Abstract
Relapse of malignancy after allogeneic hematopoietic cell transplantation (allo-HCT) remains a therapeutic challenge. Blockade of the CTLA4 molecule can effectively augment antitumor immunity mediated by autologous effector T cells. We have assessed the safety and preliminary efficacy of a neutralizing, human anti-CTLA4 monoclonal antibody, ipilimumab, in stimulating the graft-versus-malignancy (GVM) effect after allo-HCT. Twenty-nine patients with malignancies that were recurrent or progressive after allo-HCT, received ipilimumab as a single infusion at dose cohorts between 0.1 and 3.0 mg/kg. Dose-limiting toxicity was not encountered, and ipilimumab did not induce graft-versus-host disease (GVHD) or graft rejection. Organ-specific immune adverse events (IAE) were seen in 4 patients (grade 3 arthritis, grade 2 hyperthyroidism, recurrent grade 4 pneumonitis). Three patients with lymphoid malignancy developed objective disease responses following ipilimumab: complete remission (CR) in 2 patients with Hodgkin disease and partial remission (PR) in a patient with refractory mantle cell lymphoma. At the 3.0 mg/kg dose, active serum concentrations of ipilimumab were maintained for more than 30 days after a single infusion. Ipilimumab, as administered in this clinical trial, does not induce or exacerbate clinical GVHD, but may cause organ-specific IAE and regression of malignancy. This study is registered at http://clinicaltrials.gov under NCI protocol ID P6082.
Introduction
Adoptive immunotherapy in the form of allogeneic hematopoietic cell transplantation (allo-HCT) can cure several hematologic malignancies. However, relapse or progression of malignancy (RM) is an important cause of treatment failure and mortality after allo-HCT.1,2 RM may be a particularly challenging problem in patients with advanced malignancies who underwent transplantation and in patients who underwent transplantation using reduced-intensity conditioning (RIC) regimens.2 Inadequate costimulation of T cells may be one mechanism underlying failure of adoptive immunotherapy after allo-HCT. Expression of CTLA4 is induced on T cells upon activation. It competes with costimulatory receptor CD28 for the B7 ligands CD80 and CD86 on antigen-presenting cells. Through this and other mechanisms, CTLA4 functions as an important negative regulator of the duration and intensity of antigen-specific T-cell responses.3,4 CTLA4 is an important mediator of peripheral self-tolerance and tolerance to tumor antigens. Mice genetically devoid of CTLA4 develop fatal lymphoproliferation and autoimmunity.5 Antibody-mediated blockade of CTLA4 in murine models can result in tumor regression and seems to augment the efficacy of antitumor vaccines.6,7
Ipilimumab is a fully human immunoglobulin G1 (IgG1) monoclonal antibody that antagonizes CTLA4 (Medarex, Bloomsbury, NJ, and Bristol-Myers Squibb, Wallingford, CT). Human clinical trials of this antibody in several solid tumors, especially in advanced melanoma have demonstrated regression of malignancy that can be durable.8-20 These responses often occur in conjunction with organ-specific autoimmune phenomena (immune adverse events [IAE]). Tumor regressions seen can be very delayed and may even be preceded by initial disease progression, emphasizing the immune mechanism underlying the responses seen.
T cell–replete allo-HCT relies predominantly upon antigen-specific T-cell responses to generate a graft-versus-malignancy (GVM) effect. CTLA4 blockade in this context could potentially augment the GVM effect and reverse or prevent RM. Furthermore, the existence of differences in histocompatibility antigens between donor and recipient may result in greater efficacy for this strategy than in the autologous setting. However, immune complications unique to allo-HCT (eg, graft-versus-host disease [GVHD] and graft rejection) may potentially also be stimulated. In a murine model of major histocompatibility complex (MHC) disparate allogeneic transplantation, the use of an antagonistic anti-CTLA4 antibody early in the course of the transplantation led to increased GVHD or graft rejection depending upon the intensity of conditioning. However, the delayed administration of the same antibody produced only limited GVHD, while resulting in a powerful enhancement of the graft-versus-leukemia effect against host-derived acute myeloid leukemia (AML) cells.21
To assess the efficacy of ipilimumab as a means of augmenting GVM reactions, it is first important to establish a safe dose of the antibody in the setting of allo-HCT. Here we report the results of a dose-escalation trial designed to assess the safety and preliminary efficacy of ipilimumab in patients with RM after allo-HCT.
Methods
Eligibility criteria
All patients were older than 14 years of age and had previously undergone allo-HCT from a matched sibling or matched unrelated donor for a malignant disease. Patients were eligible if they demonstrated RM more than 90 days after their last infusion of allogeneic cells (allo-HCT or donor lymphocyte infusion [DLI]) and less than 3 years after withdrawal of all prophylactic immunosuppression. Disease-specific criteria for RM were used. Patients were required to have measurable or evaluable disease, except patients with acute leukemia or aggressive non-Hodgkin lymphoma (NHL) who could be induced into a complete remission (CR) with conventional therapy before study entry. Patients were required to have greater than 50% donor T-cell chimerism at the time of study entry and have no ongoing clinically significant GVHD. They were required to be off all immunosuppressive medications for the treatment or prophylaxis of GVHD for more than 6 weeks before study entry (later amended to > 4 weeks) and have no prior history of severe (grade 3 or 4) acute GVHD. Other eligibility criteria included absolute lymphocyte count greater than 250/mm3, Eastern Cooperative Oncology Group (ECOG) PS 0-2, life expectancy greater than 3 months, satisfactory vital organ function unless demonstrated to be secondary to malignancy (creatinine < 2.0 mg/dL, bilirubin < 2.0 mg/dL, aspartate transaminase [AST]/alanine transaminase [ALT] < 3× upper limit of normal). Patients with serious active infections, HIV, any condition requiring immunosuppressive therapy, any autoimmune or second malignant condition within the last 5 years, and pregnant females were excluded.
Study design and treatment plan
This trial (CTEP 6082) was an open-label dose-escalation study conducted at 4 centers. The study was approved by the institutional review boards of each participating center, and all patients were treated under written informed consent in accordance with the Declaration of Helsinki. Ipilimumab was administered in a single dose over 90 minutes. Ipilimumab was supplied by Cancer Therapy Evaluation Program (CTEP), National Cancer Institute (NCI; Bethesda, MD). A modified Fibonacci dose-escalation design was used. Ipilimumab dose was escalated using the following planned cohorts: 0.1, 0.33, 0.66, 1.0, and 3.0 mg/kg. At least 3 patients were enrolled per dose cohort with additional patients enrolled in the case of dose-limiting toxicity (DLT) or to substitute for any patients within the cohort who were removed from study within 8 weeks after dosing. An additional 12 patients were planned to enroll at the maximum tolerated dose (MTD) or the maximum planned dose in the study (3.0 mg/kg) if MTD was not reached, to provide a total of 15 patients to further define toxicity and describe preliminary efficacy at this dose level. DLT was defined as the development of grade 3 or 4 acute GVHD, graft rejection, the development of any unexpected grade greater than 3 or 4 toxicity felt to be related to ipilimumab, or the development of greater than grade 3 dysfunction of a vital organ felt to be secondary to an IAE. All patients in cohorts below 3.0 mg/kg were observed for 60 days before enrolling patients at the next dose level. The protocol was modified to allow repeat dosing at the same dose level for patients who demonstrated an objective response to the first dose and then showed evidence of disease progression. If there was no evidence of disease response and no immunologic adverse effect at 60 days after the initial ipilimumab infusion, donor leukocyte infusions (DLI) could be administered. Planned dosing for DLI (CD3+ cells × 106/kg) was 0.5, 1.0, and 5.0 at 60-day intervals, as long as no disease response or clinically significant GVHD occurred. Patients were evaluated for GVHD and other toxicity by clinical assessment and laboratory testing at baseline and at 1, 2, 4, 6, 8, 10, and 12 weeks after ipilimumab infusion and then monthly through 6 months and every 3 months through 12 months. Clinical response evaluations were performed at baseline and 1, 2, 3, 6, 9, and 12 months after infusion.
Pharmacokinetics
Blood samples were drawn immediately before the dose and at set time points thereafter. Plasma concentrations of ipilimumab were determined by Medarex using a quantitative enzyme-linked immunosorbent assay (ELISA).
Statistical considerations
This trial was designed as a phase 1 dose escalation to determine the MTD of ipilimumab administered as a single dose to patients who had previously undergone allo-HCT. A total of 15 patients were also planned to accrue at either the MTD or at 3.0 mg/kg if MTD was not a lower dose. The incidence of grade DLT after ipilimumab infusion was the primary end point analyzed. If no DLT was observed in the 15 patients treated at this dose level, the possibility that the underlying rate of DLT was greater than 20% could be excluded with greater than 95% confidence.
Results
Patient characteristics
A total of 29 patients treated were accrued between December 2003 and June 2007. Numbers of patients treated at each dose level were as follows: 4 patients at 0.1 mg/kg, 3 patients at 0.33 mg/kg, 4 patients at 0.66 mg/kg, 3 patients at 1.0 mg/kg, and 15 patients at 3.0 mg/kg. Their demographic information is shown in Table 1. Hodgkin disease (HD) was the most common underlying malignancy. No patients had ex vivo T-cell depletion during allo-HCT. Eight patients had received antithymocyte globulin as part of their transplant preparative regimen. Median donor T-cell chimerism on the day of ipilimumab infusion was 100% (range, 76%-100%). Eight patients had failed prior DLI. Median time between the patient's last allogeneic cell infusion and the treatment with ipilimumab was 366 days (range, 125-2368 days). Twenty-eight patients received a single infusion, and 1 patient received 2 infusions (initial treatment followed by retreatment at disease progression).
Patient characteristics
| Characteristic . | Value . |
|---|---|
| Median age, y (range) | 43 (21-65) |
| Sex (M/F), n (%) | 23/6 (79/21) |
| Diagnosis, n (%) | |
| Hodgkin disease | 14 (48) |
| Myeloma | 6 (21) |
| AML | 2 (6) |
| CML | 2 (6) |
| CLL | 2 (6) |
| NHL | 1 (3) |
| Breast cancer | 1 (3) |
| Renal cell cancer | 1 (3) |
| Donor, n (%) | |
| HLA-identical | 19 (65) |
| Matched unrelated | 10 (35) |
| Preparative regimen, n (%) | |
| Myeloablative | 6 (21) |
| Reduced intensity | 23 (79) |
| Characteristic . | Value . |
|---|---|
| Median age, y (range) | 43 (21-65) |
| Sex (M/F), n (%) | 23/6 (79/21) |
| Diagnosis, n (%) | |
| Hodgkin disease | 14 (48) |
| Myeloma | 6 (21) |
| AML | 2 (6) |
| CML | 2 (6) |
| CLL | 2 (6) |
| NHL | 1 (3) |
| Breast cancer | 1 (3) |
| Renal cell cancer | 1 (3) |
| Donor, n (%) | |
| HLA-identical | 19 (65) |
| Matched unrelated | 10 (35) |
| Preparative regimen, n (%) | |
| Myeloablative | 6 (21) |
| Reduced intensity | 23 (79) |
Toxicity
The adverse events experienced by the patients treated at each dose cohort are shown in Table 2. DLT as defined was not encountered, and an MTD was not reached with a single dose up to 3.0 mg/kg (one patient who developed a >grade 3 IAE did so after retreatment with ipilimumab). Although multiple grade 1 and 2 toxicities were recorded, in almost all cases no clear relationship to the study agent was evident. Thus, 15 patients in all were treated at the originally planned highest dose (3.0 mg/kg).
Adverse events by grade and dose level
| Dose level, mg/kg . | Toxicity grade . | |||
|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | |
| 0.1 | Pharyngitis, eyelid blisters, tachycardia, dizziness, headache | Leucocytosis, diarrhea, shoulder pain, bronchitis (2), vaginosis | Arthritis*, pneumonitis | |
| 0.33 | Palpitations, chest pain, diarrhea (2), nausea, cough | Blurring of vision, abdominal pain/constipation, hypercalcemia, hyperglycemia, knee swelling | Anemia, thrombocytopenia, pneumonia, neutropenia/fever | Infection, hip fracture |
| 0.66 | Hypothyroidism, elevated AST, hyperglycemia, acute GVHD (skin) | Hyperthyroidism*, cellulitis, bone pain, cough, pneumonia (2) | Anemia | |
| 1.0 | Anemia, thrombocytopenia, elevated ALT/AST, elevated alkaline phosphatase | Hypotension, urinary retention | Elevated AST/ALT | Neutropenia (transient)* |
| 3.0 | Chills, fatigue (2), fever (2)*, night sweats, anemia, thrombocytopenia, petichiae, skin dryness, rash, hypercalcemia, hypocalcemia, hypokalemia, hypernatremia, hyponatremia, muscle cramps*, dizziness (2)*, headache, insomnia, photophobia, colitis*, diarrhea*, elevated alk. phos., elevated AST/ALT*, nausea (2), vomiting*, increased creatinine, vaginosis, pharyngitis*, agitation | Fatigue (2), Fever (2 + 1)*, sweats*, insomnia, pruritis*, rash/desquamation*, abdominal pain, backache*, arthritis*, headache, sensory neuropathy, cough, dyspnea, pneumonia, anxiety | Leucocytosis, fever, neutropenic fever, dyspnea, mucositis | Pneumonitis* |
| Dose level, mg/kg . | Toxicity grade . | |||
|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | |
| 0.1 | Pharyngitis, eyelid blisters, tachycardia, dizziness, headache | Leucocytosis, diarrhea, shoulder pain, bronchitis (2), vaginosis | Arthritis*, pneumonitis | |
| 0.33 | Palpitations, chest pain, diarrhea (2), nausea, cough | Blurring of vision, abdominal pain/constipation, hypercalcemia, hyperglycemia, knee swelling | Anemia, thrombocytopenia, pneumonia, neutropenia/fever | Infection, hip fracture |
| 0.66 | Hypothyroidism, elevated AST, hyperglycemia, acute GVHD (skin) | Hyperthyroidism*, cellulitis, bone pain, cough, pneumonia (2) | Anemia | |
| 1.0 | Anemia, thrombocytopenia, elevated ALT/AST, elevated alkaline phosphatase | Hypotension, urinary retention | Elevated AST/ALT | Neutropenia (transient)* |
| 3.0 | Chills, fatigue (2), fever (2)*, night sweats, anemia, thrombocytopenia, petichiae, skin dryness, rash, hypercalcemia, hypocalcemia, hypokalemia, hypernatremia, hyponatremia, muscle cramps*, dizziness (2)*, headache, insomnia, photophobia, colitis*, diarrhea*, elevated alk. phos., elevated AST/ALT*, nausea (2), vomiting*, increased creatinine, vaginosis, pharyngitis*, agitation | Fatigue (2), Fever (2 + 1)*, sweats*, insomnia, pruritis*, rash/desquamation*, abdominal pain, backache*, arthritis*, headache, sensory neuropathy, cough, dyspnea, pneumonia, anxiety | Leucocytosis, fever, neutropenic fever, dyspnea, mucositis | Pneumonitis* |
Events that the investigator felt were at least possibly related to ipilimumab.
GVHD
No patients developed grade 3 or 4 acute GVHD after ipilimumab alone. Three patients developed minor ocular dryness and erythema at 2, 6, and 8 months from a single ipilimumab infusion without subsequent DLI. They did not require systemic therapy. Two patients with preexisting minor active chronic GVHD not requiring immunosuppressive therapy at baseline (1 oral, 1 ocular) showed no exacerbation of symptoms after ipilimumab infusion. One patient developed no GVHD after ipilimumab, but developed grade 1 skin GVHD after DLI given at 2 months after ipilimumab for progressive disease.
Organ-specific IAE
Four patients developed IAE distinct from GVHD that were potentially attributable to ipilimumab.
Patient 0102 (age 52, Hispanic female, AML, dose level 0.1 mg/kg) developed grade 3 polyarthropathy with nodules clinically consistent with rheumatoid arthritis 3 months after a single infusion of ipilimumab. The episode occurred approximately 4 weeks after DLI given for RM (3 months after ipilimumab); therefore, the role of ipilimumab versus DLI in the etiology of this episode is difficult to distinguish. The patient responded to corticosteroid therapy with a complete regression of her symptoms. Pre-ipilimumab serum was retrospectively analyzed and found to be positive for rheumatoid factor at a titer of 1:640. The patient had no prior history of rheumatoid arthritis.
Patient 0311 (age 48, white male, chronic lymphocytic leukemia [CLL], dose level 0.66 mg/kg) developed laboratory evidence of hyperthyroidism associated with the production of thyroid-stimulating hormone (TSH) receptor stimulating antibody within 4 weeks of a single ipilimumab infusion. The patient was referred to endocrinology and was monitored without therapy for the next month. He demonstrated laboratory evidence of progressive hyperthyroidism associated with rising titers of anti-TSH receptor antibodies at 2 months after infusion. The patient was then treated with methimazole, which resulted in improvement of his serum-free T4 and T3 levels by 4 months after ipilimumab infusion. He never developed clinical symptoms of hyperthyroidism. Assessment of baseline thyroid function on blood drawn immediately before ipilimumab infusion revealed normal T4, TSH, and a borderline level of TSH-receptor receptor antibody.
Patient 2521 (white male, age 45, HD, dose level 3.0 mg/kg) complained of dyspnea on exertion 10 weeks after a single dose of ipilimumab. Computed tomography (CT) scan showed no new abnormalities, but pulmonary function resting revealed an obstructive defect that responded to inhaled corticosteroids. No infectious etiology was identified.
Patient 3520 (white male, age 35, HD, dose level 3.0) had demonstrated clinical and radiologic regression of malignancy followed later by disease progression after his first infusion of 3.0 mg/kg ipilimumab. He was retreated with the same dose of ipilimumab 4 months after his first infusion. He was noted to have new bilateral ground glass opacification in bilateral upper lung fields on a routine reassessment CT scan at 4 weeks after the second infusion. Bronchoscopy revealed no infective etiology except β-hemolytic Streptococcus from a single culture and transbronchial biopsy showed inflammatory changes. The infiltrates worsened despite antimicrobial therapy. He was commenced on methylprednisolone with marked radiologic improvement at 2- and 4-week reassessments (Figure 1). Complete tapering of the patient's corticosteroid therapy over the next month was associated with significant exacerbation of pulmonary infiltrates and dyspnea leading to intubation while radiologic evidence of his HD resolved completely. Repeat transbronchial biopsy showed acute and chronic inflammatory changes only. Reinstitution of corticosteroids accompanied by infliximab (single dose 10 mg/kg) resulted in rapid improvement and eventual complete resolution of dyspnea and pulmonary infiltrates. However, despite a much slower taper, discontinuation of corticosteroid therapy was associated with recurrence of grade 4 pneumonitis. The patient responded completely to treatment with methylprednisone accompanied with a 4-week course of infliximab (10 mg/kg per week) and mycophenylate mofetil. Slow tapering of corticosteroid therapy followed by maintenance therapy with low-dose methylprednisolone and mycophenylate mofetil has prevented subsequent recurrence of the patient's pneumonitis.
Pneumonitis after retreatment with ipilimumab. CT scans of the chest from patient 3520 (dose level 3.0 mg/kg). Scans show (A) an extensive inflammatory infiltrate that developed approximately 6 weeks after retreatment with ipilimumab and (B) complete resolution of changes after corticosteroid therapy.
Pneumonitis after retreatment with ipilimumab. CT scans of the chest from patient 3520 (dose level 3.0 mg/kg). Scans show (A) an extensive inflammatory infiltrate that developed approximately 6 weeks after retreatment with ipilimumab and (B) complete resolution of changes after corticosteroid therapy.
Regression of malignancy
Three patients demonstrated objective disease responses after ipilimumab alone (summarized in Table 3).
Objective disease responses
| Patient (dose level, mg/kg) . | Age . | Sex . | Diagnosis . | Prior DLI . | Response to ipilimumab . | GVHD after ipilimumab . | Other toxicity . |
|---|---|---|---|---|---|---|---|
| 0414 (1.0) | 64 | M | NHL (mantle cell) | Yes (×2) | PR | No | Transient neutropenia |
| 2517 (3.0) | 21 | M | HD | Yes | CR | No | No |
| 3520 (3.0) | 40 | M | HD | No | CR* | No | Grade 4 pneumonitis* |
| Patient (dose level, mg/kg) . | Age . | Sex . | Diagnosis . | Prior DLI . | Response to ipilimumab . | GVHD after ipilimumab . | Other toxicity . |
|---|---|---|---|---|---|---|---|
| 0414 (1.0) | 64 | M | NHL (mantle cell) | Yes (×2) | PR | No | Transient neutropenia |
| 2517 (3.0) | 21 | M | HD | Yes | CR | No | No |
| 3520 (3.0) | 40 | M | HD | No | CR* | No | Grade 4 pneumonitis* |
Negative effect observed after retreatment with ipilimumab.
Patient 0414 had failed prior therapy for multiply relapsed mantle cell NHL before undergoing matched unrelated donor allo-HCT. Upon RM after allo-HCT, he received 2 DLI without evidence of disease response. He had rapidly growing left parotid and right axillary masses before enrollment on study. Administration of ipilimumab was followed approximately 4 weeks later with a febrile illness that was associated with significant clinical and radiologic regression of his lymphoma resulting in a partial remission (PR) that was persistent at 2 months after ipilimumab (Figure 2). At 3 months after ipilimumab, the responses in the parotid and axillary masses were maintained, but the patient developed a new positron emission tomography (PET)–avid lesion within the abdomen. The patient was then taken off the study to pursue alternative therapeutic options.
Regression of malignancy in a patient with mantle cell lymphoma. CT scans from patient 0414 (dose level 1.0 mg/kg) showing left parotid (A,B), and right axillary (C,D) nodal masses before (A,C) and 1 month after (B,D) ipilimumab infusion.
Regression of malignancy in a patient with mantle cell lymphoma. CT scans from patient 0414 (dose level 1.0 mg/kg) showing left parotid (A,B), and right axillary (C,D) nodal masses before (A,C) and 1 month after (B,D) ipilimumab infusion.
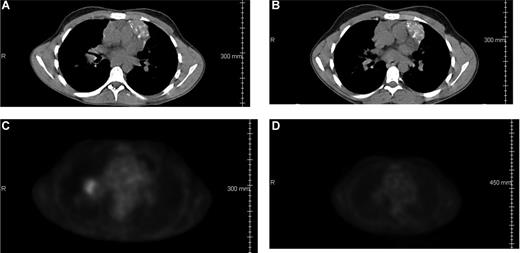
Patient 2517 had failed a prior autologous transplantation for primary refractory HD before undergoing a matched related donor allo-HCT. His disease relapsed within 6 months of the transplantation. He was treated with gemcitabine monotherapy followed by DLI. However, there was evidence of disease progression within 2 months of DLI. The patient had evidence of PET-avid right hilar adenopathy immediately before receiving ipilimumab at 3.0 mg/kg. CR was achieved on the 1-month reassessment after ipilimumab (Figure 3). The CR has been durable for 37 months at the time of this report.
Regression of malignancy in a patient with HD. CT scans (A,B) and PET scans (C,D) from patient 2517 (dose level 3.0 mg/kg) showing right hilar adenopathy before (A,C) and 2 months after (B,D) ipilimumab infusion.
Regression of malignancy in a patient with HD. CT scans (A,B) and PET scans (C,D) from patient 2517 (dose level 3.0 mg/kg) showing right hilar adenopathy before (A,C) and 2 months after (B,D) ipilimumab infusion.
Patient 3520 had a history of relapsed HD and had previously failed autologous transplantation. Matched related donor allo-HCT did not achieve CR, and there was frank malignant progression 6 months after transplantation. The patient was treated with ipilimumab at the 3.0 mg/kg dose and developed partial regression of PET-avid disease at 1- and 2-month evaluations following ipilimumab. However, disease progression was evident at the 3-month evaluation following ipilimumab, and he was retreated with 3.0 mg/kg ipilimumab approximately 4 months after the first infusion. The second infusion was associated with a corticosteroid-responsive noninfectious pneumonitis, which progressed to grade 4 severity when the corticosteroids were tapered and discontinued (see section on IAE above). However, by 3 months after the second infusion, the patient achieved a CR that was durable for 9 months thereafter.
Two additional patients with HD treated at the highest dose level (3.0 mg/kg) who had evidence of rapid disease progression before ipilimumab achieved disease stabilization for 3 and 6 months, respectively, after the infusion.
DLI
Nine patients received at least one DLI in the study after showing persistence or progression of malignancy after ipilimumab (first DLI, 0.5 × 107 CD3+ cells/kg; 2 at dose ipilimumab cohort 0.1 mg/kg, 2 at 0.66 mg/kg, 2 at 1.0 mg/kg, and 3 at 3.0 mg/kg). The first DLI was administered at a median of 2 months after ipilimumab (range, 2-4 months). Three patients also received a second DLI (1.0 × 107 CD3+ cells/kg) at a median of 4 months (range, 4-9 months) after ipilimumab. No patient who received DLI after failing ipilimumab demonstrated an objective disease response.
Survival
A Kaplan-Meier plot of estimated overall survival of all 29 patients treated in the study is shown in Figure 4. Median overall survival from the time of ipilimumab therapy was 24.7 months.
Kaplan-Meier plot of overall survival from the time of ipilimumab therapy for all patients (n = 29).
Kaplan-Meier plot of overall survival from the time of ipilimumab therapy for all patients (n = 29).
Pharmacokinetics
The concentration of ipilimumab at various time points after single infusion at the dose levels assessed is shown in Figure 5. All doses used achieved a peak concentration of ipilimumab greater than 1 μg/mL. At the highest dose studied (3.0 mg/kg), ipilimumab levels remained at concentrations above 10 μg/mL for at least 30 days and above 1 μg/mL for 60 days.
Serum ipilimumab levels after a single infusion of doses from 0.1 to 3.0 mg/kg.
Discussion
This report documents the results of the first clinical trial to use CTLA4 blockade as a means of augmenting the GVM effect after allo-HCT. We show that a single infusion of ipilimumab at doses up to 3 mg/kg can be administered safely to patients who have RM after allo-HCT. In particular, ipilimumab did not result in stimulation or exacerbation of acute or chronic GVHD in these patients. No patient developed typical clinical features of acute or chronic GVHD after ipilimumab alone. The eligibility criteria of the trial may have limited the risk of severe GVHD. Specifically, patients were required to have not experienced prior grade 3 or 4 acute GVHD and were required to have tolerated discontinuation of all immunosuppressive medications for a minimum of 4 to 6 weeks before study entry. It is unclear whether ipilimumab would also not induce or exacerbate GVHD in patients who had experienced prior severe acute GVHD or those patients who were still on immunosuppressive therapy at the time of treatment. It is notable, however, that several patients treated in this study had experienced some prior acute and/or chronic GVHD before ipilimumab therapy, and all patients had at least 50% donor T-cell chimerism at the time of ipilimumab infusion (median, 100%). The lack of GVHD as a complication of ipilimumab therapy in this trial cannot be explained by subtherapeutic dosing. Ipilimumab is a fully human monoclonal antibody and has a relatively prolonged half-life in vivo.8,11,15 Serial estimation of serum ipilimumab concentrations in our patients demonstrated that at doses greater than 1.0 mg/kg, serum ipilimumab levels remained above 10 μg/mL for several days (more than 30 days after a dose of 3.0 mg/kg). Small et al observed similar levels of ipilimumab were seen after a single dose of ipilimumab at 3.0 mg/kg.15 Serum ipilimumab levels greater than 0.1 μg/mL have been shown to saturate binding sites on recombinant CTLA4 in ELISA studies, and levels greater than 2 μg/mL achieve binding saturation for CTLA4 expressed through transfection on a T-cell hybridoma.22 Maximal inhibition of the binding of CTLA4 expressed upon these cells to CD80 and CD86 can be achieved by concentrations of ipilimumab as low as 6 and 1 μg/mL, respectively.22 CTLA4 molecules are heavily overexpressed on such transduced cell lines compared with that seen on T cells after activation in vivo. Thus it is likely that ipilimumab levels achieved in our patients at the higher dose cohorts would induce complete blockade of the CTLA4 expressed on their T cells in vivo after antigen-induced activation. Furthermore, clinical effects other than GVHD, namely clinical regression of malignancy and organ-specific IAEs were induced at these dose levels in our trial. It is possible that ipilimumab did not initiate or exacerbate GVHD in our study because of the required separation between the last infusion of donor cells and ipilimumab administration (minimum 90 days, median 366 days). The etiology of the clinical syndrome of GVHD is complex.23 Available data suggest that the presence of both recipient-derived antigen-presenting cells and possibly cytokine release associated with regimen-related toxicity may be components that participate in the generation of clinical acute GVHD.23 The lack of one or both of these components due to the relatively prolonged time between last donor-cell infusion and ipilimumab administration may have contributed to the lack of clinical acute GVHD seen after ipilimumab despite the other immune effects observed. Delayed administration of anti-CTLA4 antibody was associated with augmentation of the GVM effect without exacerbation of lethal GVHD in a murine model of MHC-mismatched allo-HCT.21 In a recent study of a murine model of minor histocompatibility antigen-mismatched allo-HCT,24 early CTLA4 blockade induced acute GVHD. However, delayed CTLA4 blockade did not result in GVHD, but resulted instead in potentially lethal host-derived autoimmune effects, as well as significant augmentation of resistance to challenge with syngeneic leukemia cells. Both autoimmune effects and antileukemia activity were mediated by host-derived T cells in this model. However, these effects were dependent upon the coexistence of donor-derived T cells, as neither effect was seen after syngeneic hematopoietic transplantation. These findings seen in experimental animal models of allo-HCT are consistent with the observations in our clinical trial. However, it is unlikely that the organ-specific IAE or the objective responses seen in patients treated on our study were mediated exclusively by host-derived T cells. All patients demonstrating these effects had greater than 80% donor T-cell chimerism on the date of ipilimumab therapy, and in only 2 patients were any host-derived T cells detectable. Furthermore, only one of the patients demonstrating IAEs or objective responses had any increase in host-derived chimerism on serial assessment after ipilimumab upon development of the immune effect (patient 0102). These findings would argue against the possibility that the immune phenomena observed in our trial were effected solely by host-derived T cells, but the possibility cannot be excluded with certainty.
Organ-specific IAEs were seen in 4 of 29 (14%) patients treated in our study. Such IAEs have been described in trials of use of ipilimumab and another anti-CTLA4 antibody in patients with cancer who had not undergone allo-HCT.8-17,19,20 As in those reports, the IAEs seen in our study were highly responsive to immunosuppressive therapy, although prolonged therapy and multiple immunosuppressive agents were required in one patient. IAEs have been found to be more common in trials using repeated dosing of ipilimumab, and their occurrence is associated with regression of malignancy. Consistent with those findings, the most severe IAE in our study occurred in a patient after he was retreated with ipilimumab. He also experienced a durable CR in his malignancy after the second infusion. The frequency of the IAEs seen in patients in our study does not appear to differ significantly from other trials in which primarily a single dose of ipilimumab up to 3 mg/kg has been studied. All patients treated in this study had greater than 50% donor T-cell chimerism at the time of ipilimumab infusion. Therefore, these data imply that despite the presence of disparity in minor histocompatibility antigens between donor and recipient, the frequency of and severity of IAE after ipilimumab may not be significantly greater in patients after allo-HCT than in patients who have not undergone allo-HCT.
Objective responses were encountered in 3 patients treated on our protocol (17% of the 18 patients treated at >1 mg/kg). The objective responses seen in our study occurred in patients with lymphoid malignancies and included 2 durable CRs in patients with HD. This finding may be attributable to chance, as HD was the most frequent malignancy treated on our study, and the total number of patients with each diagnosis in our study was small. However, objective responses have been reported in patients with lymphoma18 in a trial where the antibody was used after failure of idiotype vaccination. Further investigation of ipilimumab in patients receiving allo-HCT in HD and NHL is warranted. Once the safety of ipilimumab after allo-HCT is fully established, its efficacy in the prophylaxis of relapse in high-risk patients may be explored.
Thus, in conclusion, our study suggests that the use of ipilimumab up to 3 mg/kg as administered in our study is safe and can produce antitumor responses. It forms a platform for the exploration of true efficacy when used after allo-HCT through formal phase 2 studies in specific malignancies. Furthermore, repeated dosing and higher dosing may also now be explored as strategies to increase the efficacy of the antibody in this setting.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We acknowledge the contribution of Howard Streicher, MD, Pharmaceutical Management Branch, National Cancer Institute (NCI; Bethesda, MD) during the design and analysis of the study. Minya Pu and Karen Messer from the Biostatistics Core at the Moores Cancer Center, University of California, San Diego helped with the statistical analysis.
This study was supported by National Institutes of Health (NIH) award RO1 CA 9389-01A1 (A.B.).
National Institutes of Health
Authorship
Contribution: A.B. designed and performed research, analyzed data, and wrote the paper; B.M., S.C., and L.V. performed research and analyzed data; M.P., E.C., S.R.S., L.E.M., H.K.H., J.R.M., and E.P.A. performed research; I.L. contributed investigational agent; R.J.S. performed research, analyzed data, and contributed to manuscript; and E.D.B. helped design research, performed research, and contributed to the manuscript.
Conflict-of-interest disclosure: I.L. is employed by and owns stock in Medarex, Inc. E.D.B. owns stock in Medarex, Inc. The other authors declare no competing financial interests.
Correspondence: Asad Bashey, Blood and Marrow Transplant Group of Georgia at Northside Hospital, 5670 Peachtree Dunwoody Road NE, Suite 1000, Atlanta, GA 30342; e-mail: abashey@bmtga.com.