Abstract
Dendritic cell (DC) targeting in vivo has recently been shown to confer strong and protective cytotoxic T lymphocyte (CTL)–based immunity in tumor murine models. Our group has recently demonstrated in preclinical models that the infusion of genetically modified lymphocytes (GMLs) expressing the self/tumor antigen TRP-2 is able to elicit functional TRP-2–specific effectors with antitumor activity by targeting DCs in vivo. Here we have analyzed vaccine- and tumor-specific immune responses of 10 melanoma patients treated with autologous GMLs expressing the cancer germline gene MAGE-A3. Three of 10 patients treated with MAGE-A3–GML showed an increase of circulating anti–MAGE-A3 T cells, and developed skin delayed-type hypersensitivity to MAGE-A3. Interestingly, in 2 of these patients, with progressive and measurable tumors at study entry, anti–MAGE-A3 T cells were detected not only in the blood but also within tumors resected after vaccination. These results demonstrate that the infusion of MAGE-A3–GML elicits antitumor T cells, which are capable of trafficking to inflamed tissues and of infiltrating tumors. Clinical studies on a larger group of patients are needed to evaluate the clinical efficacy of such a strategy.
Introduction
Since tumor-associated antigens (TAAs)1 have been identified, several small-scale therapeutic vaccination and adoptive transfer studies2,3 have been performed. These trials have explored different vaccine formulations such as antigenic peptides, proteins, or peptide-pulsed dendritic cells.2,4,5 Although immune responses have been observed repeatedly, overall, these treatments have induced tumor regression in only 15% to 20% of the vaccinated patients.2,3 Several factors could be responsible for this limited efficacy, some of them related to the lack of induction of the antivaccine immune response, others related to the large variety of immune escape mechanisms operating in advanced tumors.6–8
In recent years, vaccination approaches based on dendritic cell (DC) targeting in vivo have successfully been investigated in tumor murine models.9 Most of them are based on the use of model TAA-bound antibodies recognizing phagocytic receptors on DC subsets.9 We have recently developed an approach of active vaccination based on a novel in vivo DC targeting strategy by the use of genetically modified lymphocytes (GMLs) expressing a defined TAA.10 Indeed, murine GMLs expressing the TRP-2 tumor antigen proved able to efficaciously load CD11c+CD8α+ dendritic cells within secondary lymphoid organs,10 thus eliciting TRP-2–specific effectors that could control the growth of B16F1 melanomas. The rationale of this strategy was based on clinical observations made on patients who were able to mount a strong transgene-specific response, following the infusion of genetically modified T cells.11–14 Here, we present the first results of the clinical translation of this approach in 10 patients affected by stage IIIc/IV melanoma expressing gene MAGE-A3.1 The infusion of autologous GMLs expressing MAGE-A3 and TK elicited circulating anti–MAGE-A3 T lymphocytes in 3 patients. Moreover, we demonstrate that these anti–MAGE-A3 T cells are able to migrate to the tumor site and to contribute to the inflammatory response associated with recognition of the tumor antigen in peripheral tissues (ie, delayed-type hypersensitivity [DTH]). These results, if confirmed on a larger group of patients, will allow treatment of patients affected by hematologic and solid tumors expressing MAGE-A3.
Methods
Clinical protocol
This is a pilot study of active vaccination for patients affected by malignant melanoma (stage IIIc/IV AJCC) with expression of MAGE-A3 tumor antigen, evaluated by reverse-transcription–polymerase chain reaction (RT-PCR) analysis, who have already received one line of chemotherapy and/or biochemotherapy for metastatic disease. The study was approved by the institutional review board (IRB) of the Scientific Institute S. Raffaele and by the Italian Institute of Health (ISS; Rome, Italy) and patient informed consent was obtained in accordance with the Declaration of Helsinki. Treatment consists of 5 intravenous infusions (at biweekly intervals) of escalating numbers (15, 30, and 50 × 106 × 3) of autologous lymphocytes transduced to express MAGE-A3 alone (CIP-18 and CIP-19) or in combination with TK (all the others). The 5 infusions correspond to a cycle. After the completion of the 10-week treatment, the investigator could administer (if clinically indicated) as maintenance therapy additional infusions at the maximum dose level (50 × 106) (at monthly intervals; exceptions to this rule are indicated). The primary end point of the study was safety. Secondary end points were in vivo (ie, DTH) and in vitro immune responses to the vaccine (HSV-TK and MAGE-A3). Based on the results described in this paper, a phase 1/2 clinical trial sponsored by MolMed has recently started.
Patient characteristics
All patients had progressive disease at study entry and had failed standard therapies, with the exception of patient CIP-19, who was clinically disease-free following biochemotherapy and surgery (Table 1). All patients received the first cycle of treatment. Some patients received additional injections, as indicated in Table 1. Before and after treatment, the patients were tested for their ability to mount a DTH reaction to the MAGE-A3 and TK gene products, and for the presence of circulating anti–MAGE-A3 and anti-TK T cells. Four patients (CIP-6, -13, -21, and -23) received only the first cycle of treatment, whereas the others (CIP-5, -11, -18, -19, -25, and -26) were given additional injections. Patients CIP-5, -6, -11, -13, -21, -23, -25, and 26 were treated with M3TN-transduced lymphocytes. Patients CIP-18 and CIP-19 were treated only with M3-CSM–transduced lymphocytes. DTH was carried out by subcutaneous injection of 1 to 2 × 106 autologous mock-GMLs or M3-GMLs. Twenty-four and 48 hours after injections, erythema and/or induration of at least 5 mm was scored as positive in the absence of reaction at the control injection site. Tumor sites were evaluated by physical examinations and scans performed before and after treatments. Standard definitions of response according to World Health Organization (WHO) criteria were used.15 Adverse effects were recorded using WHO toxicity criteria. Patient HLA typing was performed to identify the restriction elements involved in the elicited anti-TK and anti–MAGE-A3 immune responses (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Main patient characteristics
| Patient (CIP) . | Disease status* . | AJCC staging . | Clinical outcome* . | Prior treatments . | Infusions† . | Total GMLs infused, ×107 . | Vector used . |
|---|---|---|---|---|---|---|---|
| CIP-5 | PD | M1b | SD (50 mo) | Chemotherapy (CVD), surgery, biochemotherapy (IFNα2b, IL2, CVD) | 5 + 10 | 69,5 | LM3TN/M3-CSM |
| CIP-6 | PD | M1c | PD | Adjuvant therapy (IFNα2b), biochemotherapy (IFNα2b, IL2, CVD) | 5 | 19,5 | LM3TN |
| CIP-11 | PD | M1c | PD | Surgery, chemotherapy (DTIC) | 5 + 3 | 34,5 | LM3TN |
| CIP-13 | PD | M1c | PD | Adjuvant therapy (IFNα2b), chemotherapy (DTIC, CVD) | 5 | 19,5 | LM3TN |
| CIP-18 | PD | M1b | PD | Adjuvant therapy (IFNα2b) | 5 + 3 | 34,5 | M3-CSM |
| CIP-19 | NED | NED | NED (61 mo+) | Biochemotherapy (IFNα2b, IL2, CVD), surgery, biotherapy (IFNα2b and IL2) | 5 + 9 | 64,5 | M3-CSM |
| CIP-21 | PD | M1c | PD | Surgery, polichemotherapy with CDDP+DTIC | 5 | 19,5 | LM3TN |
| CIP-23 | PD | M1c | PD | Surgery, biochemotherapy (DTIC and IFNα2b), vaccination with pulsed DCs, biochemotherapy (fotemustine, IFNα2b and IL2), surgery | 5 | 19,5 | LM3TN |
| CIP-25 | PD | M1b | PD | Biochemotherapy (DTIC, CDDP +IFNα2b), immunotherapy (idiotype mAb + low doses IL2), radiotherapy + DTIC, mochemotherapy (taxanes) | 5 + 4 | 39,5 | LM3TN |
| CIP-26 | PD | IIIc | CR (6 + 3 mo) | Surgery, ILP, biotherapy (IFNα2b and IL2), ILP, surgery | 5 + 6 | 49,5 | LM3TN |
| Patient (CIP) . | Disease status* . | AJCC staging . | Clinical outcome* . | Prior treatments . | Infusions† . | Total GMLs infused, ×107 . | Vector used . |
|---|---|---|---|---|---|---|---|
| CIP-5 | PD | M1b | SD (50 mo) | Chemotherapy (CVD), surgery, biochemotherapy (IFNα2b, IL2, CVD) | 5 + 10 | 69,5 | LM3TN/M3-CSM |
| CIP-6 | PD | M1c | PD | Adjuvant therapy (IFNα2b), biochemotherapy (IFNα2b, IL2, CVD) | 5 | 19,5 | LM3TN |
| CIP-11 | PD | M1c | PD | Surgery, chemotherapy (DTIC) | 5 + 3 | 34,5 | LM3TN |
| CIP-13 | PD | M1c | PD | Adjuvant therapy (IFNα2b), chemotherapy (DTIC, CVD) | 5 | 19,5 | LM3TN |
| CIP-18 | PD | M1b | PD | Adjuvant therapy (IFNα2b) | 5 + 3 | 34,5 | M3-CSM |
| CIP-19 | NED | NED | NED (61 mo+) | Biochemotherapy (IFNα2b, IL2, CVD), surgery, biotherapy (IFNα2b and IL2) | 5 + 9 | 64,5 | M3-CSM |
| CIP-21 | PD | M1c | PD | Surgery, polichemotherapy with CDDP+DTIC | 5 | 19,5 | LM3TN |
| CIP-23 | PD | M1c | PD | Surgery, biochemotherapy (DTIC and IFNα2b), vaccination with pulsed DCs, biochemotherapy (fotemustine, IFNα2b and IL2), surgery | 5 | 19,5 | LM3TN |
| CIP-25 | PD | M1b | PD | Biochemotherapy (DTIC, CDDP +IFNα2b), immunotherapy (idiotype mAb + low doses IL2), radiotherapy + DTIC, mochemotherapy (taxanes) | 5 + 4 | 39,5 | LM3TN |
| CIP-26 | PD | IIIc | CR (6 + 3 mo) | Surgery, ILP, biotherapy (IFNα2b and IL2), ILP, surgery | 5 + 6 | 49,5 | LM3TN |
MAGE-A3 expression was evaluated by RT-PCR analysis on a tumor biopsy or surgical specimen. Bold text in table body indicates responding patients.
CIP indicates cancer immunotherapy patient; CVD: CDDP, vinblastin, and DTIC; ILP, isolated limb perfusion; PD, progressive disease; SD, stable disease; NED, no evidence of disease; and CR, complete response.
Disease status at study entry and clinical outcome.
Five administrations of escalating doses of GML (first cycle of treatment) plus additional infusions performed in some patients.
Cell lines, reagents, and retroviral vectors
The human melanoma cell lines CIP-5, CIP-26, and MSR3-mel were established in our laboratory and cultured in IMDM supplemented with 10% FBS. CIP-5-mel expresses HLA-A and -C alleles but not the HLA-B alleles (data not shown) that were restored by IFN-γ treatment (data not shown). Patient peripheral blood mononuclear cells (PBMCs) for immunomonitoring were harvested from blood samples collected 15 days after each infusion during the first cycle of treatment, and 1 month after each infusion for the additional treatments. Exceptions to this rule are indicated. MAGE-3.A26 and MAGE-1.A26 tetramers were done as described.16 TK.B7 pentamer and MelanA/MART-1.A2 tetramer were from Proimmune (Oxford, United Kingdom) and Beckman Coulter (Hialeah, FL), respectively. All tetramers were coupled to PE-conjugated monoclonal Abs against human CD3, CD4, and CD8, and isotype controls were from BD Biosciences Pharmingen (San Jose, CA). MAGE-3250-258, MAGE-1273-282, TK.B7,14 and MelanA/MART-126-35 analog17 peptides were synthesized by Espikem (Florence, Italy) and by PRIMM (Milan, Italy) and used at 20 mM. Lymphocytes were cultured in IMDM 10% HS. The retroviral vectors SFCMM3 and M3-CSM have previously been described.18,19 The M3TN retroviral vector codes for MAGE-3 and for the HSV-TK/neo (TN) fusion protein. Retroviral transduction of lymphocytes and lymphoblastoid B-cell lines was performed as previously described.18,19
Tetramer staining, flow cytometry, and sorting of tetramer+ effectors
Tetramer staining was performed incubating PBMCs or restimulated lymphocytes for 15 minutes at room temperature. Staining with anti-CD8, anti-CD3, and anti-CD4 mAbs was performed for 20 minutes at 4°C. Tetramer+ cells were sorted by anti-PE microbeads and restimulated with irradiated (100 Gy) autologous Epstein-Barr virus (EBV) pulsed with the relevant peptide and allogeneic EBV in the presence of IL-2 (100 U/mL) and IL-7 (10 ng/mL).
Enzyme-linked immunospot assay
PBMCs before and after treatment (2 × 105/well) and targets expressing TK or MAGE-3 or mock-transduced (0.75-1 × 105/well) were added in several replicates to nitrocellulose-bottomed 96-well plates (Millipore, Billerica, MA) coated with 10 mg/mL anti–human IFN-γ mAb (1-D1K; Mabtech, Nacka Strand, Sweden). Biotinylated secondary mAb to IFN-γ (7-B6-1; Mabtech), HRP-streptavidin (1:2000), and AEC-substrate solution (Zymed Laboratories, South San Francisco, CA) were then used according to the manufacturers' recommendations. Spots were evaluated and counted using a computer-assisted video imaging analysis system (Axioplan 2; Zeiss, Heidelberg, Germany).20 Spots released by stimulators and responders alone were subtracted. We scored as positive the samples with several spots greater than 5.
Semiquantitative assessment of TK- and MAGE-A3–specific T cells among peripheral and tumor-infiltrating lymphocytes
Multiple microcultures were performed for a semiquantitative assessment of TK- and MAGE-A3–specific T-cell precursors before and after treatment and among TILs. Peripheral blood leukocytes (PBLs) before and after treatment (105) or TILs were cultured with irradiated (30 Gy) autologous target cells (105) expressing either HSV-TK or MAGE-A3 in 96-well round-bottomed plates. Forty-eight hours later, rhIL-2 at 10 U/mL was added. For TILs, rhIL-2 (20 U/mL) was added from the beginning of the stimulation. On days 12 to 14, each microwell was divided into 4 equal samples and challenged in duplicate against HSV-TK–transduced, MAGE-A3–transduced, or mock-transduced autologous targets (0.5 × 105). Twenty-four hours later, 100 μL supernatant from each microculture was harvested and the IFN-γ content was measured by enzyme-linked immunosorbent assay (ELISA) assay (BD Biosciences Pharmingen) according to the manufacturer's recommendations. Microcultures were scored positive if the release of IFN-γ on target cells expressing HSV-TK or MAGE-A3 was greater than 50% the IFN-γ release on mock-transduced target cells. The spontaneous release of IFN-γ by stimulators and responders alone was subtracted.
Both enzyme-linked immunospot assay (ELISPOT) and microculture experiments were done twice on the same blood sample to confirm the results. The frequencies of anti–MAGE-A3 T cells were determined by the Poisson distribution analysis: frequency of anti–MAGE-A3 precursors =−ln [(number of wells tested − number of positive wells)/number of wells tested]/number of CD3+ T cells/well.21
Analysis of anti–MAGE-A3.A26 and antitumor T cells by mixed lymphocyte peptide culture
Mixed lymphocyte peptide culture (MLPC) was assessed as previously described.16 Briefly, PBMCs were thawed and incubated for 60 minutes at room temperature at 107 cells/mL in the presence of the relevant peptide (20 μM). Then, the cells were washed and plated (1-2 × 105 cells/well) in the presence of rhIL-2 (20 U/mL), and rhIL-7 (10 ng/mL). On day 7, 50% of the medium was replaced by fresh medium containing peptide. Tetramer labeling was performed on day 14. Frequencies of anti–MAGE-3.A26 and antitumor MLPCs were determined by the Poisson distribution analysis.
Analysis of antitumor T cells by mixed lymphocyte tumor cell culture and transfection of Cos-7 cells
PBMCs (5000-20 000 cells/well) were stimulated with irradiated (100 Gy) autologous tumor cells (1000 cells/well) in 96-well round-bottomed plates in the presence of 5 ng/mL rhIL-7.22 rhIL-2 (10 U/mL) was added 72 hours later. Mixed lymphocyte tumor cell cultures (MLTCs) were restimulated every week. At day 21, aliquots of them were used in an IFN-γ assay in duplicate against autologous tumor, mock-transduced autologous target cells (0.5 × 105), K562 (0.5 × 105), or Cos-7 transfected with HLA-A2603 and MelanA/MART-1. A second IFN-γ assay was performed at day 27. Transfection of Cos-7 cells was performed by the DEAE-dextran-chloroquine method as previously described.23 Frequencies of antitumor and anti-MelanA/MART-1.A26 T cells were determined by the Poisson distribution analysis. For blocking experiments the following mAbs were used: anti–MHC-I (W6/32), anti–HLA-DR (L243), anti–HLA-DP (B7/21 clone), and anti-NGFr (negative control).
TCR analysis on peripheral and tumor-infiltrating MAGE-A3.A26 tetramer+ lymphocytes
Peripheral and tumor-infiltrating MAGE-3.A26 tetramer+ lymphocytes were analyzed for the expression of TCR Vβ chain using the TCR Vβ repertoire Kit (IOTest Beta Mark; Beckman Coulter) and by RT-PCR. Total RNA was extracted by TRIzol (Invitrogen, Frederick, MD). cDNA was amplified using upstream primer specific for Vβ7 and a downstream C primer of TCR V region. PCR products were sequenced to obtain the complete identification of the CDR3 region. Alignment was performed using the International Immunogenetics Database (http://imgt.cines.fr; accession no. X61444).24–25
Immunohistochemical analysis
Immunohistochemistry was performed on 5-μm tissue sections previously fixed in buffered formalin and embedded in paraffin. Immunostainings were performed with a nonbiotin detection system (Refine polymer; Novocastra, Newcastle upon Tyne, United Kingdom) and diaminobenzidine development. The following Abs were used: anti–HLA-I (clone HC10; gift of Soldano Ferrone, Cancer Institute, University of Pittsburgh, Pittsburgh, PA), anti-CD8 (clone 1A5), anti–β2-microglobulin (polyclonal; Dako, Carpinteria, CA), pan anti–HLA-I (W6/32), and anti–HLA-A30 (One Lambda). Staining was then performed with the indicated antibodies for 30 minutes and analyzed with a Nikon DS-5M CCD camera (Calenzano, Italy), using a 20×/0.75 NA objective on a Nikon Eclipse 80i microscope. Images were taken and deconvoluted with the software provided by Nikon.
Statistics
Statistical significance (P < .05) was determined by performing ANOVA test followed by Dunnett as posttest, or by 2-tailed Student t test.
Results
Toxicity and clinical responses to infusion of GMLs
No toxicity or severe side effects attributable to the infusion of transduced lymphocytes were observed. Although this study was not designed to assess clinical end points, patients' progress was carefully monitored. In 3 patients, we observed indication of possible clinical benefit (Table 1). Patient CIP-5 experienced a long-lasting stable disease (SD; 50 months); patient CIP-26 achieved 2 complete responses (a first CR of 6 and a second CR of 3 months, following relapse); patient CIP-19, disease free at study entry, remained disease free after 61 months of follow-up (NED, 61 months+). Importantly, patients did not receive other therapies during the vaccination course.
Ex vivo characterization of anti-TK immune responses
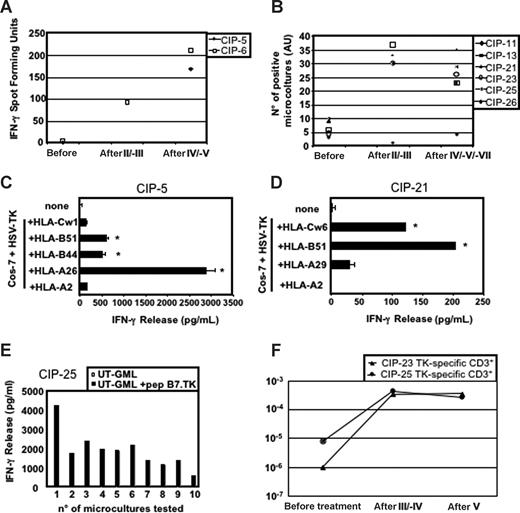
During the first cycle of vaccination, 7 of 8 patients developed TK-specific T cells detectable either with an ex vivo IFN-γ ELISPOT assay or with a semiquantitative recall assay consisting of an IFN-γ release assay performed by ELISA after in vitro stimulation of multiple groups of 105 PBLs with TK-GML (Figure 1A,B). Only one patient failed to respond to the TK antigen (CIP-11; Figure 1B). These TK-specific responses were characterized further for some patients, according to prevaccination and postvaccination PBL availability.
Characterization of TK-specific immune responses. (A) Ex vivo IFN-γ ELISPOT assay and (B) IFN-γ ELISA-based semiquantitative recall assays on multiple groups (105) of PBMCs collected before and after treatment. (C,D) HLA-I restriction of the anti-TK T cells in patients CIP-5 (C) and CIP-21 (D). Microcultures containing TK-specific effectors were challenged with Cos-7 cells transfected with available HLA-I alleles together with HSV-TK cDNAs. Supernatants were then assayed for IFN-γ release (*P < .01). Error bars represent the SD of experimental replicates. (E) Microcultures from the postfourth blood sample of patient CIP-25, a patient expressing the HLA-B7 allele, recognized autologous untransduced (UT)–GMLs pulsed with the TK279-288 epitope previously described.14 (F) Limiting dilution analysis estimating the frequency of anti-TK T cells in patients CIP-23 and CIP-25.
Characterization of TK-specific immune responses. (A) Ex vivo IFN-γ ELISPOT assay and (B) IFN-γ ELISA-based semiquantitative recall assays on multiple groups (105) of PBMCs collected before and after treatment. (C,D) HLA-I restriction of the anti-TK T cells in patients CIP-5 (C) and CIP-21 (D). Microcultures containing TK-specific effectors were challenged with Cos-7 cells transfected with available HLA-I alleles together with HSV-TK cDNAs. Supernatants were then assayed for IFN-γ release (*P < .01). Error bars represent the SD of experimental replicates. (E) Microcultures from the postfourth blood sample of patient CIP-25, a patient expressing the HLA-B7 allele, recognized autologous untransduced (UT)–GMLs pulsed with the TK279-288 epitope previously described.14 (F) Limiting dilution analysis estimating the frequency of anti-TK T cells in patients CIP-23 and CIP-25.
TK recognition was usually restricted by HLA class I (HLA-I) molecules, such as HLA-A26, -B44, and -B51 in patient CIP-5 (Figure 1C), HLA-B51 and Cw6 in patient CIP-21 (Figure 1D), or HLA-B7 in patient CIP-25 with the TK279-288 epitope, which we described previously14 (Figure 1E). In some patients, we also detected anti-TK CD4+ T cells (data not shown), as previously described by others.12
In CIP-23 and CIP-25 patients, we estimated prevaccination and postvaccination frequencies of anti-TK T cells using limiting dilution analysis. In patient CIP-23, the frequency rose from 10−6 of the CD3 cells before vaccination to 3.5 and 3.7 × 10−4 after the third and fifth infusion, respectively (Figure 1F). A similar 100-fold increase was observed in patient CIP-25 (from 8.2 × 10−6 before treatment to 4.6 × 10−4 and 2.8 × 10−4 in the postfourth and postfifth infusions, respectively; Figure 1F). In agreement with the results of the microcultures assay (ie, 0.046% of anti-TK T cells), in the postfourth infusion blood sample of this patient, 0.05% of the CD3+ cells were stained by a pentamer specific for the HLA-B7–restricted TK279-288 peptide (data not shown), indicating that all TK-specific effectors recognized this epitope.
Overall, these results also confirm in metastatic melanoma patients the immunogenicity of TK-expressing GMLs previously observed in leukemic patients.14
Enumeration and characterization of circulating anti–MAGE-A3 T cells
We measured the number of circulating anti–MAGE-A3 T cells in patients before and after treatments. In 2 of 10 patients (CIP-6 and -18) MAGE-A3–specific T cells were detected by ex vivo IFN-γ ELISPOT assay, whereas for the other patients we applied the semiquantitative recall assay also used for the detection of TK-specific T cells. We estimated the frequencies of anti–MAGE-A3 T cells only for the latter patients (Table 2).
Antivaccine immune responses
| Patient (CIP) . | Immune responses . | T-cell frequency anti–MAGE-A3* . | ||||||
|---|---|---|---|---|---|---|---|---|
| Ex vivo . | In vivo (DTH) . | |||||||
| TK . | M3 . | TK . | M3 . | Pre . | Post . | |||
| CIP-5 | + | + | + | + | 3.8 × 10−7 | 3.0 × 10−5 | ||
| CIP-6 | + | − | +/− | − | NA | NA | ||
| CIP-11 | − | − | − | − | 5.2 × 10−7 | < 5.2 × 10−7 | ||
| CIP-13 | + | − | − | − | 1.6 × 10−6 | 7.9 × 10−7 | ||
| CIP-18 | +/− | − | NA | NA | ||||
| CIP-19 | + | + | 3.9 × 10−7 | 1.7 × 10−5 | ||||
| CIP-21 | + | − | − | − | 9.3 × 10−7 | < 9.3 × 10−7 | ||
| CIP-23 | + | − | − | − | 5.2 × 10−7 | 5.8 × 10−7 | ||
| CIP-25 | + | − | − | − | 9.5 × 10−7 | 5.4 × 10−7 | ||
| CIP-26 | + | + | + | + | 2.2 × 10−6 | 1.8 × 10−5 | ||
| Patient (CIP) . | Immune responses . | T-cell frequency anti–MAGE-A3* . | ||||||
|---|---|---|---|---|---|---|---|---|
| Ex vivo . | In vivo (DTH) . | |||||||
| TK . | M3 . | TK . | M3 . | Pre . | Post . | |||
| CIP-5 | + | + | + | + | 3.8 × 10−7 | 3.0 × 10−5 | ||
| CIP-6 | + | − | +/− | − | NA | NA | ||
| CIP-11 | − | − | − | − | 5.2 × 10−7 | < 5.2 × 10−7 | ||
| CIP-13 | + | − | − | − | 1.6 × 10−6 | 7.9 × 10−7 | ||
| CIP-18 | +/− | − | NA | NA | ||||
| CIP-19 | + | + | 3.9 × 10−7 | 1.7 × 10−5 | ||||
| CIP-21 | + | − | − | − | 9.3 × 10−7 | < 9.3 × 10−7 | ||
| CIP-23 | + | − | − | − | 5.2 × 10−7 | 5.8 × 10−7 | ||
| CIP-25 | + | − | − | − | 9.5 × 10−7 | 5.4 × 10−7 | ||
| CIP-26 | + | + | + | + | 2.2 × 10−6 | 1.8 × 10−5 | ||
Bold text in table body indicates responding patients.
CIP indicates cancer immunotherapy patient; M3, MAGE-3; and NA, not applicable.
The frequencies of MAGE-A3 precursors were determined by the Poisson distribution analysis as reported in “Semiquantitative assessment of TK- and MAGE-A3–specific T cells among peripheral and tumor-infiltrating lymphocytes.”
Patients CIP-6, -11, -13, -21, -23, and -25, had no detectable anti–MAGE-A3 immune response before and after therapy (Table 2). Patient CIP-18 had a transient 4.5-fold increase of MAGE-A3–specific T cells after 2 infusions, which was no longer detected after the subsequent rounds of vaccination (data not shown). Patients CIP-5, -19, and -26 had an increase of circulating anti–MAGE-A3 T cells (Table 2). The clinical and immunologic follow-up of these 3 patients is reported in the subsequent subheadings.
Patient CIP-19
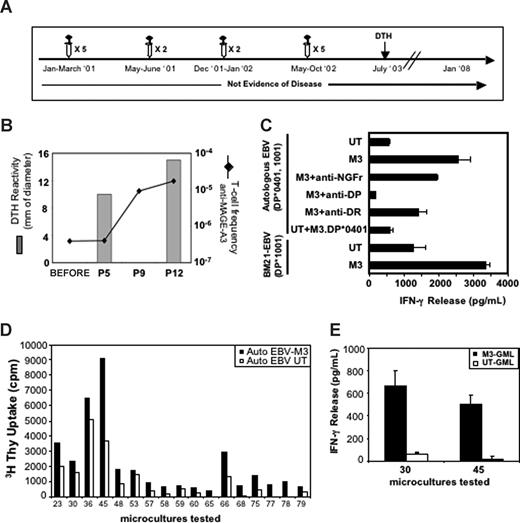
Patient CIP-19, treated when disease-free but at high risk of relapse, showed an increase of blood anti–MAGE-A3 T cells detectable between the fifth and ninth infusion of GMLs (Figure 2A,B). The frequency of anti–MAGE-A3 increased further, reaching 1.7 × 10−5 of the CD3+ cells after the 12th vaccination. This represented a 50-fold increase over the prevaccination frequency (Figure 2B). Microcultures displaying reactivity to MAGE-A3 contained CD4+ and CD8+ T cells (data not shown). One example is the CD4+ microculture no. 8, with an IFN-γ response to MAGE-A3–transduced autologous EBV-B cells, inhibited by anti-DP but not anti-DR or an irrelevant control mAbs (Figure 2C). As patient CIP-19 was typed HLA-DP*1001/DP*0401, effector cells were stimulated with either autologous EBV-B cells pulsed with a known M3.DP*0401 peptide26 or with allogeneic MAGE-A3–transduced EBV-B cells sharing the HLA-DP*1001 allele. The cells recognized the MAGE-A3+ HLA-DP*1001 EBV-B cells, but not the M3.DP*0401 peptide (Figure 2C). Furthermore, the recognition of the MAGE-A3+ HLA-DP*1001 EBV-B cells was abolished by the addition of anti-DP mAb (data not shown). We conclude that these T cells recognized a yet unknown MAGE-A3 epitope presented by HLA-DP*1001. The increase of circulating MAGE-A3–specific effectors paralleled the development of DTH reactivity to MAGE-A3 (Figure 2B). Indeed, a strong DTH reactivity was detected after 5 infusions, which further increased after subsequent injections (10 mm erythema and induration after fifth, 15 mm after 12th, and 25 mm 9 months after 14th; Figure 3B and data not shown). Limiting dilution analysis of T cells isolated from a skin biopsy of the last DTH reaction provided CD4+ T-cell clones (data not shown) that proliferated (Figure 2D) and released IFN-γ (Figure 2E) when challenged with MAGE-A3–expressing cells. The detection of DTH reactivity against MAGE-A3 9 months after the last infusion indicates that this approach is able to establish long-term memory T cells, in agreement with the results obtained in the mouse models.10
Enumeration and characterization of circulating anti–MAGE-A3 T cells in patient CIP-19 and analysis of anti–MAGE-A3 T cells infiltrating the DTH. (A) Clinical evolution of patient CIP-19 and timing of treatments. (B) Anti–MAGE-A3 T-cell frequency and DTH reactivity after indicated treatments are shown. The frequency of total anti–MAGE-A3 T cells was measured by IFN-γ release assay performed on multiple cultures of PBMCs (105) stimulated and then tested against M3-GMLs and UT-GML. After 12 infusions, we detected a strong increase of circulating anti–MAGE-A3 T cells (1.73 × 10−5). This increase paralleled the development of a strong MAGE-A3–specific DTH reaction. (C) Antigen specificity of a selected CD4+ microculture. Microculture no. 8 was tested against MAGE-A3–transduced (M3) or untransduced (UT) autologous EBV in the presence or in the absence of anti-DR, anti-DP, and anti-NGFr (used as control antibody) mAbs. Anti–MAGE-A3 T cells were also tested against autologous EBV pulsed with the M3.DP*0401 peptide26 and against allogeneic MAGE-A3–transduced or untransduced EBV (BM21-EBV) sharing the HLA-DP*1001 allele. Microculture no. 8 specifically recognized the allogeneic HLA-DP*1001 EBV expressing MAGE-3 but not the M3.DP*0401 peptide. Error bars represent SD of experimental replicates. (D,E) MAGE-A3–specific long-term T-cell memory. (D) CD3+CD4+ T-cell clones from a punch biopsy of an anti–MAGE-A3 DTH performed 9 months after the 14th infusion, proliferated in the presence of MAGE-A3–transduced autologous EBV cells. (E) Upon in vitro expansion, clones no. 30 and no. 45 specifically released IFN-γ in response to MAGE-A3–expressing target cells.
Enumeration and characterization of circulating anti–MAGE-A3 T cells in patient CIP-19 and analysis of anti–MAGE-A3 T cells infiltrating the DTH. (A) Clinical evolution of patient CIP-19 and timing of treatments. (B) Anti–MAGE-A3 T-cell frequency and DTH reactivity after indicated treatments are shown. The frequency of total anti–MAGE-A3 T cells was measured by IFN-γ release assay performed on multiple cultures of PBMCs (105) stimulated and then tested against M3-GMLs and UT-GML. After 12 infusions, we detected a strong increase of circulating anti–MAGE-A3 T cells (1.73 × 10−5). This increase paralleled the development of a strong MAGE-A3–specific DTH reaction. (C) Antigen specificity of a selected CD4+ microculture. Microculture no. 8 was tested against MAGE-A3–transduced (M3) or untransduced (UT) autologous EBV in the presence or in the absence of anti-DR, anti-DP, and anti-NGFr (used as control antibody) mAbs. Anti–MAGE-A3 T cells were also tested against autologous EBV pulsed with the M3.DP*0401 peptide26 and against allogeneic MAGE-A3–transduced or untransduced EBV (BM21-EBV) sharing the HLA-DP*1001 allele. Microculture no. 8 specifically recognized the allogeneic HLA-DP*1001 EBV expressing MAGE-3 but not the M3.DP*0401 peptide. Error bars represent SD of experimental replicates. (D,E) MAGE-A3–specific long-term T-cell memory. (D) CD3+CD4+ T-cell clones from a punch biopsy of an anti–MAGE-A3 DTH performed 9 months after the 14th infusion, proliferated in the presence of MAGE-A3–transduced autologous EBV cells. (E) Upon in vitro expansion, clones no. 30 and no. 45 specifically released IFN-γ in response to MAGE-A3–expressing target cells.
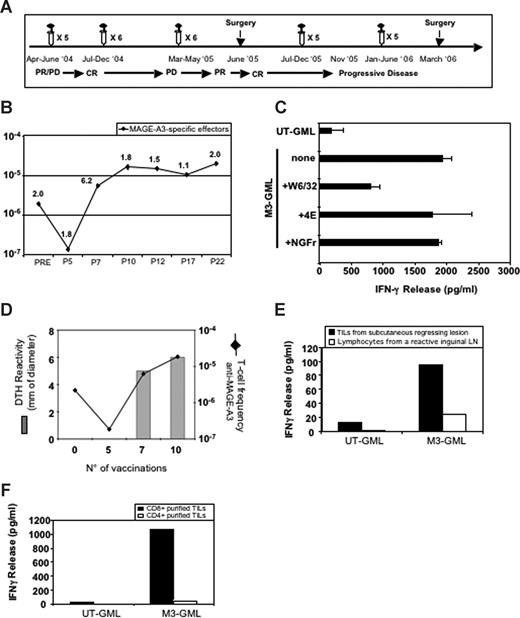
Enumeration and characterization of circulating anti–MAGE-A3 T cells in patient CIP-26 and analysis of anti–MAGE-A3 T cells infiltrating tumor lesions. (A) Clinical evolution of patient CIP-26 and timing of treatments. PD indicates progressive disease; CR, complete response; and PR, partial response. (B) Frequency of circulating anti–MAGE-A3 T cells estimated before, during, and after treatment. The frequency of total anti–MAGE-A3 T cells was measured as described in Figure 2. P indicates after infusion; P30*, this blood sample was withdrawn in 2007, 1 year after the 30th vaccination. (C) HLA restriction of a representative MAGE-A3–specific CD8+ microculture. The microculture released IFN-γ against autologous M3-GMLs preincubated with 4E mAb (recognizing HLA-B and -C alleles) or with anti-NGFr mAb (control mAb). Release of IFN-γ was instead inhibited in the presence of W6/32 mAb (anti–HLA-I mAb). Error bars represent SD of experimental replicates. (D) Relationship between the increase of MAGE-A3–specific effectors and the development of MAGE-A3–specific DTH reaction. (E) Ex vivo IFN-γ release assay performed on anti–MAGE-A3 T cells from a regressing tumor lesion and a regional lymph node collected in 2005. T cells recognized autologous M3-GMLs but not mock-transduced lymphocytes (UT-GMLs). (F) Only CD8+ purified TILs specifically released IFN-γ when challenged with M3-GMLs.
Enumeration and characterization of circulating anti–MAGE-A3 T cells in patient CIP-26 and analysis of anti–MAGE-A3 T cells infiltrating tumor lesions. (A) Clinical evolution of patient CIP-26 and timing of treatments. PD indicates progressive disease; CR, complete response; and PR, partial response. (B) Frequency of circulating anti–MAGE-A3 T cells estimated before, during, and after treatment. The frequency of total anti–MAGE-A3 T cells was measured as described in Figure 2. P indicates after infusion; P30*, this blood sample was withdrawn in 2007, 1 year after the 30th vaccination. (C) HLA restriction of a representative MAGE-A3–specific CD8+ microculture. The microculture released IFN-γ against autologous M3-GMLs preincubated with 4E mAb (recognizing HLA-B and -C alleles) or with anti-NGFr mAb (control mAb). Release of IFN-γ was instead inhibited in the presence of W6/32 mAb (anti–HLA-I mAb). Error bars represent SD of experimental replicates. (D) Relationship between the increase of MAGE-A3–specific effectors and the development of MAGE-A3–specific DTH reaction. (E) Ex vivo IFN-γ release assay performed on anti–MAGE-A3 T cells from a regressing tumor lesion and a regional lymph node collected in 2005. T cells recognized autologous M3-GMLs but not mock-transduced lymphocytes (UT-GMLs). (F) Only CD8+ purified TILs specifically released IFN-γ when challenged with M3-GMLs.
Patient CIP-26
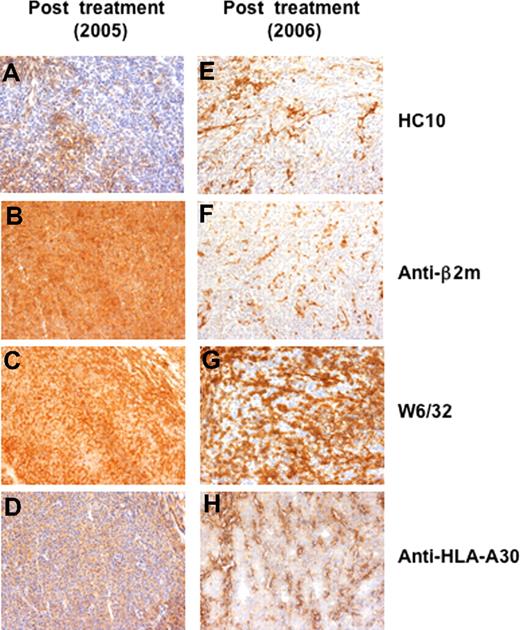
Patient CIP-26 (Figure 3A) had a decrease of circulating anti–MAGE-A3 T cells during the first cycle of treatment (2.2 × 10−6 in the pretreatment vs 1.8 × 10−7 in the postfifth samples; Figure 3B), followed by a progressive increase reaching 1.8 × 10−5 in the post-10th blood sample (Figure 3B). Concomitantly, the patient displayed a complete clinical response (Figure 3A) documented with 2 consecutive positron emission tomography (PET)/computed tomography (CT) scans (Figure S1) and associated with vitiligo on the limb previously showing subcutaneous metastases (data not shown). Analysis of microcultures revealed that the MAGE-A3–specific effectors were HLA-A restricted as IFN-γ release was partly blocked by mAb W6/32, but not by mAb 4E, which recognizes only HLA-B and -C alleles (Figure 3C). The patient developed a positive DTH reaction after the seventh infusion concurrently with the increased level of circulating MAGE-A3–specific effectors (5 and 6 mm erythema and induration after the seventh and tenth infusion, respectively) (Figure 3D). Thereafter, the anti–MAGE-A3 T cells persisted at the level of approximately 1.5 × 10−5, even though the patient relapsed (Figure 3A,B). Subcutaneous and lymph node tumor lesions were resected in June 2005 and in March 2006 (Figure 3A). To enumerate anti–MAGE-A3 T cells among the tumor infiltrating lymphocytes (TILs), we applied the same approach as for the blood lymphocytes. MAGE-A3–specific T cells were 5 to 10 times more frequent in the 2 tumor lesions (0.02% and 0.009%, respectively) than in the blood (0.002%). Interestingly, we compared the specificity of TILs from the 2005 regressing subcutaneous tumor with that of lymphocytes of a reactive inguinal lymph node resected at the same time. Only the TILs released IFN-γ in the presence of M3-GMLs (Figure 3E). Among those TILs, CD8+ T cells were the main MAGE-A3 effectors, as shown with purified CD4+ and CD8+ T-cell populations (Figure 3F). TILs recognized M3-GMLs in HLA-A–restricted manner (data not shown), as already observed in the periphery. We evaluated by immunohistochemistry the expression of HLA-I molecules on the 2 tumor lesions (ie, 2005 and 2006). The first tumor was poorly stained by mAb HC-10 (Figure 4A), which recognizes HLA-B and -C free heavy chains,27 but was stained by anti–β2-microglobulin mAb (Figure 4B), W6/32 (pan HLA-I mAb; Figure 4C), and an anti–HLA-A30 mAb (Figure 4D), suggesting that it was mainly expressing the HLA-A molecules. Tumor cells from the second lesion (2006) were not stained by mAb HC-10 (Figure 4E) and by the anti–β2-microglobulin mAb (Figure 4F). Differently from the first lesion, this tumor displayed some areas not stained by both W6/32 and anti–HLA-A30 mAbs (Figure 4G,H). These results are compatible with the hypothesis that the induced HLA-A–restricted MAGE-A3–specific T cells could have exerted an in vivo antitumor activity (Figure 3E), and selected an HLA-I–loss tumor variant.
Immunohistochemical analysis of tumor lesions of patient CIP-26 collected during the treatment. (A-G) Melanoma lesions collected during the treatment were stained with HC10 mAb (anti–HLA-B and -C), anti–β2-microglobulin mAb, W6/32 (anti–HLA-I) mAb and anti–HLA-A30 mAb. (A,B) The regressing tumor nodule collected in 2005 after 18 vaccinations, was almost completely negative for HC10 (A), but contained tumor areas stained with anti–β2-microglobulin mAb (B), W6/32 mAb (C) and with an anti–HLA-A30 mAb (D). A progressing tumor lesion collected in 2006, after 22 vaccinations, was not stained by mAb HC10 (E) and by anti–β2-microglobulin mAb (F). Some tumor areas from the progressing lesion were not stained by mAb W6/32 (G) and by an anti–HLA-A30 mAb (H). Objectives, ×200.
Immunohistochemical analysis of tumor lesions of patient CIP-26 collected during the treatment. (A-G) Melanoma lesions collected during the treatment were stained with HC10 mAb (anti–HLA-B and -C), anti–β2-microglobulin mAb, W6/32 (anti–HLA-I) mAb and anti–HLA-A30 mAb. (A,B) The regressing tumor nodule collected in 2005 after 18 vaccinations, was almost completely negative for HC10 (A), but contained tumor areas stained with anti–β2-microglobulin mAb (B), W6/32 mAb (C) and with an anti–HLA-A30 mAb (D). A progressing tumor lesion collected in 2006, after 22 vaccinations, was not stained by mAb HC10 (E) and by anti–β2-microglobulin mAb (F). Some tumor areas from the progressing lesion were not stained by mAb W6/32 (G) and by an anti–HLA-A30 mAb (H). Objectives, ×200.
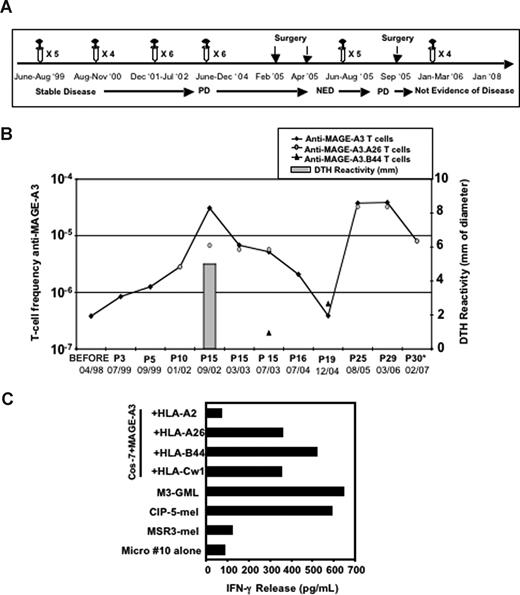
Patient CIP-5
This patient received the first cycle of 5 vaccinations with M3-TK-GML, and the blood frequency of anti–MAGE-A3 T cells increased from 4 × 10−7 to 1.2 × 10−6 of the CD3+ cells (Figure 5B). Since the DTH reactivity observed after this first cycle of treatment was directed mainly against TK (data not shown), the patient was further vaccinated with GMLs expressing only MAGE-A3 (Figure 5A). After 10 additional infusions of M3-GMLs (after 15th, July 2002), the frequency of MAGE-A3–specific T cells increased 100-fold compared with the pretreatment blood sample (Figure 5B). Both purified CD8+ and CD4+ recognized MAGE-A3 (data not shown). This response paralleled the development of a MAGE-A3–specific DTH reaction (5 mm erythema and induration; Figure 5B). The CD8+ effectors recognized the autologous tumor and were directed against epitopes of MAGE-A3 presented by different autologous HLA-I alleles (Figure 5C). Some microcultures, such as microculture no. 10, released IFN-γ when stimulated with Cos-7 cells transfected with MAGE-A3 and HLA-A26, -B44, or -Cw1 but not -A2 constructs (Figure 5C). To identify the peptides presented by HLA-A26 and -B44 molecules, 3 MAGE-A3 cDNA fragments (Figure S2A) were cotransfected with the HLA alleles in Cos-7 cells (Figure S2B,C) and incubated with the different T-cell populations (ie, MAGE-A3.A26 and MAGE-A3.B44). The pattern of recognition of the different fragments indicated that a region between BamHI and EcoRI restriction sites encoded the antigenic peptides. By screening this region for peptides carrying HLA-A*2603 and HLA-B*4402 binding motifs,28 one nonapeptide was identified, FVQENYLEY (amino acids 250-258). This peptide, referred to as M3250-258, sensitized Cos-7 cells transfected with either HLA-A*2603 or HLA-B*4402 to the recognition by MAGE-A3.A26 and MAGE-A3.B44 T cells, respectively (Figure S2B,C). The identification of this epitope allowed evaluation of whether one of these populations had undergone a predominant expansion. Multiple cultures of PBMCs (105) were stimulated with the peptide M3250-258 and tested by tetramer staining or IFN-γ release assay on Cos-7 cells transfected with MAGE-A3 and HLA-A*2603 or HLA-B*4402. There was a close match between the blood frequencies of anti–MAGE-A3 T cells and those of the anti-M3250-258 T cells restricted by HLA-A26 (Figure 5B), whereas the anti–MAGE-A3.B44 T cells were detected at lower levels (between 1.9 × 10−7 and 6.3 × 10−7 T cells; Figure 5B), thus indicating that in this patient most of the anti–MAGE-A3 effectors recognized this peptide presented by HLA-A26. The MAGE-A3–specific immune response of patient CIP-5 was long-lasting, as the induced anti–MAGE-A3 memory T cells were still detectable in vitro 1 year after the 15th infusion, performed in 2002 (Figure 5B). From July 2002 to June 2004 patient CIP-5 discontinued the therapy (Figure 5A). Nevertheless, we were still able to detect circulating MAGE-A3–specific effectors (ranging from 5.2 × 10−6 to 2.07 × 10−6; Figure 5B) and the patient remained clinically stable for 50 months (Figure 5A). Then the frequency of the anti–MAGE-A3 T cells declined and the disease progressed (Figure 5A). To boost the anti–MAGE-A3 immune response, the patient received a new cycle of 15 infusions and the anti–MAGE-A3 T cells rose up again to 3.76 × 10−5 (Figure 5B). These results further support the concept that M3-GMLs are able to induce anti–MAGE-A3 T cells and strongly suggest that the maintenance of such levels (10−5) of circulating anti–MAGE-A3 T cells requires multiple and frequent booster injections. To estimate the blood frequencies of T cells against tumor antigens other than MAGE-A3 we set up MLTCs, taking advantage of an autologous tumor cell line (CIP-5-mel) established from a metastasis resected prior to vaccination. Tumor-specific T cells were already present at a frequency of 10−4 before vaccination (Figure S3), and this frequency did not increase after vaccination (Figure S3). We identified some of the target antigens: the cancer germline protein MAGE-A11 and the melanocytic differentiation antigen MelanA/MART-1.23,29 We characterized CD8+ T cells recognizing a MAGE-A1 peptide presented by HLA-A26 (C.T., S. Tanzarella, R.F., and V.R., manuscript in preparation) and MelanA/MART-1 peptides presented by either HLA-A2 or HLA-A26. Using multiple MLTCs or multiple mixed lymphocyte-peptide cultures, we estimated the frequencies of blood T cells recognizing MAGE-A1.A26, MelanA/MART-1.A2, and MelanA/MART-1.A26 (Figure S3), with the latter showing an increase 1 year after the 15th infusion (up to 10−4; Figure S3). The patient had an abdominal lymph node metastasis resected in 1998, before study, and other abdominal metastases were resected in 2005, after a long-lasting stable disease status. This allowed us to perform a comparative analysis of TILs collected before and after treatment and to evaluate the number and function of anti–MAGE-A3 (ie, antivaccine) and other antitumor effectors inside the lesions (Figure 5A; Table 2). Histologic analysis of the 2005 lesions showed approximately 90% of necrotic tumor cells, with living cells maintaining the expression of MAGE-A3, as evaluated by RT-PCR analysis (data not shown). TILs from the pretreatment lesion did not contain detectable anti–MAGE-A3.A26 and anti–MAGE-A3.B44 T cells (Table 3; Figure S4A). But the postvaccination metastasis contained 0.04% of anti–MAGE-A3.A26 T cells (Table 3; Figure S4B) and only 0.00074% of anti–MAGE-A3.B44 T cells (Table 3). Most of the anti–MAGE-A3.A26 TILs were functional, producing IFN-γ when stimulated with Cos-7 cells transfected with HLA-A26 and MAGE-A3 cDNAs or with the autologous tumor cells (Figure S4C). The frequencies of the other antitumor T cells were similar (0.02%) within the metastases removed before and after vaccination (Table 3), with the exception of anti–MelanA/MART-1.A26–specific T cells increasing from less than 0.00075% to 0.0041% (Table 3). To prove that at least some of the blood anti–MAGE-A3 T cells were recruited at the tumor site, we used tetramers to sort anti–MAGE-A3.A26 CD8+ T cells from blood and TILs (Figure S5A), and identified their TCRβ sequences. The 2 types of cells expressed the same Vβ7.1 rearrangement, as demonstrated by spectratyping analysis (data not shown) and by cDNA sequencing (Figure S5B).
Enumeration and characterization of circulating anti–MAGE-A3 T cells in patient CIP-5. (A) Clinical evolution of patient CIP-5 and timing of treatments; PD: progressive disease, NED: not evidence of disease. (B) Frequency of circulating anti–MAGE-A3 T cells estimated in patient CIP-5 during the treatment. The frequency of total anti–MAGE-A3 T cells was measured as described in Figure 2. The frequency of anti–MAGE-A3.A26 and anti–MAGE-A3.B44 T cells was measured by tetramer staining or IFN-γ release assay, performed on multiple cultures of PBMCs (105) stimulated with the peptide M3250-258. After 15 infusions, patient CIP-5 developed a strong increase of circulating anti–MAGE-A3 T cells (3.08 × 10−5) that paralleled a MAGE-A3–specific DTH reaction; P stands for after infusion. (C) HLA-I restriction of the selected CD8+ microculture no. 10. HLA-I restriction was characterized as described in Figure 1. Microculture no. 10 recognized HLA-A26, -B44 and Cw01-restricted MAGE-A3–derived epitopes and autologous tumor cells (CIP-5-mel). Autologous lymphocytes expressing MAGE-A3 (M3-GMLs) were used as positive control.
Enumeration and characterization of circulating anti–MAGE-A3 T cells in patient CIP-5. (A) Clinical evolution of patient CIP-5 and timing of treatments; PD: progressive disease, NED: not evidence of disease. (B) Frequency of circulating anti–MAGE-A3 T cells estimated in patient CIP-5 during the treatment. The frequency of total anti–MAGE-A3 T cells was measured as described in Figure 2. The frequency of anti–MAGE-A3.A26 and anti–MAGE-A3.B44 T cells was measured by tetramer staining or IFN-γ release assay, performed on multiple cultures of PBMCs (105) stimulated with the peptide M3250-258. After 15 infusions, patient CIP-5 developed a strong increase of circulating anti–MAGE-A3 T cells (3.08 × 10−5) that paralleled a MAGE-A3–specific DTH reaction; P stands for after infusion. (C) HLA-I restriction of the selected CD8+ microculture no. 10. HLA-I restriction was characterized as described in Figure 1. Microculture no. 10 recognized HLA-A26, -B44 and Cw01-restricted MAGE-A3–derived epitopes and autologous tumor cells (CIP-5-mel). Autologous lymphocytes expressing MAGE-A3 (M3-GMLs) were used as positive control.
Antitumor T-cell frequency within metastases collected before and after treatment in patient CIP-5
| T cells . | Before treatment . | After treatment . |
|---|---|---|
| Anti–MAGE-3.A26* | < 0.00075% | 0.04% |
| Anti–MAGE-3.B44* | < 0.00075% | 0.00074% |
| Anti–MelanA/MART-1.A2 | 0.0015% | 0.00158% |
| Anti–MelanA/MART-1.A26 | < 0.00075% | 0.0041% |
| Anti–MAGE-1.A26 | nd | nd |
| Other antitumor | 0.02% | 0.022% |
| T cells . | Before treatment . | After treatment . |
|---|---|---|
| Anti–MAGE-3.A26* | < 0.00075% | 0.04% |
| Anti–MAGE-3.B44* | < 0.00075% | 0.00074% |
| Anti–MelanA/MART-1.A2 | 0.0015% | 0.00158% |
| Anti–MelanA/MART-1.A26 | < 0.00075% | 0.0041% |
| Anti–MAGE-1.A26 | nd | nd |
| Other antitumor | 0.02% | 0.022% |
nd indicates not determined.
Antivaccine T cells.
Altogether these data show that GML vaccination induces functional vaccine-specific T cells capable of migrating to inflamed skin and, more importantly, to infiltrate tumor lesions.
Discussion
In this study we have analyzed vaccine- and tumor-specific immune responses of tumor-bearing patients treated with a novel vaccination strategy based on the infusion of autologous lymphocytes genetically modified to express the cancer germline gene MAGE-A3 and the viral antigen TK. The use of such a strategy relies on clinical12,14 and preclinical10 in vivo studies, demonstrating that genetically engineered lymphocytes, which are currently used as effector cells in adoptive immunotherapy protocols,13,30 can act as efficient vehicles for in vivo loading of DCs and consequently can behave as a vaccine, eliciting TAA-specific T cells.
This vaccination strategy may account for different advantages compared with other approaches. Some are related to the previously identified ability of GMLs to target DCs in vivo,10 thus exploiting a physiologic route of immunization and “selecting” the most appropriate subset of lymphoid organ-resident DCs.9 Others are associated with the unexpected finding that contacts between GMLs and DCs triggers DC activation,10 without further addition of adjuvants. Lastly, there are advantages over treatments based on subcutaneous/intradermal injection of single tumor-derived peptides which include the intravenous route of administration, allowing antigens delivery not only in the draining lymph nodes31 but in the whole lymphoid compartment, and the expression of the entire TAA protein in vivo, favoring the presentation of an array of epitopes by the autologous HLA-I and -II alleles, as observed in patients CIP-5 and -19, thus leading to the spontaneous selection of the most frequent and effective CD8+ and CD4+ T-cell repertoire, and reducing the risk of tumor escape and the induction of anergy/tolerance of TAA-specific T cells.6,7,32
The infusion of GMLs expressing MAGE-A3 and TK antigens was effective as it induced vaccine-specific CD4+ and CD8+ T cells and established antigen-specific long-term memory T cells. Indeed, all but one of the vaccinated patients (CIP-11) showed the increase of circulating TK-specific effectors (Figure 1A,B; Table 1), thus confirming that this population of patients was immunocompetent and, therefore, appropriate for testing TAA-specific responses in vivo. Of this immunocompetent population, only 3 of 9 patients were able to mount a response against the TAA, detectable in the blood. Of note, these were the only patients with limited tumor burden. The reason why the other 6 patients completely failed to show any response is currently not known. However, the careful analysis of the 3 positive patients and its correlation with the clinical course may shed some light on the possible mechanisms for tumor escape in the whole patient population.
The observed rate of immunization and the frequencies of anti–MAGE-A3 T cells in the responding patients are in the lower range of frequencies reported by others33 (ie, from > 10-2 to 10-6). However, in the majority of these studies, the induction of vaccine-specific T-cell effectors did not correlate with the clinical outcome.33 In contrast, in our study, the 3 patients showing a sustained increase of anti–MAGE-A3 T cells (ie, CIP-5, -19, and -26) experienced a more favorable clinical outcome. Based on these observations, we hypothesize that GML-elicited effector T cells could be qualitatively different compared with those elicited by other strategies. This hypothesis is supported by 2 observations. First, the 3 patients showing a sustained increase of anti–MAGE-A3 T cells also developed vaccine-specific DTH reactions, a phenomenon that has been correlated to a favorable clinical outcome34 ; second, the circulating anti–MAGE-A3 T cells were also able to infiltrate tumor lesions. In particular, in 2 patients (CIP-26 and CIP-5) we observed an enrichment of anti–MAGE-A3 T cells among TILs collected after treatment. In patient CIP-26, the anti–MAGE-A3 T cells infiltrating the tumor were 10 and 5 times more frequent than in the blood. Furthermore, the ex vivo analysis for the presence of MAGE-A3–specific effectors in both a regressing tumor lesion and a reactive inguinal lymph node clearly showed the presence of these cells only in the tumor (Figure 3E). In patient CIP-5, we identified functional MAGE-A3.A26–specific effectors only among TILs of the tumor collected after treatment. The analysis of the TCRβ sequences indicated that these effectors expressed the same Vβ7.1 rearrangement as those isolated from the blood, therefore demonstrating that the elicited anti–MAGE-A3 T cells had migrated to tumors. Based on these observations, we started collecting tumor samples of patients who did not show circulating anti–MAGE-A3 T cells to evaluate their possible presence inside the tumor. Preliminary results in patient CIP-25 indicated that some MAGE-A3–specific effectors inside the tumor were detectable after one round of in vitro stimulation, however they failed to expand after subsequent restimulations (data not shown). On the contrary, we were consistently able to expand the MAGE-A3–specific effectors from tumor lesions of patients with high frequency of anti–MAGE-A3 T cells in the blood (ie, CIP-5 and CIP-26). These results suggest the presence, in patient CIP-25, of antitumor T cells undergoing anergy within tumor. These data, if confirmed on a larger group of patients, would further extend the notion that the tumor microenvironment may be detrimental for a sustained activity of antitumor T-cell effectors.
In patient CIP-5, we observed a strong increase of the antivaccine T cells (MAGE-A3.A26; 0.04%) but only a modest amplification of the anti–MelanA/MART-1.A26 T cells (0.0041%), in a metastasis collected after treatment. An opposite scenario has recently been described in metastases of MAGE-A3–vaccinated patients, with strong increase of antitumor specificities, but a modest amplification of the MAGE-A3–specific effectors.35,36 The differences between these studies may rely on the method used to quantify antitumor immune responses, by the fitness of the elicited MAGE-A3–specific effectors or may depend on the status of tumor. Indeed, the tumor metastasis of patient CIP-5 was made up mainly of necrotic tumor cells (approximately 90%, data not shown), thus suggesting that most effector cells could have already left the tumor following extensive tumor cell killing. In this regard, it is worth remembering that these analyses “photograph” a static process occurring in the tumor. Ex vivo dynamic studies could, in the future, also contribute to unravel some important aspects related to kinetics of the immune response within the tumor microenvironment. Nevertheless, our data would suggest that different situations may take place in tumor lesions of patients treated with immunotherapeutic approaches and that the identification of the underlying mechanisms may further improve understanding the biology of the antitumor immune response and therefore the development of more effective treatments.
This study demonstrates that GMLs administered intravenously behave as a vaccine eliciting immune responses against the self-/tumor antigen MAGE-A3. Moreover, the induced anti–MAGE-A3 T cells are able to traffic through inflamed tissues and to infiltrate tumor sites. The results described in this report deserve to be investigated on a larger group of patients, particularly those affected by early-stage diseases, to evaluate the clinical efficacy of such a vaccination strategy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank M. P. Protti (Cancer Immunotherapy and Gene Therapy Program) for discussions and critical comments. In addition, we thank P. van der Bruggen for providing us with the MAGE-3.DP*0401 peptide and K. Fleishhauer and B. Mazzi for HLA typing.
This study was supported by the Italian Association for Cancer Research (AIRC; Milan, Italy) and by European Commission (EC; Brussels, Belgium) Project “Cancerimmunotherapy” LSHC-CT-2006-518334.
Authorship
Contribution: R.F. designed and performed research and analyzed data; M.B., C.B., and C.T. designed research, analyzed data, and wrote the paper; A.C., L.R., C.R., D.M., F.L., and F.C. performed research and analyzed data; S.M. and D.C. contributed vital new reagents; C.D. contributed vital new reagents and analytical tools; P.G.C. contributed vital new reagents, analyzed data, and wrote the paper; and V.R. designed and performed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: F.C. has declared financial interest in MolMed Spa, whose potential product was studied in the present work. C.T. and C.B. are employees of MolMed Spa, whose potential product was studied in the present work. The remaining authors declare no competing financial interests.
Correspondence: Claudio Bordignon, Scientific Institute S Raffaele, via Olgettina 58, 20132 Milan, Italy; e-mail: claudio.bordignon@hsr.it.
References
Author notes
*C.T. and V.R. contributed equally to this work.