T-helper (Th) cells activated by cytokines in the absence of T-cell receptor ligation are suspected to participate in inflammatory processes by production of interferon-γ (IFN-γ). Still, the relevance of such a mechanism has not been addressed in humans. Here we demonstrate that a subset of human effector-memory Th cells expressing functional interleukin-12R (IL-12R), IL-18Rα, and CCR5 ex vivo can be induced to secrete IFN-γ by cytokines signaling via the IL-2R common γ-chain in combination with IL-12 and IL-18. Cytokine-driven IFN-γ production depends on JAK3- and p38 mitogen-activated kinase signals and is sensitive to suppression by CD25++ regulatory T cells. Contrary to IFN-γ+ Th cells induced upon antigen-specific stimulation, their cytokine-activated counterparts characteristically lack expression of costimulator 4-1BB (CD137). Strikingly, the majority of Th cells infiltrating inflamed joints of rheumatoid arthritis patients is equipped with receptors prerequisite for cytokine-induced IFN-γ secretion. Among these cells, we detected a substantial fraction that secretes IFN-γ directly ex vivo but lacks 4-1BB expression, indicating that cytokine-induced IFN-γ+ Th cells operate in autoimmune inflammation. Our data provide a rationale for how human effector-memory Thcells can participate in perpetuating inflammatory processes in autoimmunity even in the absence of T-cell receptor ligation.
Introduction
Production of interferon-γ (IFN-γ) by T-helper1 (Th1) cells is a key feature of both protective and pathologic immune responses. Control and clearance of bacterial and viral infections strictly depend on antigen-specific IFN-γ–secreting Th cells.1,2 It is also undisputed that Th1 cells of the effector-memory phenotype predominate in inflamed tissues in human autoimmune diseases, such as rheumatoid arthritis (RA),3,–5 where they compose up to 80% of the T-cell infiltrate.6 Nonetheless, how such cells get activated at sites of chronic inflammation remains an unsolved issue. Recent reports have challenged the concept that IFN-γ release by T cells strictly depends on T-cell receptor (TCR) ligation: in in vitro–generated murine Th1-effector cells, IFN-γ production is inducible in a TCR-independent manner by the cytokines interleukin-12 (IL-12) and IL-18.7 IL-12R–downstream STAT4 and IL-18R–dependent GADD45-β, which activates p38 mitogen-activated protein kinase (MAPK), were identified as essential signaling components.8,9 Although infection models suggest that such a mechanism evolved to provide an early source of IFN-γ contributing to host protection,10,11 it was at the same time postulated to promote inflammatory circuits in autoimmunity.12
In contrast to murine Th1 cells, however, stimulation of human resting Th cells with IL-12 and IL-18 induces only minute amounts of IFN-γ13 but boosts cytokine production in synergy with TCR ligation.14
Th cells isolated from synovial infiltrates of RA patients exhibit a differentiated effector-memory Th1 phenotype, reflected, for example, by expression of IL-12R and IL-18R.15 Because elevated levels of several proinflammatory mediators, including IL-12 and IL-18, were found to correlate with disease severity,16,17 we wondered whether cytokine-induced IFN-γ production in human Th cells in the absence of antigenic stimulation is functional at all in vitro and of relevance in vivo in human autoimmunity.
We demonstrate here that a subset of human resting effector-memory Th cells, characterized by ex vivo expression of IL-18Rα, IL-12R, and CCR5, can be induced to secrete IFN-γ by cytokines present at sites of chronic inflammation. IL-2R γ-chain (γc) signaling via JAK3, in addition to IL-12R and IL-18R ligation, is essential for TCR-independent induction of IFN-γ production. Cytokine-induced IFN-γ+ Th cells are sensitive to suppression by CD25++ T regulatory cells and specifically lack 4-1BB (CD137) expression. Applying this activation marker, we provide evidence that almost all Th cells spontaneously secreting IFN-γ ex vivo in synovial infiltrates from RA patients are cytokine-induced, for they characteristically lack 4-1BB expression. Our results support the notion that IFN-γ production by Th cells in chronic autoimmune inflammation may be provoked solely by the cytokine environment, independent of triggering autoantigens.
Methods
Cell isolation
Peripheral blood mononuclear cells (PBMCs) were isolated from peripheral blood of healthy donors by Ficoll-Hypaque centrifugation. Th cells were purified from PBMCs using anti-CD4 multisort beads (Miltenyi Biotec, Bergisch Gladbach, Germany). Memory Th cells were subsequently obtained by depletion of naive Th cells and residual monocytes using anti-CD45RA and anti-CD14 beads, respectively. Purities were more than 98%. IL-18Rα+ and IL-18Rα− Th cells were obtained after pre-enrichment of Th cells followed by sorting of CD4+ (TT1, house conjugate) CD45RA− (clone HI100; BD Biosciences, San Jose, CA), and IL-18+/− (FAB840P; R&D Systems, Minneapolis, MN) populations on a fluorescence-activated cell sorter (FACS) DIVA cell sorter (BD Biosciences). The same protocol was applied for enrichment of CCR7+/− (3D12; BD Biosciences) memory Th cells. Monocytes were purified to more than 99% by positive selection using anti-CD14 beads (Miltenyi Biotec) in 2 rounds of magnetic enrichment. For all procedures, LS columns or the Automacs device (Miltenyi Biotec) were used. For sorting CD4+ CD25++ (regulatory) and CD25− (responder) T cells, CD4+ T cells were pre-enriched as described and stained with anti-CD25 (4E3; Miltenyi Biotec) and anti-CD4; CD25++ and CD25− populations were sorted on a FACS DIVA cell sorter (BD Biosciences) to purities more than 98%. CD25− cells were further purified according to IL-18Rα or CD45RA expression. Live IFN-γ–secreting cells were identified and/or sorted using the cytokine secretion assay according to the manufacturer's protocol (Miltenyi Biotec). Synovial fluid (SF) mononuclear cells were washed twice in phosphate-buffered saline/bovine serum albumin buffer containing 2 mM ethylenediaminetetraacetic acid; cell debris was excluded using preseparation filters (Miltenyi Biotec). Synovial membrane (SM) was minced into pieces of 1 to 5 mm3, dissociated using Gentle MACS tissue dissociator (Miltenyi Biotec) and digested for 1 hour with collagenase IA, hyaloronidase, and DNAse I (Sigma-Aldrich, St Louis, MO).
Cell culture and stimulation
All cells were cultured in RPMI 1640 media containing 0.3 mg/mL glutamine, 100 U/mL penicillin, 0.1 mg/mL streptomycin (Invitrogen, Carlsbad, CA), and 10% human AB serum (PAA Laboratories, Coelbe, Germany). Recombinant cytokines (R&D Systems) were used at 25 ng/mL unless otherwise indicated. rIL-2 (Roche Diagnostics, Indianapolis, IN) was used at 20 U/mL. TCR stimulation was performed in round-bottom polystyrol tubes (BD Biosciences Discovery Labware, Bedford, MA) coated for 4 hours (37°C) with anti-CD3 (UCHT1; BD Biosciences) and anti-CD28 (CD28.2; BD Biosciences) at 0.5 μg/mL and 2.5 μg/mL, respectively, unless otherwise stated. Cell-signaling inhibitors SB202190, SB203580 (Sigma-Aldrich), PD98059, JAK3 inhibitor I, and cyclosporine A (all Calbiochem, San Diego, CA) were used as indicated.
For generation of recall antigen-specific Th1 lines, PBMCs were cultured with 5 μg/mL CMVpp65 protein (Imtec, Berlin, Germany) and 1 μg/mL anti-CD28 (CD28.2; BD Biosciences) for 6 hours; live antigen-specific IFN-γ+ Th1 cells were stained by IFN-γ secretion assay technology (Miltenyi Biotec) in combination with CD69 (FN50; BD Biosciences) and CD4 and purified by FACS sorting. Th1 lines were expanded for 10 to 14 days in the presence of IL-7 and IL-15 (5 ng/mL each). Before further stimulation, cells were rested for 5 days in the absence of IL-7 and IL-15. For antigen-specific restimulation, 105 CMVpp65-specific Th1 cells were cultured in the presence of 5 × 105 irradiated autologous PBMCs, 5 μg/mL CMVpp65 protein, and 1 μg/mL anti-CD28.
Cytometric bead enzyme-linked immunosorbent assay, phenotyping, and intracellular cytokine staining
Simultaneous measurement of IL-2, IL-4, IL-5, IL-10, and IFN-γ in culture supernatants was performed using the cytometric bead enzyme-linked immunosorbent assay system (BD Biosciences) according to the manufacturer's protocol. For FACS surface stainings, antibodies against CD25, CD40L, CD69, CD70, CD71, HLA-DR, 4-1BB, and Ox40 were purchased from BD Biosciences. Dead cells were excluded using propidium iodide or 4,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich). For measurement of cytokines at the single-cell level, protein secretion inhibitor brefeldin A (Sigma-Aldrich) was added to the cultures for the last 4 to 12 hours. Cells were fixed in 2% formalin, permeabilized using FACSPerm solution (BD Biosciences), and stained for 30 minutes at room temperature. Anti–IFN-γ (4SB3) and anti–tumor necrosis factor-α (TNF-α; 1D12) were self-conjugated to the respective fluorochromes. Anti–IL-17 was from eBioscience (San Diego, CA). Cells were analyzed on a FACSCalibur or LSRII (BD Biosciences).
Regulatory T-cell assays
CD4+CD25++ regulatory T cells (Tregs) were preactivated overnight in anti–CD3-coated (10 μg/mL) FACS tubes in the presence of 100 U/mL rIL-2; in IFN-γ inhibition assays, carboxyfluorescein diacetate (CFDA)–labeled (5 μM, 4 minutes, 20°C) CD4+CD25−IL18Rα+ responder cells were mixed with CFDA−CD4+CD25++ Tregs or CFDA−CD4+CD25−IL18Rα+ control cells in a ratio of 1:1 in the presence of equal numbers of autologous CD14+ accessory cells. Cells were stimulated with cytokines or anti-CD2/CD3/CD28-coated T-cell activation beads (Miltenyi Biotec) and analyzed for IFN-γ production. For comparison purposes, proliferation suppression assays were performed with naive CFDA+CD4+CD25−CD45RA+ responder cells and CFDA−CD4+CD25++ Tregs or CFDA−CD4+CD25−CD45RA+ control cells in a ratio of 1:1 in the presence of equal numbers of autologous CD14+ accessory cells. Cells were stimulated with anti-CD2/CD3/CD28-coated T-cell activation beads (Miltenyi Biotec) for 3 to 4 days; proliferation was quantified according to loss of CFDA.
Immunoblotting
After stimulation, cells were washed with phosphate-buffered saline and lysed with Laemmli sample buffer (Bio-Rad, Munich, Germany). Proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and subsequently electrotransferred to nitrocellulose. Membrane was blocked with 5% bovine serum albumin and probed with pSTAT4 (pY693, clone 38; BD Biosciences) or pSTAT5 (pY694, clone 47; BD Biosciences). After incubation with horseradish peroxidase–conjugated secondary antibody, protein was detected by chemiluminescence (GE Healthcare, Little Chalfont, United Kingdom). After stripping, membranes were reprobed with STAT4 (Sigma-Aldrich) or STAT5 (clone 89; BD Biosciences) and detected as before.
Patients
Patients with a diagnosis of RA according to the criteria of the American College of Rheumatology18 were included in the study (Table 1). All patients had active disease and underwent SF aspiration or synovectomy for therapeutic purpose. Patients' samples were taken after informed consent approved by the ethical committees of the respective hospitals in accordance with the Declaration of Helsinki.
Characteristics of RA patients included in the study
| Patient no. . | Age, y . | Sex . | Treatment . | Disease duration, y . | Sample type . |
|---|---|---|---|---|---|
| 1 | 69 | F | MTX, CQ, CS | 2 | SF |
| 2 | 21 | F | Anti–TNF-α, MTX, CS | 7 | SF |
| 3 | 68 | M | MTX, CS | < 1 | SF |
| 4 | 28 | M | CsA | 1 | SF |
| 5 | 74 | M | — | 1 | SF |
| 6 | 37 | F | — | 3 | SF |
| 7 | 48 | F | CS | 16 | SF |
| 8 | 37 | F | — | 3 | SF |
| 9 | 84 | F | — | 46 | SF |
| 10 | 63 | F | CS | 2 | SF |
| 11 | 59 | M | NSAIDs | < 1 | SF |
| 12 | 40 | F | Anti–TNF-α | 13 | SF |
| 13 | 73 | F | — | 1 | SM |
| 14 | 68 | F | — | 10 | SM |
| 15 | 30 | F | MTX, CSs | 1 | SM |
| 16 | 74 | F | CSs, leflunomide | 2 | SM |
| 17 | 52 | F | Aza, CQ | 27 | SM |
| Patient no. . | Age, y . | Sex . | Treatment . | Disease duration, y . | Sample type . |
|---|---|---|---|---|---|
| 1 | 69 | F | MTX, CQ, CS | 2 | SF |
| 2 | 21 | F | Anti–TNF-α, MTX, CS | 7 | SF |
| 3 | 68 | M | MTX, CS | < 1 | SF |
| 4 | 28 | M | CsA | 1 | SF |
| 5 | 74 | M | — | 1 | SF |
| 6 | 37 | F | — | 3 | SF |
| 7 | 48 | F | CS | 16 | SF |
| 8 | 37 | F | — | 3 | SF |
| 9 | 84 | F | — | 46 | SF |
| 10 | 63 | F | CS | 2 | SF |
| 11 | 59 | M | NSAIDs | < 1 | SF |
| 12 | 40 | F | Anti–TNF-α | 13 | SF |
| 13 | 73 | F | — | 1 | SM |
| 14 | 68 | F | — | 10 | SM |
| 15 | 30 | F | MTX, CSs | 1 | SM |
| 16 | 74 | F | CSs, leflunomide | 2 | SM |
| 17 | 52 | F | Aza, CQ | 27 | SM |
MTX indicates methotrexate; CQ, chloroquine; CSs, corticosteroids; CsA, cyclosporine A; TNF-α, tumor necrosis factor-α; NSAIDs, nonsteroidal anti-inflammatory drugs; Aza, azathioprine; SF, synovial fluid; and SM, synovial membrane.
Results
Induction of IFN-γ secretion in human resting Th cells by inflammatory cytokines
At first, we tested whether purified resting human Th cells can secrete IFN-γ in response to a mixture of inflammatory mediators abundantly present at sites of inflammation, such as inflamed joints of RA patients. For that, Th cells were stimulated for 72 hours with IL-1β, IL-6, IL-7, IL-8, IL-12, IL-15, IL-17, IL-18, TNF-α, and MIP-1α (termed cytokine cocktail), followed by analysis of secreted IL-2, IL-4, IL-5, IL-10, and IFN-γ in culture supernatants. As shown in Figure 1A, only IFN-γ was induced in human Th cells upon stimulation with the cytokine cocktail. Cytometric analysis revealed that up to 7.8% (mean percentage ± SEM: 4.5% ± 0.73%) of Th cells from peripheral blood had the capacity to produce IFN-γ after stimulation with the cytokine cocktail. We observed a peak in the frequency of responding cells after approximately 36 hours, whereas TCR-activated IFN-γ+ Th cells peaked between 6 hours and 12 hours (Figure 1B-D). In contrast to what was reported for murine Th1 cells,7 we did not detect secretion of IFN-γ after stimulation with IL-12 and IL-18 alone (Figure 1B,E). The proinflammatory mediators TNF-α (Figure 1C,E) and IL-17 (Figure 1D) were expressed after TCR, but not after cytokine stimulation.
Induction of IFN-γ secretion in human resting Th cells by inflammatory cytokines. (A) Th cells were stimulated with the cytokine cocktail (IL-1β, IL-6, IL-7, IL-8, IL-12, IL-15, IL-17, IL-18, TNF-α, and MIP-1α). After 72 hours, supernatants were analyzed for secreted IL-2, IL-4, IL-5, IL-10, and IFN-γ by cytometric bead enzyme-linked immunosorbent assay. (B-D) Th cells were stimulated as indicated. Frequencies of (B) IFN-γ–, (C) IFN-γ– and TNF-α–, or (D) IFN-γ– and IL-17–expressing cells were analyzed at different time points intracellularly by FACS. (E) Th cells were stimulated for 36 hours with different cytokines or for 12 hours with αCD3 + αCD28 and assessed intracellularly for IFN-γ and TNF-α as before. One experiment of 3 (A,D) or 5 (B,C,E) is shown.
Induction of IFN-γ secretion in human resting Th cells by inflammatory cytokines. (A) Th cells were stimulated with the cytokine cocktail (IL-1β, IL-6, IL-7, IL-8, IL-12, IL-15, IL-17, IL-18, TNF-α, and MIP-1α). After 72 hours, supernatants were analyzed for secreted IL-2, IL-4, IL-5, IL-10, and IFN-γ by cytometric bead enzyme-linked immunosorbent assay. (B-D) Th cells were stimulated as indicated. Frequencies of (B) IFN-γ–, (C) IFN-γ– and TNF-α–, or (D) IFN-γ– and IL-17–expressing cells were analyzed at different time points intracellularly by FACS. (E) Th cells were stimulated for 36 hours with different cytokines or for 12 hours with αCD3 + αCD28 and assessed intracellularly for IFN-γ and TNF-α as before. One experiment of 3 (A,D) or 5 (B,C,E) is shown.
To identify the essential factors for IFN-γ induction within the inflammatory cytokine cocktail, we tested all cytokines alone and in combination (Figure 1E; and data not shown). IFN-γ synthesis was inducible only upon stimulation with the γ-chain cytokines IL-2, IL-7, IL-15, but not IL-4, in synergy with IL-12 and IL-18. Frequencies of IFNγ+ Th cells from healthy donors that could be induced by the most efficient combination of IL-15 plus IL-12 plus IL-18 varied between 0.8% and 3% (mean percentage ± SEM: 1.68% ± 0.40%).
Cytokine-induced IFN-γ secretion is confined to CCR7−CCR5+IL-18Rα+ effector-memory Th cells coexpressing functional IL-12 receptors
We then examined whether IFN-γ expression induced by cytokines in a TCR-independent fashion is a feature of a specialized subset within resting Th cells. First, we demonstrated that Th cells capable of secreting IFN-γ after cytokine activation expressed CD45RO, characterizing them as antigen-experienced Th lymphocytes (Figure 2A). Next we isolated CD45RA− human central and effector memory Th cell subsets according to CCR7 expression and analyzed them for IFN-γ synthesis after stimulation with the cytokine cocktail or αCD3 plus αCD28. CCR7− effector-memory Th cells were highly responsive to cytokine stimulation compared with CCR7+ central-memory Th cells; this dichotomy was less pronounced after TCR stimulation (Figure 2B).
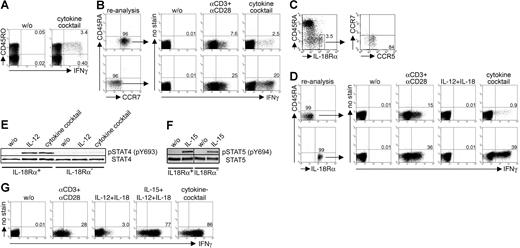
Cytokine-induced IFN-γ secretion is restricted to CCR7−CCR5+IL-18Rα+ effector-memory Th cells coexpressing functional IL-12 receptors. (A) Correlation of intracellular IFN-γ expression with CD45RO expression in Th cells after 36 hours of stimulation with the cytokine cocktail. Shown is 1 experiment of 3. (B) Purified CCR7+ and CCR7− Th cells were stimulated for 36 hours with the cytokine cocktail or for 12 hours with αCD3 + αCD28 and assessed intracellularly for IFN-γ. One representative experiment of 3 is shown. (C) Assessment of IL-18Rα–expressing memory Th cells within PBMCs and CCR5- and CCR7-expressing cells within the IL-18Rα+ Th cell population. One experiment of 5 is shown. (D) IL-18Rα+ and IL-18Rα− Th cells were analyzed intracellularly for IFN-γ after stimulation for 36 hours with the cytokine cocktail or IL-12 + IL-18 or for 12 hours with αCD3 + αCD28. One experiment of 5 is shown. (E) Detection of phosphorylated STAT4 by immunoblotting in IL-18Rα+ and IL-18R− Th cells after 1 hour of stimulation with IL-12 or with the cytokine cocktail. One experiment of 3 is shown. (F) Detection of phosphorylated STAT5 by immunoblotting in IL-18Rα+ and IL-18R− Th cells after 1 hour of stimulation with IL-15. One representative experiment of 2 is shown. (G) Cytokine-induced IFN-γ secretion in a CMVpp65-specific Th1-cell line. CMVpp65-specific Th1 cells were restimulated for 12 hours with the indicated stimuli and assessed intracellularly for IFN-γ expression. One of 2 independent experiments is shown.
Cytokine-induced IFN-γ secretion is restricted to CCR7−CCR5+IL-18Rα+ effector-memory Th cells coexpressing functional IL-12 receptors. (A) Correlation of intracellular IFN-γ expression with CD45RO expression in Th cells after 36 hours of stimulation with the cytokine cocktail. Shown is 1 experiment of 3. (B) Purified CCR7+ and CCR7− Th cells were stimulated for 36 hours with the cytokine cocktail or for 12 hours with αCD3 + αCD28 and assessed intracellularly for IFN-γ. One representative experiment of 3 is shown. (C) Assessment of IL-18Rα–expressing memory Th cells within PBMCs and CCR5- and CCR7-expressing cells within the IL-18Rα+ Th cell population. One experiment of 5 is shown. (D) IL-18Rα+ and IL-18Rα− Th cells were analyzed intracellularly for IFN-γ after stimulation for 36 hours with the cytokine cocktail or IL-12 + IL-18 or for 12 hours with αCD3 + αCD28. One experiment of 5 is shown. (E) Detection of phosphorylated STAT4 by immunoblotting in IL-18Rα+ and IL-18R− Th cells after 1 hour of stimulation with IL-12 or with the cytokine cocktail. One experiment of 3 is shown. (F) Detection of phosphorylated STAT5 by immunoblotting in IL-18Rα+ and IL-18R− Th cells after 1 hour of stimulation with IL-15. One representative experiment of 2 is shown. (G) Cytokine-induced IFN-γ secretion in a CMVpp65-specific Th1-cell line. CMVpp65-specific Th1 cells were restimulated for 12 hours with the indicated stimuli and assessed intracellularly for IFN-γ expression. One of 2 independent experiments is shown.
We wondered whether the receptors for the essential IFN-γ–inducing cytokines (γc cytokine + IL-12 + IL-18) are constitutively expressed ex vivo or up-regulated upon stimulation. IL-18Rα expression was restricted to a small subset of 3% to 12% (mean percentage ± SEM: 6.50% ± 0.96%) of human resting effector-memory Th cells ex vivo; the majority of the IL-18Rαhigh population coexpressed the Th1-associated chemokine receptor CCR5 and was largely CCR7− (Figure 2C). Next, IL-18Rα+ and IL-18R− Th cells from peripheral blood of healthy persons were tested for cytokine-induced IFN-γ expression. IL-18Rα− Th cells responded poorly to cytokine stimulation with respect to IFN-γ secretion (mean percentage ± SEM: 0.55% ± 0.19%). In contrast, the IL-18Rα+ fraction exhibited up to 40% IFN-γ+ Th cells (mean percentage ± SEM: 33.8% ± 2.5%) after stimulation with the cytokine cocktail. IL-18Rα− and IL-18Rα+ Th cells responded also differently to activation by immobilized αCD3 plus αCD28, albeit with minor discrepancy (Figure 2D).
We next examined the role of the IL-12R complex in cytokine-induced IFN-γ expression. In accordance with earlier studies,19 we were not able to detect IL-12Rβ2 expression on resting memory Th cells by flow cytometry (data not shown). To detect low but functional IL-12 receptor expression in IL-18Rα+ and IL-18Rα− memory Th cells purified from peripheral blood, we assessed IL-12R–dependent STAT4 phosphorylation (pSTAT4) by immuno-blotting. Interestingly, IL-12–induced pSTAT4 was confined almost exclusively to IL-18Rα+ Th cells, indicating that functional IL-12R complexes are constitutively expressed on IL-18Rα+, but not on IL-18Rα− Th cells (Figure 2E). As the third essential receptor component, we chose to analyze functional IL-15R expression being representative for γc cytokine receptors. Detection of phosphorylated STAT5 (pSTAT5) in IL-18Rα+ and IL-18Rα− memory Th cells after IL-15 stimulation suggested that both populations express functional IL-15R ex vivo (Figure 2F).
The observation that IFN-γ secretion was inducible predominantly in resting Th1-type effector-memory cells prompted us to analyze cytomegalovirus (CMV)–specific, proinflammatory Thcells as model Th1 cells with an in vivo history of repeated reactivation20,21 ; these cells exhibit a similar differentiation status as Th cells isolated from inflamed synovial lesions in RA, reflected, for instance, by the loss of CD27 and/or CD28.22,23 As early as 12 hours after stimulation with the minimal cytokine combination of IL-12 plus IL-18 plus IL-15, almost all CMVpp65-specific Th1 cells could be induced to secrete IFN-γ. IL-12 plus IL-18 alone induced IFN-γ secretion only in a small percentage of cells (Figure 2G), again highlighting the different requirements for IFN-γ induction in human versus murine Th cells.
Cytokine-induced IFN-γ secretion is JAK3 and p38-MAPK dependent
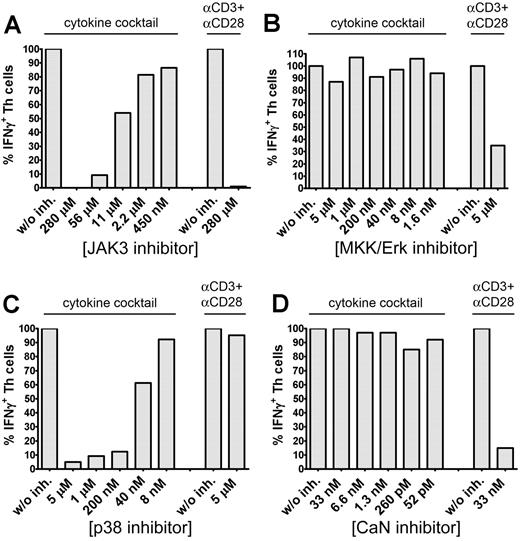
To identify essential signaling pathways involved in cytokine-induced IFN-γ secretion, memory Th cells were stimulated either with the cytokine cocktail or αCD3 plus αCD28 in the presence of various signaling inhibitors, such as JAK3 inhibitor I, MKK/Erk inhibitor PD98059, p38 inhibitor SB202190, or calcineurin inhibitor cyclosporine A (CsA) blocking nuclear factor of activated T-cell activation. JAK3 blockade concentration-dependently blocked both cytokine- and TCR-induced IFN-γ secretion (Figure 3A). MKK/Erk blockade by PD98059 did not influence cytokine-induced IFN-γ secretion but reduced frequencies of TCR-induced IFN-γ+ Th cells by approximately 65% (Figure 3B). The p38 inhibitor SB202190 concentration-dependently abrogated cytokine-dependent but did not block TCR-induced IFN-γ production (Figure 3C). The latter result was reproducible with SB203580, an alternative p38 inhibitor (data not shown). CsA almost completely suppressed TCR-induced IFN-γ secretion but did not affect IFN-γ synthesis induced by the cytokine cocktail (Figure 3D).
Cytokine-induced IFN-γ secretion is JAK3 and p38-MAPK dependent. CD45RA− memory Th cells were preincubated for 45 minutes with different concentrations of JAK3 inhibitor I (A), PD98059 (B), SB202190 (C), or CsA (D) and subsequently stimulated for 36 hours with the cytokine cocktail or for 12 hours with αCD3 + αCD28. IFN-γ expression was analyzed intracellularly by FACS. Frequencies of IFN-γ+ Th cells in the absence of an inhibitor were set to 100%. One representative experiment of 3 (B,D) or 5 (A,C) is shown.
Cytokine-induced IFN-γ secretion is JAK3 and p38-MAPK dependent. CD45RA− memory Th cells were preincubated for 45 minutes with different concentrations of JAK3 inhibitor I (A), PD98059 (B), SB202190 (C), or CsA (D) and subsequently stimulated for 36 hours with the cytokine cocktail or for 12 hours with αCD3 + αCD28. IFN-γ expression was analyzed intracellularly by FACS. Frequencies of IFN-γ+ Th cells in the absence of an inhibitor were set to 100%. One representative experiment of 3 (B,D) or 5 (A,C) is shown.
These results show that cytokine-induced IFN-γ secretion in human Th cells is dependent on both functional JAK3- and p38-MAPK pathways.
Cytokine-induced IFN-γ+ effector-memory Th cells are controlled by CD25++ Tregs
Some reports indicated that naturally occurring human CD25++ regulatory T cells can suppress production of IFN-γ and other cytokines in responder Th cells after TCR stimulation.24,25 We wondered whether cytokine-induced IFN-γ–secreting IL-18Rα+ memory-effector Th cells are equally sensitive to suppression by Tregs. Sorting strategies are depicted in Figure 4A.
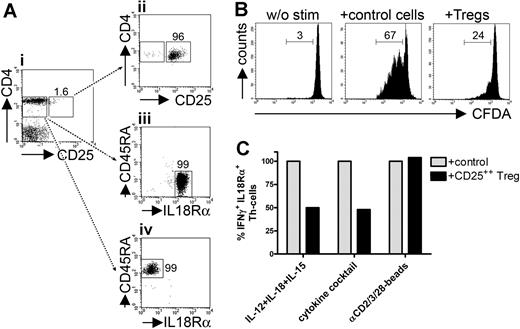
Cytokine-induced IFN-γ+ Th cells are controlled by CD25++ Tregs. (A) CD25++ Tregs (II) and CD45RA−IL-18Rα+ memory-effector (III) or CD45RA+IL-18Rα− naive (IV) responder Th cells were sorted from PBMCs (I). (B) CFDA-labeled CD45RA+IL-18Rα− naive responder Th cells were cultured with T-cell stimulation beads in the presence of equal numbers of CFDA−CD45RA+IL-18Rα− control cells or CD25++ Tregs. Proliferation was assessed after 84 hours according to loss of CFDA by FACS. (C) CFDA-labeled CD45RA−IL-18Rα+ responder Th cells were stimulated for 36 hours with IL-12 + IL-18 + IL-15 or with the cytokine cocktail or for 18 hours with T-cell stimulation beads in the presence of equal numbers of CFDA−CD45RA−IL-18Rα+ control cells or CD25++ Tregs. Frequencies of CFDA+IFN-γ+ responder cells were assessed intracellularly by FACS; cocultures with control cells were set to 100%. One representative experiment of 3 (B) or 4 (C), respectively, is shown.
Cytokine-induced IFN-γ+ Th cells are controlled by CD25++ Tregs. (A) CD25++ Tregs (II) and CD45RA−IL-18Rα+ memory-effector (III) or CD45RA+IL-18Rα− naive (IV) responder Th cells were sorted from PBMCs (I). (B) CFDA-labeled CD45RA+IL-18Rα− naive responder Th cells were cultured with T-cell stimulation beads in the presence of equal numbers of CFDA−CD45RA+IL-18Rα− control cells or CD25++ Tregs. Proliferation was assessed after 84 hours according to loss of CFDA by FACS. (C) CFDA-labeled CD45RA−IL-18Rα+ responder Th cells were stimulated for 36 hours with IL-12 + IL-18 + IL-15 or with the cytokine cocktail or for 18 hours with T-cell stimulation beads in the presence of equal numbers of CFDA−CD45RA−IL-18Rα+ control cells or CD25++ Tregs. Frequencies of CFDA+IFN-γ+ responder cells were assessed intracellularly by FACS; cocultures with control cells were set to 100%. One representative experiment of 3 (B) or 4 (C), respectively, is shown.
To demonstrate that Tregs were functional in our system, we initially performed classic proliferation suppression assays. For that, CFDA-labeled naive CD4+CD45RA+CD25− responder cells were stimulated with anti-CD2/CD3/CD28-coated T-cell stimulation beads. Proliferation was efficiently blocked by addition of Tregs compared to CD4+CD45RA+CD25− control cells (Figure 4B).
We then assessed IFN-γ synthesis after stimulation with cytokines or T-cell stimulation beads in isolated, CD45RA−IL-18Rα+CD25− responder Th cells in the presence of equal numbers of CD25++ Tregs or CD45RA−IL-18Rα+CD25− control cells. Frequencies of IFN-γ+ responder cells after stimulation with IL-12 plus IL-18 plus IL-15 were significantly reduced in the presence of CD25++ Tregs (decrease in mean percentage ± SEM: 55.4% ± 9.3%). Treg-mediated inhibition of IFN-γ production could be equally observed after stimulation with the cytokine cocktail (decrease in mean percentage ± SEM: 52.1% ± 2.6%). TCR-mediated IFN-γ induction was not suppressed by CD25++ Tregs (increase in mean percentage ± SEM: 7.55% ± 1.77%; Figure 4C).
4-1BB expression discriminates TCR- from cytokine-induced IFN-γ–secreting Th cells
To examine whether cytokine-induced Th cells operate at sites of chronic inflammation, such as arthritic joints of RA patients, we aimed at phenotypically discriminating them from their TCR-induced counterparts. To this end, we activated memory Th cells for 36 hours either with the cytokine cocktail or αCD3 plus αCD28 and analyzed the expression of a panel of activation markers on live IFN-γ–secreting Th cells by flow cytometry (Figure 5A). Among the molecules analyzed, the TNF-receptor family member 4-1BB (CD137) was the only surface marker being almost absent on cytokine-stimulated IFN-γ+ Th cells but prominently up-regulated on the majority of TCR-triggered IFN-γ+ Th cells. We then investigated the kinetics of 4-1BB expression in a more physiologic system, involving TCR stimulation by antigen processed by antigen-presenting cells instead of αCD3 plus αCD28. In model antigen CMVpp65-stimulated PBMCs, 4-1BB expression was detectable approximately after 8 hours, peaked at approximately 18 hours, and was constantly detectable for up to 48 hours on antigen-induced IFN-γ+ Th cells. As no cytokine-secreting cell could be observed after 50 hours, kinetics were terminated. In contrast to TCR-triggered Th cells, 4-1BB remained almost absent on cytokine-induced IFN-γ+ Th cells (Figure 5B). Lack of 4-1BB expression was regulated at the transcriptional level since cytokine-induced IFN-γ+ Th cells were also characterized by low 4-1BB mRNA content (data not shown). Furthermore, we demonstrated that antigen-specific restimulation of a CMVpp65-specific short-term Th1-cell line resulted in up-regulation of 4-1BB on the majority of cells, whereas cytokine-restimulated CMVpp65-specific Th cells failed to express 4-1BB (Figure 5C).
Cytokine-induced IFN-γ+ Th cells lack 4-1BB expression. (A) Expression of activation markers on live IFN-γ+ memory Th cells detected with the cytokine secretion assay after stimulation with the cytokine cocktail or αCD3 + αCD28 for 36 hours. Filled histograms represent stimulated; open histograms, unstimulated control samples. (B) PBMCs were stimulated with CMV lysate or with the cytokine cocktail. 4-1BB expression was analyzed on live IFN-γ+ Th cells at indicated time points by the cytokine secretion assay. (C) CMVpp65-specific Th1 cells were restimulated with CMVpp65 or IL-12 + IL-18 + IL-15 for 14 hours. Numbers indicate relative frequencies of 4-1BB+ and 4-1BB− cells among the IFN-γ+ Th cell population; numbers in parentheses indicate frequencies among total Th cells. (D) Memory Th cells were stimulated for 36 hours with the cytokine cocktail, followed by FACS sorting of activated cells according to IFN-γ secretion. After 2 cycles of αCD3 + αCD28 restimulation, cells were activated as indicated. Numbers indicate relative frequencies of 4-1BB+ and 4-1BB− cells among the IFN-γ+ Th cell population; numbers in parentheses indicate frequencies among total Th cells. One of 3 independent experiments is shown.
Cytokine-induced IFN-γ+ Th cells lack 4-1BB expression. (A) Expression of activation markers on live IFN-γ+ memory Th cells detected with the cytokine secretion assay after stimulation with the cytokine cocktail or αCD3 + αCD28 for 36 hours. Filled histograms represent stimulated; open histograms, unstimulated control samples. (B) PBMCs were stimulated with CMV lysate or with the cytokine cocktail. 4-1BB expression was analyzed on live IFN-γ+ Th cells at indicated time points by the cytokine secretion assay. (C) CMVpp65-specific Th1 cells were restimulated with CMVpp65 or IL-12 + IL-18 + IL-15 for 14 hours. Numbers indicate relative frequencies of 4-1BB+ and 4-1BB− cells among the IFN-γ+ Th cell population; numbers in parentheses indicate frequencies among total Th cells. (D) Memory Th cells were stimulated for 36 hours with the cytokine cocktail, followed by FACS sorting of activated cells according to IFN-γ secretion. After 2 cycles of αCD3 + αCD28 restimulation, cells were activated as indicated. Numbers indicate relative frequencies of 4-1BB+ and 4-1BB− cells among the IFN-γ+ Th cell population; numbers in parentheses indicate frequencies among total Th cells. One of 3 independent experiments is shown.
To assess the stability of 4-1BB dichotomy after repeated stimulation, we activated memory Th cells with the cytokine cocktail followed by purification of live IFN-γ secreting cells by FACS. After 1 week of rest, cells were restimulated twice with αCD3 plus αCD28 on a weekly basis. Finally, cells were either activated with αCD3 plus αCD28 or with cytokines. As demonstrated in Figure 5D, 4-1BB coexpression was almost exclusively confined to TCR-stimulated IFN-γ producers, but not to cytokine-stimulated Th cells even after intermittent TCR stimulation.
Thus, activation-induced expression of 4-1BB allows discrimination of TCR- from cytokine-induced human IFN-γ+ Th cells.
Synovial IFN-γ+ Th cells isolated ex vivo from RA patients exhibit a cytokine-induced phenotype
Extending previous reports,15,26 we could show that the majority of Th cells infiltrating inflamed joints of RA patients with active disease exhibit an effector-memory signature, being CD45RA−CD45RO+ and expressing IL-18Rα and CCR5 (Figure 6A). Based on those findings, we aimed at detecting spontaneous IFN-γ production in synovial Th cells ex vivo using the sensitive cytokine secretion assay technology; furthermore, we intended to test whether these cells are possibly triggered by inflammatory cytokines rather than autoantigens.
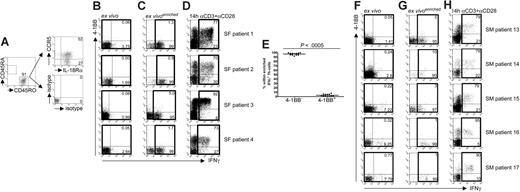
Synovial IFN-γ+ Th cells isolated ex vivo from RA patients are induced by cytokines. (A) Expression of CD45RA, CD45RO, CCR5, and IL-18Rα on SF-derived Th cells ex vivo; 1 representative patient of 6. (B,F) Live SF- or SM-derived Th cells were stained ex vivo for secreted IFN-γ and 4-1BB by the cytokine secretion assay technology. Numbers indicate frequencies among Th cells. (C,G) Magnetically enriched live IFN-γ+ Th cells were analyzed for 4-1BB expression. Numbers indicate frequencies within the IFN-γ+ population. (D,H) SF-MNCs or SM-MNCs were stimulated for 14 hours with αCD3 + αCD28. CD4+ Th cells were analyzed for 4-1BB and IFN-γ expression. Numbers indicate frequencies within the IFN-γ+ population. (B-D,F-H) Results from 4 representative SF and all analyzed SM samples. (E) Frequencies of live 4-1BB+IFN-γ+ and 4-1BB−IFN-γ+ SF-derived Th cells after magnetic enrichment according to secreted IFN-γ (all patients). P value was determined by Wilcoxon rank test.
Synovial IFN-γ+ Th cells isolated ex vivo from RA patients are induced by cytokines. (A) Expression of CD45RA, CD45RO, CCR5, and IL-18Rα on SF-derived Th cells ex vivo; 1 representative patient of 6. (B,F) Live SF- or SM-derived Th cells were stained ex vivo for secreted IFN-γ and 4-1BB by the cytokine secretion assay technology. Numbers indicate frequencies among Th cells. (C,G) Magnetically enriched live IFN-γ+ Th cells were analyzed for 4-1BB expression. Numbers indicate frequencies within the IFN-γ+ population. (D,H) SF-MNCs or SM-MNCs were stimulated for 14 hours with αCD3 + αCD28. CD4+ Th cells were analyzed for 4-1BB and IFN-γ expression. Numbers indicate frequencies within the IFN-γ+ population. (B-D,F-H) Results from 4 representative SF and all analyzed SM samples. (E) Frequencies of live 4-1BB+IFN-γ+ and 4-1BB−IFN-γ+ SF-derived Th cells after magnetic enrichment according to secreted IFN-γ (all patients). P value was determined by Wilcoxon rank test.
For that purpose, live Th cells from synovial fluid mononuclear cell (SF-MNC) infiltrates were directly stained ex vivo for secreted IFN-γ and 4-1BB to distinguish TCR-activated from cytokine-induced IFN-γ+ Th cells. Spontaneous IFN-γ–secreting Th cells could be readily detected in samples from all patients (mean percentage ± SEM: 2.32% ± 0.39%, n = 12). Figure 6B depicts the results from 4 patients. To assess 4-1BB expression accurately also in synovial infiltrates with rare frequencies of IFN-γ+ Th cells, coexpression analysis was performed on live IFN-γ+ Th cells, which were magnetically enriched by the cytokine secretion assay. Strikingly, almost the entire IFN-γ+ Th cell population (typically > 95%) lacked 4-1BB coexpression (Figure 6C,E). Figure 6E outlines the data from 12 patients analyzed; approximately 95% of the ex vivo detected, magnetically enriched IFN-γ–producing Th cells from synovial infiltrates lacked 4-1BB coexpression.
To exclude a general defect in 4-1BB expression, SF-MNCs were stimulated with αCD3 plus αCD28. As verified for effector cells from healthy donors (Figure 5), 4-1BB was highly up-regulated on the majority of TCR-activated IFN-γ–secreting Th cells from RA patients (Figure 6D).
To examine whether cytokine-induced IFN-γ+ Th cells also reside within the inflamed tissue, we analyzed Th cells freshly isolated ex vivo from SM specimens of active RA patients. Because of limited amounts of material, sufficient numbers of Th cells after tissue dissociation were found in 5 out of 9 biopsies. We detected spontaneous IFN-γ–producing Th cells in all 5 samples. Of note, the majority of SM-derived IFN-γ+ Th cells exhibited a cytokine-induced phenotype as judged by lack of 4-1BB coexpression (Figure 6F,G). Activation with αCD3 plus αCD28 revealed that the cells had the capacity to express the molecule on TCR stimulation (Figure 6H).
Our findings argue for recent cytokine rather than antigen stimulation being responsible for induction of the majority of IFN-γ+ Th cells at the site of inflammation in RA.
Discussion
In this study, we have characterized a cytokine-driven, TCR-independent mode of induction of the Th1 cytokine IFN-γ in human effector-memory Th cells. Furthermore, we provide strong evidence that this mechanism is the basis for IFN-γ production by Th cells in chronically inflamed joints of patients with RA.
In resting as well as in recently activated human Th cells, cytokine-mediated IFN-γ induction required γc cytokine signaling in addition to the engagement of IL-12R and IL-18R; this is in contrast to murine Th1 cells in which synthesis of the Th1 cytokine can be induced by IL-12 and IL-18 alone.7 Consistent with the need for γc cytokines in the stimulation cocktail, we found complete abrogation of cytokine-induced IFN-γ induction after pharmacologic blockade of γc downstream JAK3. Using the p38-specific inhibitor SB202190, we further proved that p38 MAPK-signaling is indispensable for cytokine-induced IFN-γ synthesis in human Th cells, which is in agreement with reports on murine Th1 cells stimulated by IL-12 and IL-18.27 In human NK cells, IL-2–driven IFN-γ production is mediated by the MKK/Erk pathway,28 which also operates downstream of JAK3. The fact that we did not observe a reduction in cytokine-induced IFN-γ production upon MKK/Erk blockade argues for different signaling requirements in Th cells; this notion is supported by the fact that CD8+ T cells express IFN-γ after stimulation with IL-2 and IL-12 in a MKK/Erk-independent, but p38-dependent fashion.29 Because p38 can be activated as a consequence of both IL-18R and γc ligation,27,30 it is conceivable that converging signals synergistically activate p38, thus overcoming a threshold for IFN-γ induction in human Th cells.
Some reports suggested that human CD25++ regulatory T cells do not only inhibit proliferation but also suppress production of IFN-γ and other effector cytokines in responder Th cells after TCR stimulation.24,25 It is unclear, however, whether diminished cytokine production in these experimental systems is rather a secondary event resulting from suppression of proliferation and differentiation of naive cells, leaving the question of whether this equally applies to differentiated memory Th cells. Of note, we found cytokine-induced, but not TCR-induced, IFN-γ+ effector-memory Th cells to be sensitive to suppression by Tregs. Our observation suggests that, once being activated, Tregs have the potential to control a cytokine-induced inflammatory response even in an environment containing factors such as IL-6, IL-7, or IL-15 which were reported to abrogate suppression in context with TCR-dependent activation.31,32 At the same time, as cytokine-induced IFN-γ+ Th cells exhibit a highly differentiated memory-effector signature, our data highlight the fact that Treg-mediated suppression is not restricted to naive responder Th cells during primary activation. Because the suppressive mechanism of Tregs is still incompletely understood, we can only speculate that different IFN-γ expression kinetics or signaling pathways after TCR versus cytokine stimulation account for the differential sen-sitivity to Tregs.
IFN-γ synthesis in response to cytokine-stimulation is confined to IL-18Rα+ IL-12R+ effector-memory Th cells characterized by coexpression of functional receptors for γc cytokines. In addition, these cells lack CCR7 expression and are largely CCR5+, enabling their migration to inflamed tissues.26,33 So far, functional IL-12R expression among Th cells was exclusively attributed to recently activated Th1 cells.19 We demonstrate, however, that functional receptors for IL-12 are also expressed by resting peripheral IL-18Rα+ Th cells reflected by STAT4 phosphorylation on IL-12 stimulation. Whether the observed receptor expression pattern is stable over time in vivo after initial Th1 polarization or results from recurrent antigenic reactivation is currently under investigation.
Interestingly, we find that Th cells present at sites of chronic inflammation, such as SF from RA patients, also predominantly express IL-18Rα and CCR5+ and have up-regulated IL-12R.26 As, in addition, cytokines being essential for Th cell IFN-γ induction (eg, IL-15, IL-12, and IL-18) are present in high amounts in the RA joint,17,34,35 we hypothesize that this inflammatory environment may trigger IFN-γ+ Th cells in vivo.
We identified lack of 4-1BB expression as a unique and robust phenotypic feature of cytokine-induced compared with TCR-induced IFN-γ+ Th cells. This differential expression pattern allowed us to analyze the in vivo relevance of cytokine-dependent IFN-γ induction in Th cells directly in cellular infiltrates obtained from a site of chronic inflammation. Whereas systemic levels of IFN-γ in the RA joint may be small,36 analysis at the single-cell level revealed that a substantial fraction of Th cells from both SF and inflamed tissue specimens of RA patients with active synovitis spontaneously secreted IFN-γ ex vivo. Strikingly, almost the entire population of joint-derived IFN-γ+ Th cells in RA patients lacked 4-1BB expression, indicating that they had been induced by cytokines in a TCR-independent manner. The absence of 4-1BB was not the result of an intrinsic defect, for example, resulting from chronic activation of synovial Th cells leading to anergy,37 because the activation marker could be induced among the majority of synovial IFN-γ+ Th cells by TCR-dependent activation with immobilized αCD3 and αCD28 antibodies. Thus, we demonstrate here that a TCR-independent mechanism for IFN-γ induction in human Th cells operates in vivo at a site of chronic autoimmune inflammation.
Our results are in line with the concept that discrete stages of RA are dominated by different effector mechanisms: whereas disease initiation may depend on antigen-specific activation of T and B cells, chronic autoimmune inflammation may result from inflammatory cytokine networks that are maintained in the absence of antigenic triggers38,–40 ; within such a network, cytokine-activated Th cells could not only contribute to inflammation by IFN-γ production, as our data demonstrate, but also by stimulating TNF-α release from monocytes.41,42 Against this background, it is not surprising that in established RA Th cells specific for candidate autoantigens such as collagen type II, aggrecan or gp39 is rarely found.43,,,–47
The notion that the prototype Th1 cytokine IFN-γ, irrespective of the induction mode, contributes to human autoimmune inflammation is supported by clinical trials demonstrating clear therapeutic benefit upon neutralization of IFN-γ.48,,–51 Reciprocally, administration of recombinant IFN-γ to treat malignant or infectious diseases can result in manifestation or exacerbation of autoimmune symptoms,52 an effect that was also attributed to the IFN-γ–inducing factor IL-12.53 Consequences of IFN-γ release in the inflamed tissue include induction of other proinflammatory mediators, such as IL-6, IL-8, and IL-15, as was demonstrated in coculture experiments of synovial T cells with synovial fibroblasts from active RA patients.54
Although several rodent arthritis models substantiate the view that IFN-γ contributes to joint inflammation,55,,–58 IFN-γ–secreting Th cells may also serve regulatory functions by restriction of the primary expansion of Th1 cells and suppression of Th17 cell development.59,60 In IFN-γ knockout animals, a defect in the latter mechanism is assumed to give rise to pathogenic Th17 cells.61
Yet, the finding that human RA is associated with decreased frequencies of Th17 cells at the site of inflammation, at the same time confirming the predominance of Th1 cells in the synovium,5 casts general doubts on the value of animal models to explain the respective human disease. In this context, recent studies exemplified how the induction protocol in experimental autoimmunity can dramatically affect the quality of the inflammatory response: although in experimental autoimmune uveitis active immunization with a retinal antigen emulsified with complete Freund adjuvant gave rise to pathogenic Th17 cells, disease was mainly driven by IFN-γ–producing Th1 cells when these were directly transferred or in vivo induced by antigen-loaded dendritic cells.62,63 Together with data from experimental models for multiple sclerosis,64,65 these studies suggest that both cytokines obviously bear the potential to mediate autoimmune tissue injury depending on the experimental setting.
As for human RA, our results reveal how a potentially destructive immune response can even be triggered autoantigen-independently only by the inflammatory cytokine environment. The role of such a mechanism in protective immunity that might serve as an early line of defense in infections10,11 still needs to be addressed in humans.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Katharina Raba and Thoralf Kaiser for expert FACS sorting.
This work was supported in part by the Bundesministerium für Bildung und Forschung (BMBF, Bonn, Germany; 01GI9944/DRFZ C2.4) and the Deutsche Forschungsgemeinschaft (Bonn, Germany; SFB 650 TP 11 and SFB 633 TP 10). C.R. was supported by a European Molecular Biology Organization (EMBO) fellowship (Heidelberg, Germany). S.K. was supported in part by the European Sixth Framework Program (Brussels, Belgium; DC-Thera, Project No. 512074).
Authorship
Contribution: A.S. designed the research, performed experiments, analyzed results, made the figures, and wrote the paper; A.T. designed the research and wrote the paper; M.R. and P.W. performed experiments; U.W. analyzed data and wrote the paper; J.S., W.A.S., S.R., and A.K. analyzed data; and S.K. and C.R. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Andreas Thiel, Clinical Immunology Group, German Rheumatism Research Center, Charitéplatz 1, 10117 Berlin, Germany; e-mail: thiel@drfz.de.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal