MLL5 is an MLL family protein and a candidate tumor suppressor located within the human chromosome band 7q22 that is frequently deleted in myeloid malignancies. In this issue of Blood, 3 independent studies report the first genetic analysis of MLL5 deficiency in mice. All 3 strains of MLL5 knockout mice exhibited defects in hematopoiesis, highlighting the critical role of MLL5 in hematopoietic stem cell functions.
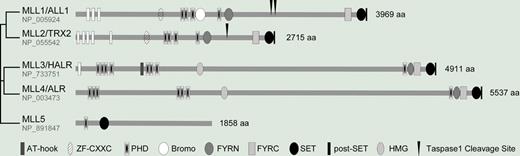
Mammalian MLL family consists of 5 members, that is, MLL1-5. This family shares a distinct SET domain along with various numbers of PHD domains. The SET of MLL1-4 encodes a histone methyl transferase (HMT) that specifically methylates histone 3 at Lysine 4 for gene activation and plant homeodomain (PHD) domains mediate protein to protein interactions. The founding member MLL1/HRX/ALL1 gene is located on the human chromosome band 11q23, of which recurring translocations result in infant and therapy-related leukemias.1 MLL2/TRX2 is highly homologous to MLL1, not only in all aligned domains, but also in shared interacting partners. For example, both MLL1 and 2 form H3K4 HMT complexes consisting of WDR5, RbBP5, and ASH2L.2,3 Furthermore, they undergo Taspase1-mediated proteolytic maturation, which renders full HMT activity.4 On the other hand, MLL3/HALR is more related to MLL4/ALR than to MLL1 and 2, and MLL3 and MLL4 were found in ASC-2–containing coactivator complexes (ASCOM).5 All 4 members share an architecturally positioned PHD-FYRN-FYRC-SET-postSET domain cluster. Unlike the MLL1-4, MLL5 is a distantly related MLL family protein that only contains a PHD and a SET domain.
MLL5 was initially identified as a candidate tumor suppressor gene located in the commonly deleted 2.5 Mb segment of human chromosome band 7q22 in myeloid malignancies.6 Building on their initial studies, Zhang and colleagues generated mice deficient in MLL5 by deleting exon 3 and 4.7 They found that MLL5−/− mice displayed some postnatal lethality and retarded growth. Importantly, they demonstrated a critical role played by MLL5 in hematopoiesis. Specifically, MLL5 deficiency in mice resulted in a decrease of long-term hematopoietic stem cells (LT-HSCs: Lin-Sca1+c-kit+CD34-Flt3−), a functional impairment of LT-HSC based on competitive repopulation assays, a reduced common lymphoid progenitor (CLP) population, disrupted myeloid differentiation, and an enhanced susceptibility to 5-fluorouracil–induced myelosuppression. Zhang et al also examined the potential role of MLL5 as a tumor suppressor gene and found that the status of MLL5 does not affect the fatal myeloproliferative phenotype induced by Kras in vivo.7 Also, MLL5−/− mice did not have an increased incidence of spontaneous tumors.
A similar targeting strategy was undertaken by Madan and colleagues to examine the in vivo function of MLL5.8 They observed male sterility as well as some postnatal lethality and retarded growth. However, upon inspection of surviving MLL5−/− mice, they detected reduced thymus, spleen, and lymph node sizes. The critical role of MLL5 in lymphopoiesis was confirmed when transplanted MLL5−/− bone marrow cells failed to competitively reconstitute Rag2−/−γc−/− mice. In addition, Madan et al8 demonstrated that MLL5 is required to maintain the quiescent state of LT-HSC whereas Zhang et al reported no cell cycle defects in the stem cell compartments.7
Lastly, Heuser and colleagues created their MLL5−/− mice by disrupting exon 3 and examined these animals in 129S6 genetic background,9 whereas the other 2 studies used MLL5−/− mice in C57Bl6 background.7,8 In addition to the incomplete postnatal lethality, male sterility, growth retardation, and decreased red blood cell counts reported in the other 2 studies, they demonstrated an increased incidence of eye infection in MLL5−/− mice due to defects in the maturation of neutrophils. In terms of HSCs, Heuser et al reported that MLL5−/− bone marrow competes poorly during primary but not secondary transplantations, and a mild but significant increase in the cycling of MLL5−/− LSK cells.9 To test the role of MLL5 as a tumor suppressor, they transduced NUP98-HOXD13 into MLL5−/− cells and did not detect an increased incidence of leukemia. Again, none of their MLL5−/− mice developed spontaneous tumors by up to 18 months of observation.
Taken together, all 3 groups of scientists simultaneously reached the same conclusion that MLL5 plays a critical role in adult hematopoiesis and is dispensable for embryonic development. In addition to regulation of LT-HSCs, MLL5 also controls the development of lymphoid (Madan et al8 ) and myeloid lineages (Zhang et al7 and Heuser et al9 ). However, the function of MLL5 in maintaining stem cell quiescence remains unsettled. Surprisingly, none of these 3 studies support an apparent function of MLL5 as a tumor suppressor in that MLL5 deficiency does not incur spontaneous tumors nor enhance the tumorigenic phenotypes initiated by Kras or NUP98-HOXD13. Thus, if MLL5 is indeed a tumor suppressor, additional mutations are likely required to uncover such a function.
Thus far, mice deficient of MLL1 or MLL2 but not of MLL3 or MLL4 have been reported.10,11 Deficiency of either MLL1 or MLL2 results in embryonic lethality, and conditional knockout of MLL1 leads to profound hematopoietic stem cell defects in part through failed maintenance of Hox gene expression.12,13 Although MLL5 deficiency also disrupts stem cell function, key downstream effectors remain elusive. Zhang et al7 reported a 2-fold reduction of Hoxb2 and Hoxb5 whereas Madan et al8 de-tected no differences in Hox gene expression in MLL5 deficient LSK cells. Two pieces of data reported here may provide potential clues as to how MLL5 functions. First, MLL5 contains alterations of a few critical amino acids within its SET domain and lacks the post-SET domain, explaining why Madan et al failed to detect any HMT activity of recombinant MLL5.8 Although these data may suggest that MLL5 functions in a different manner than MLL1-4, a proper examination of its HMT activity using in vivo assembled MLL5 complex is needed to clarify such a hypothesis. It is known that the functional assembly of MLL1-4 HMT complexes is needed for its full HMT activity. Second, Heuser et al demonstrated that MLL5−/− HSCs are extremely sensitive to DNA demethylation-induced differentiation.9 This suggests that MLL5 maintains stem cell fate directly or indirectly through DNA methylation. In summary, these studies clearly provide the first in vivo glimpse of functions concerning the mysterious MLL member MLL5. However, we are still missing much of the molecular details as to how MLL5 actually acts.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal