Abstract
These studies investigate how interactions between the BCR and FcγRIIB affect B lymphocyte stimulator (BLyS) recep-tor expression and signaling. Previous studies showed that BCR ligation up-regulates BLyS binding capacity in mature B cells, reflecting increased BLyS receptor levels. Here we show that FcγRIIB coaggregation dampens BCR-induced BLyS receptor up-regulation. This cross-regulation requires BCR and FcγRIIB coligation, and optimal action relies on the Src-homology-2 (SH2)–containing inositol 5 phosphase-1 (SHIP1). Subsequent to FcγRIIB/BCR coaggregation, the survival promoting actions of BLyS are attenuated, reflecting reduced BLyS receptor signaling capacity in terms of Pim 2 maintenance, noncanonical NF-κB activation, and Bcl-xL levels. These findings link the negative regulatory functions of FcγRIIB with BLyS-mediated B-cell survival.
Introduction
B-cell differentiation, survival, and activation are regulated by multiple surface receptors. In mature B cells, survival and activation signals from the B-cell receptor (BCR)1 can be augmented by costimulatory and innate receptors, such as CD40, toll-like receptors (TLRs), or scavenger receptors.2,3 In contrast, the low-affinity IgG receptor FcγRIIB exerts powerful negative effects when coligated with the BCR.4,5 Negative signaling via FcγRIIB helps maintain peripheral tolerance, as evidenced by the B cell–intrinsic development of fatal autoimmune glomerulonephritis in FcγRIIB knockout (KO) mice.6 In addition, FcγRIIB interactions influence the selection of high-affinity BCRs during germinal center (GC) reactions, whereby signaling via the BCR versus BCR/FcγRIIB-bound antibody engenders survival or apoptosis, respectively.4
In general, FcγRIIB coligation opposes BCR signaling, dampening calcium flux and phosphorylation events associated with BCR engagement,7-9 thus reducing the likelihood of activation or survival. The underlying mechanisms involve activation of lipid and tyrosine phosphatases. On BCR and FcγRIIB coaggregation, Lyn tyrosine kinase is activated by the BCR-mediated phosphorylation of residues within the cytoplasmic tail of FcγRIIB, generating an Src-homology-2-domain–containing inositol 5 phosphatase-1 (SHIP1) and Src-homology-2 (SH2) binding motif. This phosphorylation leads to recruitment and phosporylation of SHIP1 and its adaptor downstream of kinase-1 (Dok1). SHIP1 and Dok1 form a bidentate complex in which the Dok1 phosphotyrosine-binding domain binds to a phosphorylated SHIP1 N-P-X-pY motif, and the SHIP1-SH2 domain binds to phosphotyrosine residues in the Dok1 C-terminus. Because the SHIP1-SH2 domain is blocked by pDok1, the complex dissociates from pFcγRIIB. Recent studies have shown that this stable complex can function in trans to inhibit signaling by remotely stimulated BCRs and CXCR4, receptors whose signaling depend on generation of phosphatidylinositol-3,4,5-trisphosphate (PIP3), the substrate of SHIP1.10-14 Dok1 appears to also mediate inhibitory signaling via recruitment of p21RasGTP-ase activating protein.9 Finally, under conditions of very efficient coaggregation with BCR, pFcγRIIB can mediate the recruitment and activation of the Src-homology-2-domain-containing phosphatase-1 (SHP1), which inhibits by dephosphorylating proximal effectors in BCR signaling.12
In contrast to this detailed knowledge of proximal signals mediating FcγRIIB activity, less is understood about the downstream events ultimately impacting B-cell viability. A growing literature suggests that lymphocyte survival is regulated through cytokine receptor modulation, with tumor necrosis factor (TNF) family members playing dominant roles in B cells. For example, both CD40 and FAS14 levels shift during B-cell activation, mediating positive or negative survival effects, respectively. Similarly, B lymphocyte stimulator15 (BLyS, also known as BAFF16 ) and its receptors play crucial roles in B-cell survival.17 BLyS can bind 3 receptors, B-cell maturation antigen18-20 (BCMA), transmembrane activator and CAML interactor20,21 (TACI), and BLyS receptor 322,23 (BR3, also termed BAFFr24 ). Both BR3 and TACI are expressed by mature follicular (FO) B cells and, on BLyS binding, modulate survival and differentiation.25,26 Analogous to FcγRIIB, BLyS family members can regulate peripheral tolerance and ongoing immune responses. For example, elevated BLyS levels are associated with humoral autoimmunity and relaxed negative selection in mice and humans.17,27 In addition, GC reactions and other hallmarks of appropriate humoral immune responses are compromised in KO and mutants of BLyS ligands and receptors.28,29
Recent studies have shown that activation cues can modulate BLyS receptor expression and, hence, BLyS sensitivity. Thus, both BCR and TLR ligation increase BLyS binding capacity,30,31 reflecting up-regulation of BR3 and TACI expression. Although such positive regulatory cues can influence the nature and extent of BLyS receptor expression, potential effects of negative regulatory signals, such as those mediated by FcγRIIB, remain unexplored. Of particular interest is the recent demonstration that BLyS survival signaling requires the generation of PIP3, making it a probable candidate for FcγRIIB-mediated transinhibition.32 Herein we examine whether FcγRIIB signaling influences BLyS receptor expression and signaling. Our results indicate that FcγRIIB ligation attenuates BCR-mediated BLyS receptor up-regulation. This effect requires FcγRIIB coligation with either primary BCR isotype and operates via a SHIP-dependent mechanism. Downstream BLyS signaling pathways are dampened after FcγRIIB/BCR coligation, blunting the survival-promoting effects of BLyS. Together, these findings link the regulatory actions of FcγRIIB with downstream survival functions mediated through BLyS and its receptors.
Methods
Mice
BALB/cJ and C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME). FcγRIIB KOs on a BALB/c background were purchased from Taconic Farms (Germantown, NY). SHIP KO mice and littermate controls33 were bred and maintained in the animal colony of J.C.C. B cell–specific Bcl-xL transgenic mice34 were obtained from Dr Craig Thompson (Abramson Family Cancer Research Institute, University of Pennsylvania, Philadelphia, PA). All procedures were conducted in accordance with the Animal Welfare Act and approved by the University of Pennsylvania Institutional Animal Care and Use Committee.
B-cell subset isolation, culture, and stimulation
B cells were prepared using CD23+ magnetic activated cell-sorting enrichment (Miltenyi Biotec, Auburn, CA) and cultured at 2 × 105 cells/mL in 200 μL of medium as previously described.26,30 In addition, follicular B cells (CD23+CD21/35+), marginal zone B cells (CD23−CD21/35hi), and B1 B cells (CD19+CD23−CD43+B220lo) were fluorescence-activated cell sorted (FACS) using biotin-conjugated anti–mouse CD23, biotin-conjugated anti–mouse CD19, fluorescein isothiocyanate (FITC)–conjugated anti–mouse CD43, FITC-conjugated CD43 (BD Biosciences, San Jose, CA), phycoerythrin (PE)–Cy7-conjugated CD23, and AlexaFluor 700–conjugated B220 (eBioscience, San Diego, CA). In some experiments, B cells were loaded with 1.25 μM carboxyfluorescein diacetate, succinimidyl ester (CFSE; Invitrogen, Carlsbad, CA) and stained with TO-PRO-3 (Invitrogen) as described.30 In some experiments, cells were stained with annexin V–PE (BD Biosciences) for 15 minutes in annexin V binding buffer (BD Biosciences) before analysis on a FACS Calibur (BD Biosciences). Cells were cultured with 10 μg/mL F(ab′)2 or 10 μg/mL whole-molecule (WM) goat anti–mouse IgM (Jackson ImmunoResearch Laboratories, West Grove, PA). Allotype-specific anti–IgD Hδa/1 was provided by J. G. Monroe (Genentech, South San Francisco, CA) and used at a concentration of 10 μg/mL, and polyclonal goat anti–mouse IgD was from Bethyl Laboratories (Montgomery, TX) and used at a final dilution of 1:600. For biotin-avidin cross-linking studies, biotinylated anti-IgM (clone B7.6), biotinylated anti-FcγRIIB (clone 2.4G2), and/or biotinylated anti-CD22 (clone CY34) were used at 10 μg/mL and, after a 30-minute incubation at 37°C, immediately cross-linked with 10 μg/mL avidin.
Recombinant BLyS and BLyS receptor antibodies
Recombinant human BLyS was provided by Human Genome Sciences (Rockville, MD) and used at a final concentration of 100 ng/mL. PE-conjugated anti–mouse TACI, FITC-conjugated anti–mouse BCMA, PE-conjugated rat IgG2a isotype control, and FITC-conjugated rat IgG1 isotype control were purchased from R&D Systems (Minneapolis, MN). Unlabeled anti-BR3 or rat IgG1 isotype control was purchased from Axxora Life Sciences (San Diego, CA) and revealed using mouse anti–rat IgG1 FITC or PE from Southern Biotechnology (Birmingham, AL). AlexaFluor 647–conjugated anti–mouse BR3 and AlexaFluor 647–conjugated rat IgG1 isotype control were purchased from eBioscience. Biotinylated recombinant human BLyS was provided by Human Genome Sciences and revealed using streptavidin conjugated Red 670 (Invitrogen). Cells were stained with primary antibodies for 20 minutes in phosphate-buffered saline (PBS), 0.5% bovine serum albumin, and 2 mL of 0.5 M of ethylene diamine tetraacetic acid, followed by 10-minute incubation with secondary reagent. Samples were analyzed using a FACSCalibur (BD Biosciences) and FlowJo software (TreeStar, Ashland, OR).
Real-time polymerase chain reaction analysis
A total of 106 B cells were harvested and washed with cold PBS (Invitrogen). RNA was isolated using QIAshredder and Rneasy Mini Kit (QIAGEN, Valencia, CA) and reverse transcribed using random hexamers and Superscript II reverse transcriptase (Invitrogen). For real-time polymerase chain reaction (PCR) analysis, TaqMan Universal Master mix (Applied Biosystems, Foster City, CA) was used with primer/probe sets to murine gapdh, Pim 2, Mcl-1, or Bcl-2 (Applied Biosystems) and analyzed on an ABI 7300 real-time PCR system.
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis and Western blot analysis
Cells were harvested and washed with cold PBS (Invitrogen). Cells were lysed in a Tris-buffered saline/NaCl/Tween-20 lysis buffer solution consisting of 150 mM NaCl, 50 mM Tris-Hcl (pH 7.5; Invitrogen), and 1% Triton X-100 containing a Complete Mini Protease Inhibitor tablet (Roche Diagnostics, Basel, Switzerland). Lysates were boiled for 3 minutes in loading buffer containing 50 mM dithiothreitol and 1% sodium dodecyl sulfate (Bio-Rad, Hercules, CA). Samples were electrophoresed through 10% polyacrylamide gels using a Tris/glycine system.35 Gels were blotted onto an Immobilon-P polyvinylidene fluoride membrane (Millipore, Billerica, MA) and stained with antibodies to murine NFκB2/p100 (Cell Signaling Technology, Danvers, MA), Bcl-xL (Cell Signaling Technology), or β-actin (Sigma-Aldrich, St Louis, MO). Gel densitometry was performed using Quantity One software (Bio-Rad).
Nuclear and cytoplasmic fractionation
Briefly, cells were collected and washed with ice-cold PBS. The pellet was then incubated in 200 μL of buffer A (10 mM of N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 0.5% NP-40, 0.5 mM dichlorodiphenyltrichloroethane) for 20 minutes on ice. Cells were spun down, and the supernatant was collected as the cytoplasmic fraction. The pellet was then washed twice in buffer A and incubated in 50 μL of buffer C (20 mM of N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.9, 0.42 M NaCl, 1.5 mM MgCl, 0.2 mM of ethylenediaminetetraacetic acid, 25% glycerol, 0.5 mM of dichlorodiphenyltrichloroethane). The pellet was vortexed extensively and incubated on ice for 60 minutes. The samples were centrifuged at 12 000g for 15 minutes at 4°C, and the resulting supernatant was harvested as the nuclear fraction.
Statistics
Statistical analyses were performed using Student t test.
Results
FcγRIIB ligation attenuates BCR-mediated BLyS receptor up-regulation
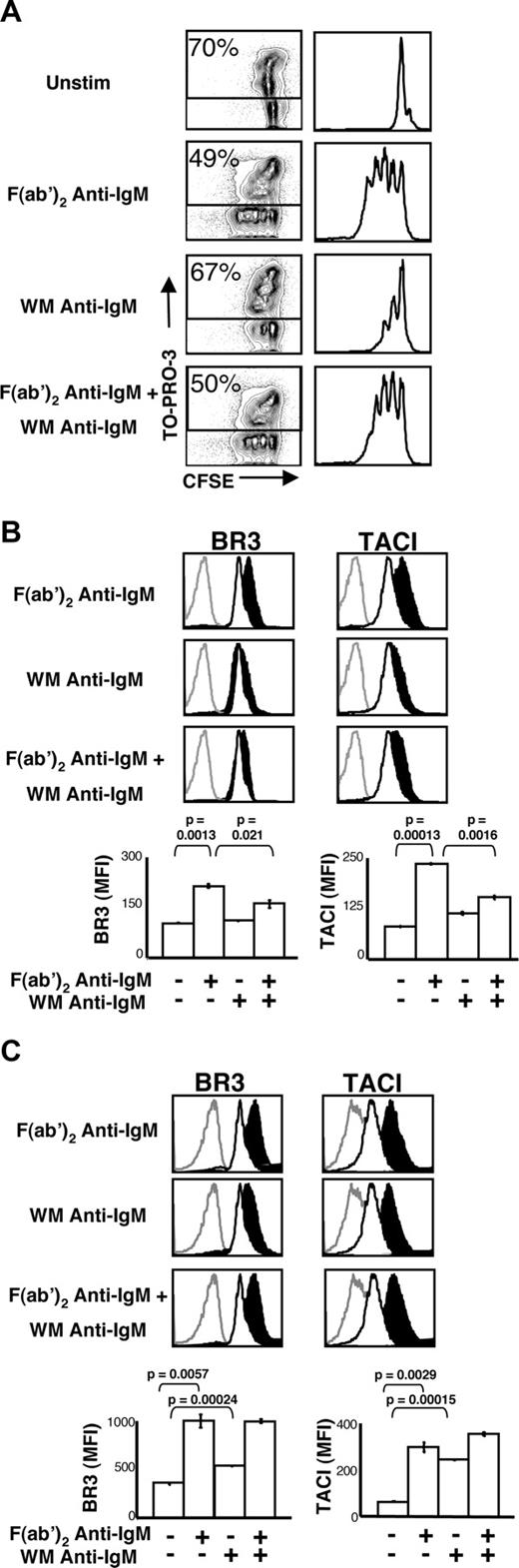
We used 2 BCR cross-linking reagents to assess the effect of FcγRIIB ligation on BLyS receptor expression: an F(ab′)2 fragment of polyclonal goat anti-IgM, which affords BCR cross-linking without concomitant FcγRIIB engagement; and its counterpart, an undigested WM anti-IgM antibody, which yields simultaneous BCR cross-linking and FcγRIIB ligation. Consistent with early findings of Phillips and Parker,36 cells stimulated with F(ab′)2 anti-IgM divided 3 or 4 times after 72 hours, whereas cells stimulated with WM anti-IgM underwent 1 or 2 divisions and exhibited a higher death rate (Figure 1A). Further, the combination of F(ab′)2 anti-IgM and WM anti-IgM showed intermediate proliferative activity.
Coligation of BCR and FcγRIIB does not increase BLyS receptor expression and attenuates BCR-induced effects. (A) Proliferation and death profiles of BALB/c CD23+ B cells 72 hours after stimulation. B cells were loaded with CFSE and cultured with indicated stimuli (concentrations of stimuli are indicated in “B-cell subset isolation, culture, and stimulation”). One minute before analysis, TO-PRO-3 was added as a vital dye. CFSE profiles of live (TO-PRO-3−) cells are shown at right. (B) CD23+ B cells were cultured with the indicated stimuli. After 24 hours, the cells were harvested and analyzed by flow cytometry for BR3 and TACI expression (BCMA expression was negligible) on live cells. Gray histograms indicate isotype control; unfilled histograms, unstimulated cell expression levels; filled black histograms, expression by stimulated cells. Median fluorescence intensities (MFI) of these populations are shown in bar graphs at bottom. (C) CD23+ B cells were cultured with the indicated stimuli. After 36 hours, the cells were harvested and analyzed by flow cytometry for BR3 and TACI expression (BCMA expression was negligible) on live cells. Gray histograms indicate isotype control staining; unfilled histograms, unstimulated cell expression levels; filled black histograms, stimulated cell expression levels. Populations' median fluorescence intensities (MFI) are shown in bar graphs at bottom. Plots are representative of at least 3 experiments; in all cases, n equals 3 or more. Significance levels are indicated by P values.
Coligation of BCR and FcγRIIB does not increase BLyS receptor expression and attenuates BCR-induced effects. (A) Proliferation and death profiles of BALB/c CD23+ B cells 72 hours after stimulation. B cells were loaded with CFSE and cultured with indicated stimuli (concentrations of stimuli are indicated in “B-cell subset isolation, culture, and stimulation”). One minute before analysis, TO-PRO-3 was added as a vital dye. CFSE profiles of live (TO-PRO-3−) cells are shown at right. (B) CD23+ B cells were cultured with the indicated stimuli. After 24 hours, the cells were harvested and analyzed by flow cytometry for BR3 and TACI expression (BCMA expression was negligible) on live cells. Gray histograms indicate isotype control; unfilled histograms, unstimulated cell expression levels; filled black histograms, expression by stimulated cells. Median fluorescence intensities (MFI) of these populations are shown in bar graphs at bottom. (C) CD23+ B cells were cultured with the indicated stimuli. After 36 hours, the cells were harvested and analyzed by flow cytometry for BR3 and TACI expression (BCMA expression was negligible) on live cells. Gray histograms indicate isotype control staining; unfilled histograms, unstimulated cell expression levels; filled black histograms, stimulated cell expression levels. Populations' median fluorescence intensities (MFI) are shown in bar graphs at bottom. Plots are representative of at least 3 experiments; in all cases, n equals 3 or more. Significance levels are indicated by P values.
Consistent with previous findings,37 BLyS binding capacity increased with BCR stimulation after 24 hours in culture (not shown), reflecting increases in both BR3 and TACI (Figure 1B). In contrast, WM anti-IgM stimulation altered neither BLyS binding capacity nor BLyS receptor levels (Figure 1B). Further, combined stimulation blunted the increases in BLyS receptor expression seen with F(ab)′2 anti-IgM alone (Figure 1B).
The lack of BLyS receptor up-regulation after BCR and FcγRIIB coligation may represent delayed expression kinetics or a complete block of receptor expression. To address these possibilities, we investigated BLyS receptor expression on B cells cultured with F(ab′)2 anti-IgM, WM anti-IgM, or both for 36 hours, the time point just before division. As shown in Figure 1C, increased BR3 and TACI expression levels are seen at 36 hours but fail to reach the levels seen induced by F(ab′)2 anti-IgM.
To more carefully define the B-cell subsets subject to this regulatory mechanism, we performed studies with FACS-sorted FO, marginal zone (MZ),38 and B1 B cells (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). As anticipated, WM anti-IgM blunted BLyS receptor up-regulation in FO B cells. MZ cells displayed similar behavior, despite considerable cell death by 24 hours after stimulation. These observations are consistent with prior reports that MZ B cells exhibit enhanced early BCR signaling events39 but then proliferate and survive poorly.38 In contrast to FO and MZ B cells, the BLyS receptor expression profiles of splenic B1 B cells were unchanged regardless of treatment. In agreement with earlier observations,26 BCMA surface expression was negligible in all primary B cells examined and did not change on treatment (data not shown).
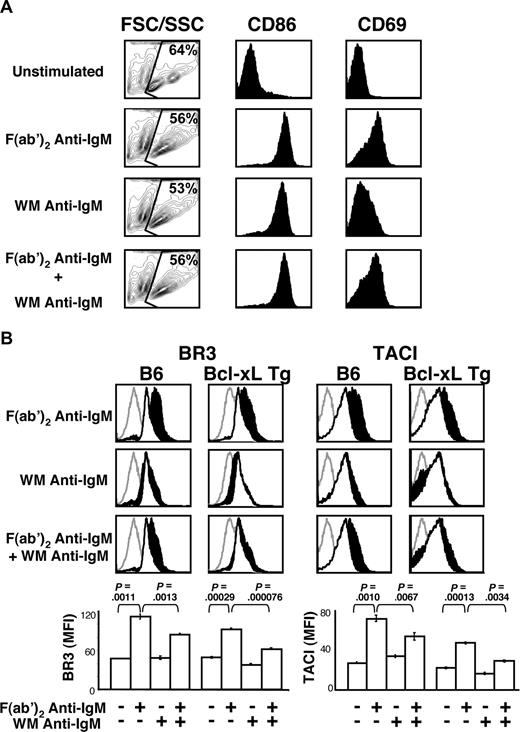
Attenuated BLyS receptor up-regulation did not reflect differences in cell-cycle status, as our analyses were carried out 24 hours after stimulation, a time point when the cells have not begun to divide (data not shown). Further, this effect does not reflect global inhibition of B-cell activation because all treatments yielded comparable levels of blast formation (Figure 2A far left column) and CD86 increases, although attenuation of CD69 up-regulation was seen in WM anti-IgM–stimulated cells (Figure 2A middle and rightmost columns). Nonetheless, it remains possible that BLyS receptor up-regulation depends uniquely on PIP3 generation, diacylglycerol generation, calcium mobilization, or Ras activation, as these events are targeted by FcγRIIB signals. Finally, because similar levels of cell death were observed across all treatments at 24 hours (Figure 2A leftmost column) and B cells from Bcl-xL transgenic mice34 yielded identical results (Figure 2B), differential cell death did not underlie these observations.
B cells stimulated with F(ab′)2 or WM anti-IgM show similar levels of activation and lack of BLyS receptor up-regulation is not because of increased cell death. (A) CD23+ B cells were cultured with the indicated stimuli (concentrations of stimuli are indicated in “B-cell subset isolation, culture, and stimulation”). After 24 hours, B cells were harvested and analyzed by FACS for forward side scatter/side scatter (FSC/SSC), CD86, and CD69 expression on live cells. Blasting profiles (far left column) have FSC displayed on the x-axis and SSC displayed on the y-axis. Live cells were gated and the percentage of live cells shown. Histograms of CD86 and CD69 expression on live cells are shown in the middle and right columns. (B) CD23+ B cells from Bcl-xL transgenic or control C57BL/6 mice were cultured with the indicated stimuli. After 24 hours, the cells were harvested and analyzed by FACS for BR3 and TACI expression on live cells. Gray histograms indicate isotype control; unfilled white histograms, levels expressed by unstimulated; filled black histograms, levels expressed by stimulated cells. Median fluorescence intensities (MFI) of respective cell populations are shown in bar graphs at bottom. Plots are representative of 3 independent experiments; in all cases, n equals 3 or more. Significance levels are indicated by P values.
B cells stimulated with F(ab′)2 or WM anti-IgM show similar levels of activation and lack of BLyS receptor up-regulation is not because of increased cell death. (A) CD23+ B cells were cultured with the indicated stimuli (concentrations of stimuli are indicated in “B-cell subset isolation, culture, and stimulation”). After 24 hours, B cells were harvested and analyzed by FACS for forward side scatter/side scatter (FSC/SSC), CD86, and CD69 expression on live cells. Blasting profiles (far left column) have FSC displayed on the x-axis and SSC displayed on the y-axis. Live cells were gated and the percentage of live cells shown. Histograms of CD86 and CD69 expression on live cells are shown in the middle and right columns. (B) CD23+ B cells from Bcl-xL transgenic or control C57BL/6 mice were cultured with the indicated stimuli. After 24 hours, the cells were harvested and analyzed by FACS for BR3 and TACI expression on live cells. Gray histograms indicate isotype control; unfilled white histograms, levels expressed by unstimulated; filled black histograms, levels expressed by stimulated cells. Median fluorescence intensities (MFI) of respective cell populations are shown in bar graphs at bottom. Plots are representative of 3 independent experiments; in all cases, n equals 3 or more. Significance levels are indicated by P values.
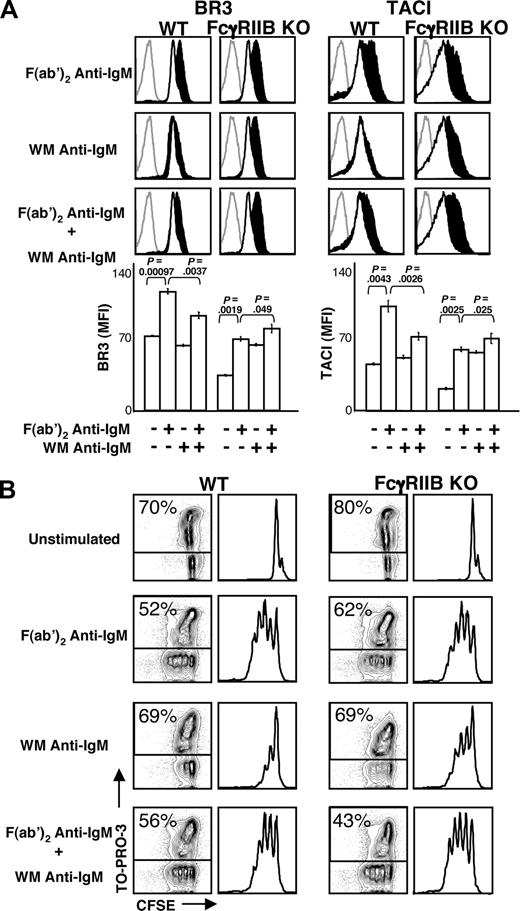
The role of FcγRIIB in dampening BLyS receptor up-regulation was interrogated directly with FcγRIIB KO mice. Both BR3 and TACI are expressed on resting FcγRIIB KO B cells, and their levels increased after stimulation with F(ab′)2 anti-IgM (Figure 3A). However, KO mice showed no attenuation of BLyS receptor up-regulation after whole anti-IgM or combined stimuli. Proliferation and viability (Figure 3B) were in accord with these findings, as robust proliferation was seen among KO B cells stimulated with WM anti-IgM either alone or in conjunction with the F(ab′)2 anti-IgM antibody. Together, these findings identify FcγRIIB as a negative regulator of the BR3 and TACI up-regulation that follows BCR cross-linking.
FcγRIIB cross-linking is required for the attenuation of BLyS receptor expression. (A) CD23+ B cells from FcγRIIB knockout (KO) or BALB/c wild-type (WT) mice were cultured with the indicated stimuli (concentrations of stimuli are indicated in “B-cell subset isolation, culture, and stimulation”). After 24 hours, the cells were harvested and analyzed by FACS for BR3 and TACI expression. Gray histograms indicate isotype controls; unfilled white histograms, expression by unstimulated cells; filled black histograms, expression by stimulated cells. Median fluorescence intensities (MFI) of respective populations are shown in bar graphs at bottom. Plots are representative of at least 3 experiments; in all cases, n equals 3 or more. Significance levels are indicated by P values. (B) Proliferation and death profiles of CD23+ B cells from FcγRIIB KO or WT mice 72 hours after stimulation. B cells were loaded with CFSE and cultured with indicated stimuli as in Figure 1. At 1 minute before analysis, TO-PRO-3 was added as a vital dye. CFSE profiles of live (TO-PRO-3−) cells are shown at right. Plots are representative of at least 3 independent experiments.
FcγRIIB cross-linking is required for the attenuation of BLyS receptor expression. (A) CD23+ B cells from FcγRIIB knockout (KO) or BALB/c wild-type (WT) mice were cultured with the indicated stimuli (concentrations of stimuli are indicated in “B-cell subset isolation, culture, and stimulation”). After 24 hours, the cells were harvested and analyzed by FACS for BR3 and TACI expression. Gray histograms indicate isotype controls; unfilled white histograms, expression by unstimulated cells; filled black histograms, expression by stimulated cells. Median fluorescence intensities (MFI) of respective populations are shown in bar graphs at bottom. Plots are representative of at least 3 experiments; in all cases, n equals 3 or more. Significance levels are indicated by P values. (B) Proliferation and death profiles of CD23+ B cells from FcγRIIB KO or WT mice 72 hours after stimulation. B cells were loaded with CFSE and cultured with indicated stimuli as in Figure 1. At 1 minute before analysis, TO-PRO-3 was added as a vital dye. CFSE profiles of live (TO-PRO-3−) cells are shown at right. Plots are representative of at least 3 independent experiments.
FcγRIIB coligation with either IgM or IgD attenuates BLyS receptor up-regulation in a SHIP1-dependent manner
Blunted BLyS receptor increases might reflect direct regulation of BLyS receptor expression by FcγRIIB signals. Alternatively, dampened BCR signal propagation might limit the usual downstream effects on BLyS receptor transcription. Indeed, most regulatory effects of FcγRIIB require BCR coligation, Lyn activation,40,41 and SHIP recruitment, presumably reflecting the need for local cooperative interactions between the membrane-proximal mediators of BCR signaling.
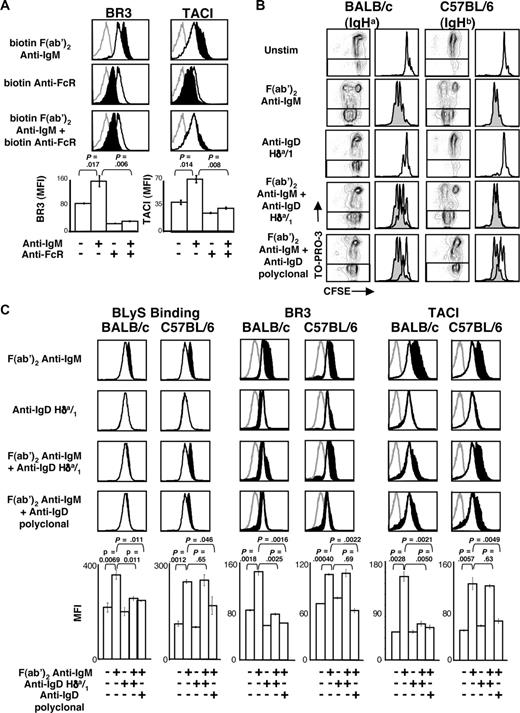
To confirm a direct role for FcγRIIB and to establish whether coligation with the BCR is required, we cultured B cells with biotinylated F(ab′)2 anti-IgM, anti-FcγRIIB, or both and cross-linked these antibodies with avidin. These combinations afforded stimulation exclusively through IgM, FcγRIIB, or both receptors. As expected, avidin cross-linked F(ab′)2 anti-IgM induced increased expression of BR3 and TACI, whereas anti-FcγRIIB cross-linking alone did not (Figure 4A). However, concomitant addition and cross-linking of both reagents abrogated the BLyS receptor increases seen with the anti-F(ab′)2 IgM alone. Thus, coaggregation of FcγRIIB with BCR is required to dampen the BCR-mediated increase in BLyS receptor surface expression.
BCR and FcγRIIB coaggregation are required for the attenuation of BLyS receptor expression. (A) CD23+ B cells were cultured with biotinylated anti-IgM, biotinylated anti-FcγRIIB, or both, and cross-linked with avidin as described in “B-cell subset isolation, culture, and stimulation.” After 24 hours, the cells were harvested and analyzed by FACS for BR3 and TACI expression on live cells. Fluorescence histograms are shown at the top, and MFIs are shown in bar graphs at the bottom. (B) CD23+ B cells from BALB/c or C57BL/6 mice were cultured with the indicated stimuli. Proliferation and death profiles of CD23+ B cells from BALB/c or C57BL/6 mice are shown at 72 hours after stimulation. B cells were loaded with CFSE and cultured with indicated stimuli. One minute before analysis, TO-PRO-3 was added as a vital dye. CFSE profiles of live (TO-PRO-3−) cells are shown at right and F(ab′)2 anti-IgM histograms shaded and overlaid to highlight effects of anti-IgD reagents. (C) CD23+ B cells from BALB/c or C57BL/6 mice were analyzed by FACS for BLyS binding or anti-BLyS receptor staining on live cells at 24 hours after stimulation. Gray histograms indicate isotype control staining; unfilled histograms, unstimulated cell levels; filled black histograms, stimulated cell levels. Median fluorescence intensities (MFI) of populations are shown in bar graphs at the bottom. Plots are representative of at least 3 experiments; in all cases, n equals 3 or more. Significance levels are indicated by P values.
BCR and FcγRIIB coaggregation are required for the attenuation of BLyS receptor expression. (A) CD23+ B cells were cultured with biotinylated anti-IgM, biotinylated anti-FcγRIIB, or both, and cross-linked with avidin as described in “B-cell subset isolation, culture, and stimulation.” After 24 hours, the cells were harvested and analyzed by FACS for BR3 and TACI expression on live cells. Fluorescence histograms are shown at the top, and MFIs are shown in bar graphs at the bottom. (B) CD23+ B cells from BALB/c or C57BL/6 mice were cultured with the indicated stimuli. Proliferation and death profiles of CD23+ B cells from BALB/c or C57BL/6 mice are shown at 72 hours after stimulation. B cells were loaded with CFSE and cultured with indicated stimuli. One minute before analysis, TO-PRO-3 was added as a vital dye. CFSE profiles of live (TO-PRO-3−) cells are shown at right and F(ab′)2 anti-IgM histograms shaded and overlaid to highlight effects of anti-IgD reagents. (C) CD23+ B cells from BALB/c or C57BL/6 mice were analyzed by FACS for BLyS binding or anti-BLyS receptor staining on live cells at 24 hours after stimulation. Gray histograms indicate isotype control staining; unfilled histograms, unstimulated cell levels; filled black histograms, stimulated cell levels. Median fluorescence intensities (MFI) of populations are shown in bar graphs at the bottom. Plots are representative of at least 3 experiments; in all cases, n equals 3 or more. Significance levels are indicated by P values.
To rule out potential contributions from other regulators of BCR signaling that might fortuitously coaggregate in this system, we investigated the role of CD2242-44 in BLyS receptor regulation with a biotin-conjugated anti-CD22 antibody. This yielded similar levels of BR3 and TACI as cells receiving anti-IgM alone (Figure S2). When added together, anti-CD22 augmented the effect of anti-IgM, in accord with previous studies showing that CD22 ligation can enhance the anti-IgM response.45,46 This may reflect sequestration of CD22, thereby blunting its constitutive negative effects.
To determine whether FcγRIIB coligation with either primary BCR isotype could dampen BLyS receptor up-regulation, we repeated our experiments using the IgHa-specific anti-IgD antibody, Hδa/1. This reagent would coaggregate IgD and FcγRIIB on B cells from BALB/c (IgHa) but not C57BL/6 (IgHb) mice, thus allowing the C57BL/6 cells to serve as controls for nonspecific effects of the reagent. To confirm that Hδa/1 would mirror the biologic actions of WM anti-IgM in an allotype-specific manner, we assessed its effects on proliferation and viability among B cells from BALB/c or C57BL/6 mice. As expected, B cells from both strains responded similarly to the F(ab′)2 anti-IgM antibody (Figure 4B), whereas stimulation with Hδa/1 yielded moderate proliferation only in BALB/c B cells. Furthermore, F(ab′)2 anti-IgM–induced proliferation was attenuated only in the BALB/c cells when combined with Hδa/1.
Both C57BL/6 and BALB/c B cells expressed resting BLyS binding, BR3, and TACI levels that increased after F(ab′)2 anti-IgM stimulation (Figure 4C). Stimulation with Hδa/1 did not alter BLyS binding or receptor expression on BALB/c B cells, although in some experiments, BR3 levels dropped slightly (data not shown). Consistent with the notion that downstream signaling for IgM and IgD is similar,47,48 F(ab′)2 anti-IgM and Hδa/1 together blunted BLyS binding, BR3, and TACI up-regulation in BALB/c, but not C57BL/6 B, cells. A polyclonal anti-IgD antibody was used as a control for Hδa/1 and blunted receptor expression on BALB/c and C57BL/6 B cells. This transinhibition observed when Hδa/1 was combined with IgM cross-linking did not reflect residual anti–light chain activity, as Ig-κ KO mice ruled out this possibility. At 24 hours after stimulation with F(ab′)2 anti-IgM and Hδa/1, the BLyS binding levels of Ig-κ KO B cells were equivalent to the levels observed with wild-type control cells (data not shown).
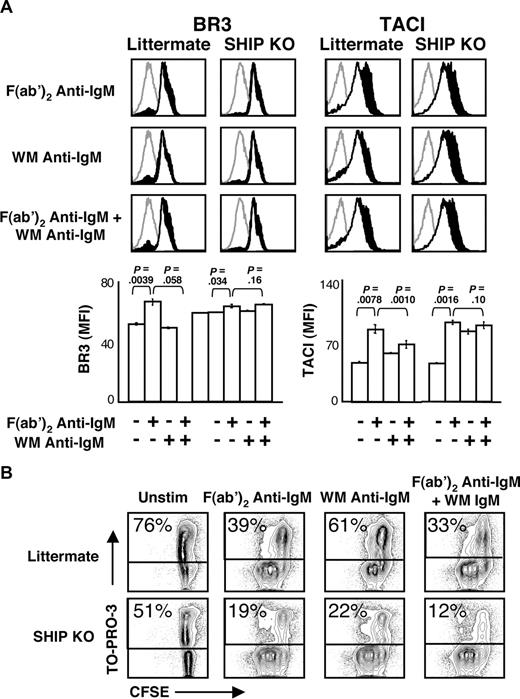
Most inhibitory effects of FcγRIIB require immunoreceptor tyrosine-based inhibitory motif phosphorylation and consequent recruitment and function of SHIP1.10 In accord with this mechanism, stimulation of SHIP1 KO B cells failed to diminish BLyS receptor up-regulation compared with littermate controls (Figure 5A). Interestingly, BR3 expression on SHIP KO B cells is somewhat higher than in wild-type mice and changed little after this stimulation. This may reflect freedom from a basal level of SHIP and, by extension, FcγRIIB regulation. In contrast, TACI levels increased dramatically regardless of stimulation. These data suggest a possible role for SHIP1 in the regulation of TACI but not BR3 up-regulation. Further investigation is needed to address these differential roles, and a B cell–specific SHIP KO mouse may be required to definitively address this question.
SHIP activation is required for attenuation of BLyS receptor expression. (A) CD23+ B cells from SHIP KO or littermate control mice were cultured with the indicated stimuli (concentrations of stimuli are indicated in “B-cell subset isolation, culture, and stimulation”). Cells were analyzed by FACS for BLyS receptor expression on live cells at 24 hours after stimulation. Histograms are shown at the top. Gray histograms indicate isotype control; unfilled histograms, unstimulated cell levels; filled black histograms, stimulated cell levels. Median fluorescence intensities (MFI) of populations are shown in bar graphs at bottom. Plots are representative of 2 independent experiments; in all cases, n equals 3 or more. Significance levels are indicated by P values. (B) Proliferation and death profiles of CD23+ B cells from SHIP KO or littermate control mice 72 hours after stimulation. B cells were loaded with CFSE and cultured with indicated stimuli. One minute before FACS analysis, TO-PRO-3 was added as a vital dye. Plots are representative of 2 independent experiments.
SHIP activation is required for attenuation of BLyS receptor expression. (A) CD23+ B cells from SHIP KO or littermate control mice were cultured with the indicated stimuli (concentrations of stimuli are indicated in “B-cell subset isolation, culture, and stimulation”). Cells were analyzed by FACS for BLyS receptor expression on live cells at 24 hours after stimulation. Histograms are shown at the top. Gray histograms indicate isotype control; unfilled histograms, unstimulated cell levels; filled black histograms, stimulated cell levels. Median fluorescence intensities (MFI) of populations are shown in bar graphs at bottom. Plots are representative of 2 independent experiments; in all cases, n equals 3 or more. Significance levels are indicated by P values. (B) Proliferation and death profiles of CD23+ B cells from SHIP KO or littermate control mice 72 hours after stimulation. B cells were loaded with CFSE and cultured with indicated stimuli. One minute before FACS analysis, TO-PRO-3 was added as a vital dye. Plots are representative of 2 independent experiments.
To further confirm the role of SHIP1 in B-cell activation, we assessed the proliferation and viability of B cells after culture with F(ab′)2 anti-IgM, WM anti-IgM, or the combination of the 2 stimuli (Figure 5B). As expected, cells from littermate control mice stimulated with WM anti-IgM antibody divided only 1 or 2 times, whereas the SHIP KO cells divided 3 or 4 times.
FcγRIIB coligation impacts BLyS signaling and responsiveness
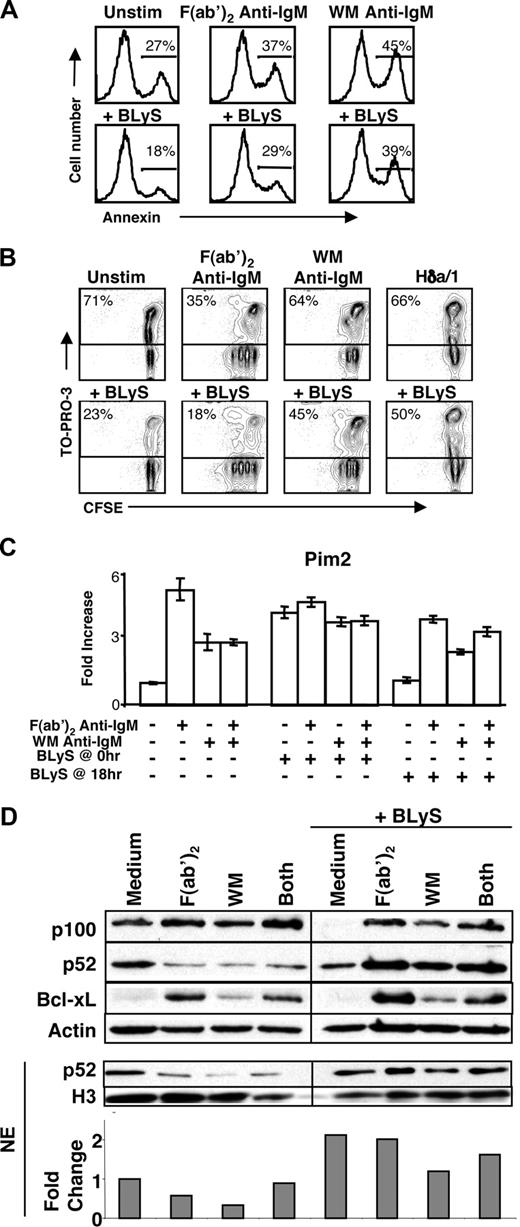
BR3 levels determine the survival, lifespan, and competitive fitness of primary B cells in vitro and in vivo.17 Accordingly, we addressed the biologic relevance of BCR and FcγRIIB coligation on BLyS-induced survival and signaling. To determine whether FcγRIIB/BCR coaggregation impacts BLyS-induced survival, B cells were stimulated with various BCR stimuli with or without BLyS for 24 hours (Figure 6A) or 72 hours (Figure 6B) and responses assessed. Consistent with prior reports, BLyS enhanced the survival of both quiescent and F(ab′)2 anti-IgM–stimulated B cells.37 In contrast, cells stimulated with either WM anti-IgM or Hδa/1 responded less strongly, and a similar attenuation in BLyS-mediated survival was observed in cultures stimulated with combined F(ab′)2 and WM anti-IgM (data not shown). Because resting and WM anti-IgM–stimulated B cells express equivalent levels of BLyS receptor, these results indicate that FcγRIIB signals mediated by SHIP1 probably inhibit BLyS responses by 2 distinct mechanisms: inhibition of receptor up-regulation and inhibition of BLyS receptor signaling.
BCR and FcγRIIB coligation reduces cellular responsiveness to BLyS. (A) Annexin V staining of C57BL/6 CD23+ B cells cultured for 24 hours with various stimuli (concentrations of stimuli are indicated in “B-cell subset isolation, culture, and stimulation”). B cells were stained with annexin V and analyzed by flow cytometry. (B) Proliferation and death profiles of CD23+ B cells cultured with and without rBLyS for 72 hours. B cells were loaded with CFSE and cultured with indicated stimuli. One minute before FACS analysis, TO-PRO-3 was added as a vital dye. Plots are representative of at least 3 independent experiments. (C) Pim 2 message levels after 36-hour culture with the indicated stimuli, with and without the addition of rBLyS at time 0 hour (hr) or time 18 hours (concentrations of stimuli are indicated in “B-cell subset isolation, culture, and stimulation”). (D) Top blot shows p100 processing and Bcl-xL levels, as assessed by Western blot after 24 hours of culture with the indicated anti-IgM stimuli and the addition of recombinant human BLyS at 0 hours. NE indicates nuclear extract. Bottom blot and densitometry show nuclear p52 levels after the same culture period, normalized to histone H3. Data are representative of at least 3 independent experiments.
BCR and FcγRIIB coligation reduces cellular responsiveness to BLyS. (A) Annexin V staining of C57BL/6 CD23+ B cells cultured for 24 hours with various stimuli (concentrations of stimuli are indicated in “B-cell subset isolation, culture, and stimulation”). B cells were stained with annexin V and analyzed by flow cytometry. (B) Proliferation and death profiles of CD23+ B cells cultured with and without rBLyS for 72 hours. B cells were loaded with CFSE and cultured with indicated stimuli. One minute before FACS analysis, TO-PRO-3 was added as a vital dye. Plots are representative of at least 3 independent experiments. (C) Pim 2 message levels after 36-hour culture with the indicated stimuli, with and without the addition of rBLyS at time 0 hour (hr) or time 18 hours (concentrations of stimuli are indicated in “B-cell subset isolation, culture, and stimulation”). (D) Top blot shows p100 processing and Bcl-xL levels, as assessed by Western blot after 24 hours of culture with the indicated anti-IgM stimuli and the addition of recombinant human BLyS at 0 hours. NE indicates nuclear extract. Bottom blot and densitometry show nuclear p52 levels after the same culture period, normalized to histone H3. Data are representative of at least 3 independent experiments.
To explore which aspects of BLyS signaling and function are impacted by FcγRIIB coligation, we assessed several downstream mediators of BLyS-mediated survival. Because Pim 2 transcription increases after BLyS stimulation,26,49-51 we investigated Pim 2 expression after F(ab′)2 or WM anti-IgM treatments. Pim 2 message levels were analyzed by real-time PCR after a 36-hour stimulation. BLyS was added to the cultures either at the start (t = 0 hours) or at the time when surface BLyS receptor signatures had changed (t = 18 hours; Figure 6C). We observed maximal Pim 2 expression when BLyS was added at the beginning of culture, consistent with a role for Pim 2 in BLyS-mediated B-cell survival.52 However, when BLyS was added at 18 hours after initial stimulation, only cells stimulated with F(ab′)2 anti-IgM maintained Pim 2 expression, whereas those receiving WM anti-IgM did not.
BLyS activates the nonclassical NF-κB pathway in FO B cells, affording p100 processing to the active p52 form.53 We therefore assessed total and nuclear p52 levels after BCR and FcγRIIB coligation in concert with BLyS (Figure 6D).53 BLyS induced p100 processing after 24 hours of culture, regardless of F(ab′)2 or WM anti-IgM stimulation. However, less p52 processing was consistently observed in the total cell extract or nuclear extract after WM anti-IgM stimulation. We also assessed the levels of Bcl-xL in B cells after 24 hours of culture with and without the addition of BLyS. Similarly, Bcl-xL levels increased in B cells stimulated with either antibody, but F(ab′)2 anti-IgM yielded significantly more Bcl-xL than WM anti-IgM stimulation. Further, the addition of BLyS to cultures stimulated with both F(ab′)2 anti-IgM and WM anti-IgM yielded an intermediate level of Bcl-xL expression. We also investigated the expression of Mcl-1 and Bcl-2 under these conditions (Figure S3). As previously reported,26,49 both Mcl-1 and Bcl-2 increase in response to BLyS alone. Surprisingly, these increases were both blocked by either form of anti-IgM alone, precluding any role for FcγRIIB coligation in the regulation of Bcl-2 and Mcl-1 responses to BLyS signaling.
Discussion
These studies examine how FcγRIIB signaling influences BLyS receptor levels and responsiveness. The results indicate that FcγRIIB coaggregation dampens BCR-mediated BLyS receptor up-regulation. This requires coligation of FcγRIIB with either IgM or IgD and operates via a SHIP1-dependent mechanism. Moreover, downstream BLyS signaling is reduced after FcγRIIB/BCR coaggregation, negatively impacting the survival-promoting effects of BLyS. Together, these findings link the negative regulatory activities of FcγRIIB with the survival functions of BLyS and its receptors.
Several mechanisms contribute to this relationship. Because B cells with reduced functional BR3 levels compete poorly for survival,54 attenuated BLyS responsiveness may directly reflect lowered BLyS receptor levels and signaling. In addition, FcγRIIB signals may block BLyS signaling by reducing levels of active downstream effectors or second messenger, most notably, PIP3. BLyS-mediated survival requires PIP3, a critical second messenger that is hydrolyzed by SHIP.32,55 Similarly, signals that directly maintain antiapoptotic mediators or cross-talk between downstream signaling systems, such as adaptor proteins or components within the NF-κB pathways, may be impacted. In addition, the direct up-regulation of apoptotic factors by FcγRIIB ligation may counter survival signals delivered by BLyS. FcγRIIB ligation also activates SHIP256 in blasting B cells. Although not interrogated here, a connection with SHIP2 might follow anti-IgM stimulation, influencing the ability of BLyS to mediate cell-cycle status among activated B cells. Finally, FcγRIIB-mediated SHP1 activation may play a role in this response. These possibilities are not mutually exclusive, and it is probable that all may contribute to the overall reduction in BLyS-induced survival.
It is noteworthy that both the BLyS-BLyS receptor axis and FcγRIIB signaling are involved in balancing BCR-mediated selection processes among developing and activated B cells. Indeed, either FcγRIIB deficiency or BLyS overexpression can yield systemic humoral autoimmunity, and elevated BLyS levels are associated with several autoimmune syndromes.27 Thus, one route to immune dysfunction may involve situations in which small perturbations in one of these systems are magnified by concomitant dysregulation in the other. Several observations are consistent with this possibility. For example, a critical factor in peripheral tolerance is the silencing of autoreactive B cells from the transitional stage through anergy, and anergic B cells can be rescued in an environment containing high levels of BLyS,50,57 even though SHIP is activated in these cells.58,59 Similarly, consistent with this idea, the expression of FcγRIIB is down-regulated on memory and plasma cells in systemic lupus erythematosus patients, perhaps allowing BLyS-mediated survival of self-reactive cells that would otherwise die.60
Another intersection between FcγRIIB and BLyS signaling is in the GC reaction. Experimental manipulation of either system impacts GC maintenance and the selection of somatically hypermutated B-cell clones.6,28,29 It is tempting to speculate that FcγRIIB-mediated BLyS receptor down-regulation plays a role in determining competitive advantage in the GC. Proper BLyS levels and BR3 function are crucial for normal GC reactions,28,29 and GC B cells express BR3 (J.E.C., unpublished data, 2007). Thus, if BLyS is a survival signal for which GC B cells compete, then the ratio of BCR to FcγRIIB signals may determine BR3 levels and, hence, the relative ability to capture BLyS and survive.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Thi-Sau Migone (Human Genome Sciences) for supplying rhBLyS, Drs Fred D. Finkelman and John G. Monroe for anti-IgD reagents, Patrick J. O'Neill for technical assistance, and Drs Avinash Bhandoola and Jean Scholz for helpful discussions and critical reading of the manuscript.
This work was supported by US Public Health Service research grants R01 AG013983 (J.C.C.) and AI0544898 (M.P.C.) and training grants T32-AI-055428 (J.E.C.) and HL07971 (J.E.S.). J.C.C. is an Ida and Cecil Green endowed Professor of Immunology.
National Institutes of Health
Authorship
Contribution: J.E.C. designed and performed the research, analyzed the data, and wrote the paper; J.E.S. performed the research, analyzed data, and wrote portions of the paper; J.C.C. contributed critical reagents and provided help in preparing the manuscript; and M.P.C. designed the research, guided the data analysis, and wrote portions of the paper.
Conflict-of-interest disclosure: M.P.C. holds a sponsored research agreement with Human Genome Sciences. The remaining authors declare no competing financial interests.
Correspondence: Michael P. Cancro, 284 John Morgan, Department of Pathology and Laboratory Medicine, University of Pennsylvania School of Medicine, 3620 Hamilton Walk, Philadelphia, PA 19104; e-mail: cancro@mail.med.upenn.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal