Abstract
We made a disease-specific comparison of unrelated cord blood (CB) recipients and human leukocyte antigen allele–matched unrelated bone marrow (BM) recipients among 484 patients with acute myeloid leukemia (AML; 173 CB and 311 BM) and 336 patients with acute lymphoblastic leukemia (ALL; 114 CB and 222 BM) who received myeloablative transplantations. In multivariate analyses, among AML cases, lower overall survival (hazard ratio [HR] = 1.5; 95% confidence interval [CI], 1.0-2.0, P = .028) and leukemia-free survival (HR = 1.5; 95% CI, 1.1-2.0, P = .012) were observed in CB recipients. The relapse rate did not differ between the 2 groups of AML (HR = 1.2; 95% CI, 0.8-1.9, P = .38); however, the treatment-related mortality rate showed higher trend in CB recipients (HR = 1.5; 95% CI, 1.0-2.3, P = .085). In ALL, there was no significant difference between the groups for relapse (HR = 1.4, 95% CI, 0.8-2.4, P = .19) and treatment-related mortality (HR = 1.0; 95% CI, 0.6-1.7, P = .98), which contributed to similar overall survival (HR = 1.1; 95% CI, 0.7-1.6, P = .78) and leukemia-free survival (HR = 1.2; 95% CI, 0.9-1.8, P = .28). Matched or mismatched single-unit CB is a favorable alternative stem cell source for patients without a human leukocyte antigen–matched related or unrelated donor. For patients with AML, decreasing mortality, especially in the early phase of transplantation, is required to improve the outcome for CB recipients.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) with bone marrow (BM) or peripheral blood, the curative treatment of choice for acute leukemia, is limited by the inadequate supply of human leukocyte antigen (HLA)–identical related donors. Bone marrow from HLA-matched unrelated donors has been a major alternative graft source.1-3 Umbilical cord blood (CB), an alternative stem cell source to BM or peripheral blood stem cells, has been used primarily in children,4-10 but its use in adults is increasing.11,12
Clinical comparison studies of cord blood transplantation (CBT) and bone marrow transplantation (BMT) for leukemia from unrelated donors in adult recipients showed comparable outcomes.11-13 Recipients of CBT showed delayed neutrophil recovery and lower incidence of acute graft-versus-host disease (GVHD).11-13 Overall treatment-related mortality (TRM) was reported to be similar12 or higher11 compared with HLA-matched BM. Acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL) are different disease entities that require different chemotherapy regimens for treatment. However, previous comparison studies have included both diseases because of limitation in the number of CBTs given to adults.
In addition, the study periods of previous studies encompass the pioneering period of CBT, when the general practice was to use these grafts in patients in whom there were no other curative options and when the relevance of cell dose and HLA matching had not yet been recognized.6,7,14
Accumulation of a larger number of CBT results enabled us to make a controlled comparison with unrelated BMTs. To avoid the inclusion of the pioneering period of CBT, the subjects were limited to those who received transplantations in and after 2000.
Methods
Collection of data and data source
The recipients' clinical data were provided by the Japan Cord Blood Bank Network (JCBBN) and the Japan Marrow Donor Program (JMDP).15 Peripheral blood stem cell donation from unrelated donors is not permitted in Japan. All 11 CB banks in Japan are affiliated to JCBBN. Both JCBBN and JMDP collect recipients' clinical information at 100 days after transplantation. Patients' information on survival, disease status, and long-term complications, including chronic GVHD and second malignancies, are renewed annually by follow-up forms. This study was approved by the data management committees of JMDP and JCBBN.
Patients
Between January 2000 and December 2005, a total of 1690 adult patients at least 16 years of age with acute leukemia (999 AML, 261 CB and 738 BM; and 691 ALL, 178 CB and 513 BM) received first HSCT with myeloablative conditioning either CB or BM from unrelated donors. Of these, patients who received a single CB unit with 0 to 2 HLA mismatches, or HLA-A, -B, -C, and DRB1 allele-matched BM from unrelated donors were analyzed. HLA matching of CB was performed using low-resolution molecular typing methods for HLA-A and -B, and high-resolution molecular typing for HLA-DRB1. Of 1023 BM recipients with complete HLA high-resolution data, the following recipients with HLA HLA-A, -B, -C, and DRB1 allele mismatches were excluded: 306 recipients with 1 of 8 mismatches (39 for HLA-A, 6 for HLA-B, 137 for HLA-C, and 124 for HLA-DRB1), 150 recipients with 2 of 8 mismatches (36 for 2 class I antigens, and 114 for class I and class II antigens), 33 recipients with 3 of 8 mismatches, and 1 recipient with 4 of 8 mismatches. Of 390 recipients of CB with complete HLA data, 95 recipients with 3 mismatches and 8 patients with 4 mismatches were excluded. A total of 484 patients with AML (173 CBTs and 311 BMTs) and 336 patients with ALL (114 CBTs and 222 BMTs) were the subjects for the analyses. Eighty-five centers performed 287 CBTs analyzed in this study, and 114 centers performed 533 BMTs.
Definitions
Neutrophil recovery was defined by an absolute neutrophil count of at least 500 cells/mm3 for 3 consecutive points; platelet recovery was defined by a count of at least 50 000 platelets/mm3 without transfusion support. Diagnosis and clinical grading of acute GVHD were performed according to the established criteria.16 Relapse was defined as a recurrence of underlying hematologic malignant diseases. Treatment-related death was defined as death during a continuous remission. Leukemia-free survival (LFS) was defined as survival in a state of continuous remission.
Statistical analysis
Separate analyses were performed for AML and ALL. Descriptive statistical analysis was performed to assess patient baseline characteristics, diagnosis, disease classification, disease status at conditioning, donor-patient ABO mismatches, preparative regimen, and GVHD prophylaxis. The 2-sided χ2 test was used for categorical variables, and the 2-sided Wilcoxon rank sum test was used for continuous variables. Cumulative incidence curves were used in a competing-risks setting to calculate the probability of neutrophil and platelet recovery, acute and chronic GVHD, relapse, and TRM.17 For neutrophil and platelet recovery, death before neutrophil or platelet recovery was the competing event; for GVHD, death without GVHD and relapse were the competing events; for relapse, death without relapse was the competing event; and, for TRM, relapse was the competing event. Gray test was used for group comparison of cumulative incidence.18 Overall survival (OS) and LFS were calculated using the Kaplan-Meier method. The log-rank test was used for group comparisons. Adjusted comparison of the stem cell source on OS and LFS was performed with the use of the Cox proportional-hazards regression model. For other outcomes, the Fine and Gray proportional-hazards model for subdistribution of a competing risk was used.19 Adjusted probabilities of OS and DFS were estimated using the Cox proportional-hazards regression model, with consideration of other significant clinical variables in the final multivariate models. The variables considered were the patient's age at transplantation, patient's sex, donor-patient sex mismatch, donor-patient ABO mismatch, disease status at conditioning, and t(9;22) chromosome abnormality or others for ALL, cytogenetic information and French-American-British (FAB) classification of M5/M6/M7 or others for AML, the conditioning regimen, and the type of prophylaxis against GVHD. Factors differing in distribution between CB and BM recipients (P < .10) and factors known to influence outcomes (such as patient age at transplantation and chromosome abnormalities and FAB classification of leukemia) were included in the final models. Variables with more than 2 categories were dichotomized for the final multivariate model. The cutoff points of the variables were chosen to make optimal use of the information, with the proviso that smaller groups contain at least 20% of the patients. Variables were dichotomized as follows: patient age greater or younger than 45 years at transplantation, female donor to male recipient donor-recipient sex mismatch versus others for donor-recipient sex matching, donor-recipient ABO major mismatch versus others for ABO matching, M5/M6/M7 FAB classification versus others for classification of AML, chromosome abnormality other than favorable abnormalities for cytogenetics of AML, cyclophosphamide and total body irradiation (TBI) or busulfan and cyclophosphamide or others for conditioning regimen of AML, cyclophospohamide and TBI, or others for conditioning regimen of ALL, and cyclosporine-based versus tacrolimus-based prophylaxis against GVHD. Disease status at transplantation was categorized as first complete remission (1CR), second or later complete remission (2CR), or more advanced disease; which was included in the final model using dichotomized dummy variables. All P values were 2-sided.
The statistical power to detect hazard ratios (HRs) of 2.0 and 1.5 (a regression coefficient equal to 0.6931 and 0.4055, respectively) on Cox regression of the log hazard ratio at a .05 significance level adjusted for event rate were 99% and 78%, respectively, for 484 patients with AML and 97% and 60%, respectively, for 336 patients with ALL. The levels of statistical power for subgroup analyses were as follows: 54% and 22% for 1CR, 51% and 21% for 2CR, 96% and 58% for more advanced in AML patients, 62% and 26% for 1CR, 47% and 20% for 2CR, and 67% and 29% for more advanced in ALL patients.20
Results
Patient characteristics
The characteristics of the patients are shown in Table 1. There was no significant difference in recipients' age at transplantation in AML (median age, CB vs BM = 38 vs 38 years, P = .61) and in ALL (median age, CB vs BM = 34 vs 32 years, P = .29). The female/male ratio was higher (CB vs BM = 54% vs 38% in AML patients, and CB vs BM = 54% vs 38% in ALL patients, P < .001 and P = .005, respectively) in CB recipients, resulting in the lower donor-patient sex match rate (CB vs BM = 48% vs 69% in AML patients, and CB vs BM = 46% vs 65% in ALL patients, P < .001 and P = .002, respectively) in CB recipients. The proportion of ALL patients with Philadelphia chromosome abnormality was higher (CB vs BM = 38% vs 23%) in CB recipients. CB recipients were likely to have more advanced disease status at transplantation (relapse or induction failure, CB vs BM = 47% vs 31% in AML patients, and CB vs BM = 26% vs 19% in ALL patients), and the difference was significant in AML (P = .003). HLA-A, -B (low-resolution typing), and -DRB1 (high-resolution typing) was mismatched in 93% of both AML and ALL among CB recipients, whereas HLA -A, -B, -C, and -DRB1 were all genotypically matched for BM recipients. The ABO-matched donor-patient pair proportion was consistently lower for CB (CB vs BM = 34% vs 59% in AML patients and CB vs BM = 32% vs 58% in ALL patients).
Characteristics of recipients of cord blood or bone marrow from unrelated donors in 484 patients with acute myeloid leukemia and 336 patients with acute lymphoblastic leukemia
| Characteristic . | Acute myeloid leukemia . | Acute lymphoblastic leukemia . | ||||
|---|---|---|---|---|---|---|
| U-CBT . | U-BMT . | P . | U-CBT . | U-BMT . | P . | |
| No. of transplantations | 173 | 311 | 114 | 222 | ||
| Median patient age at transplantation, y (range) | 38 (16-69) | 38 (16-60) | .61 | 34 (16-58) | 32 (16-59) | .29 |
| Patient sex, n (%) | ||||||
| Male | 80 (46) | 194 (62) | < .001 | 52 (46) | 137 (62) | .005 |
| Female | 93 (54) | 117 (38) | 62 (54) | 85 (38) | ||
| Sex matching, n (%) | < .001 | .002 | ||||
| Matched | 83 (48) | 216 (69) | 52 (46) | 145 (65) | ||
| Male to female | 44 (25) | 57 (18) | 35 (31) | 42 (19) | ||
| Female to male | 46 (27) | 37 (12) | 27 (24) | 35 (16) | ||
| Unknown | 0 (0) | 1 (0) | 0 (0) | 0 (0) | ||
| Disease classification | ||||||
| AML (French-American-British) | .045 | |||||
| M0 | 17 (10) | 26 (8) | ||||
| M1 | 30 (17) | 38 (12) | ||||
| M2 | 52 (30) | 88 (28) | ||||
| M3 | 4 (2) | 25 (8) | ||||
| M4 | 27 (16) | 55 (18) | ||||
| M5 | 23 (13) | 41 (13) | ||||
| M6 | 3 (2) | 18 (6) | ||||
| M7 | 2 (1) | 5 (2) | ||||
| Others/unknown | 15 (9) | 15 (5) | ||||
| Cytogenetics | .042 | |||||
| Favorable* | 19 (11) | 66 (21) | ||||
| Normal | 74 (43) | 116 (37) | ||||
| Other | 57 (33) | 95 (31) | ||||
| Unknown | 23 (13) | 34 (11) | ||||
| ALL cytogenetics | .022 | |||||
| t(9;22) | 43 (38) | 52 (23) | ||||
| t(4;11) | 2 (2) | 3 (1) | ||||
| Others | 22 (19) | 51 (23) | ||||
| Normal | 27 (24) | 85 (38) | ||||
| Unknown | 20 (18) | 31 (14) | ||||
| Disease status | .003 | .33 | ||||
| First CR | 50 (29) | 130 (42) | 63 (55) | 130 (59) | ||
| Second or after CR | 39 (23) | 82 (26) | 21 (18) | 48 (22) | ||
| Relapse/induction failure | 81 (47) | 95 (31) | 30 (26) | 42 (19) | ||
| Unknown | 3 (2) | 4 (1) | 0 (0) | 2 (1) | ||
| HLA matching† | ||||||
| 0 mismatched loci | 12 (7) | 8 (7) | ||||
| 1 mismatched locus | 35 (20) | 25 (22) | ||||
| 2 mismatched loci | 126 (73) | 81 (71) | ||||
| ABO matching | < .001 | < .001 | ||||
| Matched | 59 (34) | 185 (59) | 37 (32) | 128 (58) | ||
| Minor mismatch | 48 (28) | 57 (18) | 30 (26) | 48 (22) | ||
| Major mismatch | 37 (21) | 59 (19) | 24 (21) | 41 (18) | ||
| Bidirectional | 28 (16) | 8 (3) | 23 (20) | 3 (1) | ||
| Unknown | 1 (1) | 2 (1) | 0 (0) | 2 (1) | ||
| Nucleated cells infused per 107/kg, median (range) | 2.44 (1.65-5.49) | 26.3 (2.10-58.8) | < .001 | 2.48 (1.51-4.06) | 28.2 (2.30-79.0) | < .001 |
| Preparative regimen | < .001 | .38 | ||||
| CY + TBI | 43 (25) | 142 (46) | 42 (37) | 92 (41) | ||
| CY + CA + TBI | 62 (36) | 41 (13) | 31 (27) | 53 (24) | ||
| CY + BU + TBI | 7 (4) | 36 (12) | 3 (3) | 5 (2) | ||
| Other TBI regimen | 42 (24) | 33 (11) | 34 (30) | 54 (24) | ||
| BU + CY | 18 (10) | 55 (18) | 4 (4) | 12 (5) | ||
| Other non-TBI regimen | 1 (1) | 4 (1) | 0 (0) | 6 (3) | ||
| GVHD prophylaxisis | < .001 | < .001 | ||||
| Cyclosporine A + sMTX | 103 (60) | 131 (42) | 65 (57) | 100 (45) | ||
| Cyclosporine A ± other | 20 (12) | 4 (1) | 6 (5) | 3 (1) | ||
| Tacrolimus + sMTX | 34 (20) | 168 (54) | 26 (23) | 106 (48) | ||
| Tacrolimus ± other | 15 (9) | 5 (2) | 16 (14) | 11 (5) | ||
| Others | 1 (1) | 3 (1) | 1 (1) | 2 (1) | ||
| Characteristic . | Acute myeloid leukemia . | Acute lymphoblastic leukemia . | ||||
|---|---|---|---|---|---|---|
| U-CBT . | U-BMT . | P . | U-CBT . | U-BMT . | P . | |
| No. of transplantations | 173 | 311 | 114 | 222 | ||
| Median patient age at transplantation, y (range) | 38 (16-69) | 38 (16-60) | .61 | 34 (16-58) | 32 (16-59) | .29 |
| Patient sex, n (%) | ||||||
| Male | 80 (46) | 194 (62) | < .001 | 52 (46) | 137 (62) | .005 |
| Female | 93 (54) | 117 (38) | 62 (54) | 85 (38) | ||
| Sex matching, n (%) | < .001 | .002 | ||||
| Matched | 83 (48) | 216 (69) | 52 (46) | 145 (65) | ||
| Male to female | 44 (25) | 57 (18) | 35 (31) | 42 (19) | ||
| Female to male | 46 (27) | 37 (12) | 27 (24) | 35 (16) | ||
| Unknown | 0 (0) | 1 (0) | 0 (0) | 0 (0) | ||
| Disease classification | ||||||
| AML (French-American-British) | .045 | |||||
| M0 | 17 (10) | 26 (8) | ||||
| M1 | 30 (17) | 38 (12) | ||||
| M2 | 52 (30) | 88 (28) | ||||
| M3 | 4 (2) | 25 (8) | ||||
| M4 | 27 (16) | 55 (18) | ||||
| M5 | 23 (13) | 41 (13) | ||||
| M6 | 3 (2) | 18 (6) | ||||
| M7 | 2 (1) | 5 (2) | ||||
| Others/unknown | 15 (9) | 15 (5) | ||||
| Cytogenetics | .042 | |||||
| Favorable* | 19 (11) | 66 (21) | ||||
| Normal | 74 (43) | 116 (37) | ||||
| Other | 57 (33) | 95 (31) | ||||
| Unknown | 23 (13) | 34 (11) | ||||
| ALL cytogenetics | .022 | |||||
| t(9;22) | 43 (38) | 52 (23) | ||||
| t(4;11) | 2 (2) | 3 (1) | ||||
| Others | 22 (19) | 51 (23) | ||||
| Normal | 27 (24) | 85 (38) | ||||
| Unknown | 20 (18) | 31 (14) | ||||
| Disease status | .003 | .33 | ||||
| First CR | 50 (29) | 130 (42) | 63 (55) | 130 (59) | ||
| Second or after CR | 39 (23) | 82 (26) | 21 (18) | 48 (22) | ||
| Relapse/induction failure | 81 (47) | 95 (31) | 30 (26) | 42 (19) | ||
| Unknown | 3 (2) | 4 (1) | 0 (0) | 2 (1) | ||
| HLA matching† | ||||||
| 0 mismatched loci | 12 (7) | 8 (7) | ||||
| 1 mismatched locus | 35 (20) | 25 (22) | ||||
| 2 mismatched loci | 126 (73) | 81 (71) | ||||
| ABO matching | < .001 | < .001 | ||||
| Matched | 59 (34) | 185 (59) | 37 (32) | 128 (58) | ||
| Minor mismatch | 48 (28) | 57 (18) | 30 (26) | 48 (22) | ||
| Major mismatch | 37 (21) | 59 (19) | 24 (21) | 41 (18) | ||
| Bidirectional | 28 (16) | 8 (3) | 23 (20) | 3 (1) | ||
| Unknown | 1 (1) | 2 (1) | 0 (0) | 2 (1) | ||
| Nucleated cells infused per 107/kg, median (range) | 2.44 (1.65-5.49) | 26.3 (2.10-58.8) | < .001 | 2.48 (1.51-4.06) | 28.2 (2.30-79.0) | < .001 |
| Preparative regimen | < .001 | .38 | ||||
| CY + TBI | 43 (25) | 142 (46) | 42 (37) | 92 (41) | ||
| CY + CA + TBI | 62 (36) | 41 (13) | 31 (27) | 53 (24) | ||
| CY + BU + TBI | 7 (4) | 36 (12) | 3 (3) | 5 (2) | ||
| Other TBI regimen | 42 (24) | 33 (11) | 34 (30) | 54 (24) | ||
| BU + CY | 18 (10) | 55 (18) | 4 (4) | 12 (5) | ||
| Other non-TBI regimen | 1 (1) | 4 (1) | 0 (0) | 6 (3) | ||
| GVHD prophylaxisis | < .001 | < .001 | ||||
| Cyclosporine A + sMTX | 103 (60) | 131 (42) | 65 (57) | 100 (45) | ||
| Cyclosporine A ± other | 20 (12) | 4 (1) | 6 (5) | 3 (1) | ||
| Tacrolimus + sMTX | 34 (20) | 168 (54) | 26 (23) | 106 (48) | ||
| Tacrolimus ± other | 15 (9) | 5 (2) | 16 (14) | 11 (5) | ||
| Others | 1 (1) | 3 (1) | 1 (1) | 2 (1) | ||
U-CBT, indicates unrelated cord blood transplantation; U-BMT, unrelated bone marrow transplantation; CR, complete remission; HLA, human leukocyte antigen; CY, cyclophosphamide; CA, cytarabine; BU, oral busulfan; TBI, total body irradiation; and sMTX, short-term methotrexate.
Favorable abnormal karyotypes are defined as t(8;21), inv16,or t(15;17).
Number of mismatches was counted among HLA-A, -B (low-resolution typing), and DRB1 (high-resolution typing).
A preparative regimen with TBI and cyclophosphamide was used in almost all patients, and cytosine arabinoside was supplemented for CB recipients with AML (36%) in addition to TBI and cyclophosphamide. For GVHD prophylaxis, tacrolimus (CB vs BM = 29% vs 56% in AML patients, and CB vs BM = 37% vs 53% in ALL patients) and short-term methotrexate (CB vs BM = 80% vs 96% in AML patients, and CB vs BM = 80% vs 93% in ALL patients) were used preferentially in BM recipients. The median follow-up period for survivors was 1.9 years (range, 0.1-6.2 years) for CB recipients and 1.4 years (range, 0.3-4.5 years) for BM recipients.
Outcome
OS.
For patients with AML, the unadjusted probabilities of OS were lower for CB recipients at 1 year (51% vs 69%) and 2 years (43% vs 60%) compared with BM recipients (P < .001). For patients with ALL, there were no significant differences between the 2 groups (CB vs BM = 66% vs 66% at 1 year, 49% vs 57% at 2 years, P = .40).
Among patients with AML, the use of CB remained a significant risk factor for overall mortality after adjustment for other factors (HR = 1.5; 95% confidence interval [CI], 1.0-2.0; P = .028; Table 2). However, in patients with ALL, the use of CB was not a significant factor for overall mortality on multivariate analysis (HR = 1.1; 95% CI, 0.7-1.6; P = .78). The adjusted probability of OS was significantly lower for CB recipients (57% vs 69% at 1 year, and 48% vs 59% at 2 years, P = .010; Figure 1A) compared with BM recipients for patients with AML, whereas the adjusted probability of OS was similar (69% vs 64% at 1 year, and 52% vs 53% at 2 years, P = .99; Figure 1B) between the groups for patients with ALL.
Results of multivariate analysis of outcomes in 173 recipients of cord blood and 311 recipients of bone marrow with acute myeloid leukemia, and 114 recipients of cord blood and 222 recipients of bone marrow with acute lymphoblastic leukemia
| Outcome . | Acute myeloid leukemia . | Acute lymphoblastic leukemia . | ||
|---|---|---|---|---|
| RR (95% CI) . | P . | RR (95% CI) . | P . | |
| Overall survival* | ||||
| BM | 1.00 | 1.00 | ||
| CB | 1.45 (1.04-2.01) | .028 | 1.06 (0.71-1.57) | .78 |
| Leukemia-free survival† | ||||
| BM | 1.00 | 1.00 | ||
| CB | 1.48 (1.09-2.01) | .012 | 1.22 (0.85-1.76) | .28 |
| Relapse‡ | ||||
| BM | 1.00 | 1.00 | ||
| CB | 1.21 (0.79-1.87) | .38 | 1.42 (0.84-2.41) | .19 |
| TRM§ | ||||
| BM | 1.00 | 1.00 | ||
| CB | 1.47 (0.95-2.28) | .085 | 1.01 (0.59-1.73) | .98 |
| Neutrophil recovery‖ | ||||
| BM | 1.00 | 1.00 | ||
| CB | 0.41 (0.33-0.51) | < .001 | 0.37 (0.29-0.48) | < .001 |
| Platelet recovery¶ | ||||
| BM | 1.00 | 1.00 | ||
| CB | 0.34 (0.27-0.44) | < .001 | 0.43 (0.33-0.56) | < .001 |
| Acute GVHD# | ||||
| BM | 1.00 | 1.00 | ||
| CB | 0.80 (0.56-1.15) | .23 | 0.61 (0.39-0.95) | .028 |
| Chronic GVHD** | ||||
| BM | 1.00 | 1.00 | ||
| CB | 0.94 (0.63-1.42) | .79 | 1.08 (0.66-1.77) | .77 |
| Chronic GVHD, extensive type†† | ||||
| BM | 1.00 | 1.00 | ||
| CB | 0.36 (0.18-0.72) | .004 | 0.58 (0.28-1.20) | .14 |
| Outcome . | Acute myeloid leukemia . | Acute lymphoblastic leukemia . | ||
|---|---|---|---|---|
| RR (95% CI) . | P . | RR (95% CI) . | P . | |
| Overall survival* | ||||
| BM | 1.00 | 1.00 | ||
| CB | 1.45 (1.04-2.01) | .028 | 1.06 (0.71-1.57) | .78 |
| Leukemia-free survival† | ||||
| BM | 1.00 | 1.00 | ||
| CB | 1.48 (1.09-2.01) | .012 | 1.22 (0.85-1.76) | .28 |
| Relapse‡ | ||||
| BM | 1.00 | 1.00 | ||
| CB | 1.21 (0.79-1.87) | .38 | 1.42 (0.84-2.41) | .19 |
| TRM§ | ||||
| BM | 1.00 | 1.00 | ||
| CB | 1.47 (0.95-2.28) | .085 | 1.01 (0.59-1.73) | .98 |
| Neutrophil recovery‖ | ||||
| BM | 1.00 | 1.00 | ||
| CB | 0.41 (0.33-0.51) | < .001 | 0.37 (0.29-0.48) | < .001 |
| Platelet recovery¶ | ||||
| BM | 1.00 | 1.00 | ||
| CB | 0.34 (0.27-0.44) | < .001 | 0.43 (0.33-0.56) | < .001 |
| Acute GVHD# | ||||
| BM | 1.00 | 1.00 | ||
| CB | 0.80 (0.56-1.15) | .23 | 0.61 (0.39-0.95) | .028 |
| Chronic GVHD** | ||||
| BM | 1.00 | 1.00 | ||
| CB | 0.94 (0.63-1.42) | .79 | 1.08 (0.66-1.77) | .77 |
| Chronic GVHD, extensive type†† | ||||
| BM | 1.00 | 1.00 | ||
| CB | 0.36 (0.18-0.72) | .004 | 0.58 (0.28-1.20) | .14 |
RR indicates relative risk; CI, confidence interval; BM, bone marrow; CB, cord blood; and GVHD, graft-versus-host disease.
For overall survival, other significant variables for AML were patient age more than 45 years at transplantation, more advanced disease status at conditioning, M5/M6/M7 French-American-British classification, and female donor to male recipient donor-recipient sex mismatch; other significant variables for ALL were second or after complete remission disease status, more advanced disease status, and Philadelphia chromosome abnormality.
For leukemia-free survival, other significant variables for AML were patient age more than 45 years at transplantation, more advanced disease status at conditioning, M5/M6/M7 French-American-British classification, and female donor to male recipient donor-recipient sex mismatch; other significant variables for ALL were second or after complete remission disease status, more advanced disease status, and Philadelphia chromosome abnormality.
For relapse, other significant variables for AML were more advanced disease status at conditioning, donor-recipient ABO major mismatch, chromosome abnormality other than favorable abnormalities, and cyclophosphamide and total body irradiation or busulfan and cyclophosphamide conditioning regimen; other significant variables for ALL were second or after complete remission disease status, more advanced disease status, and cyclophosphamide and total body irradiation conditioning.
For TRM, other significant variables for AML were patient age more than 45 years at transplantation, second or after complete remission disease status, more advanced disease status, and chromosome abnormality other than favorable abnormalities; other significant variables for ALL were patient age more than 45 years at transplantation, more advanced disease status at conditioning, and conditioning other than cyclophosphamide and total body irradiation.
For neutrophil recovery, other significant variables for AML were second or after complete remission disease status and more advanced disease status; other significant variables for ALL were more advanced disease status at conditioning and cyclosporine-based GVHD prophylaxis.
For platelet recovery; other significant variables for AML were second or after complete remission disease status, more advanced disease status, female donor to male recipient donor-recipient sex mismatch, and tacrolimus-based GVHD prophylaxis; other significant variables for ALL were more advanced disease status at conditioning and conditioning other than cyclophosphamide and total body irradiation.
For acute GVHD, no other significant variables were identified for both AML and ALL.
For chronic GVHD, other significant variables for AML were more advanced disease status and conditioning other than cyclophosphamide and total body irradiation or busulfan and cyclophosphamide; there were no other significant variables identified for ALL.
For extensive chronic GVHD, there were no other significant variables identified for AML; another significant variable for ALL was patient male sex.
Adjusted OS and LFS of recipients with AML or ALL of CB or BM from unrelated donors. For patients with AML, adjusted probabilities of (A) OS (CB vs BM = 48% vs 59% at 2 years, P = .010) and (C) LFS (CB vs BM = 42% vs 54% at 2 years, P = .004) were both lower in CB recipients. For patients with ALL, the adjusted probabilities of (B) OS (CB vs BM = 52% vs 53% at 2 years, P = .99) and (D) LFS (CB vs BM = 46% vs 44% at 2 years, P = .41) were similar between CB recipients and BM recipients.
Adjusted OS and LFS of recipients with AML or ALL of CB or BM from unrelated donors. For patients with AML, adjusted probabilities of (A) OS (CB vs BM = 48% vs 59% at 2 years, P = .010) and (C) LFS (CB vs BM = 42% vs 54% at 2 years, P = .004) were both lower in CB recipients. For patients with ALL, the adjusted probabilities of (B) OS (CB vs BM = 52% vs 53% at 2 years, P = .99) and (D) LFS (CB vs BM = 46% vs 44% at 2 years, P = .41) were similar between CB recipients and BM recipients.
Results of the subgroup analyses showed that the difference in survival among AML patients was prominent in patients demonstrating 1CR at transplantation (RR = 2.9, 95% CI = 1.4-6.2, P = .005; Table 3).
Results of multivariate analysis of overall survival according to disease status at transplantation
| Overall survival . | First complete remission . | Second or after complete remission . | More advanced . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n . | RR (95% CI) . | P . | n . | RR (95% CI) . | P . | n . | RR (95% CI) . | P . | |
| AML | |||||||||
| UBMT | 130 | 1.00 | 82 | 1.00 | 95 | 1.00 | |||
| UCBT | 50 | 2.92 (1.38-6.18) | .005 | 39 | 1.24 (0.51-3.04) | .63 | 81 | 1.29 (0.84-1.98) | .25 |
| ALL | |||||||||
| UBMT | 130 | 1.00 | 48 | 1.00 | 42 | 1.00 | |||
| UCBT | 63 | 1.60 (0.84-3.05) | .16 | 21 | 0.62 (0.22-1.74) | .36 | 30 | 0.80 (0.38-1.69) | .57 |
| Overall survival . | First complete remission . | Second or after complete remission . | More advanced . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n . | RR (95% CI) . | P . | n . | RR (95% CI) . | P . | n . | RR (95% CI) . | P . | |
| AML | |||||||||
| UBMT | 130 | 1.00 | 82 | 1.00 | 95 | 1.00 | |||
| UCBT | 50 | 2.92 (1.38-6.18) | .005 | 39 | 1.24 (0.51-3.04) | .63 | 81 | 1.29 (0.84-1.98) | .25 |
| ALL | |||||||||
| UBMT | 130 | 1.00 | 48 | 1.00 | 42 | 1.00 | |||
| UCBT | 63 | 1.60 (0.84-3.05) | .16 | 21 | 0.62 (0.22-1.74) | .36 | 30 | 0.80 (0.38-1.69) | .57 |
RR indicates relative risk; CI, confidence interval; UBMT, unrelated bone marrow transplantation; and UCBT, unrelated cord blood transplantation.
LFS.
For patients with AML, the unadjusted probabilities of LFS were significantly lower for CB recipients at 1 year (43% vs 62%) and 2 years (36% vs 54%) compared with BM recipients (P < .001). For patients with ALL, the unadjusted probabilities of LFS were lower with marginal significance for CB recipients at 1 year (52% vs 58%) and 2 years (45% vs 51%) compared with BM recipients (P = .06).
Among patients with AML, the use of CB remained as a significant risk factor for treatment failure (ie, relapse or death) after adjustment for other factors (HR = 1.5; 95% CI, 1.1-2.0; P = .012; Table 2). However, in patients with ALL, the use of CB was not a significant factor for treatment failure by multivariate analysis (HR = 1.2; 95% CI, 0.9-1.8; P = .28). The adjusted probability of LFS was significantly lower for CB recipients (51% vs 62% at 1 year, and 42% vs 54% at 2 years, P = .004; Figure 1C) compared with BM recipients for patients with AML, whereas the adjusted probability of LFS was similar (53% vs 53% at 1 year, and 46% vs 44% at 2 years, P = .41; Figure 1D) between the groups for patients with ALL.
Relapse
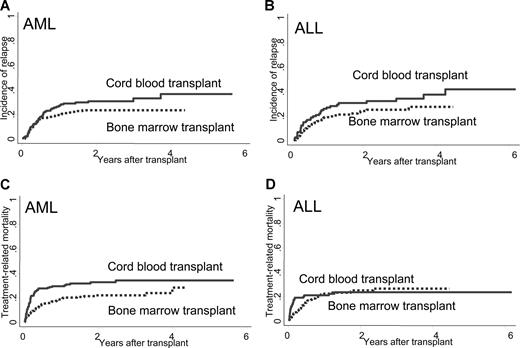
On univariate analyses, the cumulative incidence of relapse was higher for CB recipients with marginal significance in both AML (27% vs 20% at 1 year, and 31% vs 24% at 2 years) and ALL (27% vs 19% at 1 year, and 31% vs 24% at 2 years) (P = .067, and .085, respectively; Figure 2A,B).
Cumulative incidence of relapse or TRM of recipients of CB or BM among patients with AML or ALL. For patients with AML, the cumulative incidence of (A) relapse (CB vs BM = 31% vs 24% at 2 years, P = .068) and (C) TRM (CB vs BM = 33% vs 22% at 2 years, P = .004) was higher in CB recipients. For patients with ALL, the cumulative incidence of relapse (B) was higher in CB recipients with marginal significance (CB vs BM = 31% vs 24% at 2 years, P = .085), but the incidence of TRM (D) was similar in CB and BM recipients (CB vs BM = 24% vs 25% at 2 years, P = .83).
Cumulative incidence of relapse or TRM of recipients of CB or BM among patients with AML or ALL. For patients with AML, the cumulative incidence of (A) relapse (CB vs BM = 31% vs 24% at 2 years, P = .068) and (C) TRM (CB vs BM = 33% vs 22% at 2 years, P = .004) was higher in CB recipients. For patients with ALL, the cumulative incidence of relapse (B) was higher in CB recipients with marginal significance (CB vs BM = 31% vs 24% at 2 years, P = .085), but the incidence of TRM (D) was similar in CB and BM recipients (CB vs BM = 24% vs 25% at 2 years, P = .83).
On multivariate analyses adjusted by other factors, there was no significantly higher risk of relapse for CB recipients with either AML (RR = 1.2, 95% CI = 0.8-1.9, P = .38) or ALL (RR = 1.4, 95% CI = 0.8-2.4, P = .19; Table 2).
TRM
For patients with AML, the unadjusted cumulative incidence of TRM was significantly higher for CB recipients at 1 year (30% vs 19%) and 2 years (33% vs 22%) compared with those for BM recipients (P = .004; Figure 2C). For patients with ALL, the cumulative incidence of TRM was similar between the 2 groups (CB vs BM = 21% vs 23% at 1 year, 24% vs 25% at 2 years, P = .83; Figure 2D).
On multivariate analyses adjusted by other factors, the risk for TRM was higher for CB recipients compared with that for BM recipients among patients with AML (RR = 1.5, 95% CI = 1.0-2.3, P = .085; Table 2) with marginal significance. For patients with ALL, the risk for TRM was similar between CB and BM recipients (RR = 1.0, 95% CI = 0.6-1.7, P = .98).
Cause of death
Recurrence of the primary disease was the leading cause of death in each group (CB vs BM = 37% vs 33% in patients with AML and 36% vs 41% in patients with ALL). The following causes were infection and organ failure in all groups (Table 4).
Causes of death after transplantation of unrelated cord blood or unrelated bone marrow among patients with acute myeloid leukemia or acute lymphoblastic leukemia
| Cause of death . | Acute myeloid leukemia . | Acute lymphoblastic leukemia . | ||
|---|---|---|---|---|
| UCBT . | UBMT . | UCBT . | UBMT . | |
| Recurrence of disease | 35 (37) | 34 (33) | 18 (36) | 34 (41) |
| Graft failure/rejection | 3 (3) | 4 (4) | 0 (0) | 3 (4) |
| Graft-versus-host disease | 6 (6) | 7 (7) | 3 (6) | 5 (6) |
| Infection | 22 (23) | 19 (18) | 13 (26) | 11 (13) |
| Idiopathic pneumonia | 4 (4) | 4 (4) | 2 (4) | 6 (7) |
| Organ failure | 17 (18) | 17 (16) | 8 (16) | 10 (12) |
| Secondary cancer | 0 (0) | 1 (1) | 0 (0) | 0 (0) |
| Other causes | 5 (5) | 5 (5) | 2 (4) | 4 (5) |
| Unknown/data missing | 2 (2) | 13 (13) | 4 (8) | 10 (12) |
| Total | 94 (100) | 104 (100) | 50 (100) | 83 (100) |
| Cause of death . | Acute myeloid leukemia . | Acute lymphoblastic leukemia . | ||
|---|---|---|---|---|
| UCBT . | UBMT . | UCBT . | UBMT . | |
| Recurrence of disease | 35 (37) | 34 (33) | 18 (36) | 34 (41) |
| Graft failure/rejection | 3 (3) | 4 (4) | 0 (0) | 3 (4) |
| Graft-versus-host disease | 6 (6) | 7 (7) | 3 (6) | 5 (6) |
| Infection | 22 (23) | 19 (18) | 13 (26) | 11 (13) |
| Idiopathic pneumonia | 4 (4) | 4 (4) | 2 (4) | 6 (7) |
| Organ failure | 17 (18) | 17 (16) | 8 (16) | 10 (12) |
| Secondary cancer | 0 (0) | 1 (1) | 0 (0) | 0 (0) |
| Other causes | 5 (5) | 5 (5) | 2 (4) | 4 (5) |
| Unknown/data missing | 2 (2) | 13 (13) | 4 (8) | 10 (12) |
| Total | 94 (100) | 104 (100) | 50 (100) | 83 (100) |
Data are presented as n (%).
UCBT indicates unrelated cord blood transplantation; and UBMT, unrelated bone marrow transplantation.
Other outcomes of transplantation
Neutrophil and platelet recovery.
The unadjusted cumulative incidence of neutrophil recovery or platelet recovery at day 100 was significantly lower in CB recipients for both AML (77% vs 94%) and ALL (80% vs 97%) compared with that among BM recipients (P < .001 for both). On multivariate analyses, neutrophil recovery was significantly lower among CB recipients for both AML (RR = 0.4, 95% CI = 0.3-0.5, P < .001) and ALL (RR = 0.4, 95% CI = 0.3-0.5, P < .001; Table 2).
The unadjusted cumulative incidence of platelet recovery greater than 50 000/μL at 4 months was significantly lower among CB recipients for both AML (59% vs 85%) and ALL (61% vs 83%) compared with that of BM recipients (P < .001 for both). The difference was also significant on multivariate analyses for both AML (RR = 0.3, 95% CI = 0.3-0.4, P < .001) and ALL (RR = 0.4, 95% CI = 0.3-0.6, P < .001; Table 2).
Acute GVHD.
The unadjusted cumulative incidence of grade 2 to 4 acute GVHD was lower among CB recipients compared with that among BM recipients (32% vs 35% in AML, 28% vs 42% in ALL); the difference was significant in patients with ALL (P = .39 in AML, P = .008 in ALL). The difference was also significant on multivariate analyses in ALL (RR = 0.6, 95% CI = 0.4-1.0, P = .028). There was no significant difference in patients with AML (RR = 0.8, 95% CI = 0.6-1.2, P = .23; Table 2).
Chronic GVHD.
The unadjusted cumulative incidence of chronic GVHD at 1 year after transplantation did not significantly differ between CB recipients and BM recipients in both AML (28% vs 32%, P = .46) and ALL (27% vs 30%, P = .50). The cumulative incidence of extensive-type chronic GVHD was significantly lower among CB recipients compared with that among BM recipients in both AML (8% vs 20%, P < .001) and ALL (10% vs 17%, P = .034). On multivariate analyses, the risk of developing chronic GVHD was similar in CB recipients and BM recipients in both AML (RR = 0.9, 95% CI = 0.6-1.4, P = .79) and ALL (RR = 1.1, 95% CI = 0.7-1.8, P = .77). The risk of developing extensive chronic GVHD was lower in CB recipients compared with BM recipients (RR = 0.4, 95% CI = 0.2-0.7, P = .004 in AML, and RR = 0.6, 95% CI, 0.3-1.2, P = .14 in ALL) and was significantly different in patients with AML (Table 2).
Discussion
The objective of our study was to investigate the outcomes of HLA-A, -B, low-resolution, and -DRB1 high-resolution 0 to 2 mismatched single-unit unrelated CBT in adult patients with acute leukemia compared with those of HLA-A, -B, -C, and -DRB1 (8 of 8) allele-matched unrelated BMT. Although AML and ALL are different diseases, previous comparisons of unrelated BMT and unrelated CBT did not separate these 2 diseases. Our report is the first to show the result of disease-specific analyses with a sufficient number of patients.
For AML patients, the recipients of CB were more likely to have advanced leukemia at the time of transplantation, as reported previously, suggesting that CB was used as an alternative stem cell source in the later phase of unrelated donor searches, especially in adults.11,12,14 A larger proportion of CB recipients with ALL had the Philadelphia chromosome abnormality, which correlates with highly aggressive ALL and usually requires urgent transplantation, in which CB has an advantage over BM.21
Different outcomes of mortality were found between AML and ALL in a controlled comparison using multivariate analyses. Whereas significantly lower OS and LFS rates were observed in CB recipients with AML, rates of overall mortality and treatment failure were similar between CB and BM recipients with ALL. The relapse rate was not different between CBT and BMT in patients with both AML and ALL, which was consistent with previous reports.11-13 In adult patients with ALL, a previous report showed no difference in the outcome of related compared with unrelated BM or peripheral blood transplantation in 1CR.22 Favorable disease status at transplantation could be a more important factor affecting outcome rather than the type of stem cell source or donor type in patients with ALL. It is notable that TRM in HLA allele-matched unrelated BM recipients with AML was quite low in our study. This is probably associated with the low incidence of acute and chronic GVHD in the Japanese population, which is thought to be the result of genetic homogeneity.23-26 Among patients with AML, although the difference was not statistically significant, a higher trend of TRM observed in CB recipients might be associated with higher overall and TRM rates in CB recipients. Reasons for higher TRM could include the graft source and delayed neutrophil recovery. Better supportive care is required after CBT for patients going through a prolonged neutropenic period. Development of better graft engineering or better conditioning regimens would help to decrease the TRM rate in CB recipients. Because relapse was the major cause of death in all groups, any attempt to decrease TRM should preserve the antileukemia effect to improve OS and LFS. Another reason for the higher TRM could be a higher risk patient population, higher risk for both disease status and comorbid conditions, requiring rapid transplantation. Searching for unrelated donors earlier and providing transplantation earlier in the disease course could help to decrease TRM in CB recipients.
Neutrophil and platelet recovery was slower in CB recipients with either AML or ALL, consistent with the results of previous reports.11,12,27 Multiple studies have reported lower incidence of acute GVHD in CB recipients.8-10,12,13 In our study, particularly in patients with ALL, the risk of developing grade 2 to 4 acute GVHD in CB recipients was lower compared with BM recipients, which was reported to be lower compared with the incidence reported from Western countries.23-25 The risk of developing chronic GVHD was similar between CB and BM recipient with either disease, but the risk of developing extensive-type chronic GVHD was lower in CB recipients; the difference was significant in patients with AML. It is notable that there was no increase in the incidence of acute or chronic GVHD in CB recipients among patients with either AML or ALL, despite HLA disparity.
For differences in outcomes between AML and ALL, one possibility is a difference of treatment before conditioning therapy. Most AML patients received a more intense treatment for induction and consolidation therapy compared with that for ALL. There was no adjustment made for previous treatment, and this could be the reason for higher mortality in CBT, which requires a longer time for neutrophil recovery. Another possible cause of the difference in outcomes is the difference in conditioning regimens. Preparative regimens were similar between CB and BM recipients among ALL patients. However, in patients with AML, the proportion of standard regimens, such as cyclophosphamide and TBI or busulfan and cyclophosphamide, was smaller among CB recipients. These differences in the distribution of preparative regimens were also seen in a previous report.11 Although the final model was adjusted for conditioning regimens, we cannot rule out the possibility of an effect that larger CB recipients received additional or different chemotherapeutic agents compared with BM recipients among patients with AML. Although the difference was small, the median age of CB recipients with AML was 4 years older than CB recipients with ALL (median age, 38 vs 34 years, P = .021), which might have affected the higher mortality rate among CB recipients with AML. It is also possible that some unknown biologic aspects have contributed to these differences, and this would require further evaluation in future studies.
Further subgroup analyses indicated that the superiority of HLA allele-matched BM versus CB for OS was mostly found in patients with AML showing 1CR at conditioning. However, because of the limited numbers of patients in these subgroup analyses and the possibility of an unidentified bias in stem cell source selection, our findings should be verified by further analysis in a larger population.
In conclusion, we found different outcomes between patients with AML and ALL, indicating the importance of disease-specific analyses in alternative donor studies. HLA-A, -B low-resolution, and -DRB1 high-resolution 0 to 2 mismatched single-unit CB is a favorable alternative stem cell source for patients without a suitable related or 8 of 8 matched unrelated BM donor. In the absence of a suitable donor, unrelated CBT should be planned promptly to transplant the patient while in a better disease status and better clinical condition. For patients with AML, decreasing mortality, especially in the early phase of transplantation, is required to improve the outcome for CB recipients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all the staff members of the collaborating institutes of the Japan Cord Blood Bank Network and Japan Marrow Donor Program for their assistance and cooperation and Dr Takakazu Kawase for validating human leukocyte antigen data of the Japan Marrow Donor Program.
This work was supported by a Research Grant for Tissue Engineering (H17-014), a Research Grant for Allergic Disease and Immunology (H20-015), and a Research Grant for Cancer (H19-1) from the Japanese Ministry of Health, Labor, and Welfare.
Authorship
Contribution: Y.A. and R.S. designed the study and wrote the paper; Y.A. analyzed results and made the figures; S. Kato and Y.M. designed the research; T.-N.I., H.A., and M. Takanashi reviewed and cleaned the Japan Cord Blood Bank Network data and reviewed the results; S. Taniguchi, S. Takahashi, S. Kai, H.S., Y. Kouzai, M.K., and T.F. submitted and cleaned the data; and S.O., M. Tsuchida, K.K., Y.M., and Y. Kodera reviewed and cleaned the Japan Marrow Donor Program data and reviewed the results.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of members from the Japan Marrow Donor Program and the Japan Cord Blood Bank Network can be found in the Supplemental Appendix (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Correspondence: Yoshiko Atsuta, Department of Hematopoietic Stem Cell Transplantation Data Management, Nagoya University School of Medicine, 1-1-20 Daiko-Minami, Higashi-ku Nagoya, 461-0047 Japan; e-mail: y-atsuta@med.nagoya-u.ac.jp.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal