs 22.1 ± 2.5 μg/L, n = 6 and 7, respectively, P < .001; Figure 5B). Since thrombin does not interact with antithrombin in the presence of PPACK, all TAT in the sample was likely generated before the blood entered the sampling port. Thus, while reflecting thrombin generation, TAT levels are likely an underestimation of the total thrombin. Platelet activation at the thrombus surface, as assessed by the release of platelet α-granule βTG, was 86% lower at 30 minutes in samples taken distal to thrombi in aXIMab-treated animals than in vehicle-treated controls (Figure 5C). Systemic βTG levels in aXIMab-treated animals measured at 60 minutes were 42% less (23.0 ± 2.1 IU/mL, n = 6) in aXIMab-treated animals than in controls (39.5 ± 5.5 IU/mL, n = 7; P < .05; Figure 5D). Local D-dimer levels were not changed at 60 minutes compared with baseline systemic values in either aXIMab-treated animals (2.4 ± 0.2 and 2.2 ± 0.2 μg/mL, respectively, n = 6) or control animals (2.3 ± 0.2 and 2.4 ± 0.4 μg/mL, respectively, n = 7; Figure 5E). Systemic D-dimer levels assessed at 60 minutes also were unchanged from baseline systemic values in both groups (2.3 ± 0.2 and 2.4 ± 0.3 μg/mL, aXIMab-treated vs control animals). This confirms previous reports from similar baboon studies showing that systemic D-dimer levels do not increase within 60 minutes after thrombus initiation, unless a thrombolytic agent is administered.37,38 These data indicate that (1) FXI plays an important role in thrombin generation and platelet activation during acute, arterial-type thrombus formation, and (2) endogenous local thrombolysis appears to be limited during the time course of these studies, regardless of FXI activity.
FXI inhibition reduces thrombin generation and platelet activation. Local and systemic TAT (A,B) and βTG (C,D) levels were monitored during thrombus formation. Blood samples were also tested for the fibrinolysis product D-dimer (E). Local values are those taken from the near wall, low flow concentration boundary layer, 1 cm distal to the growing thrombus, over the course of 10 minutes before its designated time; systemic samples were taken from the arteriovenous shunt proximal to the thrombogenic device. Zero time points in all groups are from samples taken systemically immediately before each study. FXI inhibition (n = 6) was associated with a reduction in local thrombin formation and platelet activation, which translated into lower systemic TAT and βTG levels in aXIMab-treated animals than in collagen controls (n = 7), within 60 minutes after treatment. No significant change in D-dimer release during acute thrombus formation (≤ 60 minutes) was detected in aXIMab-treated and control animals. The silicone rubber tubing without the collagen-coated graft segment (n = 5) did not induce significant increases in coagulation or platelet activation during these studies. Values are means plus or minus SEM.
FXI inhibition reduces thrombin generation and platelet activation. Local and systemic TAT (A,B) and βTG (C,D) levels were monitored during thrombus formation. Blood samples were also tested for the fibrinolysis product D-dimer (E). Local values are those taken from the near wall, low flow concentration boundary layer, 1 cm distal to the growing thrombus, over the course of 10 minutes before its designated time; systemic samples were taken from the arteriovenous shunt proximal to the thrombogenic device. Zero time points in all groups are from samples taken systemically immediately before each study. FXI inhibition (n = 6) was associated with a reduction in local thrombin formation and platelet activation, which translated into lower systemic TAT and βTG levels in aXIMab-treated animals than in collagen controls (n = 7), within 60 minutes after treatment. No significant change in D-dimer release during acute thrombus formation (≤ 60 minutes) was detected in aXIMab-treated and control animals. The silicone rubber tubing without the collagen-coated graft segment (n = 5) did not induce significant increases in coagulation or platelet activation during these studies. Values are means plus or minus SEM.
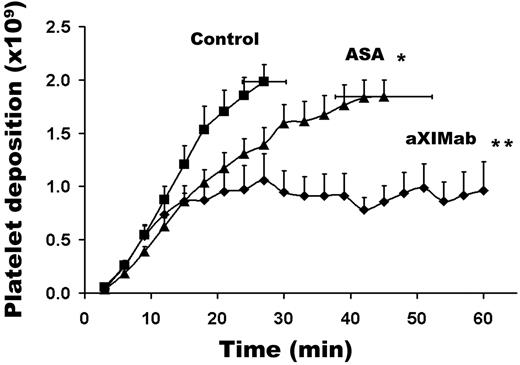
Treatment with aXIMab prevents vascular graft occlusion
Even though macroscopic thrombi formed rapidly in 4-mm id vascular grafts, none of these grafts occluded during 60 minutes of blood perfusion. The effects of inhibiting FXI activity on thrombus formation and occlusion were therefore evaluated using smaller diameter (2-mm id), collagen-coated vascular grafts that accumulated thrombi under higher shear conditions (2120 s−1). The initial platelet accumulation rate was similar in aXIMab-treated and vehicle-treated baboons. Within 12 to 15 minutes after initiation of blood perfusion, platelet deposition was reduced in all aXIMab-treated animals (Figure 6), and the number of platelets in the graft decreased abruptly, which can be explained by reduced thrombus stability and net loss of thrombus material to embolization. Treatment with aXIMab prevented graft occlusion over 60 minutes in all experiments (5 of 5 grafts remained patent), but vehicle treatment in controls did not prevent graft occlusion, which occurred in 8 of 9 grafts within 27.0 plus or minus 3.3 minutes (P < .01; Figure 6; Table 1). Fibrin accumulation was also lower with aXIMab treatment than with vehicle treatment (0.18 ± 0.02 vs 0.30 ± 0.04 mg, respectively; P < .01; Table 1). As expected, treatment with high-dose ASA reduced platelet deposition in the 2-mm id grafts, but did not completely interrupt occlusive thrombus formation. The time to graft occlusion was prolonged by ASA by an average of 18 minutes in 4 grafts, while 2 additional grafts remained patent throughout the 60 minutes of the study. High-dose ASA was therefore less effective in preventing graft oc-clusion than aXIMab (P < .05 for aXIMab vs ASA). These data indicate that FXI plays an important role in the throm-bogenic process that leads to acute occlusion of small caliber vascular grafts.
FXI inhibition limits vascular graft-associated platelet deposition under high arterial shear. Effects of FXI inhibition or ASA administration on platelet deposition on collagen-coated (2-mm id) vascular grafts are shown. Collagen-coated vascular graft segments were placed in permanent arteriovenous shunts in untreated (n = 9), ASA-treated (n = 6), and aXIMab-treated (n = 5) animals. Blood was allowed to flow through the grafts at a rate of 100 mL/min, producing an average initial wall shear rate of 2120 s−1. The flow was maintained by the pulsatile arterial pressure until the graft occluded (defined as ≤ 20 mL/min flow rate). Thrombi that formed in the grafts in the aXIMab-treated animals were unstable, embolized more frequently than in ASA-treated animals, and did not occlude the grafts for at least 60 minutes. Significance levels (*P = .05; **P < .01) pertain to comparisons with untreated controls, using the log-rank test, with the nonoccluded devices being censored. Values are means plus or minus SEM.
FXI inhibition limits vascular graft-associated platelet deposition under high arterial shear. Effects of FXI inhibition or ASA administration on platelet deposition on collagen-coated (2-mm id) vascular grafts are shown. Collagen-coated vascular graft segments were placed in permanent arteriovenous shunts in untreated (n = 9), ASA-treated (n = 6), and aXIMab-treated (n = 5) animals. Blood was allowed to flow through the grafts at a rate of 100 mL/min, producing an average initial wall shear rate of 2120 s−1. The flow was maintained by the pulsatile arterial pressure until the graft occluded (defined as ≤ 20 mL/min flow rate). Thrombi that formed in the grafts in the aXIMab-treated animals were unstable, embolized more frequently than in ASA-treated animals, and did not occlude the grafts for at least 60 minutes. Significance levels (*P = .05; **P < .01) pertain to comparisons with untreated controls, using the log-rank test, with the nonoccluded devices being censored. Values are means plus or minus SEM.
FXI inhibition by aXIMab prevents occlusion of 2-mm id vascular grafts in baboons
| . | Number occluded . | Embolic events per experiment* . | Fibrin deposition, mg . |
|---|---|---|---|
| Control | 8/9 | ND | 0.30 ± 0.04 |
| ASA | 4/6 | 1.5 ± 0.6 | 0.23 ± 0.04 |
| aXIMab | 0/5 | 6.6 ± 0.9 | 0.18 ± 0.02† |
| . | Number occluded . | Embolic events per experiment* . | Fibrin deposition, mg . |
|---|---|---|---|
| Control | 8/9 | ND | 0.30 ± 0.04 |
| ASA | 4/6 | 1.5 ± 0.6 | 0.23 ± 0.04 |
| aXIMab | 0/5 | 6.6 ± 0.9 | 0.18 ± 0.02† |
Data shown are absolute values or means plus or minus SEM when applicable.
ND indicates none detected.
Embolic events were defined as an abrupt decrease of at least 106 platelets in the graft between 2 subsequent radioimaging frames.
P < .01 compared with control using the 2-tailed Student t test.
Treatment with aXIMab does not prolong the bleeding time in baboons
The template bleeding time is typically prolonged by both antiplatelet agents and anticoagulants in baboons,31,39 yet FXI inhibition by aXIMab had no effect on the standard template bleeding, which was similar in aXIMab- and vehicle-treated animals (3.5 ± 0.3 vs 3.4 ± 0.2 minutes, n = 18 and 14, respectively). In comparison, single-dose ASA pretreatment nearly doubled the bleeding time to 6.4 plus or minus 0.7 minutes (n = 10, P < .01). No rebleeding from the template bleeding-time wounds, petechiae, hematomas, or other adverse bleeding events were noted in any of the aXIMab- or ASA-treated animals during 1-week follow-up periods of observation. Taken together, these results show that inhibition of FXI activity by a neutralizing monoclonal antibody more effectively reduces acute occlusive arterial-type thrombus propagation, with less effect on primary hemostasis, than an antihemostatic dose of ASA, in baboons.
Discussion
The present findings document that FXI is an essential contributor to local thrombin generation on the surface of thrombi during arterial-type thrombogenesis. FXI inhibition in baboons slowed platelet accumulation onto a thrombogenic surface (collagen-coated vascular grafts) under moderate arterial shear conditions (Figure 4). While FXI inhibition did not demonstrably affect platelet adhesion to collagen in the ex vivo chamber perfusion studies, it clearly reduced centripetal accumulation of platelet aggregates over longer periods of time in the 2-mm id grafts. The stability of thrombi formed under higher shear flow was noticeably dependent on FXI activity (Table 1; Figure 6). Indeed, under high shear, repeated loss of platelets from the graft was distinctly evident in aXIMab-treated animals, a mechanism which likely contributed to preventing graft occlusion. These findings are consistent with the antithrombotic and antiocclusive phenotype observed in FXI-deficient mice after ferric chloride–induced arterial injury and thrombosis.17,18,40
FXI promotes clot resistance to fibrinolysis through thrombin-mediated activation of the metalloproteinase TAFI, which proteolytically modifies fibrin, making it resistant to plasmin.41,42 In addition, inhibition of thrombin generation by anticoagulants enhances clot lysis due to a slower forming, less dense fibrin network.43,44 Both processes may account, at least in part, for the enhanced lysis of blood clots formed in the presence of an anticoagulant anti–factor XI antibody in the clamped jugular vein of rabbits.11 During the present studies in baboons, the fibrin degradation product D-dimer was measured by local blood sampling in the vicinity of fibrin-, platelet-, and leukocyte-rich arterial-type thrombi. Unlike the robust changes in TAT and βTG levels, there were no changes in local D-dimer levels in vehicle- or aXIMab-treated baboons. This finding suggests that endogenous fibrinolysis may not be a dominant process on the surface of thrombi during acute arterial thrombogenesis, which is consistent with the observation that TAFI deficient mice that were expected to have a fibrinolytic phenotype were not protected against injury-induced arterial thrombus formation.45 Since adjacent endothelial cells capable of secreting tissue plasminogen activator are absent from the graft, the data do not rule out the possibility of reduced TAFI activity and more efficient fibrinolysis during inhibition of FXI. Our data suggest, however, that the relative reduction in deposited fibrin associated with FXI inhibition was not caused by an increase in fibrinolysis.
The inhibitory effect of aXIMab on thrombogenesis and TAT formation suggests that sustained thrombus propagation on the luminal flow surface depends on FXI-mediated thrombin generation. FXI-dependent thrombin generation could occur via continued FXI activation by FXIIa, thrombin, and/or autoactivation.7,46 FXIIa appears to play an important role in experimental thrombosis in mice.47 However, the flow chamber data (Figure 2B) indicates that FXI promotes thrombosis independent of FXIIa, under shear flow conditions in the presence of blood cells. Although thrombin-mediated FXI activation in static plasma assays in vitro has been questioned,48 our flow-augmented thrombosis model clearly demonstrates an FXII-independent thrombogenic pathway for FXI. Since inhibition of the contact pathway (FXII) as well as the extrinsic TF pathway did not affect thrombus formation, initiation of coagulation in this ex vivo model must depend on coagulation factor autoactivation, delivery of coagulation proteases by platelets, or an as yet unrecognized mechanism.
Whereas FXI activity decreased to less than 1% for more than a week after aXIMab injection, total FXI:Ag levels temporarily increased severalfold above baseline after the disappearance of FXI activity in the baboon circulation. Whether this increase was due to a longer half-life of the circulating immune complexes or up-regulation of FXI synthesis and/or secretion remains to be explored.
The physiologic function and regulation of FXI have been difficult to elucidate. The bleeding tendency seen in FXI deficiency suggests that FXI supports hemostasis after injury to anatomical areas with pronounced fibrinolytic activity.5 In contrast to patients with hemophilia A or B, patients with FXI deficiency rarely experience spontaneous bleeding, and trauma-related bleeding is typically milder. All conventional anticoagulant, antiplatelet, and fibrinolytic agents impair hemostasis, and most produce a significant risk of life-threatening bleeding. While bleeding time prolongation is indicative of a hemostatic defect, the test is not particularly sensitive to coagulation abnormalities in humans. However, bleeding time has been useful and predictive of the antihemostatic effects of antithrombotic agents, when baseline and treatment values are compared in baboons.31 In the present study, bleeding time was significantly prolonged by ASA but not sensitive to the presumably modest hemostatic abnormality produced by FXI inhibition. Clearly, development of agents that could inhibit thrombosis without impairing hemostasis would significantly improve the safety profile of antithrombotic therapy. The results of the present study suggest that a pharmacologic inhibitor of FXI may represent an example of such a strategy.
In summary, this study confirms that FXI activity is not essential for primary hemostasis in otherwise healthy primates. We introduce a pharmacologically active monoclonal antibody that, by binding to the A3 domain of FXI, can achieve sustained FXI blockade and represents a new strategy for limiting acute arterial-type thrombus growth and blood vessel occlusion. Though we did not establish a role for FXI in endogenous thrombolysis, we demonstrate that vaso-occlusive thrombus propagation is driven by FXI-dependent thrombin generation, platelet activation, and fibrin formation in primates. Furthermore, we provide evidence for an FXII- and FVII-independent pathway for fibrin formation in whole blood under flow that requires FXI.
Presented in part at the 49th annual meeting of the American Society of Hematology, Atlanta, GA, December 11, 2007.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Daniel Cawley for his help in generating aXIMab and Tonya Swanson for her technical assistance with the primates.
This work was supported in part by American Heart Association grants 0850056Z (A.G.), 0665512Z (O.J.T.M.), and 0760063Z (S.R.), and National Institutes of Health (NIH) grants HL073813, HL068571 (A.G), and HL58837, HL081326 (D.G.), and grants VUMC33680-R (A.G.), 5-24958-G1 (S.R.H.), and RR-00163 (to the Oregon National Primate Research Center). E.I.T., S.H., and T.C.W. are supported by NIH training grants.
National Institutes of Health
Authorship
Contribution: A.G., S.R.H., and E.I.T. designed research; E.I.T., U.M.M., T.C.W., S.H., O.J.T.M., S.R., and D.G. performed research; E.I.T., U.M.M., O.J.T.M., S.R., and D.G. collected the data; E.I.T., D.G., S.R.H., and A.G. analyzed the data; and E.I.T., D.G., S.R.H., and A.G. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: András Gruber, MD, Department of Biomedical Engineering, Oregon Health & Science University School of Medicine, 3303 SW Bond Avenue, CH13B, Portland, OR 97239; e-mail: grubera@ohsu.edu.

![Figure 1. Thrombogenic vascular graft and blood collection device. Thrombogenesis started and thrombi developed in a collagen-coated (4-mm internal diameter [id]) expanded-polytetrafluoroethylene vascular graft that was deployed for 60 minutes into a chronic high flow arteriovenous shunt in healthy baboons. Blood samples were drawn from the coagulation marker concentration boundary layer by a syringe pump 1 cm downstream from acutely developing thrombi. PPACK anticoagulant was infused 3 mm proximal to the sample port to prevent the sample port from occluding during the 1-hour study. Blood flow through the graft was maintained at a fixed rate of 100 mL/min for the entirety of each study by proximal clamping.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/4/10.1182_blood-2008-06-163675/4/m_zh80020929330001.jpeg?Expires=1767705319&Signature=coq-vuoLxOyQg05r70qnLK9J-i9m1DS7S0gnEcL6lCSfaRFIy2gLllYfciJFOpanxj6mQtj1teJLatgGkmFv7X4T0JOOplIuoag2TM5kMAvV3Vhj2ZUBJ968bqCp9wO2VAtgyLgQ8H-~mv7bEnf7FmBNrzzv1MQX4yJdkvjT3-Dy5P3WGyqdFciEq4ngRK7FD1txoj21cs0m2vhFh47-T25JU~kj8xi88liR0f3Gjg8Pm9xrA0i9AFaKg3PIXomrMtBV1dJxOGoWWENWKLdEc1cnwkh-xEzyp3CxomR8q-LSex37L3r1aHUomwBgQwV3WjpDA~d88miHMu5DOalg~w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal