Abstract
Specification of endothelial cell (EC) fate during vascular development is controlled by distinct key regulators. While Notch plays an essential role in induction of arterial phenotypes, COUP-TFII is required to maintain the venous EC identity. Homeodomain transcription factor Prox1 functions to reprogram venous ECs to lymphatic endothelial cells (LECs). Here, we report that the venous EC fate regulator COUP-TFII is expressed in LECs throughout development and physically interacts with Prox1 to form a stable complex in various cell types including LECs. We found that COUP-TFII functions as a coregulator of Prox1 to control several lineage-specific genes including VEGFR-3, FGFR-3, and neuropilin-1 and is required along with Prox1 to maintain LEC phenotype. Together, we propose that the physical and functional interactions of the 2 proteins constitute an essential part in the program specifying LEC fate and may provide the molecular basis for the hypothesis of venous EC identity being the prerequisite for LEC specification.
Introduction
Lymphatic endothelial cells (LECs) are derived from venous endothelial cells (ECs) during mammalian development1,2 : a subset of ECs in the cardinal vein expresses the homeodomain transcriptional factor Prox1 and migrates out to form the primitive lymphatic vessels and Prox1-deficient mice fail to form the lymphatic system. Furthermore, when ectopically expressed in postdevelopmental cultured blood vascular ECs (BECs), Prox1 can repress BEC-specific markers and up-regulate LEC-specific genes.3-10 These findings indicate that Prox1 plays as the master regulator for lymphatic system development by reprogramming cell fate of BECs to LECs. Chicken ovalbumin upstream promoter transcription factor II (COUP-TFII) is an orphan nuclear receptor and modulates transcriptional activities of its interacting partners as a coregulator to control a broad range of developmental processes.11 Although COUP-TFII is abundantly expressed in various cell types, it is only expressed in venous, but not arterial, ECs in the vascular system.12 Importantly, EC-specific genetic ablation of COUP-TFII resulted in both loss of the venous EC identity and acquisition of arterial phenotypes and, conversely, EC-specific ectopic expression of COUP-TFII disturbed normal arteriovenous specification, demonstrating that COUP-TFII functions as the key regulator to specify the venous EC identity.12 A previous LEC-lineage tracing study has proposed that the venous EC identity is a necessary prerequisite for establishment of LEC fate.13 Here we report that the venous cell fate regulator COUP-TFII physically and functionally interacts with the lymphatic master regulator Prox1 to augment and maintain LEC phenotypes.
Methods
Endothelial cell isolation for our study has been approved by the Institutional Review Board of the University of Southern California (no. HS-06-00292 to Y.H.). All microarray data have been deposited with Gene Expression Omnibus (GEO) under accession number GSE12846. For complete methods information, see Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Results and discussion
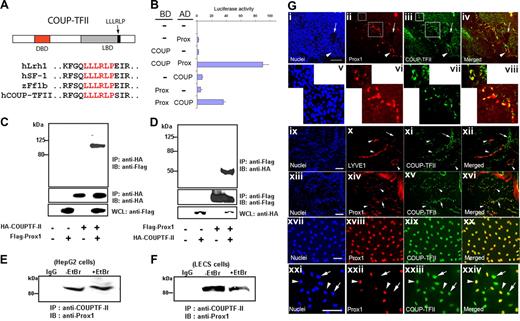
Previously, 3 nuclear receptors (Lrh1, HNF4α, and SF-1/ff1b) have been identified to interact with Prox1 through their amino acid motif (LLLRLP) in nonendothelial cell types.14-17 To identify additional Prox1-interacting proteins that are expressed in endothelial cells, we set out to search for proteins containing the LLLRLP motif using the BLAST program (National Center for Biotechnology Information, National Institutes of Health [NIH], Bethesda, MD) and found that the same motif is present in COUP-TFII protein (Figure 1A). To investigate interaction of COUP-TFII with Prox1 protein, we performed the mammalian 2-hybrid assay using COUP-TFII and Prox1 proteins that are fused with either the GAL4 DNA-binding domain (BD) or the VP16 activation domain (AD). We found that whereas either fusion protein alone did not show any activation of the luciferase reporter in HEK293 cells, 2 fusion proteins together could yield a significant activation (Figure 1B). We next performed coimmunoprecipitation (Co-IP) studies by transfecting the expression vectors for Flag-tagged Prox1 and/or HA-tagged COUP-TFII into HEK293 cells and by precipitating protein complexes with an anti-HA antibody. Western blotting analyses with an anti-Flag antibody showed that Flag-Prox1 protein can form a stable complex with HA-COUP-TFII protein (Figure 1C). Conversely, when we pulled down protein complex with an anti-Flag antibody and analyzed by Western blotting assays with an anti-HA antibody, we found that HA-COUP-TFII protein was also precipitated with Flag-Prox1 (Figure 1D). We then performed Co-IP assays against endogenous Prox1 and COUP-TFII proteins using an anti–COUP-TFII antibody and were able to precipitate the COUP-TFII/Prox1 protein complex from whole-cell lysates of HepG2 and primary LECs in the absence or presence of ethidium bromide (EtBr), which disrupts DNA-mediated protein-protein interactions (Figure 1E,F). Together, these data demonstrate that Prox1 and COUP-TFII physically interact and form a stable complex in various cell types including primary human LECs.
Interaction between Prox1 and COUP-TFII proteins and their colocalization in LECs. (A) Sequence alignments of human Lrh1, SF-1, zebrafish ff1b, and COUP-TFII show that COUP-TFII harbors the LLLRLP sequence motif for interaction with Prox1.15-17 DBD indicates DNA-binding domain; and LBD, ligand-binding domain. (B) Mammalian 2-hybrid assay demonstrating a physical interaction of Prox1 and COUP-TFII proteins. Expression constructs for human full-length Prox1 and mouse COUP-TFII (67∼414 aa) proteins fused with either BD (GAL4-DNA–binding domain) or AD (VP16-activation domain) were transfected with a reporter vector into HEK293 cells and their activities on the expression of the luciferase reporter were measured after 48 hours. (C,D) Coimmunoprecipitation of Prox1 and COUP-TFII proteins; whole-cell lysates (WCL) from HEK293 cells that were transfected with the control or expression vector of Flag-Prox1 and/or HA-COUP-TFII were immunoprecipitated (IP) with anti-HA (C) or anti-Flag (D) antibodies and immunoblotted (IB) with anti-Flag and anti-HA antibodies, respectively. Endogenous Prox1 from HepG2 (E) or primary human LEC cells (F) were immunoprecipitated using an anti–COUP-TFII antibody in the absence or presence of ethidium bromide (EtBr, 100 μg/mL). IgG, a control isotype antibody. (G) Coexpression of Prox1 and COUP-TFII in developing and postdevelopmental lymphatic vessels. i-iv: COUP-TFII is expressed in Prox1-positive ECs of the cardinal vein (arrowhead) and in sprouting LECs of mouse embryo (E11.5). v-viii: Enlarged images of 2 newly formed lymphatic vessels and budding LECs (shown in boxes in ii and iii). Neither Prox1 nor COUP-TFII is expressed in the dorsal aorta (arrow). Bar, 100 μm. Human foreskin sections were stained for LYVE-1/COUP-TFII (ix-xii) and Prox1/COUP-TFII (xiii-xvi) and Prox1- or LYVE-positive LECs (arrowheads) clearly express COUP-TFII, whereas venous ECs (arrows) express only COUP-TFII. Expression of COUP-TFII and Prox1 in cultured LECs and BECs isolated from human foreskins: LECs express both Prox1 and COUP-TFII (xvii-xx) and the COUP-TFII expression levels are comparable in Prox1-positive LECs (arrowheads) versus Prox1-negative BECs (arrows) in a mixed culture of both cell types (xxi-xxiv). Bars, 100 μm.
Interaction between Prox1 and COUP-TFII proteins and their colocalization in LECs. (A) Sequence alignments of human Lrh1, SF-1, zebrafish ff1b, and COUP-TFII show that COUP-TFII harbors the LLLRLP sequence motif for interaction with Prox1.15-17 DBD indicates DNA-binding domain; and LBD, ligand-binding domain. (B) Mammalian 2-hybrid assay demonstrating a physical interaction of Prox1 and COUP-TFII proteins. Expression constructs for human full-length Prox1 and mouse COUP-TFII (67∼414 aa) proteins fused with either BD (GAL4-DNA–binding domain) or AD (VP16-activation domain) were transfected with a reporter vector into HEK293 cells and their activities on the expression of the luciferase reporter were measured after 48 hours. (C,D) Coimmunoprecipitation of Prox1 and COUP-TFII proteins; whole-cell lysates (WCL) from HEK293 cells that were transfected with the control or expression vector of Flag-Prox1 and/or HA-COUP-TFII were immunoprecipitated (IP) with anti-HA (C) or anti-Flag (D) antibodies and immunoblotted (IB) with anti-Flag and anti-HA antibodies, respectively. Endogenous Prox1 from HepG2 (E) or primary human LEC cells (F) were immunoprecipitated using an anti–COUP-TFII antibody in the absence or presence of ethidium bromide (EtBr, 100 μg/mL). IgG, a control isotype antibody. (G) Coexpression of Prox1 and COUP-TFII in developing and postdevelopmental lymphatic vessels. i-iv: COUP-TFII is expressed in Prox1-positive ECs of the cardinal vein (arrowhead) and in sprouting LECs of mouse embryo (E11.5). v-viii: Enlarged images of 2 newly formed lymphatic vessels and budding LECs (shown in boxes in ii and iii). Neither Prox1 nor COUP-TFII is expressed in the dorsal aorta (arrow). Bar, 100 μm. Human foreskin sections were stained for LYVE-1/COUP-TFII (ix-xii) and Prox1/COUP-TFII (xiii-xvi) and Prox1- or LYVE-positive LECs (arrowheads) clearly express COUP-TFII, whereas venous ECs (arrows) express only COUP-TFII. Expression of COUP-TFII and Prox1 in cultured LECs and BECs isolated from human foreskins: LECs express both Prox1 and COUP-TFII (xvii-xx) and the COUP-TFII expression levels are comparable in Prox1-positive LECs (arrowheads) versus Prox1-negative BECs (arrows) in a mixed culture of both cell types (xxi-xxiv). Bars, 100 μm.
We next investigated the expression pattern of COUP-TFII in LECs during lymphatic development by immunostaining. Cardinal vein–derived sprouting LECs and newly formed lymphatic vessels of mouse embryos (E11.5) showed a strong coexpression of COUP-TFII and Prox1 (Figure 1Gi-viii). LYVE-1 or Prox1-positive lymphatic vessels of human neonatal foreskins clearly expressed COUP-TFII (Figure 1Gix-xvi). Furthermore, cultured LECs expressed both Prox1 and COUP-TFII (Figure 1Gxvii-xx) and the expression levels of COUP-TFII in primary LECs and BECs isolated from neonatal human foreskins are comparable (Figure 1Gxxi-xxiv). Taken together, these data indicate that Prox1 and COUP-TFII are coexpressed in LECs throughout development.
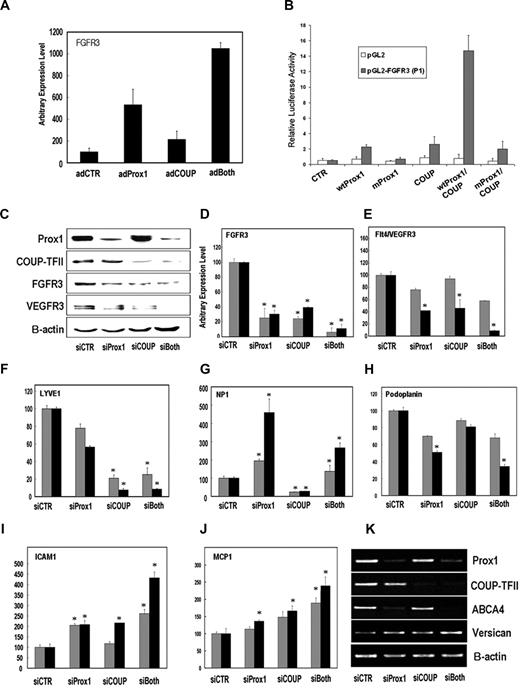
Since COUP-TFII has been shown to coregulate the expression of target genes of its interacting proteins,11 we asked whether COUP-TFII modulates the expression of Prox1-target genes. Adenoviral expression of Prox1 and/or COUP-TFII in primary human BECs revealed that COUP-TFII and Prox1 coactivated the expression of FGFR-3, a Prox1 target gene that is more prominently expressed in LECs compared with BECs6 (Figure 2A). Furthermore, luciferase assays using a 3-kb FGFR-3 promoter construct demonstrated that the proximal promoter can mediate their cooperative transcriptional activation (Figure 2B). Notably, a DNA-binding defective mutant of Prox16,8 failed to show the activation, suggesting that DNA binding of Prox1 is necessary for their coregulation of FGFR-3 (Figure 2B). We then performed siRNA-mediated knockdown of Prox1 and/or COUP-TFII in cultured LECs to investigate the necessity of COUP-TFII to maintain the expression of LEC-associated genes.3-10 Knockdown of Prox1 and COUP-TFII resulted in a concerted down-regulation of FGFR-3 and VEGFR-3 (Figure 2C-E). In comparison, some LEC-associated genes are regulated by either protein alone: while LYVE-1 was predominantly regulated by COUP-TFII, podoplanin and ABCA4 were mainly regulated by Prox1 (Figure 2F,H,K). Furthermore, ICAM1, MCP-1, and versican, which are more abundantly expressed in BECs than in LECs,5,7,8,10 were cooperatively up-regulated by knockdown of Prox1 and COUP-TFII (Figure 2I,J,K), suggesting that their expression in LECs are suppressed by both Prox1 and COUP-TFII. Interestingly, the expression of neuropilin-1 was counter-regulated by the 2 proteins in LECs (Figure 2G). We then investigated alteration of genome-wide gene expression profile by knockdown of Prox1 and/or COUP-TFII in primary LECs using microarrays and found that COUP-TFII variously modulates the expression of many Prox1 target genes, which further corroborates their functional relationship as coregulators. The microarray data are summarized in the supplemental data (Figure S1, Tables S1Table S2. Genes that are upregulated by knockdown of Prox1 and/or COUP-TFII for 48 hours in cultured LECs (PDF, 114 KB)–S3). Taken together, we conclude that COUP-TFII functions as an important coregulator of Prox1 and plays an essential role in establishing LEC fate.
Concerted regulation of EC-lineage genes by Prox1 and COUP-TFII. (A) FGFR-3 expression is cooperatively activated by Prox1 and COUP-TFII in primary human BECs that were transduced for 48 hours with a control (AdCTR), Prox1 (AdProx1), COUP-TFII (AdCOUP), or both (adBoth) adenovirus based on quantitative real-time reverse transcription–polymerase chain reaction (RT-PCR). (B) A 3-kb proximal promoter construct of mouse FGFR-3 can deliver a Prox1/COUP-TFII–mediated concerted activation when the expression vectors for control, wild-type Prox1 (wt Prox1), DNA-binding defective mutant Prox1 (mutProx1)6,8 and/or wild-type COUP-TFII (COUP) were transfected into HEK293 with a control empty (pGL2) or FGFR-3 promoter (pGL2-FGFR3) vector.6 Activation was determined by luciferase activity after normalization against total protein amount. (C-K) Both Prox1 and COUP-TFII are required to maintain the optimal expression of EC-lineage genes in primary LECs. siRNA duplexes against Prox1 and/or COUP-TFII were transfected in primary LECs for 48 to 72 hours and regulation of EC lineage genes was determined. (C) Protein levels of Prox1, COUP-TFII, FGFR3, VEGFR-3, and β-actin were determined by Western blotting analyses in primary LECs upon knockdown of Prox1 and/or COUP-TFII for 72 hours. Messenger RNA levels of FGFR-3 (D), VEGFR-3 (E), LYVE-1 (F), neuropilin-1 (NP-1, G), podoplanin (H), ICAM-1 (I), and MCP-1 (J) were determined in primary LECs, of which Prox1 and/or COUP-TFII were knocked down for 48 hours ( ) or 72 hours (■) by quantitative real-time RT-PCR. All data are expressed as an average (SD, *P < .05) compared with the corresponding control siRNA samples. (K) Messenger RNA levels of Prox1, COUP-TFII, ABCA4, versican, and β-actin were determined by semiquantitative conventional RT-PCR analyses in primary LECs after knockdown of Prox1 and/or COUP-TFII for 72 hours.
) or 72 hours (■) by quantitative real-time RT-PCR. All data are expressed as an average (SD, *P < .05) compared with the corresponding control siRNA samples. (K) Messenger RNA levels of Prox1, COUP-TFII, ABCA4, versican, and β-actin were determined by semiquantitative conventional RT-PCR analyses in primary LECs after knockdown of Prox1 and/or COUP-TFII for 72 hours.
Concerted regulation of EC-lineage genes by Prox1 and COUP-TFII. (A) FGFR-3 expression is cooperatively activated by Prox1 and COUP-TFII in primary human BECs that were transduced for 48 hours with a control (AdCTR), Prox1 (AdProx1), COUP-TFII (AdCOUP), or both (adBoth) adenovirus based on quantitative real-time reverse transcription–polymerase chain reaction (RT-PCR). (B) A 3-kb proximal promoter construct of mouse FGFR-3 can deliver a Prox1/COUP-TFII–mediated concerted activation when the expression vectors for control, wild-type Prox1 (wt Prox1), DNA-binding defective mutant Prox1 (mutProx1)6,8 and/or wild-type COUP-TFII (COUP) were transfected into HEK293 with a control empty (pGL2) or FGFR-3 promoter (pGL2-FGFR3) vector.6 Activation was determined by luciferase activity after normalization against total protein amount. (C-K) Both Prox1 and COUP-TFII are required to maintain the optimal expression of EC-lineage genes in primary LECs. siRNA duplexes against Prox1 and/or COUP-TFII were transfected in primary LECs for 48 to 72 hours and regulation of EC lineage genes was determined. (C) Protein levels of Prox1, COUP-TFII, FGFR3, VEGFR-3, and β-actin were determined by Western blotting analyses in primary LECs upon knockdown of Prox1 and/or COUP-TFII for 72 hours. Messenger RNA levels of FGFR-3 (D), VEGFR-3 (E), LYVE-1 (F), neuropilin-1 (NP-1, G), podoplanin (H), ICAM-1 (I), and MCP-1 (J) were determined in primary LECs, of which Prox1 and/or COUP-TFII were knocked down for 48 hours ( ) or 72 hours (■) by quantitative real-time RT-PCR. All data are expressed as an average (SD, *P < .05) compared with the corresponding control siRNA samples. (K) Messenger RNA levels of Prox1, COUP-TFII, ABCA4, versican, and β-actin were determined by semiquantitative conventional RT-PCR analyses in primary LECs after knockdown of Prox1 and/or COUP-TFII for 72 hours.
) or 72 hours (■) by quantitative real-time RT-PCR. All data are expressed as an average (SD, *P < .05) compared with the corresponding control siRNA samples. (K) Messenger RNA levels of Prox1, COUP-TFII, ABCA4, versican, and β-actin were determined by semiquantitative conventional RT-PCR analyses in primary LECs after knockdown of Prox1 and/or COUP-TFII for 72 hours.
Previous studies1,2,12 have shown that the nuclear receptor COUP-TFII is a key regulator of the venous EC identity and that LECs are differentiated from venous ECs through the function of Prox1. Moreover, a lineage-tracing study has proposed that the venous EC identity is a necessary prerequisite for LEC specification.13 Here, we demonstrate that COUP-TFII physically interacts with Prox1 and is required to modulate Prox1-mediated lineage-specific gene expression in LECs. Our findings strongly agree with the previous hypothesis13 of the venous identity being a prerequisite for LEC specification. Furthermore, we propose that the physical and functional interaction of Prox1 and COUP-TFII may be an important constitute in the program specifying for LEC fate during vascular development. Since COUP-TFII is the first Prox1-interacting partner identified to date that has been reported to play an essential role in EC fate determination, it will be of great interest to better understand the molecular mechanism underlying Prox1 and COUP-TFII–mediated transcriptional regulation during the lymphatic reprogramming of venous EC during development.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs You-Hua Xie (Shanghai Institute for Biological Sciences, Shanghai, People's Republic of China) and Eckardt Treuter (Karolinska Institutet, Stockholm, Sweden) for Flag-Prox1 vectors.
This study was supported by the CIRM fellowship (San Francisco, CA), a predoctoral NIH Training Grant (National Institutes of Health, Bethesda, MD), the Margaret Early Foundation Award (Los Angeles, CA), American Heart Society (Burlingame, CA), Concern Foundation (Beverly Hills, CA), American Cancer Society (Atlanta, GA), March of Dimes Foundation (White Plains, NY), and NIH/NHLBI (National Heart, Lung, and Blood Institute; 1R21HL082643-01).
National Institutes of Health
Authorship
Contribution: S.L., J.K., J.Y., S.K.G., S.C.C., S.R., B.A., and J.L. performed experiments and analyzed data; and S.L. and Y.-K.H. designed research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Young-Kwon Hong, PhD, 1450 Biggy Street NRT6501, Mail Code 9601, Los Angeles, CA 90033; e-mail: young.hong@usc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal