Abstract
Neurotrophins (NTs) and their receptors play a key role in neurogenesis and survival. The TRK (tropomyosin-related kinase) receptor protein tyrosine kinases (TRKA, TRKB, TRKC) are high-affinity NT receptors that are expressed in a variety of human tissues. Their role in normal and malignant hematopoiesis is poorly understood. In a prospective study involving 94 adult patients we demonstrate for the first time cell-surface expression of the 3 TRKs and constitutive activation in blasts from patients with de novo or secondary acute leukemia. At least one TRK was expressed in 55% of the analyzed cases. We establish a clear correlation between the TRK expression pattern and FAB classification. Although only few point mutations were found in TRK sequences by reverse-transcriptase–polymerase chain reaction (RT-PCR), we observed coexpression of BDNF (ligand for TRKB) in more than 50% of TRKB+ cases (16/30). Activation of TRKA or TRKB by NGF and BDNF, respectively, efficiently rescued murine myeloid cells from irradiation-induced apoptosis. Coexpression of TRKB/BDNF or TRKA/NGF in murine hematopoietic cells induced leukemia. Moreover, activation of TRKs was important for survival of both human and murine leukemic cells. Our findings suggest that TRKs play an important role in leukemogenesis and may serve as a new drug target.
Introduction
Current concepts of leukemogenesis postulate a collaboration of “class I” mutations that result in, for example, constitutively activated protein-tyrosine kinases (PTKs) with “class II” mutations of transcription factors such as AML/Runx or ETS proteins. In this scenario, class I mutations (such as BCR/ABL and FLT3-ITD) promote proliferation but generally do not inhibit differentiation, whereas the reverse is true for class II mutations.1 Although the molecular analysis of patient samples has supported this concept in human acute myeloid leukemia (AML), there still remains a substantial proportion of patients in whom both types of mutations have not yet been demonstrated
There is growing evidence for involvement of multiple PTK oncogenes, their immediate downstream targets (eg, phosphatidylinositol 3-kinase = PI3K), or of proteins regulating their function in hematologic malignancies.2–5 The fact that cytogenetic remissions can be achieved in the majority of patients with chronic myeloid leukemia (CML) demonstrates a causal role of the BCR-ABL oncoprotein in this disease.6 Analysis of activated PTK is also of clinical relevance.7,8 At least one-third of AML patients carry mutated FLT3 alleles and have unfavorable prognosis.8,9 It is thus important to identify other PTKs that are activated in the remaining patients. Moreover, coactivation of receptor PTKs has been suggested to be important for tumor development and to affect the tumor cell response to targeted therapy.10 Oncogenic transformation by PTKs occurs in different ways,11 for example, by genomic rearrangements, such as chromosomal translocations, gain-of-function (GOF) mutations, PTK overexpression, or small deletions in receptor PTKs and cytoplasmic PTKs. Autocrine and/or paracrine loops have been suggested as important mechanisms for aberrant kinase activation in human solid tumors12 and leukemia,13 and may have therapeutic potential.14 However, few experimental studies convincingly demonstrate the oncogenic potential of autocrine/paracrine circuits of PTKs in animal models,12,15 and a prognostic role of autocrine loops in human leukemia has not been demonstrated.
The neurotrophins (NTs), which include nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), NT-3, NT-4, and NT-6, play a major role in neuronal survival. NTs are unique in that they use 2 different classes of receptors: the TRK (tropomyosin-related kinase) receptor protein tyrosine kinases (TRKA, TRKB, TRKC) and the low-affinity NGF receptor (LNGFR = p75NTR),16 a member of the tumor necrosis factor cytokine receptor family. The biologically active receptors for NGF, BDNF, and NT-3 are TRKA, TRKB, and TRKC, respectively. NT-3 can bind to all of the TRK receptors, and NT-4 binds preferentially to TRKB. NT binding to TRK receptors leads to dimerization of receptors and kinase activation. LNGFR may modulate the activity of this signaling complex but is not required for its function.16 Of note, human embryonic stem cell survival has recently been shown to be mediated through activation of TRK receptors by NTs.17 Members of the TRK family have been found in several nonneural cell types,18,19 and may also play a crucial role in initiation, progression, and metastasis of many tumors in humans (eg, neuroblastoma, medullary thyroid carcinoma and breast cancer).20–23 In addition, some data indicate relevance of TRK receptors as prognostic factors.20 For example, TRKB is associated with bad prognosis in Wilms' tumor.24 However, relatively little is known about the mechanisms of oncogenesis mediated by altered TRK signaling.25,26
Many PTK oncogenes are derived from genes (eg, Abl, FLT3, c-Kit, and PDGFR-β) normally involved in the regulation of hematopoiesis or hematopoietic cell function.2 TRK receptors and their respective ligands are also expressed at various stages of hematopoiesis.27,28 A role for neurotrophins in hematopoiesis has yet to be confirmed using conditional knockouts. Nevertheless, recent data suggested important functions of TRK signaling in hematopoiesis. TRKs promote proliferation and survival of lymphocytes and monocytes/macrophages.29 TRKB expression is greatest in precursor CD4−CD8− thymocytes and progressively declines throughout the T-cell differentiation pathway.30 Importantly, there is increasing evidence for involvement of TRK receptors in leukemogenesis. A cryptic translocation t(12;15) (p13;q25), which resulted in the chimeric transcript TEL-TrkC, was found in an AML patient.25,31 Furthermore, a deleted form of TRKA (ΔTrkA), in which 75 amino acids are lacking in the extracellular domain, was identified in another AML patient.32 In mouse models, we found that ΔTrkA is a very potent oncogene that transforms cells mainly via PI3K and mTOR.33 Another study revealed the induction of TRKA and a contribution of NGF to survival signaling in human cord blood cells transduced with retroviral vectors encoding the AML1-ETO oncogene.34 In addition, we had evidence to suggest that a cytoplasmically deleted form of LNGFR may contribute to leukemia in mice.35 Furthermore, we observed expression of LNGFR in patients with acute leukemia (AL), preferentially in common acute lymphoblastic leukemia (ALL).36 Taken together, these data suggest a previously underestimated role of NT signaling in leukemogenesis. However, with the exception of one report showing expression of TRKA mRNA in primary leukemic cells in 44% of AML patients,37 the expression pattern of other TRKs and NTs and their potential prognostic relevance have not been reported in primary leukemic cells.
Here, we screened AL patients for expression of TRKA, TRKB, and TRKC. A distinct expression pattern of these receptors in different leukemia subtypes as well as expression of NTs was observed. Coexpression of TRKB and BDNF was associated with poor outcome and induced leukemia in a murine model. Moreover, TRK signaling was important for maintenance of leukemic cells in vitro. This study expands current concepts of leukemogenesis and encourages further evaluation of NT receptor signaling as a drug target in leukemia therapy.
Methods
TRK and NT expression in leukemic blasts of AL patients
We studied tumor specimens from AL patients who had been diagnosed in the Hannover Medical School from 2004 to 2006. Blood and/or bone marrow (BM) samples from AL patients were collected at diagnosis after informed consent was obtained in accordance with the Declaration of Helsinki. Mononuclear cells from all samples studied were immediately isolated by centrifugation over Ficoll gradient and freshly used or stored at −180°C until further use. The following monoclonal antibodies were used: anti-TRKA (clone H10; Biodesign, Saco, ME), anti-TRKB (clone 75133; R&D, Minneapolis, MN), anti-TRKC (clone 75219; R&D), anti-BDNF (CALBIOCHEM, San Diego, CA), and anti–NT-3 (clone 41512; R&D). A polyclonal antibody against human NGF (R&D) was used for detection of NGF. Antibodies were validated on cell lines that expressed retrovirally encoded TRK receptors and NTs. The study was approved by the ethics committee of the Hannover Medical School. The majority of patients were treated according to previously described protocols.38 For surviving patients, the median follow-up period after diagnosis was 30 months. In all cases, cytomorphologic classification according to French-American-British (FAB) criteria was made on bone marrow and/or peripheral blood smears.
Retroviral vectors, vector production, retroviral transductions, in vivo tumorigenesis assays, and tumor phenotyping
A retroviral vector encoding full-length human TRKA was kindly provided by Dr Gary Reuther (University of South Florida, Tampa, FL).32 Plasmid SF91.IRES-EGFP.WPRE, which mediates efficient transgene expression in hematopoietic cells, has been described.33 The retroviral vector is referred to as SF91-IE. A polymerase chain reaction (PCR) fragment containing the cDNA of human TRKB or BDNF was generated and cloned into the NotI site before the IRES-EGFP cassette of SF91-IE. Resulting vectors encoding TRKB or BDNF were named SF91-TRKB and SF91-BDNF, respectively. Cell-free high-titer supernatants containing the ecotropic envelope protein were generated as described.33 Retroviral transductions, in vivo tumorigenesis assays, and tumor phenotyping were performed as previously described (Document S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).33
Radiation-induced apoptosis assay and leukemic cell growth assays
32D cells (2.5 × 105) were starved from IL-3 and serum for 3 hours, placed in 24-well plates, and exposed to 5 Gy γ-irradiation. Immediately after irradiation, cells were supplemented with NGF, BDNF (each 100 ng/mL), IL-3 (2 ng/mL), or no factor. Cell viability was analyzed using the annexin-V assay. Cells staining negative for both annexin-V and propidium iodide were counted as viable cells. To analyze clonal growth, 2 × 102 or 2 × 103 murine cells were plated per dish in M3234 media (StemCell Technologies, Vancouver, BC) in the presence of signal transduction inhibitors. The assays were plated as duplicates or quadruplicates, and colonies were counted on day 6. Mononuclear cells from patients were cultured in RPMI 1640 supplemented with 10% fetal calf serum (FCS) and exposed to inhibitors and/or idarubicin.39 Inhibitors K252a, AG879 (Calbiochem) and anti–human BDNF antibody (Promega, Mannheim, Germany) were used.
Western blot analysis
For signal transduction analysis, leukemic cell extracts were prepared following established protocols.33 Cell lysates were used as indicated in results. Antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
RNA extraction and reverse transcriptase–PCR, small interfering RNA–mediated knockdown, BDNF–enzyme-linked immunosorbent assay, and statistical analysis
Please refer to Document S1.
Results
Frequent expression of TRK receptors in leukemic blasts of AL patients
We analyzed expression of TRKs in AL patients. Ninety-four adult patients (42 female, 52 male) with a mean age of 54.3 years and diagnosis of primary or secondary AML (87%), ALL (12%), or acute undifferentiated leukemia (AUL) (1%) were enrolled after informed consent. The patients' clinical characteristics are summarized in Table 1. Expression of TRKA, TRKB, and TRKC was detected by flow cytometry using monoclonal antibodies. If more than 20% of leukemic blasts expressed at least one of the TRK receptors, cases were considered TRK+ (Figure 1A,B; Figure S1).42 Thus, 55% of the analyzed cases expressed at least one TRK receptor, without statistically meaningful differences in expression rates between AML (43/82) and ALL (8/11). We observed expression of TRKA on AML blasts, which is in agreement with a previous study that demonstrated expression of TRKA on the RNA level.37
Patient characteristics according to TRK expression
| Characteristic . | Total . | TRK−, % of total=column 2 . | TRK+, % of total=column 2 . | P, between TRK+ and TRK− . |
|---|---|---|---|---|
| Patients, no. (%) | 94 | 42 (45) | 52 (55) | |
| Age, y | ||||
| Mean | 54.3 | 52.9 | 55.5 | .41 |
| Range | 16-79 | 20-77 | 16-79 | |
| Sex, no. | .17 | |||
| Male | 52 | 27 | 25 | |
| Female | 42 | 15 | 27 | |
| Leukemia, no. (%) | .358 | |||
| AML | 82 | 39 (48) | 43 (52) | |
| ALL | 11 | 3 (27) | 8 (73) | |
| AUL | 1 | 0 | 1 | |
| Diagnosis (AML), no. (%) | .057 | |||
| De novo | 58 | 32 (55) | 26 (45) | |
| Post-MDS/secondary | 24 | 7 (29) | 17 (71) | |
| FAB subtype AML | .003 | |||
| M0 | 3 | 3 | 0 | |
| M1 | 25 | 16 | 9 | |
| M2 | 17 | 10 | 7 | |
| M3 | 1 | 1 | 0 | |
| M4 | 21 | 9 | 12 | |
| M5 | 13 | 0 | 13 | |
| M6 | 1 | 0 | 1 | |
| M7 | 1 | 0 | 1 | |
| % Blasts | ||||
| Bone marrow | 73 | 76 | 70 | .280 |
| Peripheral blood | 44 | 49 | 39 | .173 |
| WBC counts, ×109/L | ||||
| Mean | 24.4 | 61.0 | .113 (cutoff: 20.000)40 | |
| Range | 0.8-159.0 | 0.6-454.0 | ||
| ECOG status | .764 | |||
| 0 | 22 | 10 | 12 | |
| 1 | 52 | 25 | 27 | |
| 2 | 13 | 7 | 6 | |
| 3 | 1 | 0 | 1 | |
| Cytogenetics, no. (%) | .481 | |||
| Normal karyotype | 39 | 17 (44) | 22 (56) | |
| t(8;21) (q22;q22) | 1 | 1 | 0 | |
| inv(16) (p13q12) | 1 | 1 | 0 | |
| t(15:17)(q22;q11-21) | 1 | 1 | 0 | |
| t(11q23) | 2 | 1 | 1 | |
| Complex karyotype* | 21 | 11 | 10 | |
| Other aberrations | 24 | 8 | 16 | |
| FLT3-ITD−, no. (%) | 50 | 26 (52) | 24 (48) | .363 |
| FLT3-ITD+, no. (%) | 17 | 6 (35) | 11 (65) | |
| Missing | 17 (18) |
| Characteristic . | Total . | TRK−, % of total=column 2 . | TRK+, % of total=column 2 . | P, between TRK+ and TRK− . |
|---|---|---|---|---|
| Patients, no. (%) | 94 | 42 (45) | 52 (55) | |
| Age, y | ||||
| Mean | 54.3 | 52.9 | 55.5 | .41 |
| Range | 16-79 | 20-77 | 16-79 | |
| Sex, no. | .17 | |||
| Male | 52 | 27 | 25 | |
| Female | 42 | 15 | 27 | |
| Leukemia, no. (%) | .358 | |||
| AML | 82 | 39 (48) | 43 (52) | |
| ALL | 11 | 3 (27) | 8 (73) | |
| AUL | 1 | 0 | 1 | |
| Diagnosis (AML), no. (%) | .057 | |||
| De novo | 58 | 32 (55) | 26 (45) | |
| Post-MDS/secondary | 24 | 7 (29) | 17 (71) | |
| FAB subtype AML | .003 | |||
| M0 | 3 | 3 | 0 | |
| M1 | 25 | 16 | 9 | |
| M2 | 17 | 10 | 7 | |
| M3 | 1 | 1 | 0 | |
| M4 | 21 | 9 | 12 | |
| M5 | 13 | 0 | 13 | |
| M6 | 1 | 0 | 1 | |
| M7 | 1 | 0 | 1 | |
| % Blasts | ||||
| Bone marrow | 73 | 76 | 70 | .280 |
| Peripheral blood | 44 | 49 | 39 | .173 |
| WBC counts, ×109/L | ||||
| Mean | 24.4 | 61.0 | .113 (cutoff: 20.000)40 | |
| Range | 0.8-159.0 | 0.6-454.0 | ||
| ECOG status | .764 | |||
| 0 | 22 | 10 | 12 | |
| 1 | 52 | 25 | 27 | |
| 2 | 13 | 7 | 6 | |
| 3 | 1 | 0 | 1 | |
| Cytogenetics, no. (%) | .481 | |||
| Normal karyotype | 39 | 17 (44) | 22 (56) | |
| t(8;21) (q22;q22) | 1 | 1 | 0 | |
| inv(16) (p13q12) | 1 | 1 | 0 | |
| t(15:17)(q22;q11-21) | 1 | 1 | 0 | |
| t(11q23) | 2 | 1 | 1 | |
| Complex karyotype* | 21 | 11 | 10 | |
| Other aberrations | 24 | 8 | 16 | |
| FLT3-ITD−, no. (%) | 50 | 26 (52) | 24 (48) | .363 |
| FLT3-ITD+, no. (%) | 17 | 6 (35) | 11 (65) | |
| Missing | 17 (18) |
Complex karyotype was defined as 3 or more cytogenetic abnormalities in the absence of t(8;21), inv(16), t(15;17), or t(11q23).41
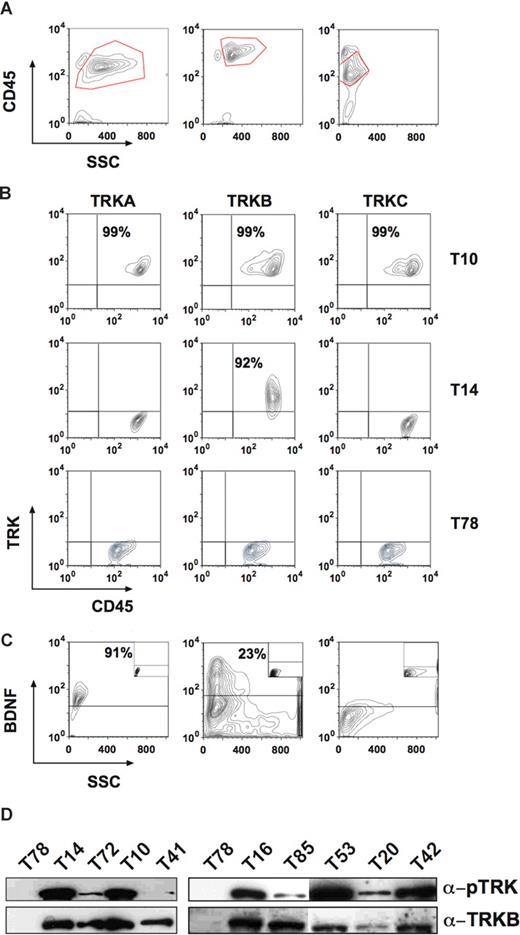
Expression of TRK receptors and NTs in patients with AL. (A) Showing the gate for the blast population. Left panel: patient T10 (AML M5). Middle panel: T14 (T-cell ALL). Right panel: patient T78 (AML M1). (B) Top row showing expression of all 3 TRK receptors in the blasts of patient T10. Middle row: patient T14 expressed only TRKB. Bottom row: patient T78 was negative for TRK receptors. (C) BDNF was also expressed in patients T10 (left panel) and T14 (middle panel), but not in patient T78 (right panel). The isotype controls are shown as insets. (D) Constitutive phosphorylation of p145TRK in primary leukemic cells. Total cell lysates were blotted and probed with an anti-pTRK (E-6) antibody detecting phosphorylated forms of all 3 TRK receptors. The blot was stripped and reprobed with the anti-TRKB (794) antibody. Please see flow cytometry diagrams in Figures S1 and S3 showing expression of TRKs and BDNF in the blasts of the patients not shown here.
Expression of TRK receptors and NTs in patients with AL. (A) Showing the gate for the blast population. Left panel: patient T10 (AML M5). Middle panel: T14 (T-cell ALL). Right panel: patient T78 (AML M1). (B) Top row showing expression of all 3 TRK receptors in the blasts of patient T10. Middle row: patient T14 expressed only TRKB. Bottom row: patient T78 was negative for TRK receptors. (C) BDNF was also expressed in patients T10 (left panel) and T14 (middle panel), but not in patient T78 (right panel). The isotype controls are shown as insets. (D) Constitutive phosphorylation of p145TRK in primary leukemic cells. Total cell lysates were blotted and probed with an anti-pTRK (E-6) antibody detecting phosphorylated forms of all 3 TRK receptors. The blot was stripped and reprobed with the anti-TRKB (794) antibody. Please see flow cytometry diagrams in Figures S1 and S3 showing expression of TRKs and BDNF in the blasts of the patients not shown here.
For the first time, we found expression of TRKB and TRKC in human leukemia. Interestingly, whereas TRKB can be expressed alone in blasts, TRKA or TRKC expression always occurred concomitantly with TRKB. About 18% of leukemia cases coexpressed all TRK receptors. Coexpression of 2 or more TRK receptors (ie, TRKA + TRKB, TRKB + TRKC and TRKA + TRKB + TRKC) was observed in AML, whereas ALL blasts exclusively expressed only TRKB. Although TRK mRNA was found in hematopoietic cells from healthy volunteers,27,37 we did not detect TRK receptors on the surface of normal mononuclear cells by flow cytometry (data not shown), which is in agreement with previous studies.43
Correlation of TRK expression and FAB leukemia classification
Next, we analyzed the relationship between patient age, FAB subtype, white blood cell (WBC) counts, ECOG status, cytogenetics, FLT3 mutation, and TRK expression.40,41 In agreement with recent publications,8 we found internal tandem duplications of the juxtamembrane region of the FLT3 receptor (FLT3-ITD) in 25% (17/67) of AML patients. There was no significant correlation of patient age, WBC counts, ECOG status, FLT3-ITD, or cytogenetics with TRK expression (Table 1). However, in contrast to a previous study,37 we established a clear correlation of TRK expression pattern and the FAB classification (Table 1). In particular, TRKA was expressed in 21 (62%) of 34 myelomonocytic/monocytic leukemias (AML M4 and M5), whereas only 5 (10%) of 48 nonmyelomonocytic/monocytic leukemias were positive (P < .001). The same observation was made for TRKB and TRKC (71% vs 38% [P < .005] and 47% vs 8% [P < .001], respectively). Coexpression of at least 2 of the 3 TRK receptors was observed in 62% (21/34) of patients with AML M4 and M5, but in only 13% (6/48) of other subtypes (P < .001). The latter cases were often secondary AML after a myelodysplastic syndrome (MDS). Thus, our data show that TRK expression in AML is closely linked to monocytic differentiation (AML M4 and M5), unless the AL developed on the basis of MDS. Corroboratively, we found expression of TRKA in more than 98% of THP1 cells (a commonly used human monoblastic leukemia cell line) by flow cytometry (data not shown).
Expression of NTs in leukemia and constitutive activation of TRK in leukemic cells
To assess potential mutations and deletions, we sequenced the second Ig-like domain, transmembrane domain, and whole intracellular domain (focusing on the kinase domain) of TRKA, TRKB, and TRKC receptors following reverse-transcriptase (RT)–PCR of RNA isolated from leukemic cells. Mutations, small deletions, and duplications in these regions have been reported as potential mechanisms for constitutive activation of TRKs.32,44 RT-PCR and direct sequencing of PCR fragments were successfully performed in 98% and 97% of patients, respectively. Unexpectedly, only 4 different point mutations (TRKB: T573I, Y707N, and V684I; TRKC: Y800H) were found in 4 patients by RT-PCR. We did not observe deletions or duplications in TRKs. As oncogenic TRKA was originally cloned in a patient with colon carcinoma as a TPM3/TRK transcript caused by translocation within chromosome 1,45 we also searched for the TPM3/TRK translocation in our patients, yet without success (n = 32).
Therefore, we investigated other major mechanisms by which TRK expression could contribute to transformation or differentiation. Autocrine and/or paracrine loop has been suggested as an important mechanism of PTK activation in human cancers.12,13 Elevated expression of TRKB and/or BDNF has also been reported to occur frequently in multiple myeloma (24 and 12 of 25 cases studied, respectively), promoting myeloma cell survival.46 Thus, we next investigated expression of NTs in blasts from patients with AL. We chose flow cytometry to detect intracellular expression of NGF, BDNF, or NT-3. Coexpression of BDNF (primary ligand for TRKB) was observed in more than half of TRKB+ cases (53.3%, 16/30) (Figure 1C; Figure S1). Moreover, we found expression of NGF or NT-3 in 2 patients expressing TRKs (Figure S2). Importantly, we observed constitutive phosphorylation of TRKs in all analyzed primary leukemia samples (n = 13, Figure 1D), suggesting a role of TRK signaling in leukemic transformation.
Coexpression of TRKB and BDNF is associated with poor survival
The good response rate on days 15 and 21 after induction therapy was not significantly different according to TRK expression (62% vs 81% for TRK− and TRK+, respectively, P = .16). However, we found that expression of TRKs and BDNF was associated with bad prognosis (Table 2). Patients whose blasts express TRKA had a shorter median survival compared with patients whose blasts do not (312 vs 547 days; hazard ratio, 1.44; P = .23). Interestingly, in agreement with other reports,47 it seems that expression of LNGFR is associated with better prognosis (Figure S3). These differences may reach statistical significance if investigated in larger cohorts.
Univariate Cox regression analysis for TRKs and BDNF
| . | Hazard ratio (95% CI) . | P . | Median survival, d . | |
|---|---|---|---|---|
| Receptor positive . | Receptor negative . | |||
| TRKA | 1.44 (0.80-2.62) | .23 | 312 | 547 |
| TRKB | 1.17 (0.66-2.07) | .60 | 359 | 547 |
| TRKC | 1.32 (0.70-2.50) | .40 | 359 | 535 |
| BDNF | 1.38 (0.68-2.82) | .37 | 225 | 480 |
| TRKB / BDNF | 2.26 (1.07-4.80) | .03 | 212 | 547 |
| . | Hazard ratio (95% CI) . | P . | Median survival, d . | |
|---|---|---|---|---|
| Receptor positive . | Receptor negative . | |||
| TRKA | 1.44 (0.80-2.62) | .23 | 312 | 547 |
| TRKB | 1.17 (0.66-2.07) | .60 | 359 | 547 |
| TRKC | 1.32 (0.70-2.50) | .40 | 359 | 535 |
| BDNF | 1.38 (0.68-2.82) | .37 | 225 | 480 |
| TRKB / BDNF | 2.26 (1.07-4.80) | .03 | 212 | 547 |
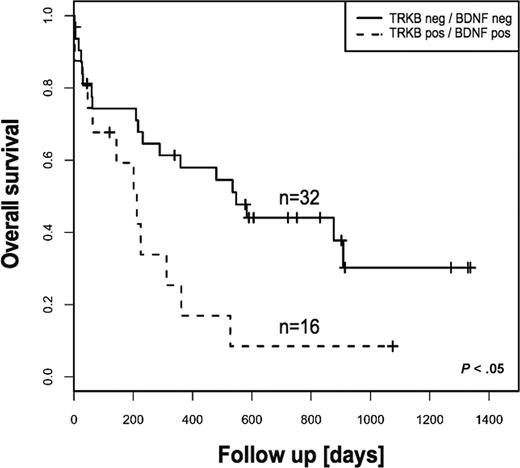
The survival difference was even more pronounced for patients whose blasts coexpress TRKB and BDNF (Table 2). These patients had significantly shorter overall survival compared with patients whose blasts express neither TRKB nor BDNF (8% vs 30% at 3 years, respectively, P < .05) (Figure 2). However, event-free survival displayed no statistical difference. Importantly, there was no significant difference regarding incidence of FLT3-ITD in both groups of patients. Although this finding is reminiscent of recent data demonstrating association of the TRKB/BDNF autocrine survival pathway with poor outcome in human solid tumors,24 we are not aware of a previous report demonstrating a prognostic relevance of an autocrine or paracrine loop in AL.
Kaplan-Meier estimates of overall survival for patients with AML according to TRKs and BDNF status. Coexpression of TRKB and BDNF was associated with statistically significant poor outcome. The log-rank test was used to compare differences between survival curves.
Kaplan-Meier estimates of overall survival for patients with AML according to TRKs and BDNF status. Coexpression of TRKB and BDNF was associated with statistically significant poor outcome. The log-rank test was used to compare differences between survival curves.
Activation of TRK signaling protects myeloid cells from apoptosis and supports proliferation
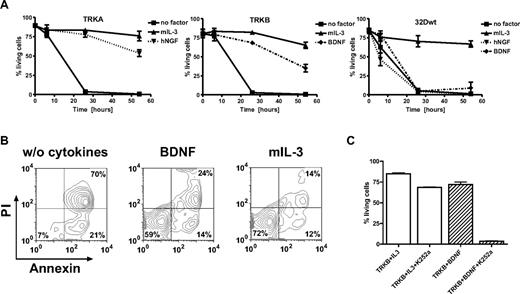
On cell types other than neuronal cells including mast cells, keratinocytes, monocytes and B cells, TRK signals potentially stimulate cell proliferation through antiapoptotic effects.18,48 To examine the role of TRKs in the regulation of apoptosis in myeloid cells, we used 32D cells transduced with retroviral vectors expressing TRKA or TRKB.33 We tested whether stimulation of 32D/TRKA cells by NGF could rescue cells from irradiation-induced apoptosis. 32D/TRKA cells were highly sensitive to irradiation, with less than 10% viable cells 26 hours after irradiation. Upon NGF exposure, up to 88% of 32D/TRKA cells escaped apoptosis (Figure 3A). Similar data were obtained with 32D cells expressing TRKB after stimulation with BDNF (Figure 3A,B). For yet unknown reasons, activation of TRKB was not as potent as TRKA in protecting the cells from apoptosis at a later time point (54 hours).Exposure to NGF or BDNF rescued less than 8% of nontransduced 32D cells from apoptosis (Figure 3A). Importantly, treatment of 32D TRKB cells with K252a (a widely used TRK inhibitor17,49,50 ) significantly counteracted the antiapoptotic effect of BDNF activation (Figure 3C), suggesting that kinase activity of TRKs is absolutely required for their antiapoptotic function. Moreover, activation of TRKA by NGF or TRKB by BDNF also supported proliferation of 32D cells in liquid culture (> 1 month) and methylcellulose in the absence of IL-3 (data not shown).
Ligand-dependent resistance to radiation-induced apoptosis. (A) 32D cells expressing TRKA, TRKB, and 32D wild-type cells. Cells were starved for 3 hours and exposed to 5 Gy irradiation. Cells that were annexin-V and propidium iodide (PI) negative were counted as viable cells. Viability was calculated as the percentage of these cells over the total cell population. (B) BDNF prevented apoptosis of 32D cells expressing TRKB almost as efficiently as IL-3. Cells were analyzed by flow cytometry 26 hours after irradiation. Combined annexin-V and PI staining was used to distinguish early apoptotic (annexin-V+/PI−) and later apoptotic cells (annexin-V+/PI+). (C) K252a dramatically inhibited antiapoptotic effects of BDNF-mediated activation of TRKB, whereas only slight inhibition was observed if cells were cultured in the presence of IL-3 alone. Results presented are the mean plus or minus SD of at least 2 independent experiments. NGF/BDNF concentration was 100 ng/mL, murine IL-3 2 ng/mL.
Ligand-dependent resistance to radiation-induced apoptosis. (A) 32D cells expressing TRKA, TRKB, and 32D wild-type cells. Cells were starved for 3 hours and exposed to 5 Gy irradiation. Cells that were annexin-V and propidium iodide (PI) negative were counted as viable cells. Viability was calculated as the percentage of these cells over the total cell population. (B) BDNF prevented apoptosis of 32D cells expressing TRKB almost as efficiently as IL-3. Cells were analyzed by flow cytometry 26 hours after irradiation. Combined annexin-V and PI staining was used to distinguish early apoptotic (annexin-V+/PI−) and later apoptotic cells (annexin-V+/PI+). (C) K252a dramatically inhibited antiapoptotic effects of BDNF-mediated activation of TRKB, whereas only slight inhibition was observed if cells were cultured in the presence of IL-3 alone. Results presented are the mean plus or minus SD of at least 2 independent experiments. NGF/BDNF concentration was 100 ng/mL, murine IL-3 2 ng/mL.
Coexpression of TRKA/NGF and TRKB/BDNF efficiently transforms hematopoietic cells and induces leukemia in mouse models
To address the in vivo leukemogenic potential of autocrine activation of TRKA and TRKB, we first used a model based on 32D cells as previously described.33 Retroviral vector–mediated expression of NGF in 32D/TRKA cells caused growth-factor independence in vitro. Even without prior selection for growth-factor independence in vitro, retroviral vector–mediated coexpression of TRKA and NGF or TRKB and BDNF in 32D cells elicited a fatal AML in all syngeneic C3H/Hej recipients (n = 4/4 for TRKA/NGF, n = 2/2 for TRKB/BDNF). In contrast, only 4 of 26 animals that received a transplant of cells expressing TRKA alone developed leukemia, and none of the animals that received a transplant of cells expressing TRKB or NGF or BDNF alone (n = 6) developed donor cell–derived leukemia. Interestingly, in agreement with the less potent antiapoptotic effect of TRKB observed in 32D cells, transformation induced by autocrine activation of TRKB required a longer latency in comparison with TRKA/NGF (13 vs 3.5 weeks). Importantly, whereas only 14% of cells were positive for TRKB/BDNF on the day of transplantation (Figure 4A), the majority of leukemic cells recovered from animals with overt disease coexpressed both vectors (Figure 4B), strongly suggesting selection for leukemic transformation by autocrine activation. The developing leukemias led to elevated WBC counts (Figure 4C), splenomegaly, and hepatomegaly. Cytology and histopathology revealed extensive infiltration of blasts in the BM, spleen, and liver (Figure 4D-H), and in some cases also in the lung and kidney (data not shown). As in the patient samples, constitutive activation of TRKB was observed, suggesting a crucial contribution of TRK signaling to leukemogenesis (Figure 4I).
Development of leukemia in mouse no. 681 that received a transplant of TRKB- and BDNF-modified hematopoietic cells (32D). FACS analysis showing coexpression of TRKB and BDNF before transplantation (A) and in myeloid blasts recovered from the animal (B). (C) Peripheral blood smear showing marked leukocytosis consisting predominantly of immature myeloid cells. (D) Cytospin of BM showing myeloblasts with an abundant cytoplasm (×1000). (E,F) Diffuse myeloblastic infiltration in spleen and complete effacement of its normal structure (×100, ×1000). (G,H) Extensive infiltration of leukemic cells in liver, primarily in portal areas, but also diffusely in the sinusoid (×100, ×1000). (I) Constitutive activation of TRKB in leukemic cells and blocking of the TRKB/BDNF autocrine loop by anti–BDNF-neutralizingantibody and K252a. Leukemic cells were treated with either 20 μg/mL anti-BDNF antibody or 100 nM K252a for 12 hours. Total cell lysates (300 μg) from untreated and treated cells were immunoprecipitated with the anti-TRK (C-14), separated on SDS–polyacrylamide gel electrophoresis (PAGE), blotted, and probed with an anti-pTRK (E-6) antibody (top and middle panels with short and long exposition, respectively). The blot was stripped and reprobed with the anti-TRKB (H181) antibody. Arrows point to the phosphorylated forms of TRK. Note different expression pattern of human TRKB in human cells (only 145-kDa form in Figure 1) and rodent cells (both 145-kDa and 120-kDA forms) as previously described.51 (J) Antihuman BDNF antibody (10 μg/mL and 20 μg/mL) inhibited growth of leukemic cells in colony-forming assays. Results are presented as the average percentage of colonies formed in the presence of antibody (100% value derived from untreated control). Results presented are the mean plus or minus SD of 2 independent experiments.
Development of leukemia in mouse no. 681 that received a transplant of TRKB- and BDNF-modified hematopoietic cells (32D). FACS analysis showing coexpression of TRKB and BDNF before transplantation (A) and in myeloid blasts recovered from the animal (B). (C) Peripheral blood smear showing marked leukocytosis consisting predominantly of immature myeloid cells. (D) Cytospin of BM showing myeloblasts with an abundant cytoplasm (×1000). (E,F) Diffuse myeloblastic infiltration in spleen and complete effacement of its normal structure (×100, ×1000). (G,H) Extensive infiltration of leukemic cells in liver, primarily in portal areas, but also diffusely in the sinusoid (×100, ×1000). (I) Constitutive activation of TRKB in leukemic cells and blocking of the TRKB/BDNF autocrine loop by anti–BDNF-neutralizingantibody and K252a. Leukemic cells were treated with either 20 μg/mL anti-BDNF antibody or 100 nM K252a for 12 hours. Total cell lysates (300 μg) from untreated and treated cells were immunoprecipitated with the anti-TRK (C-14), separated on SDS–polyacrylamide gel electrophoresis (PAGE), blotted, and probed with an anti-pTRK (E-6) antibody (top and middle panels with short and long exposition, respectively). The blot was stripped and reprobed with the anti-TRKB (H181) antibody. Arrows point to the phosphorylated forms of TRK. Note different expression pattern of human TRKB in human cells (only 145-kDa form in Figure 1) and rodent cells (both 145-kDa and 120-kDA forms) as previously described.51 (J) Antihuman BDNF antibody (10 μg/mL and 20 μg/mL) inhibited growth of leukemic cells in colony-forming assays. Results are presented as the average percentage of colonies formed in the presence of antibody (100% value derived from untreated control). Results presented are the mean plus or minus SD of 2 independent experiments.
We next assessed the ability of autocrine activation of TRKB to transform primary murine hematopoietic cells. Hematopoietic stem/progenitor cell–enriched lineage-negative (Lin−) BM cells were transduced with retroviral vectors expressing TRKB (SF91-TRKB), BDNF (SF91-BDNF), or both. Three of 6 animals coexpressing TRKB and BDNF developed lymphoblastic leukemia within 14 weeks after transplantation (Figure S4). The other 3 remained healthy for another 5 months, but had no or less than 5% transgene expression. All animals (n = 6) transduced with TRKB or BDNF alone showed normal hematopoiesis. Similarly, we observed development of myeloid leukemia in 1 of 3 animals that received a transplant of Lin− cells coexpressing TRKA and NGF (data not shown). Thus, in agreement with our clinical observation, activation of TRKs induced both myeloid and lymphoblastic leukemia in our mouse models. Moreover, our data (patients and mouse) support the view that LNGFR is not absolutely required for TRK-mediated responses.52
Autocrine loop TRKB/BDNF is a survival factor for murine leukemic cells
Leukemic cells from mouse no. 681 (Figure 4) grew factor-independently. Importantly, supernatant collected from no. 681's cells supported growth of 32D cells expressing TRKB without cytokine supplementation. BDNF was measured to be more than 300 pg/mL by enzyme-linked immunosorbent assay (ELISA; in comparison, concentration of BDNF in serum of human healthy control was 27 pg/mL46 ) and no membrane-bound BDNF was found by fluorescence-activated cell sorting (FACS) analysis (data not shown). This strongly suggests existence of a TRKB/BDNF autocrine loop in no. 681's cells.
We examined the ability of a neutralizing antibody to BDNF, or the TRK inhibitor K252a, to interfere with the autocrine activation loop. In the absence of treatment, autophosphorylation of TRKB was detected in leukemic cells isolated from mouse no. 681, suggesting that the autocrine loop is constitutively active. Both anti–BDNF-neutralizing antibody and K252a dramatically blocked phosphorylation of TRKB (Figure 4I). If leukemia development is mediated through activation of TRKs by NT, then pharmacological inhibition of TRK signaling or blocking the action of NT should reduce leukemia cell survival. To test this hypothesis, survival of leukemic cells isolated from mouse no. 681 was measured by colony formation in the presence or absence of BDNF-neutralizing antibody and K252a.17 Anti–human BDNF antibody showed a dose-dependent effect on leukemic cell survival (Figure 4J). K252a induced up to 50% growth inhibition of leukemic cells (data not shown). In contrast, addition of either the anti–BDNF-neutralizing antibody (Figure 4J) or K252a did not alter the survival of control cells. Further, we generated 2 lentiviral vectors expressing small interfering RNAs (siRNAs) targeted against different regions on the TRKB mRNA sequence.53 Compared with control vector–transduced cells, siRNA expression reduced colony formation up to 13-fold, and induced cell death in the absence of IL-3 (data not shown). Moreover, leukemic cells from mice coexpressing TRKA/NGF were very sensitive to treatment of K252a and AG879, a TRKA inhibitor (Figure S5). Collectively, these data reveal that an autocrine loop involving TRK receptors is a major survival factor for leukemic cells in the murine model.
TRK signaling is important for survival of human AML cells
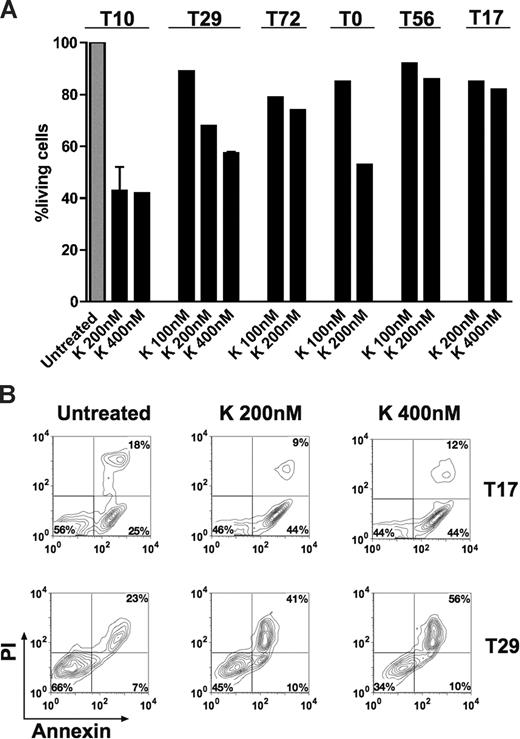
We evaluated apoptosis in cultured leukemic cells obtained from 4 patients with AML. Cells were cultured in RPMI 1640 (10% FCS) and exposed to the TRK inhibitor K252a (100-400 nM) for approximately 18 hours. We found that K252a induced apoptosis over basal levels in all cases and led to up to 65% reduction of living cells by FACS analysis (Figure 5A,B), whereas K252a had minimal effect on leukemic cells from patients T17 and T56 not expressing TRKs (Figure 5A,B). Moreover, we also observed dephosphorylation of TRK proteins after treatment (data not shown), suggesting that the apoptotic effect of K252a was mainly due to inhibition of TRK signaling. In patient T72 (coexpressing TRKB/BDNF only), a moderate reduction of living cells was observed upon anti-BDNF antibody treatment alone or in combination with K252a (Figure S6). This indicates that survival activity of a portion of leukemic cells is indeed due to an autocrine loop of TRKB/BDNF. However, no or less apoptotic effect of anti-BDNF antibody was observed in cells from patients T0 (negative for BDNF) and T10 (expressing TRKs and BDNF; Figure S6). In the latter case, it is possible that the potential effect of the anti-BDNF antibody was compensated by signaling driven from TRKA and/or TRKC. K252a also enhanced idarubicin-induced apoptosis in leukemic cells from patients T72 and T0 (data not shown). However, cells from patients with ALL (T14 and T20) were resistant or less sensitive to K252a treatment compared with AML patients (data not shown), suggesting different sensitivities to TRK signal transduction inhibition among patients. Generally, the growth inhibition effects of K252a and anti-BDNF antibody we observed in human leukemic cells (Figure 5; Figure S6) are less strong than in murine leukemic cells (Figure 4), reflecting the more complex leukemogenesis in humans. However, our findings collectively suggest that TRK signaling is involved in survival of human blasts, particularly in AML.
K252a induces apoptosis of primary AML cells. Cell viability was analyzed using the annexin-V assay. Results are presented as the percentage of living cells in the presence of K252a (100% value derived from untreated control). (A) Cells were from patient T72 expressing TRKB and BDNF, both patients T10 and T29 expressing TRKs (TRKA, TRKB, and TRKC) and BDNF, and T0 (only TRKs). Patients T17 and T56 were negative for TRKs and NTs. Results presented for T10 (K 200 nM) and T29 (K 400 nM) are the mean plus or minus SD of 2 independent experiments. Note that treatment schedule was not completely applied to all patients tested due to limited number of cells. The concentrations of K252a that we used have been well documented for TRK inhibition in the literature.17,49,50 K indicates K252a. (B) Flow cytometric diagram of apoptosis of blasts from patient T17 and T29, cultured with K252a for 18 hours before analysis.
K252a induces apoptosis of primary AML cells. Cell viability was analyzed using the annexin-V assay. Results are presented as the percentage of living cells in the presence of K252a (100% value derived from untreated control). (A) Cells were from patient T72 expressing TRKB and BDNF, both patients T10 and T29 expressing TRKs (TRKA, TRKB, and TRKC) and BDNF, and T0 (only TRKs). Patients T17 and T56 were negative for TRKs and NTs. Results presented for T10 (K 200 nM) and T29 (K 400 nM) are the mean plus or minus SD of 2 independent experiments. Note that treatment schedule was not completely applied to all patients tested due to limited number of cells. The concentrations of K252a that we used have been well documented for TRK inhibition in the literature.17,49,50 K indicates K252a. (B) Flow cytometric diagram of apoptosis of blasts from patient T17 and T29, cultured with K252a for 18 hours before analysis.
Discussion
NTs and their receptors regulate proliferation, differentiation, and death of normal and neoplastic neuronal cells and also have been implicated in the formation of various human cancers.16,24 Although expression of TRKs has been shown in different stages of hematopoiesis, their functional role has remained largely unclear. By RT-PCR, expression of TRKs and NTs in adult human BM cells was much lower than in fetal BM cells, suggesting down-regulation in adult hematopoiesis.27 Consistently, we and others did not observe surface expression of TRKs in normal mononuclear cells.43 In contrast, expression of TRKA has been shown in different leukemia cell lines.43 Multiple myeloma cells expressed TRKB, and responded to BDNF by activating MAPK and PI3K/Akt signaling cascades.46 So far only one group analyzed expression of TRK in human AML, detecting TRKA mRNA in leukemic cells of less than half of the patients tested.37 However, expression on the protein level and a potential correlation with leukemia subtypes or prognosis were not assessed. Using stringent criteria, our study provides direct evidence for frequent and high expression of TRK receptors in human AL. As the TRK expression pattern depended not only on the leukemia subtype but also was correlated with prognosis, we hypothesize that this receptor system may play a pathogenetic role in human leukemia. The mechanisms underlying aberrant expression of TRKs and BDNF in leukemic cells are unknown. By real-time RT-PCR, we observed up-regulation of TRKA and BDNF but not TRKB and TRKC in analyzed samples (n = 4, data not shown), suggesting that posttranscriptional events may contribute the expression of TRKB and TRKC.
One characteristic finding in the present study is that TRK expression is associated with myelomonocytic and monocytic leukemia (Figure 1; Table 1). It is possible that the expression of different members of the TRK family simply reflects the cell of origin, without a significant role in tumor biology. However, although TRKA can be found in B lymphocytes,18 T lymphocytes,29 and monocytes/macrophages,48,54 we observed TRKA expression only in AML patients by flow cytometry. Therefore, it is more likely that differential expression of TRK receptors and activation of their respective signal transduction pathways directly affect the biologic behavior of the cells, which leads to differentiation, survival, and/or proliferation. For example, tumors with functional TRKB may be particularly aggressive because TRKB provides a growth advantage and may protect them from chemotherapy.24 In the present study, expression of TRKs was associated with shorter survival, particularly for patients coexpressing TRKB and BDNF. Our data thus support the concept that TRKB may play an important role in human tumorigenesis.24,51 In neuroblastoma, TRKB expression has been demonstrated preferentially in aggressive, MYCN-amplified tumors, and is associated with a poor prognosis. Activation of the BDNF/TRKB signaling pathway in neuroblastoma cells expressing TRKB increases cell survival,55 and may protect neuroblastoma cells from chemotherapy and thereby contribute to a more chemoresistant phenotype.56 In a recent functional genomic screen for genes that suppress anoikis, TRKB was identified as an oncoprotein associated with metastatic capacity.21 Overexpression of TRKB rendered nonmalignant epithelial cells anoikis (apoptosis) resistant and highly tumorigenic. Consistent with the model that suppression of anoikis facilitates metastasis, TRKB-expressing cells formed highly invasive and metastatic tumors in nude mice, with very short latencies.21 TRKB kinase activity is required and also sufficient for anoikis suppression, tumor formation, and experimental metastasis.51 Consistently, we found that TRKB kinase activity was absolutely required for its antiapoptotic effect (Figure 3D), potentially a major mediator for transformation induced by TRK signaling. We also demonstrated that targeting TRKs by TRK inhibitor or siRNA efficiently inhibited growth of leukemic cells in vitro (Figures 4,5). These results suggest that targeting the enzymatic activity of TRKB or its downstream effectors might be beneficial in therapy of both solid tumors and leukemia.
Autocrine circuits of tyrosine kinases such as FLT313 or KDR14 are potentially involved in human leukemia. Multiple myeloma cells were found to express TRKB, with potential survival signaling induced by autocrine BDNF.46 In all of these cases, a prognostic role remains to be determined. In addition, there are so far no animal studies that directly confirm their tumorigenic potential, although blocking autocrine loop function/activity has been shown to be important for treatment of leukemia in vitro and in animal models.14 Here, we found a high incidence of expression of TRKB/BDNF and its association with poor prognosis in our patient cohort (Figure 2). Our mouse models showed direct evidence for the leukemogenic potential of autocrine activation of TRKA/NGF and TRKB/BDNF (Figure 4). Consistent with recent publications showing less than 2% of point mutations in more than 90 tyrosine kinases (including TRKs) in more than 300 AML patients,57,58 we found very few point mutations in TRK receptors, indirectly supporting the hypothesis that autocrine activation represents a major mechanism for transformation by TRKs, and probably also for many other receptor PTKs. However, reflecting the finding that NGF and BDNF are also expressed by stromal cells in bone marrow, and the recent finding that TRK receptors can also be activated in the absence of NTs,49,59 we do not rule out the possibility of other mechanisms for activation of TRKs in leukemogenesis (eg, paracrine or intracrine mechanism). In fact, we also observed constitutive activation of TRKs in patients expressing TRKs only (T20, T42 in Figure 1). Interestingly, autocrine activation of TRK has also been reported in inflammatory cells including monocytes and has been suggested to be an important factor in the establishment of autoimmune diseases.48 Moreover, recent data demonstrate that NGF is an autocrine factor essential for the survival of macrophages infected with HIV. These cells take advantage of their autocrine NGF, survive for a very long period of time, and continuously produce virus particles. Thus, exploring the effects of inhibiting autocrine TRK signaling may open new therapeutic strategies for both leukemia and latent HIV infection.54
Development of AML is believed to require the cooperation of class I and class II mutations.1 Our finding that more than half of the patients investigated showed expression/mutations of TRKs reduces the proportion of patients in whom so far no activation of PTKs (class I) has been identified. Therapies targeting receptor PTKs have provided remarkable responses in both hematologic cancers and solid tumors, but their clinical efficacy has been limited in many cases, and few, if any, patients are cured. Recently, Stommel et al have identified obstacles to meaningful response to single-agent therapies targeting receptor PTKs using a glioblastoma model.10 They found 3 or more activated receptor PTKs in each tumor, and up to 10 activated receptor PTKs in some cases. Combinations of PTK inhibitors, but not single agents, inhibited PI3K signaling and related sequelae. Consistently, single-agent targeted therapies for AML patients with FLT3-ITD or c-Kit mutations showed only moderate efficacy in some cases.8 In the present study, we found a significant proportion of AML patients with both FLT3-ITD and TRK expression. We speculate that assessment of TRK expression and signaling in these patients, and combination of targeted therapies or multifunctional kinase inhibitors directed at activated receptor PTKs, may improve outcome.60
In summary, we demonstrate that primary AL cells frequently express TRK receptors and BDNF on the protein level. We found a significant correlation with TRK expression and morphologic subtypes as established in the FAB classification, and poor outcome in patients with coexpression of TRKB and BDNF. The leukemogenic activity of this autocrine loop was confirmed in mouse models. Moreover, activation of TRKs was an important survival factor for leukemic cells from both patients and mice. Collectively, our findings suggest that TRKs play an important role in leukemogenesis with autocrine stimulation as a potential mechanism for oncogenic activity. Thus, TRKs and their downstream signaling partners might serve as novel therapeutic targets in AL. To improve classification and treatment of hematologic malignancies, we would recommend the prospective assessment of NTs, their receptors, and downstream pathways in larger cohorts and additional malignancies of hematopoietic origin.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are very grateful to Stefan Bartels and Ludwig Hoy for help with statistical analysis; Axel Schambach for providing vector backbones; Michael Morgan for providing reagents and critical reading of this paper; Peter Horn and Martin Sauer for providing cells; Vanessa Prox, Christine Garen, Rene Kirstein, Ellen Neumann, Elke Stürmer, Elvira Lux, and Cindy Elfers for technical assistance; and Rolf Baumann, Hans Grundtke, Jörg Frühauf, Anne Koop, and Bernd Polivka (all MHH) for irradiation of animals and cells. We also thank Dr D. Martin-Zanca for providing cDNA of TPM3/TRK.
This study was supported by the Deutsche Krebshilfe (Bonn, Germany; grant: 10-2090-Li I) and by the Deutsche Forschungsgemeinschaft (DFG, Bonn, Germany; excellence cluster REBIRTH). C.B. was also supported by the National Cancer Institute (R01-CA107492-01A2). M.R. is a student of the MD/PhD program at Hannover Medical School (MHH), and received support from the Deutsche José Carreras Leukämie-Stiftung (München, Germany; grant: DJCLS F05/10). C.K. was supported by DFG (KO 3582/1-1).
National Institutes of Health
Authorship
Contribution: Z.L., J.M., A.G., and C.B. designed the study; Z.L. and C.B. interpreted the data and wrote the paper; Z.L., M.R., T.N., and M.Y. performed immunophenotyping, mutation analysis, colony assays, apoptosis assays, and animals studies; G.B., C.K., M.H., H.D., and Z.L. collected patients' data and samples; M.R. and J.M. contributed Western blot analysis; N.N., G.G., L.W., J.K., and B.S. performed cytogenetic and molecular genetic analysis and provided samples; and G.B. performed statistical analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Zhixiong Li or Christopher Baum, Department of Experimental Hematology, OE6960, Hannover Medical School, Carl-Neuberg-Straße 1, 30625 Hannover, Germany; e-mail: li.zhixiong@mh-hannover.de or baum.christopher@mh-hannover.de.
References
Author notes
*Z.L., G.B., and M.R. contributed equally to this work.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal