Response
The pathways that regulate apoptosis are complex and multi-layered. It is well known, for example, that death induced by Fas can in some cells involve mitochondria, while in others does not. In the granzyme system there are cell lines that die primarily through the granzyme B pathway and those that are sensitive to granzyme A. Consequently, we do not find it too surprising that Waterhouse et al have found lines that do not polarize mitochondria.
Clearly there are differences between the target cells used in either study. For example, the kinetics of apoptosis induction differ markedly in the 2 systems. We see mitochondrial membrane potential loss very early in our cells, whereas they state that it is more than an hour before they observe cytochrome c loss, although this is difficult to appreciate from their data as the cc–green fluorescent protein (GFP) appears throughout the cell at time zero. It is also unclear what effector molecules are involved, as in their previous work Waterhouse et al have reported that some of their killer lines use primarily perforin. In addition, one interesting difference between our targets and those used by Waterhouse et al is the high density of mitochondria in the HeLa cells. Clearly the HeLa already have a large number of mitochondria adjacent to the synapse.
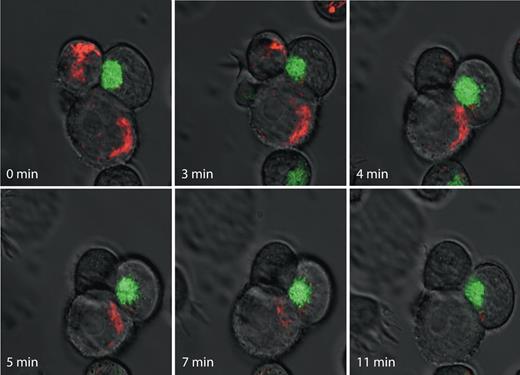
Waterhouse et al imply that our work is less reliable because we used single time points and cannot determine whether target cells eventually undergo apoptosis. The advantage of our approach is that we can score many conjugates. As well, we have also performed time lapse photography and some representative stills of one particularly interesting group of cells is shown in Figure 1. The killer cell has been transfected with grB-GFP so the granules are green. Initially the granules are polarized toward the upper target. We first observe a movement of the mitochondria labeled with tetramethylrhodamine ethyl ester (TMRE) toward the synapse and mitochondrial depolarization clearly occurs. The granules then redirect toward the lower target. Only then do the mitochondria migrate to the synapse, and ultimately TMRE is lost. In this case, there is a clear correlation with mitochondrial targeting to the synapse followed closely by the initiation of apoptosis, as recorded by loss of mitochondrial electrochemical potential.
Time-lapse imaging of mitochondrial polarization in target cells during cytotoxic lymphocyte-induced death. YTS human natural killer (NK) cells stably expressing human grB-EGFP chimeric protein (green) were mixed with human 721.221 B cells stained with the potentiometric dye TMRE (red) and were spotted on a glass coverslip and immediately placed on a heated microscope stage. Images were acquired using a Zeiss Axiovert confocal microsope (Jena, Germany). Images were acquired at 37°C on an LSM 510 laser scanning confocal microscope mounted on a Zeiss Axiovert 100M microscope fitted with a Plan Neofluar oil objective (40×/1.3 NA) and analyzed with LSM5 software (Zeiss). Representative images at indicated time points are shown.
Time-lapse imaging of mitochondrial polarization in target cells during cytotoxic lymphocyte-induced death. YTS human natural killer (NK) cells stably expressing human grB-EGFP chimeric protein (green) were mixed with human 721.221 B cells stained with the potentiometric dye TMRE (red) and were spotted on a glass coverslip and immediately placed on a heated microscope stage. Images were acquired using a Zeiss Axiovert confocal microsope (Jena, Germany). Images were acquired at 37°C on an LSM 510 laser scanning confocal microscope mounted on a Zeiss Axiovert 100M microscope fitted with a Plan Neofluar oil objective (40×/1.3 NA) and analyzed with LSM5 software (Zeiss). Representative images at indicated time points are shown.
The wording of the Waterhouse et al letter implies our results are suspect, but we believe that they just reflect differences in cell type. A heterogeneous cell type–dependent response to cytotoxic lymphocytes is not surprising and likely reflects the diverse signaling pathways that distinguish one cell type from another. This is a common theme in biology, underlying all aspects of cell and tissue specificity. We believe it is naive to think that there is a single route to death used by killer lymphocytes, as survival requires multiple backup protection mechanisms.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Chris Bleackley, University of Alberta, 4-63 Medical Sciences Building, Edmonton, AB Canada T6G 2H7; e-mail: chris.bleackley@ualberta.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal