Abstract
Oxygen-dependent antimicrobial activity of human polymorphonuclear leukocytes (PMNs) relies on the phagocyte nicotinamide adenine dinucleotide phosphate (NADPH) oxidase to generate oxidants. As the oxidase transfers electrons from NADPH the membrane will depolarize and concomitantly terminate oxidase activity, unless there is charge translocation to compensate. Most experimental data implicate proton channels as the effectors of this charge compensation, although large-conductance Ca2+-activated K+ (BK) channels have been suggested to be essential for normal PMN antimicrobial activity. To test this latter notion, we directly assessed the role of BK channels in phagocyte function, including the NADPH oxidase. PMNs genetically lacking BK channels (BK−/−) had normal intracellular and extracellular NADPH oxidase activity in response to both receptor-independent and phagocytic challenges. Furthermore, NADPH oxidase activity of human PMNs and macrophages was normal after treatment with BK channel inhibitors. Although BK channel inhibitors suppressed endotoxin-mediated tumor necrosis factor-α secretion by bone marrow-derived macrophages (BMDMs), BMDMs of BK−/− and wild-type mice responded identically and exhibited the same ERK, PI3K/Akt, and nuclear factor-κB activation. Based on these data, we conclude that the BK channel is not required for NADPH oxidase activity in PMNs or macrophages or for endotoxin-triggered tumor necrosis factor-α release and signal transduction BMDMs.
Introduction
Many important compositional, physiologic, and biochemical features of the polymorphonuclear leukocyte (PMN) oxidase are now understood.1 Assembled and active at the membrane of the cell surface or phagosome, the phagocyte oxidase operates as an electron transferase, shuttling electrons from cytoplasmic nicotinamide adenine dinucleotide phosphate (NADPH) across the membrane to oxygen, which is reduced to superoxide anion, the proximal product of the active enzyme.2 Uncompensated, the directional electron flow would eventually depolarize the membrane to the equilibrium potential of electron transfer and prematurely terminate oxidase activity and superoxide anion generation. Evidence indicates that voltage-gated proton channels compensate most, if not all, of the charge and thus support continued oxidase activity.3 An alternative mechanism for these events has been proposed, whereby a flux of K+ into the phagosome mediates charge compensation for oxidase-triggered electron flow, raises the pH of phagosome, and triggers the release of cationic granule proteases.4 According to this model, K+ flux is mediated through the large conductance Ca++-activated K+ channels (BK channel), and there is no role for proton channels, as the authors reported that Zn++, at concentrations in excess of those that nearly completely block proton channel activity,5 did not inhibit phagocyte oxidase activity.6 We reasoned that, if BK channels contributed a functionally significant compensatory force during phagocyte oxidase activation, then the activities of BK channels and the NADPH oxidase would exhibit parallel behaviors; inhibition of BK channels would compromise the capacity to offset the obligatory negative charge accumulation during oxidase activation and thus prematurely terminate the oxidase or alter the kinetics of its activation. To test this prediction directly, we sought to clarify the relevance of BK channels to normal phagocyte function, NADPH oxidase activity of PMNs and macrophages, and tumor necrosis factor-α (TNF-α) production by endotoxin-stimulated macrophages.
Methods
Mice
All experiments were performed with litter- or age-matched wild-type (WT; BK+/+) and BK channel gene-deficient (BK−/−) 2- to 3-month-old male or female mice of the hybrid SV129/C57BL6 background in accordance with the German legislation on protection of animals.7 The animal experiments were approved by the institutional review board of the Max Delbrück Center.
Superoxide in bone marrow PMNs and BMDMs from BK+/+ and BK−/− mice
Mice were killed, femurs and tibias were dissected, and bone marrow was flushed with ice-cold sterile phosphate-buffered saline. PMNs were further isolated by Ficoll-Hypaque density gradient centrifugation and red blood cell lysis, whereas bone marrow-derived macrophages (BMDMs) were generated as described in this subsection. Superoxide anion was measured as described earlier, using the superoxide dismutase-inhibitable reduction of ferricytochrome c assay.8,9 In this study, 2.5 × 105 cells were incubated in a total assay volume of 200 μL with either buffer control, 25 ng/mL phorbol 12-myristate 13-acetate (PMA), or 100 μg/mL opsonized zymosan, respectively. When indicated, cells were preincubated with 100 nM iberiotoxin (IbTX; I2141; Sigma-Aldrich Laborchemikalien, Seelze, Germany) or 1 μM paxilline (P-2928; Sigma-Aldrich Laborchemikalien) for 10 minutes before PMA stimulation. All experiments were set up in duplicates. The samples were incubated in 96-well plates at 37°C for up to 60 minutes, and the absorption of samples, with and without 300 U/mL superoxide dismutase (SOD), was scanned repetitively at 550 nm using a Microplate Autoreader (Molecular Devices, Sunnyvale, CA). PMA, ferricytochrome c, zymosan, and SOD were from Sigma-Aldrich Laborchemikalien.
BK channel activity
BK channel activity was measured by the whole-cell perforated-patch configuration in freshly isolated tibial artery smooth muscle cells and in BMDMs from WT and BK−/− mice. The cell isolation procedure was described previously.10 Membrane currents were recorded with an Axopatch 200B amplifier (Molecular Devices), low-pass filtered at 1 kHz, and sampled at 2 kHz. Data were acquired and analyzed with a CED1401 interface and CED Patch and Voltage Clamp Software, version 6.08 (Cambridge Electronic Design, Cambridge, United Kingdom). Patch clamp recording micropipettes of 2 to 4 MΩ were prepared from borosilicate glass capillary tubes (1.5 mm outer diameter, 0.86 mm inner diameter) using a Sutter programmable puller (model P-97; Sutter Instrument, Novato, CA). The pipette solution contained 110 mM K-aspartate, 30 mM KCl, 10 mM NaCl, 1 mM MgCl2, 10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, and 0.05 mM ethyleneglycoltetraacetic acid (made to pH 7.2 with KOH). Pipette saline was supplemented with 250 μg/mL amphotericin B (freshly prepared from dimethyl sulfoxide stocks within 2-3 hours of the experiment) to obtain perforated patch recordings. Whole cell access was achieved by amphotericin B within 10 to 15 minutes after seal formation. The external solution contained 134 mM NaCl, 6 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 10 mM glucose (made to pH 7.4 with NaOH). Tibial artery smooth muscle cells were voltage-clamped at a holding potential of −40 mV and depolarized for 400 ms to voltages between −80 and 100 mV. Voltage pulses were imposed in steps of 20 mV and applied every 10 seconds. BMDMs were voltage clamped at a holding potential of −40 mV and depolarized for 300 to 400 ms to voltages between −100 mV and 140 mV (20-mV voltage steps every 10 seconds) or by linear voltage ramps (300 ms every 30 seconds). Cells were differentiated in culture medium for 7 to 8 days. Currents were obtained in control solution and after exposure for 10 to 20 minutes to 100 nM IbTX. All recordings were done at room temperature (2°C-24°C).
Respiratory burst measurement by DHR oxidation to rhodamine
We assessed PMNs and monocyte oxidase activity using dihydrorhodamine 123 (DHR) as a fluorescent probe.11,12 Bone marrow PMNs or BMDMs (1 × 107/mL Hanks balanced salt solution) from WT or BK−/− mice were loaded with DHR (1 μM, Invitrogen, Carlsbad, CA) for 10 minutes at 37°C. 2.5 × 105 cells or 100 μL of human whole blood were incubated with buffer control, 25 ng/mL PMA, or 100 μg/mL opsonized zymosan, respectively. Reactions were stopped after 30 minutes by adding 400 μL of ice-cold phosphate-buffered saline/1% bovine serum albumin. Samples were analyzed using a FACScan (BD Biosciences, San Jose, CA). Data were collected from 10 000 cells per sample and analyzed with Cell-quest Pro software (BD Biosciences). To assay human PMNs and monocytes in whole blood, gating algorithms were applied. The shift of green fluorescence in the FL-1 mode was determined. For each condition, the mean fluorescence intensity (MFI), representing the amount of generated hydrogen peroxide and the light scatter characteristics, is reported.
Specific PMNs and monocyte/macrophage staining by flow cytometry
Fluorescein isothiocyanate (FITC)–conjugated antibodies were used to identify specific cell types: GR-1 for mouse PMNs, CD66b for human PMNs, F4/80 for mouse monocytes/macrophages, and CD14 for human monocytes. A total of 100 μL of heparinized whole blood or 2.5 × 105 isolated BMDMs in 100 μL HBSS was incubated with 5 μL of the indicated FITC-conjugated antibodies in the dark for 20 minutes on ice. When whole blood was used, red blood cells were lysed after the staining procedure using a commercial lysing solution (BD Biosciences) for 10 minutes at room temperature. Cells were washed and resuspended in 500 μL cell wash solution. Samples were analyzed using a FACScan. The PMNs and monocyte population were gated using a light scatter algorithm, and specific FITC-conjugated antibody staining was measured in the FL-1 channel. Data were collected from 10 000 cells per sample. GR-1 monoclonal antibody (mAb), lysing, and cell wash solutions were from BD Biosciences, the F4/80 mAb from Serotec (Oxford, United Kingdom), and CD66b mAb and CD14 mAb were from Immunotec (Marseille, France).
BMDM lipopolysaccharide stimulation
Bone marrow cells from the femurs from adult mice were cultured with cytokine growth factors as described13,14 ; namely, 4 × 106 of 8-day-old BMDMs were seeded in Dulbecco modified Eagle medium (Invitrogen) supplemented with 10% fetal bovine serum (Sigma-Aldrich Laborchemikalien), 4 mM l-glutamine (Invitrogen), 1 mM sodium pyruvate (Biochrom KG, Berlin, Germany), 100 U/mL penicillin (Invitrogen), and 100 μg/mL streptomycin (Invitrogen) in a humidified 5% CO2 atmosphere at 37°C for one day. Subsequently, cells were treated with Ultra pure lipopolysaccharide (LPS) from Salmonella enterica serovar Minnesota (EMD Chemicals, Gibbstown, NJ) with or without addition of 20 μM paxilline (P-2928; Sigma-Aldrich Laborchemikalien) or 100 nM IbTX, for 4 hours. Supernatants were harvested and enzyme-linked immunosorbent assays were performed to measure concentrations of TNF-α (BD Biosciences) according to the manufacturer's instructions. We used immunoblots to assess nuclear factor-κB (NF-κB) activation. Samples were incubated for 5 minutes at 95°C in loading buffer (250 mM Tris-HCl, pH 6.8 with 4% sodium dodecyl sulfate, 20% glycerol, 0.01% bromphenol blue, 10% β-mercaptoethanol). A total of 20 μg of protein was loaded per lane, electrophoresed on a 10% sodium dodecyl sulfate–polyacrylamide gel, and transferred to a nitrocellulose membrane. The membrane was blocked with Tris-buffered saline/Tween-20/5% skim milk for 1 hour and incubated overnight with IκBα antibodies or antibodies to phospho-ERK and phospho-Akt, respectively (New England Biolabs, Ipswich, MA). Membranes were washed and incubated with a horseradish peroxidase-labeled secondary antibody. The blot was developed by incubation in a chemiluminescence substrate (ECL, Amersham, the Netherlands) and exposed to an x-ray film. We confirmed equal protein loading in parallel experiments using total ERK antibodies.
Results
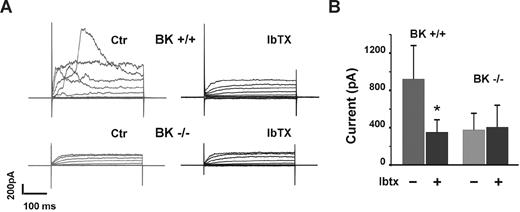
We first verified that the BK−/− mice to be used for our studies genuinely lacked BK channel functional activity by performing whole cell patch clamp recordings on isolated tibial smooth muscle cells from WT and BK−/− mice. Vascular smooth muscle cells from BK−/− mice lacked BK channel activity (Figure 1A), and the channel activity observed in WT, BK+/+ vascular smooth muscle cells was inhibited by the BK channel inhibitor IbTX (Figure 1B). Thus, in a tissue site known to express BK channels, WT mice exhibited bona fide BK channel activity that was blocked by a BK channel inhibitor, whereas vascular smooth muscle cells from BK−/− mice had no BK channel activity.
BK channel activity in vascular smooth muscle cells from WT and BK−/− mice. To confirm the presence and normal function of BK channels in WT mice, vascular smooth muscle cells from BK+/+ and BK−/− mice were subjected to patch clamp (A). Bars (mean ± SD) represent the mean current amplitudes measured at 100 mV (B). n cells ≥ 6, each bar. *P < .05.
BK channel activity in vascular smooth muscle cells from WT and BK−/− mice. To confirm the presence and normal function of BK channels in WT mice, vascular smooth muscle cells from BK+/+ and BK−/− mice were subjected to patch clamp (A). Bars (mean ± SD) represent the mean current amplitudes measured at 100 mV (B). n cells ≥ 6, each bar. *P < .05.
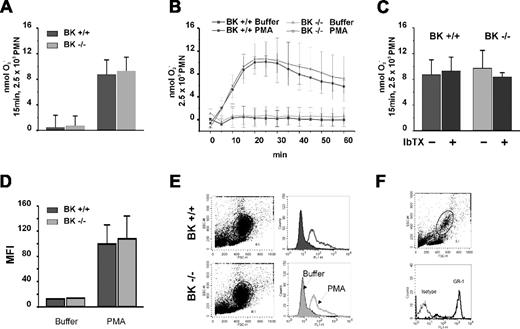
We next used SOD-inhibitable ferricytochrome c reduction to quantitate extracellular superoxide anion generation. We reasoned that charge compensation by BK channel activity might be subtle and best appreciated by examining the kinetics of phagocyte oxidase activation; we quantitated superoxide production by BK+/+ and BK−/− PMNs at multiple time points after stimulation. The net O2− generation by PMNs stimulated with PMA for 60 minutes as well as the kinetics of oxidant generation were the same for PMNs from BK−/− mice and BK+/+ mice (Figure 2A). Moreover, IbTX, which effectively blocked BK channel activity in vascular smooth muscle, had no effect on the PMA response either in BK−/− PMNs or BK+/+ PMNs (Figure 2B). To determine whether the failure of IbTX to inhibit PMN oxidase activity was limited to soluble agonist PMA stimulation, we challenged PMNs with opsonized zymosan. When we assayed superoxide generation by BK+/+ and BK−/− PMNs after zymosan, we again observed similar values and kinetics (Figure 2C). Thus, IbTX had no effect on either receptor-independent or phagocytosis-induced NADPH oxidase activation.
Superoxide in bone marrow PMNs from BK+/+ and BK−/− mice. Net superoxide production as well as the kinetics over 60 minutes by PMA-stimulated PMNs was the same in normal and BK−/− mice (A). The BK channel inhibitor IbTX had no effect on extracellular superoxide generation by either PMN population (B). Superoxide generation after opsonized zymosan was the same in BK+/+ and BK−/− cells (C). Intracellular NADPH oxidase activity, assessed as the DHR oxidation by stimulated PMNs measured and quantitated as mean fluorescent intensity (MFI), was the same in BK+/+ and BK−/− cells (D) based on the light scatter characteristics (E). PMNs specific GR-1 staining in whole blood indicated that the gated populations used to assess DHR oxidation were indeed PMNs (F).
Superoxide in bone marrow PMNs from BK+/+ and BK−/− mice. Net superoxide production as well as the kinetics over 60 minutes by PMA-stimulated PMNs was the same in normal and BK−/− mice (A). The BK channel inhibitor IbTX had no effect on extracellular superoxide generation by either PMN population (B). Superoxide generation after opsonized zymosan was the same in BK+/+ and BK−/− cells (C). Intracellular NADPH oxidase activity, assessed as the DHR oxidation by stimulated PMNs measured and quantitated as mean fluorescent intensity (MFI), was the same in BK+/+ and BK−/− cells (D) based on the light scatter characteristics (E). PMNs specific GR-1 staining in whole blood indicated that the gated populations used to assess DHR oxidation were indeed PMNs (F).
Recognizing that our assays quantitated only extracellular superoxide production and thereby failed to detect intracellular oxidase activity, we assessed intracellular oxidant generation by BK+/+ and BK−/− PMNs by measuring the oxidation of DHR to rhodamine in response to PMA (Figure 2D,E). The PMNs from BK−/− mice and from BK+/+ mice responded in identical fashion, generating the same amounts of oxidants intracellularly after PMA stimulation. Again, opsonized zymosan gave lower but similar results when both groups were compared (data not shown). PMN-specific GR-1 staining in whole blood confirmed that the gated populations we used to assess DHR oxidation were indeed PMNs (Figure 2F). Taken together, these data demonstrate that PMN NADPH oxidase activity was normal in the absence of BK channel activity.
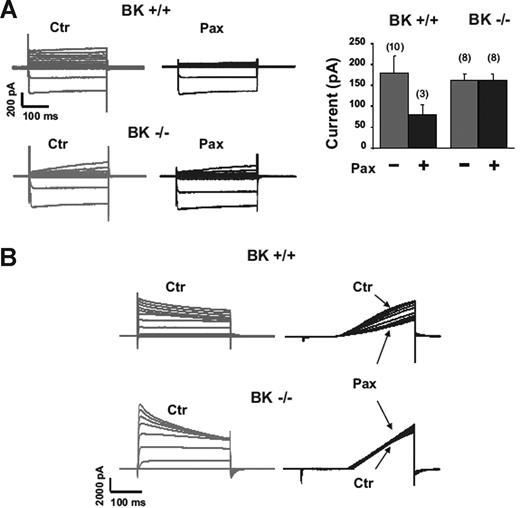
We reasoned that, despite their lack of significance for PMNs, BK channels might participate in the function of other innate immune cells, such as macrophages. We first assessed currents in BMDMs using the perforated patch configuration of the patch clamp technique as previously described.15 Inward currents were apparent in all cells at membrane potentials negative to −40 mV, namely, in 45 cells from 7 BK+/+ mice and 18 cells from 3 BK−/− mice. Outward currents were quite small in the majority of cells (Figure 3 group A cells), whereas only 10% to 20% of cells showed large outward currents (Figure 3 group B cells). The basic characteristics of the currents and passive electrical parameters of the cells are shown in Table S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). We first tested the effects of 100 nM IbTX in 11 BK+/+ cells. In only 2 cells did IbTX induce a clear current reduction within 10 to 20 minutes. However, the interpretation of the results was difficult because of channel “run down” in our preparation, which was significant during long-lasting recordings (>10 minutes). When we used 1 μM paxilline in 7+/+ BK and 9−/− BK cells, we observed a reduction in outward current within 3 to 5 minutes in only 4 of 7 +/+ cells but not in −/− cells (Figure 3A group A cells; Figure 3B group B cells). However, the paxilline-responsive cells showed suspicious low channel noise at positive potentials (140 mV), which is not typical for large-conductance channels. These data indicate that paxilline-inhibitable channels were present in only a very small minority of BMDMs and did not exhibit features typical of BK channels.
Paxilline-sensitive outward currents in BMDMs from BK+/+ mice, but not from BK−/− mice. (A) Group A cells. (B) Group B cells. Bars (mean ± SD) represent the mean current amplitudes measured at 140 mV (A). The number of cells is given in parentheses.
Paxilline-sensitive outward currents in BMDMs from BK+/+ mice, but not from BK−/− mice. (A) Group A cells. (B) Group B cells. Bars (mean ± SD) represent the mean current amplitudes measured at 140 mV (A). The number of cells is given in parentheses.
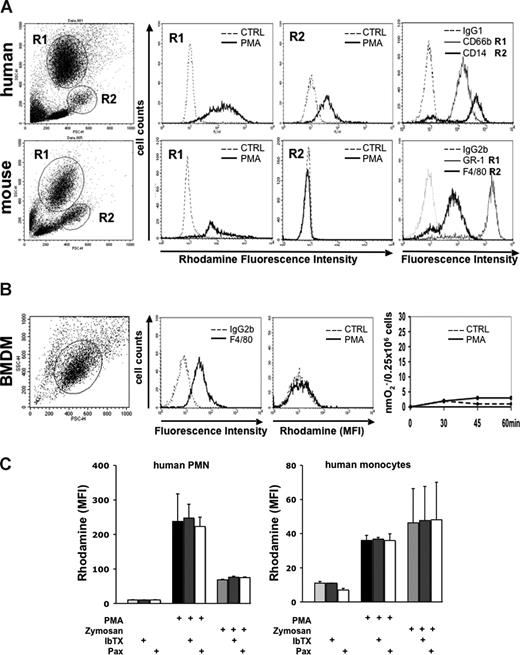
When we assessed PMA-stimulated respiratory burst activity in BMDMs and in mouse whole blood with gating on monocytes, we observed no measurable ROS generation in either cell population. Appropriate control experiments were performed in parallel that demonstrated that monocytes in human whole blood and mouse PMNs prepared from the same bone marrow exhibited a strong response to activation (Figure 4A,B). Similar observations describing the lack of respiratory burst activity in BMDMs were reported by other investigators.16 When we blocked BK channels in human whole blood experiments using IbTX or paxilline, we observed no inhibition of DHR production either to PMA or to opsonized zymosan. (Figure 4C).
NADPH oxidase activity in stimulated human and murine phagocytes in the absence and presence of BK channel inhibitors. (A) Respiratory burst by rhodamine generation in PMNs and monocytes assayed in either human or mouse whole blood by flow cytometry. Whole blood (100 μL) was loaded with DHR after red blood cell lysis. Generation of rhodamine was measured in FL-1 channel (rhodamine fluorescence intensity). To ensure that the gated cells were specifically either PMNs or monocytes, staining with the indicated antibodies and controls was performed. One of 2 independent experiments is shown. (B) Respiratory burst in BMDMs. BMDMs were differentiated from WT bone marrow. Cells stained positive for the macrophage marker F4/80, but not for the isotype control antibody. PMA stimulation did not evoke generation of reactive oxygen species, as measured either by rhodamine generation (second panel from right) or ferricytochrome c reduction (right panel). A representative of 3 independent experiments is shown. (C) Effect of IbTX and paxilline on rhodamine production of human PMNs and monocytes assayed in whole blood by flow cytometry. A total of 100 μL of whole blood was loaded with DHR after red blood cell lysis. Samples were preincubated with IbTX and paxilline, respectively. After 10 minutes, cells were stimulated with either PMA or opsonized zymosan. Generation of rhodamine was measured in FL-1 channel and is reported as rhodamine mean fluorescence intensity (MFI). Neither IbTX nor paxilline inhibited rhodamine production in human PMNs and monocytes (n = 2).
NADPH oxidase activity in stimulated human and murine phagocytes in the absence and presence of BK channel inhibitors. (A) Respiratory burst by rhodamine generation in PMNs and monocytes assayed in either human or mouse whole blood by flow cytometry. Whole blood (100 μL) was loaded with DHR after red blood cell lysis. Generation of rhodamine was measured in FL-1 channel (rhodamine fluorescence intensity). To ensure that the gated cells were specifically either PMNs or monocytes, staining with the indicated antibodies and controls was performed. One of 2 independent experiments is shown. (B) Respiratory burst in BMDMs. BMDMs were differentiated from WT bone marrow. Cells stained positive for the macrophage marker F4/80, but not for the isotype control antibody. PMA stimulation did not evoke generation of reactive oxygen species, as measured either by rhodamine generation (second panel from right) or ferricytochrome c reduction (right panel). A representative of 3 independent experiments is shown. (C) Effect of IbTX and paxilline on rhodamine production of human PMNs and monocytes assayed in whole blood by flow cytometry. A total of 100 μL of whole blood was loaded with DHR after red blood cell lysis. Samples were preincubated with IbTX and paxilline, respectively. After 10 minutes, cells were stimulated with either PMA or opsonized zymosan. Generation of rhodamine was measured in FL-1 channel and is reported as rhodamine mean fluorescence intensity (MFI). Neither IbTX nor paxilline inhibited rhodamine production in human PMNs and monocytes (n = 2).
To examine a BK channel–dependent immune response independent of the NADPH oxidase, we studied LPS-mediated TNF-α secretion in BMDMs because pharmacologic inhibitors of BK channel activity had been reported to decrease LPS-elicited TNF-α secretion by human macrophages, thus implicating an essential role for BK channels in this process.17 Using the BK channel inhibitors IbTX and paxilline, we observed that the dose-dependent LPS-induced TNF-α production in BMDM from BK+/+ mice was abrogated by these compounds (Figure 5A). To test the role of BK channels directly, we measured TNF-α release in response to LPS by BMDM from BK+/+ and BK−/− mice. Studying a total of 9 BMDM preparations from 9 mice of each group showed very consistent data within a given mouse (data not shown). However, we observed large mouse-to-mouse variation, and more importantly, no significant differences between BMDM from BK+/+ and BK−/− mice (Figure 5B,C). Papavlassopoulos et al demonstrated that LPS-induced NF-κB activation in human and rat macrophages was inhibited by BK channel blockade and proposed that BK channels might participate in TNF-α production by controlling NF-κB–dependent gene transcription.17 However, similar NF-κB activation occurred in BK+/+ and BK−/− BMDMs (Figure 5D). Moreover, no differences in Akt and ERK phosphorylation were observed between BK+/+ and BK−/− BMDMs, indicating that BK channels do not control TNF-α release or signal transduction pathways that are implicated in TNF-α generation and release in BMDMs.
Contribution of BK channels to TNF-α release and signaling from LPS-stimulated BMDM. (A) BK+/+ BMDMs were stimulated with indicated concentrations of LPS resulting in a dose-dependent TNF-α release. TNF-α release was inhibited by paxilline and IbTX in a dose-dependent fashion. Bars (mean ± SD from 3 experiments). (B,C) LPS stimulation (10 ng/mL) triggered similar TNF-α release in BK+/+ and BK−/− BMDMs as shown by individual results (B) and mean plus or minus SD (C) from 9 experiments. (D) IκB-α degradation, Akt phosphorylation, and ERK phosphorylation were similar in BK+/+ and BK−/− BMDMs, as assayed by immunoblots.
Contribution of BK channels to TNF-α release and signaling from LPS-stimulated BMDM. (A) BK+/+ BMDMs were stimulated with indicated concentrations of LPS resulting in a dose-dependent TNF-α release. TNF-α release was inhibited by paxilline and IbTX in a dose-dependent fashion. Bars (mean ± SD from 3 experiments). (B,C) LPS stimulation (10 ng/mL) triggered similar TNF-α release in BK+/+ and BK−/− BMDMs as shown by individual results (B) and mean plus or minus SD (C) from 9 experiments. (D) IκB-α degradation, Akt phosphorylation, and ERK phosphorylation were similar in BK+/+ and BK−/− BMDMs, as assayed by immunoblots.
Discussion
Our data demonstrate that BK channels were not required for normal PMN NADPH oxidase activity. Thus, these data together with 2 previous reports do not confirm the suggestion that the large-conductance Ca2+-activated K+ channels are essential for PMN antimicrobial action.4,6 Femling et al did not detect immunochemical or electrophysiologic evidence for BK channels in human PMNs.18 Furthermore, the BK channel inhibitors IbTX and paxilline do not inhibit any component of outward currents or interfere with the capacity of PMNs to kill ingested Staphylococcus aureus, although the widely recognized flavoprotein inhibitor diphenylene iodonium both blocks NADPH oxidase activity and inhibits killing of S aureus.15 Searching for BK channels in human and murine PMNs, Essin et al performed patch-clamp recordings on PMA-stimulated PMNs in normal and BK−/− mice and found no evidence of BK-channel currents. Furthermore, IbTX does not inhibit PMNs-mediated bacterial killing, and BK−/− mice are no more susceptible to bacterial infection than are BK+/+ mice.15,18 Taken together, the data presented here and the earlier reports demonstrate conclusively that BK channels do not play an important role in 2 pivotal PMNs functions, namely, NADPH oxidase activity and bacterial killing. In the current study, we observed no differences in PMA or opsonized zymosan-mediated degranulation by BK+/+ and BK−/− PMNs, providing additional evidence for normal antimicrobial responses in the absence of functional BK channels (Figure S1).
Macrophages respond to microbes and microbial products not only by generating reactive oxygen and nitrogen species but also with the robust release of a wide range of inflammatory mediators, including TNF-α, which amplify and modulate the inflammatory response.19 Prior studies using pharmacologic compounds implicated a BK channel dependence of LPS-induced macrophage activation, in particular with respect to NF-κB activation and TNF-α production.17,20 When we explored the potential role of BK channels using in vitro–differentiated BMDM from BK+/+ and BK−/− mice, we found no biologic significance for this channel; neither TNF-α release nor the initiation of signaling pathways, such as NF-κB, ERK, and phosphoinositide-3 kinase/Akt, was dependent on the presence of BK channels. Phosphoinositide-3 kinase has recently been implicated as a negative regulator of TLR4-mediated TNF-α release in human alveolar macrophages.21 Although all BMDMs expressed a macrophage marker after differentiation, we recorded paxilline-sensitive outward currents in only a small minority of BK+/+ BMDMs. BK channels were observed earlier in human macrophages, and interestingly, the BK channel inhibitor effect on TNF-α and NF-κB was also obtained by studying macrophages from either human blood or rat lungs.17,22,23 It is therefore conceivable that differences between in vitro–differentiated mouse BMDMs, and peripheral human and rat macrophage account for the observed differences. This assumption is further supported by the fact that mouse blood macrophages, but not BMDMs, initiated a respiratory burst to a strong activator such as PMA. Nevertheless, we think that our data sound a note of caution with respect to exclusive reliance on pharmacologic compounds because, even in our studies of BMDMs, paxilline initially suggested some role of BK channels in TNF-α release.
In conclusion, we demonstrate unequivocally that BK channels are not required for NADPH oxidase activity in innate immune cells. Furthermore, our data indicate that BK channels are not required for the release of TNF-α or initiation of important intracellular signaling pathways in murine BMDMs stimulated with LPS. Additional studies are necessary to define potential roles for BK channels in the immune responses of macrophages derived from other body sites, as many phenotypic characteristics of macrophages are tissue-specific.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (M.G., R.K., and F.C.L.), the National Institutes of Health (grants AI 34879-19 and AI 070958; W.M.N.), and the Veterans Administration (Merit Review). F.C.L. is the Helen C. Levitt Visiting Professor at the University of Iowa.
National Institutes of Health
Authorship
Contribution: K.E. and M.G. performed the electrophysiology; S.R. and R.K. performed the PMNs burst studies; P.R. and M.S. developed the BK-deficient mice; P.W., E.B., and I.B.A. performed the BMDM studies; and W.M.N. and F.C.L. conceived the study. All authors participated in the preparation of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: William M. Nauseef, Inflammation Program and Department of Medicine, Roy J. and Lucille A. Carver College of Medicine, University of Iowa, 2501 Crosspark Road, D160 MTF, Coralville, IA 52241; e-mail: william-nauseef@uiowa.edu.
References
Author notes
*F.C.L., W.M.N., and R.K. contributed equally to this work.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal