Transfusion-related acute lung injury (TRALI) is the leading cause of transfusion death. We hypothesize that TRALI requires 2 events: (1) the clinical condition of the patient and (2) the infusion of antibodies against MHC class I antigens or the plasma from stored blood. A 2-event rat model was developed with saline (NS) or endotoxin (LPS) as the first event and the infusion of plasma from packed red blood cells (PRBCs) or antibodies (OX18 and OX27) against MHC class I antigens as the second event. ALI was determined by Evans blue dye leak from the plasma to the bronchoalveolar lavage fluid (BALF), protein and CINC-1 concentrations in the BALF, and the lung histology. NS-treated rats did not evidence ALI with any second events, and LPS did not cause ALI. LPS-treated animals demonstrated ALI in response to plasma from stored PRBCs, both prestorage leukoreduced and unmodified, and to OX18 and OX27, all in a concentration-dependent fashion. ALI was neutrophil (PMN) dependent, and OX18/OX27 localized to the PMN surface in vivo and primed the oxidase of rat PMNs. We conclude that TRALI is the result of 2 events with the second events consisting of the plasma from stored blood and antibodies that prime PMNs.
Introduction
Transfusion-related acute lung injury (TRALI) is the leading cause of transfusion mortality in the United States.1,2 TRALI is the acute onset of noncardiogenic pulmonary edema as documented by chest radiograph and profound hypoxemia, in accordance with the definition of acute lung injury (ALI), that occurs within 6 hours of transfusion.3,4 TRALI may occur with or without conditions that predispose the patient to ALI, and may be the worsening of pulmonary function in patients with preexisting ALI.3,4 All blood products have been implicated in TRALI, but components that contain large amounts of plasma are mainly responsible.5,6 The current incidence of TRALI has been estimated as 1/7900 to 1/1330 in the United Kingdom and the United States with lesser incidences in Europe.5,,–8 Current mortality rates vary from 5% to 35% with the lesser mortality rates predominating.5,,–8
The pathophysiology of TRALI has not been elucidated despite numerous studies.9,,,,–14 The first mechanism proposed was the infusion of donor antibodies directed against the HLA class I or granulocyte-specific antigens on the recipient's leukocytes with animal models composed of an in vivo murine model and an isolated, perfused rabbit lung that provided physiologic relevance.9,,–12,14 In addition, the neutrophil (PMN) was proposed to be the effector cell, identical to other forms of ALI and the acute respiratory distress syndrome (ARDS).9,,–12,14 However, look-back studies of donors with specific antibodies directed against HLA or granulocyte antigens demonstrated that the infusion of donor antibodies into a recipient that expressed the cognate antigen resulted in TRALI in a minority of these patients, implying that the clinical condition of the recipient may be important for the development of TRALI.15,–17 A 2-event model was proposed identical to that of ARDS such that the first event was the underlying clinical condition of the patients and the second event was the infusion of biologic response modifiers (BRMs), including lipids or antibodies directed against the antigens expressed on the recipient's PMNs.13,18,,–21 Two clinical studies and an animal model consisting of isolated perfused rat lungs provided supportive evidence and implicated new mediators including soluble CD40 ligand (sCD40L), which like lipids accumulates during the routine storage of cellular components.13,18,,,–22 However, there are several problems with the current animal models, including inconsistencies with clinical TRALI, the lack of a dose-response to the antibody used, and a mortality rate of 50%.9 Moreover, isolated perfused lung models suffer from several inherent deficiencies, including the inability to excrete or to modify the introduced mediators, introduction of human PMNs, and the use of tubing on the perfusion circuits that have the capacity to prime human PMNs.11,–13,19 We hypothesize that TRALI is the result of 2 distinct clinical events, and both antibodies and the plasma and lipids from stored but not fresh cellular components cause ALI as second events in an in vivo model of PMN-mediated ALI.
Methods
Materials
All chemicals were purchased from Sigma-Aldrich unless otherwise stated (St Louis, MO). CINC-1 enzyme-linked immunosorbent assays (ELISAs) were obtained from R&D Systems (Minneapolis, MN) and the rat BNP-32 ELISA was purchased from AssayPro (St Charles, MO). OX18 and OX27 antibodies were obtained from AbD Serotec (Raleigh, NC) or Abcam (Cambridge, MA). The rat PMN antisera, Fc block, and the fluorescent goat anti–rabbit antibodies were purchased from Accurate Chemical (Westbury, NY). PE50 tubing, HistoPrep, and Tissue-Tek OCT Compound were obtained from Fisher Scientific (Pittsburgh, PA).
Packed red blood cell collection
Whole blood (450 mL) was collected from 10 healthy donors after informed consent was obtained in accordance with the Declaration of Helsinki under a protocol approved by the Colorado Multiple Internal Review Board at the University of Colorado Denver (UCD). Each unit was then divided by equal weight with one-half being leukoreduced (LR-PRBCs) via filtration (Pall BPF4 leukoreduction filter; Pall, Westbury NY), and stored in AS-5 at 4°C according to AABB standards.23 Sterile couplers were used to obtain serial samples throughout the storage interval on day 1 (D1), D28, and D42 (the last day a unit can be transfused). The plasma fraction was isolated via centrifugation and stored at −80°C.23 Prior to infusion, the PRBC plasma was heated to 56°C for 30 minutes to obviate the nonspecific effects of human complement and fibrinogen, which include widespread thrombus formation.
MPO assay
MPO was measured in homogenized lungs by the reduction of o-dianisidine at 460 nm in a Thermomax Microplate reader (Molecular Dynamics, Sunnyvale, CA).24
Two-event in vivo model
Male Sprague Dawley rats (Harlan, Indianapolis, IN) underwent ALI via a treatment protocol approved by the Animal Care and Use Committee, UCD. Rats were injected intraperitoneally with 2 mg/kg lipopolysaccharide (LPS, Salmonella enteritides) or an equal volume of 0.9% saline (NS) and returned to their cage. After 2 hours, they were anesthetized with 60 mg/kg pentobarbital, and the femoral vessels were cannulated with PE50 tubing filled with 50/50 heparin-saline. The heart rate and the mean arterial blood pressure (MAP, arterial line) were monitored using a small animal monitor (ProPac; Protocol Systems, Beaverton, OR). Blood was removed (5-10 minutes) equal to 5% to 10% of the total blood volume (total microliters of blood = kilograms of body weight × 6025 ) followed by infusion of an identical volume as the shed blood with NS, 4.5 μM lysophosphatidylcholines (LPCs; positive control), the heat-treated plasma fraction from PRBCs or LR-PRBCs, OX18 or OX27 antibodies to rat MHC class I loci, or mouse IgG isotypic controls at a rate of 4 mL/hour (15-30 minutes) through the femoral vein, and followed by 30 mg/kg Evans blue dye (EBD; 10 mg/mL). The rats were then placed prone in their cages for 6 hours, and then reanesthetized to draw blood followed by humane killing with pentobarbital. A bronchoalveolar lavage (BAL) was performed with 3 washes of NS (5 mL), and the BAL fluid (BALF) and blood were centrifuged, and the cell-free BALF and plasma were stored at −80°C. In selected experiments, the lungs were removed and stored at −80°C for further use. The mortality rate for rats that died prior to 6 hours was 9.1%, similar to the 5% to 10% seen clinically.5,6,10,18,26,27
Measurement of pulmonary edema
Pulmonary edema (lung leak) was measured as the percentage of the plasma EBD that leaked into the BALF, as previously described.28 The amount of EBD in the BALF compared with the plasma was measured at 620 nm.28 The total protein was also measured in the BALF by a BCA protein assay kit (Pierce, Rockford, IL).
EBD leak into the pulmonary interstitium
ALI was also quantified by the amount of remaining EBD in the lung interstitium.28 Briefly, 1 g lung tissue was washed (NS) and homogenized in 10 mL PBS, 20 mL formamide was added, and the mixture was incubated for 18 hours at 37°C. Samples were then centrifuged for 30 minutes at 5000g, and the amount of EBD in the supernatant was measured against known concentrations of EBD in 1:2 PBS/formamide at 620 nm.28
Lung histology
Selected rats underwent ALI without the infusion of EBD. Following humane killing, the lungs were exposed, a cannula was placed in the branch into the right lung, a 1:1 mixture of OCT/PBS (pH 7.0) was infused by gravity (10 cm) until the lung returned to its original size (∼ 1-2 mL), and a lobe was tied off at the bronchus. The lobe was removed, placed in HistoPrep, snap-frozen in a 2-methylbutane dry-ice bath, and stored in liquid nitrogen. The tissue was sectioned with a microtome (International Equipment, Needham Heights, MA). Individual sections were placed on slides and stained with hematoxylin and eosin (H&E). Immunohistochemistry was performed using rabbit antisera against a specific rat granulocyte surface antigen (Accurate Chemical). PMNs in the lung sections were visualized with Cy3-conjugated goat anti–rabbit IgG (red) and counterstained with Alexa 488–linked wheat germ agglutinin (WGA; green; Accurate Chemical), and nuclei were identified with bis-benzimide (blue).28 All images were photographed at 40×/1.25-0.75 oil objective using a Leica DMRXA digital microscope (Exton, PA) with images acquired using Intelligence Imaging Innovations Slidebook software (Denver, CO).
To determine the localization of the antibodies to the rat MHC class I antigens, lung sections from rats infused with 0.15 mg/kg OX27 were stained with wheat germ agglutinin (WGA) linked to Cy-3 (red, a membrane-specific stain) and with bis-benzamide (blue, nuclear stain) for 1 hour at room temperature. After washing, the sections were probed with a donkey anti–mouse antibody labeled with Alexa 488 (green).
CINC-1 measurement
Cytokine-induced neutrophil chemoattractant (CINC-1) was measured in the BALF via commercial ELISA.
Priming of PMNs by MHC class I abs
Rats were anesthetized, the femoral vessels were catheterized, and blood was collected in a heparinized syringe.29 Whole blood was added to a gradient of 12 mL Histopaque-1077 layered on 12 mL Histopaque-1119 and centrifuged at 700g for 40 minutes. The leukocyte layer was removed, centrifuged at 200g for 10 minutes at 4°C, and the pellet resuspended in Krebs ringer phosphate with 2% dextrose, pH 7.35 (KRPD). PMNs represented 99% of the isolated leukocytes by differential staining and light microscopy.
Rat PMNs (3.5 × 105 cells) were warmed to 37°C for measurement of superoxide anion (O2−) release, as described previously.19 Briefly, buffer, 4.5 μM LPCs (positive control), or MHC class I abs (OX18 and OX27) equal to 0.075 to 0.6 mg/kg antibody transfused or granulocyte-specific antibodies (1.25-10 μg/mL) were added to rat PMNs and incubated for 5 minutes. After incubation, 1 μM formyl-Met-Leu-Phe (fMLP) was added to activate the respiratory burst and the maximal rate of O2− production was measured.19 Priming was defined as augmentation of the fMLP-activated respiratory burst. For Fcγ receptor (FcγR) blocking, PMNs were incubated with Fc block (Accurate Chemical) or 1 μg CD32/106 cells (BD Biosciences, San Jose, CA) for 15 minutes at 37°C prior to adding OX18 or OX 27.
Detection of MHC class I antigens on rat PMNs by flow cytometry
Rat PMNs (106) were incubated for 30 minutes at 4°C with OX18-PE–, OX27-PE–, or PE-labeled isotype control (AbD; Serotec), fixed with fresh, cold 4% paraformaldehyde (5%), and diluted to 1%. OX18 and OX27 immunoreactivity was measured by flow cytometry.
PMN depletion
Rats were transfused with 200 μL rat PMN antiserum (Accurate Chemical) over 30 minutes and returned to their cages for 24 hours.30,31 At 24 hours, heparinized blood was obtained, smeared onto slides, and stained with a modified Wright stain. Leukocyte differentials were completed by a blinded hematopathologist to ensure PMN depletion. These rats then underwent the protocol for ALI.
Statistics
All data are presented as the mean plus or minus the standard error of the mean. Statistical differences among groups were measured by independent ANOVAs followed by either a Bonferroni or Newman-Keuls post-hoc test for multiple comparisons depending upon the equality of variance. Significance was determined at the P value less than .05 level.
Results
Plasma from stored PRBCs causes lung injury in rats
Because intraperitoneal injection of LPS causes proinflammatory activation of pulmonary endothelium resulting in PMN sequestration, LPS was used to mimic active infection, a clinical first event associated with TRALI.13,18,19,27 Rats were injected intraperitoneally with NS or 2 mg/kg LPS and the amount of myeloperoxidase (MPO) was measured in the homogenized lung tissue. LPS caused a significant increase in MPO, which is directly related to PMN accumulation versus NS-treated animals (NS: 2.7 ± 0.23 vs LPS: 4.4 ± 0.6* units/mg lung, *P < .05).
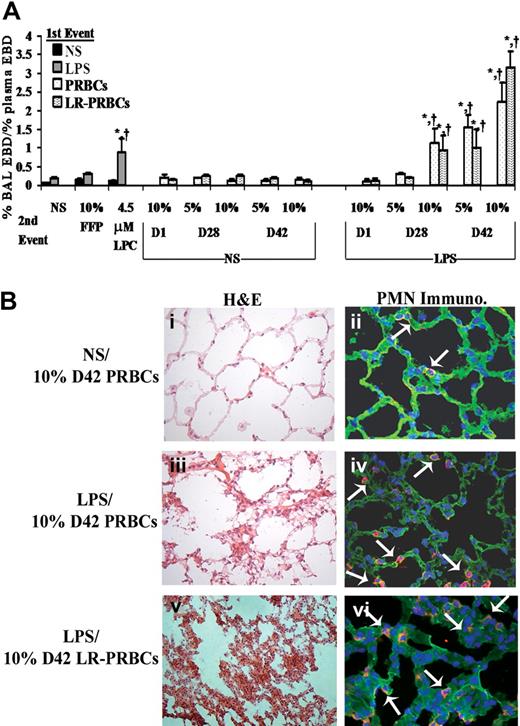
Pulmonary edema (ALI) was initially measured as the percentage of plasma EBD leak into the BALF (Figure 1A). Rats injected with NS (first event) and infused with (1) 10% fresh frozen plasma (FFP), (2) the plasma from fresh (D1) (5%-10%)FINAL (vol/vol) or stored (D28 or D42) unmodified PRBCs, or (3) the plasma from fresh (D1) or stored (D28 or D42) prestorage LR-PRBCs (5%-10%)FINAL (vol/vol) (second events) did not demonstrate ALI in this model (Figure 1A). Moreover, LPS alone did not cause EBD lung leak nor did LPS (first event) followed by the infusion of FFP, plasma from fresh PRBCs or LR-PRBCs, or 5% plasma from D28 PRBCs or LR-PRBCs (Figure 1A). However, rats injected with LPS and then transfused with the plasma from stored PRBCs, 10% plasma from D28, and 5% and 10% plasma from D42 PRBCs and LR-PRBCs developed ALI compared with LPS/NS, LPS/FFP, LPS/D1 PRBCs, and LPS/D1 LR-PRBCs (Figure 1A). As a positive control, LPCs, the lipids that accumulate during routine storage of cellular blood components and cause PMN-mediated ALI in isolated perfused rat lungs, were used as the second event and also caused significant ALI (Figure 1A).19,32 The amount of protein in the BAL was also significantly higher in the animals that evidenced ALI (LPS/NS: 10.3 ± 1.7 mg, LPS/10% FFP: 13.1 ± 0.6 mg, LPS/10% D28 PRBCs: 47.9 ± 6.0 mg,* LPS/10% D28 LR-PRBCs: 19.3 ± 0.9 mg,* LPS/5% D42 PRBCs: 28.0 ± 2.1 mg,* LPS/5% D42 LR-PRBCs: 11.6 ± 0.3 mg,* LPS/10% D42 PRBCs: 29.7 ± 1.1 mg,* and LPS/10% D42 LR-PRBCs: 49.0 ± 1.3 mg*; *P < .05 compared with both LPS/NS and LPS/10% FFP). To ensure that the pulmonary edema was not due to volume overload, rat brain natriuretic peptide-32 (BNP-32) plasma levels were measured after injury, and no significant increases were present in the treatment groups (data not shown).
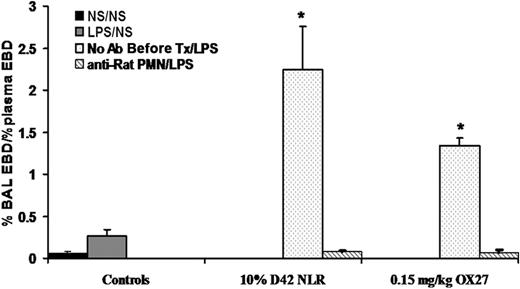
The plasma from stored PRBCs causes lung injury in a 2-event in vivo model of TRALI. (A) Sprague Dawley rats were injected intraperitoneally with 0.9% saline (NS; ■ left or NS middle) or endotoxin (LPS,  left, LPS right) 2 hours prior to transfusion (first event) and then infused with NS or 10% FFP (controls), 4.5 μM lysophosphatidylcholines (LPCs, positive control), or the plasma from D1, D28, and D42 PRBCs (
left, LPS right) 2 hours prior to transfusion (first event) and then infused with NS or 10% FFP (controls), 4.5 μM lysophosphatidylcholines (LPCs, positive control), or the plasma from D1, D28, and D42 PRBCs ( ) or LR-PRBCs (
) or LR-PRBCs ( ) at 5% or 10% of the total blood volume. The percentage of Evans blue dye (EBD) leak from the plasma to the bronchoalveolar lavage (BAL) was measured (% BAL EBD/% plasma EBD, y-axis) and is shown as a function of treatment group (x-axis). No rats treated with NS as the first event demonstrated significant EBD leak (ALI) nor did animals injected with LPS followed by NS, 10% FFP, the plasma (10%) from D1 PRBC or D1 LR-PRBCs, or the plasma (5%) from D28 PRBCs and LR-PRBCs. However in rats treated with LPS and then infused with the plasma from D28 PRBCs (10%) or D28 LR-PRBCs (10%), D42 PRBCs (5%-10%) or D42 LR-PRBCs (5%-10%), or LPCs, the lipids from stored PRBCs, significant EBD leak occurred compared with the controls (*P < .05 from LPS/NS, †P < .05 from LPS/10% FFP, n = 4 for each bar). (B) Rats were pretreated with NS or LPS 2 hours prior to infusion of plasma from D42 PRBCs or D42 LR-PRBCs at 10% of the total blood volume. After 6 hours, the rats were humanely killed, and the lungs were removed, embedded in OCT compound, and snap-frozen. The lungs were sectioned and stained with H&E (left column) or underwent immunohistochemistry to identify rat PMNs (right columns) that used a specific rabbit antirat granulocyte antibody followed by a fluorescently labeled goat antirabbit antibody (red). The sections were visualized at 40×. Rats pretreated with NS and infused with plasma (10% total blood volume) demonstrated normal pulmonary histology (i) with intact endothelium and alveoli with a few scattered PMNs (red) in the vasculature; and the lung parenchyma including the vasculature were stained with WGA (green, membrane stain) and bis-benzamide (blue, nuclear stain) (ii). In rats treated with LPS and then infused with 10% plasma from D42 PRBCs (iii,iv) or D42 LR-PRBCs (v,vi) the histology demonstrates PMN infiltration, pulmonary edema, arcuate inflammation, and hyaline membrane formation. In addition, the PMN infiltration was confirmed by the immunohistochemistry, which demonstrated widespread PMN margination into the lung (red), further demarcated with the white arrows (iv,vi; n = 2).
) at 5% or 10% of the total blood volume. The percentage of Evans blue dye (EBD) leak from the plasma to the bronchoalveolar lavage (BAL) was measured (% BAL EBD/% plasma EBD, y-axis) and is shown as a function of treatment group (x-axis). No rats treated with NS as the first event demonstrated significant EBD leak (ALI) nor did animals injected with LPS followed by NS, 10% FFP, the plasma (10%) from D1 PRBC or D1 LR-PRBCs, or the plasma (5%) from D28 PRBCs and LR-PRBCs. However in rats treated with LPS and then infused with the plasma from D28 PRBCs (10%) or D28 LR-PRBCs (10%), D42 PRBCs (5%-10%) or D42 LR-PRBCs (5%-10%), or LPCs, the lipids from stored PRBCs, significant EBD leak occurred compared with the controls (*P < .05 from LPS/NS, †P < .05 from LPS/10% FFP, n = 4 for each bar). (B) Rats were pretreated with NS or LPS 2 hours prior to infusion of plasma from D42 PRBCs or D42 LR-PRBCs at 10% of the total blood volume. After 6 hours, the rats were humanely killed, and the lungs were removed, embedded in OCT compound, and snap-frozen. The lungs were sectioned and stained with H&E (left column) or underwent immunohistochemistry to identify rat PMNs (right columns) that used a specific rabbit antirat granulocyte antibody followed by a fluorescently labeled goat antirabbit antibody (red). The sections were visualized at 40×. Rats pretreated with NS and infused with plasma (10% total blood volume) demonstrated normal pulmonary histology (i) with intact endothelium and alveoli with a few scattered PMNs (red) in the vasculature; and the lung parenchyma including the vasculature were stained with WGA (green, membrane stain) and bis-benzamide (blue, nuclear stain) (ii). In rats treated with LPS and then infused with 10% plasma from D42 PRBCs (iii,iv) or D42 LR-PRBCs (v,vi) the histology demonstrates PMN infiltration, pulmonary edema, arcuate inflammation, and hyaline membrane formation. In addition, the PMN infiltration was confirmed by the immunohistochemistry, which demonstrated widespread PMN margination into the lung (red), further demarcated with the white arrows (iv,vi; n = 2).
The plasma from stored PRBCs causes lung injury in a 2-event in vivo model of TRALI. (A) Sprague Dawley rats were injected intraperitoneally with 0.9% saline (NS; ■ left or NS middle) or endotoxin (LPS,  left, LPS right) 2 hours prior to transfusion (first event) and then infused with NS or 10% FFP (controls), 4.5 μM lysophosphatidylcholines (LPCs, positive control), or the plasma from D1, D28, and D42 PRBCs (
left, LPS right) 2 hours prior to transfusion (first event) and then infused with NS or 10% FFP (controls), 4.5 μM lysophosphatidylcholines (LPCs, positive control), or the plasma from D1, D28, and D42 PRBCs ( ) or LR-PRBCs (
) or LR-PRBCs ( ) at 5% or 10% of the total blood volume. The percentage of Evans blue dye (EBD) leak from the plasma to the bronchoalveolar lavage (BAL) was measured (% BAL EBD/% plasma EBD, y-axis) and is shown as a function of treatment group (x-axis). No rats treated with NS as the first event demonstrated significant EBD leak (ALI) nor did animals injected with LPS followed by NS, 10% FFP, the plasma (10%) from D1 PRBC or D1 LR-PRBCs, or the plasma (5%) from D28 PRBCs and LR-PRBCs. However in rats treated with LPS and then infused with the plasma from D28 PRBCs (10%) or D28 LR-PRBCs (10%), D42 PRBCs (5%-10%) or D42 LR-PRBCs (5%-10%), or LPCs, the lipids from stored PRBCs, significant EBD leak occurred compared with the controls (*P < .05 from LPS/NS, †P < .05 from LPS/10% FFP, n = 4 for each bar). (B) Rats were pretreated with NS or LPS 2 hours prior to infusion of plasma from D42 PRBCs or D42 LR-PRBCs at 10% of the total blood volume. After 6 hours, the rats were humanely killed, and the lungs were removed, embedded in OCT compound, and snap-frozen. The lungs were sectioned and stained with H&E (left column) or underwent immunohistochemistry to identify rat PMNs (right columns) that used a specific rabbit antirat granulocyte antibody followed by a fluorescently labeled goat antirabbit antibody (red). The sections were visualized at 40×. Rats pretreated with NS and infused with plasma (10% total blood volume) demonstrated normal pulmonary histology (i) with intact endothelium and alveoli with a few scattered PMNs (red) in the vasculature; and the lung parenchyma including the vasculature were stained with WGA (green, membrane stain) and bis-benzamide (blue, nuclear stain) (ii). In rats treated with LPS and then infused with 10% plasma from D42 PRBCs (iii,iv) or D42 LR-PRBCs (v,vi) the histology demonstrates PMN infiltration, pulmonary edema, arcuate inflammation, and hyaline membrane formation. In addition, the PMN infiltration was confirmed by the immunohistochemistry, which demonstrated widespread PMN margination into the lung (red), further demarcated with the white arrows (iv,vi; n = 2).
) at 5% or 10% of the total blood volume. The percentage of Evans blue dye (EBD) leak from the plasma to the bronchoalveolar lavage (BAL) was measured (% BAL EBD/% plasma EBD, y-axis) and is shown as a function of treatment group (x-axis). No rats treated with NS as the first event demonstrated significant EBD leak (ALI) nor did animals injected with LPS followed by NS, 10% FFP, the plasma (10%) from D1 PRBC or D1 LR-PRBCs, or the plasma (5%) from D28 PRBCs and LR-PRBCs. However in rats treated with LPS and then infused with the plasma from D28 PRBCs (10%) or D28 LR-PRBCs (10%), D42 PRBCs (5%-10%) or D42 LR-PRBCs (5%-10%), or LPCs, the lipids from stored PRBCs, significant EBD leak occurred compared with the controls (*P < .05 from LPS/NS, †P < .05 from LPS/10% FFP, n = 4 for each bar). (B) Rats were pretreated with NS or LPS 2 hours prior to infusion of plasma from D42 PRBCs or D42 LR-PRBCs at 10% of the total blood volume. After 6 hours, the rats were humanely killed, and the lungs were removed, embedded in OCT compound, and snap-frozen. The lungs were sectioned and stained with H&E (left column) or underwent immunohistochemistry to identify rat PMNs (right columns) that used a specific rabbit antirat granulocyte antibody followed by a fluorescently labeled goat antirabbit antibody (red). The sections were visualized at 40×. Rats pretreated with NS and infused with plasma (10% total blood volume) demonstrated normal pulmonary histology (i) with intact endothelium and alveoli with a few scattered PMNs (red) in the vasculature; and the lung parenchyma including the vasculature were stained with WGA (green, membrane stain) and bis-benzamide (blue, nuclear stain) (ii). In rats treated with LPS and then infused with 10% plasma from D42 PRBCs (iii,iv) or D42 LR-PRBCs (v,vi) the histology demonstrates PMN infiltration, pulmonary edema, arcuate inflammation, and hyaline membrane formation. In addition, the PMN infiltration was confirmed by the immunohistochemistry, which demonstrated widespread PMN margination into the lung (red), further demarcated with the white arrows (iv,vi; n = 2).
ALI was also confirmed by the lung histology and measurement of cytokine-induced neutrophil chemoattractant-1 (CINC-1), the rat homologue to IL-8, in the BALF.33,34 Treatment of rats with NS followed by 10% plasma from D42 PRBCs did not demonstrate histologic evidence of ALI (Figure 1Bi) with only a few scattered PMNs by immunohistochemistry (Figure 1Bii). However, when rats treated with LPS were then infused with 10% plasma from D42 PRBCs or D42 LR-PRBCs, there were findings consistent with ALI including PMN infiltration and inflammation, hyaline membrane formation, and PMN-mediated damage to the pulmonary endothelium, interstitium, and the alveolar air spaces (Figure 1Biii-vi). CINC-1 concentrations were also significantly increased in the BALF in animals that had ALI, namely those injected with LPS and infused with LPCs, 10% plasma from D28 PRBCs and D28 LR-PRBCs, and 5% and 10% plasma from both D42 PRBCs and D42 LR-PRBCs compared with CINC-1 levels from LPS/NS- and LPS/10% D1 PRBC– or LPS/10% D1 LR-PRBC–treated rats (P < .05; Figure 2).
CINC-1 increases in the BALF of rats treated with LPS and infused with plasma from stored PRBCs or LR-PRBCs. CINC-1 levels (ng/mL) were measured (ELISA) in the BAL fluid (BALF) from rats transfused with PRBCs ( ) or LR-PRBCs (▧) at 5% to 10% total blood volume. Rats pretreated with NS and infused with the plasma from fresh or stored PRBCs as well as LPCs, the lipids that accumulate during routine storage of PRBCs, did not evidence any significant increases in CINC-1 compared with rats pretreated with NS and infused with NS. Moreover, animals treated with LPS and then infused with NS or LPS or the plasma from fresh (D1) PRBCs and LR-PRBCs or 5% plasma from D28 PRBC or D28 LR-PRBCs also did not evidence increases in CINC-1. However, rats injected with LPS and transfused with plasma from D28 (10%) and D42 (5% and 10%) PRBCs and LR-PRBCs had a significant increase (P < .05, n = 4) in CINC-1 levels in the BALF from rats pretreated with LPS and transfused with NS (*P < .05 from LPS/NS, n = 4).
) or LR-PRBCs (▧) at 5% to 10% total blood volume. Rats pretreated with NS and infused with the plasma from fresh or stored PRBCs as well as LPCs, the lipids that accumulate during routine storage of PRBCs, did not evidence any significant increases in CINC-1 compared with rats pretreated with NS and infused with NS. Moreover, animals treated with LPS and then infused with NS or LPS or the plasma from fresh (D1) PRBCs and LR-PRBCs or 5% plasma from D28 PRBC or D28 LR-PRBCs also did not evidence increases in CINC-1. However, rats injected with LPS and transfused with plasma from D28 (10%) and D42 (5% and 10%) PRBCs and LR-PRBCs had a significant increase (P < .05, n = 4) in CINC-1 levels in the BALF from rats pretreated with LPS and transfused with NS (*P < .05 from LPS/NS, n = 4).
CINC-1 increases in the BALF of rats treated with LPS and infused with plasma from stored PRBCs or LR-PRBCs. CINC-1 levels (ng/mL) were measured (ELISA) in the BAL fluid (BALF) from rats transfused with PRBCs ( ) or LR-PRBCs (▧) at 5% to 10% total blood volume. Rats pretreated with NS and infused with the plasma from fresh or stored PRBCs as well as LPCs, the lipids that accumulate during routine storage of PRBCs, did not evidence any significant increases in CINC-1 compared with rats pretreated with NS and infused with NS. Moreover, animals treated with LPS and then infused with NS or LPS or the plasma from fresh (D1) PRBCs and LR-PRBCs or 5% plasma from D28 PRBC or D28 LR-PRBCs also did not evidence increases in CINC-1. However, rats injected with LPS and transfused with plasma from D28 (10%) and D42 (5% and 10%) PRBCs and LR-PRBCs had a significant increase (P < .05, n = 4) in CINC-1 levels in the BALF from rats pretreated with LPS and transfused with NS (*P < .05 from LPS/NS, n = 4).
) or LR-PRBCs (▧) at 5% to 10% total blood volume. Rats pretreated with NS and infused with the plasma from fresh or stored PRBCs as well as LPCs, the lipids that accumulate during routine storage of PRBCs, did not evidence any significant increases in CINC-1 compared with rats pretreated with NS and infused with NS. Moreover, animals treated with LPS and then infused with NS or LPS or the plasma from fresh (D1) PRBCs and LR-PRBCs or 5% plasma from D28 PRBC or D28 LR-PRBCs also did not evidence increases in CINC-1. However, rats injected with LPS and transfused with plasma from D28 (10%) and D42 (5% and 10%) PRBCs and LR-PRBCs had a significant increase (P < .05, n = 4) in CINC-1 levels in the BALF from rats pretreated with LPS and transfused with NS (*P < .05 from LPS/NS, n = 4).
MHC class I antibody causes lung injury in rats
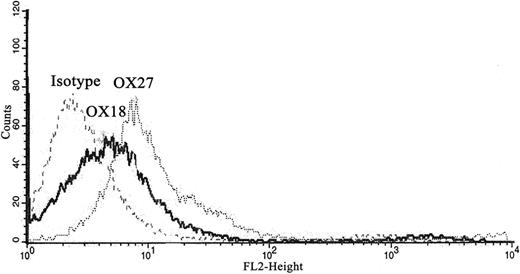
To determine antibody specificity, isolated rat PMNs were incubated with fluorescently labeled OX18 and OX27 antibodies. Compared with PMNs treated with fluorescently labeled isotypic IgG controls, there were significant increases in fluorescent intensity in the PMNs incubated with OX18 and OX27 via flow cytometry, illustrating the presence of the cognate antigen on the PMN surface (Figure 3).
Rat MHC class I Abs bind to the rat PMNs. Rat PMNs were isolated, incubated with PE-labeled isotype, OX18, or OX27, and fixed in paraformaldehyde. PMNs incubated with the OX18 and OX27 antibodies bound to the PMNs, as demonstrated by the fluorescent shift compared with the labeled isotype controls via flow cytometry (n = 3).
Rat MHC class I Abs bind to the rat PMNs. Rat PMNs were isolated, incubated with PE-labeled isotype, OX18, or OX27, and fixed in paraformaldehyde. PMNs incubated with the OX18 and OX27 antibodies bound to the PMNs, as demonstrated by the fluorescent shift compared with the labeled isotype controls via flow cytometry (n = 3).
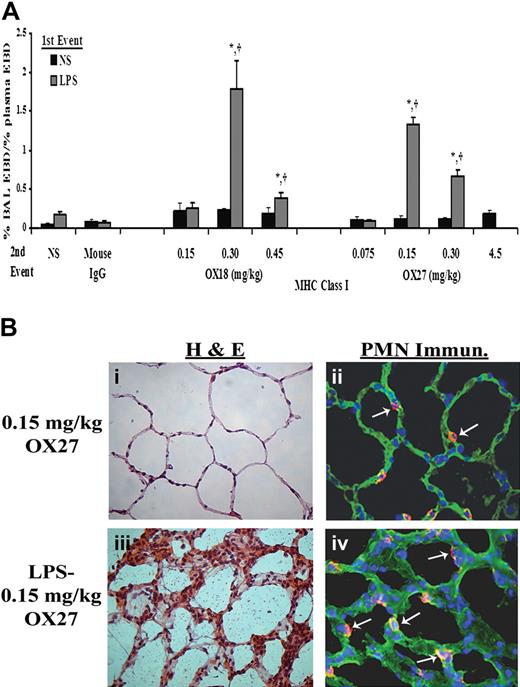
Similar to the experiments with PRBC plasma, rats injected intraperitoneally with NS followed by infusion of mouse IgG (0.30 mg/kg) or specific antibodies to the OX18 (0.15-0.45 mg/kg) or OX27 (0.075-4.5 mg/kg) did not evidence ALI (Figure 4A). Importantly, the respiratory rate and the mean arterial blood pressure (MAP) via an arterial line were monitored closely. We measured the MAPs before (76.5 ± 5.5-120.5 ± 10.5 mm/Hg), during (52.0 ± 14.0-117.7 ± 7.9 mmHg), and 6 hours after (115 ± 0.01 mmHg) the antibody infusion. The lowest MAPs were during the infusion of 4.5 mg/kg OX27 antibody, necessitating a decrease in the infusion rate to 6 mL/hour. All animals recovered, and the survival for the NS/antibody-treated animals was 100%. In contrast, rats injected with LPS and infused with OX18 and OX27 demonstrated ALI in a concentration-dependent fashion (Figure 4A). OX18 elicited ALI at concentrations of 0.3 mg/kg but did not cause injury at 0.15 mg/kg. Interestingly, higher concentrations (0.45 mg/kg) also caused ALI; however, there was less injury than in the animals receiving 0.30 mg/kg OX18 as the second event (Figure 4A). In addition, OX27 caused ALI in LPS-treated animals at 0.15 mg/kg, and concentrations less than 0.15 mg/kg did not (Figure 4A). Similar to OX18, OX27 at 0.30 mg/kg caused ALI; however, there was a lesser amount of pulmonary edema compared with the LPS/0.15 mg/kg OX27 group (Figure 4A).
MHC class I Abs cause ALI in a 2-event in vivo model. (A) Rats were injected with either NS or LPS intraperitoneally 2 hours prior to transfusion and then infused with mouse IgG (0.3 mg/kg, control) or MHC class I Abs (0.075-4.5 mg/kg OX18 or OX27), followed by Evans blue dye (EBD), which binds to albumin. Rats were humanely killed 6 hours after transfusion, a BAL was performed, and ALI was measured as the percentage of EBD leak into the BAL from the plasma (% BAL EBD/% plasma EBD, y-axis), which is depicted as a function of the treatment group (x-axis). Rats that were treated with NS (■) did not evidence significant EBD leak for any treatment group including rats transfused with 4.5 mg/kg OX27. In addition, rats injected with LPS ( ) and infused with mouse IgG did not demonstrate EBD leak, compared with the LPS/NS controls, and neither did LPS-treated rats infused with 0.15 mg/kg OX18 or 0.075 mg/kg OX27. However, rats injected with LPS then infused with 0.30 to 0.45 mg/kg OX18 and 0.15 to 0.30 mg/kg OX27 had a significant amount of lung injury compared with LPS/NS and LPS/mouse IgG (*P < .05 from LPS/NS; †P < .05 from LPS/mouse IgG, n = 4). (B) Rats were injected with saline (NS) or LPS 2 hours prior to the infusion of 0.15 mg/kg OX27. After completion of the experimental protocol, the rats were humanely killed, and the lungs were snap-frozen and fixed, embedded in paraffin, and sectioned. All images were shot at 40×, and the sections were stained with H&E (i,iii) or immunohistochemistry was performed (ii,iv) to localize rat PMNs (red) and to demonstrate the pulmonary parenchyma by staining the membranes green (WGA linked to Cy-3) and the nuclei blue (bis-benzamide). Rat lungs from animal injected with NS and then infused with OX27 demonstrated normal pulmonary histology without evidence of ALI (i) with a few PMNs visible in the lung (arrow, ii). In contrast, in rats injected with LPS and then infused with OX27 there are obvious signs of ALI with pulmonary edema, hyaline membrane formation, and arcuate inflammation with PMNs (iii). The immunohistochemistry demonstrated widespread PMN infiltration throughout the lung parenchyma as shown by the red color further demarcated by the white arrows (iv; n = 2).
) and infused with mouse IgG did not demonstrate EBD leak, compared with the LPS/NS controls, and neither did LPS-treated rats infused with 0.15 mg/kg OX18 or 0.075 mg/kg OX27. However, rats injected with LPS then infused with 0.30 to 0.45 mg/kg OX18 and 0.15 to 0.30 mg/kg OX27 had a significant amount of lung injury compared with LPS/NS and LPS/mouse IgG (*P < .05 from LPS/NS; †P < .05 from LPS/mouse IgG, n = 4). (B) Rats were injected with saline (NS) or LPS 2 hours prior to the infusion of 0.15 mg/kg OX27. After completion of the experimental protocol, the rats were humanely killed, and the lungs were snap-frozen and fixed, embedded in paraffin, and sectioned. All images were shot at 40×, and the sections were stained with H&E (i,iii) or immunohistochemistry was performed (ii,iv) to localize rat PMNs (red) and to demonstrate the pulmonary parenchyma by staining the membranes green (WGA linked to Cy-3) and the nuclei blue (bis-benzamide). Rat lungs from animal injected with NS and then infused with OX27 demonstrated normal pulmonary histology without evidence of ALI (i) with a few PMNs visible in the lung (arrow, ii). In contrast, in rats injected with LPS and then infused with OX27 there are obvious signs of ALI with pulmonary edema, hyaline membrane formation, and arcuate inflammation with PMNs (iii). The immunohistochemistry demonstrated widespread PMN infiltration throughout the lung parenchyma as shown by the red color further demarcated by the white arrows (iv; n = 2).
MHC class I Abs cause ALI in a 2-event in vivo model. (A) Rats were injected with either NS or LPS intraperitoneally 2 hours prior to transfusion and then infused with mouse IgG (0.3 mg/kg, control) or MHC class I Abs (0.075-4.5 mg/kg OX18 or OX27), followed by Evans blue dye (EBD), which binds to albumin. Rats were humanely killed 6 hours after transfusion, a BAL was performed, and ALI was measured as the percentage of EBD leak into the BAL from the plasma (% BAL EBD/% plasma EBD, y-axis), which is depicted as a function of the treatment group (x-axis). Rats that were treated with NS (■) did not evidence significant EBD leak for any treatment group including rats transfused with 4.5 mg/kg OX27. In addition, rats injected with LPS ( ) and infused with mouse IgG did not demonstrate EBD leak, compared with the LPS/NS controls, and neither did LPS-treated rats infused with 0.15 mg/kg OX18 or 0.075 mg/kg OX27. However, rats injected with LPS then infused with 0.30 to 0.45 mg/kg OX18 and 0.15 to 0.30 mg/kg OX27 had a significant amount of lung injury compared with LPS/NS and LPS/mouse IgG (*P < .05 from LPS/NS; †P < .05 from LPS/mouse IgG, n = 4). (B) Rats were injected with saline (NS) or LPS 2 hours prior to the infusion of 0.15 mg/kg OX27. After completion of the experimental protocol, the rats were humanely killed, and the lungs were snap-frozen and fixed, embedded in paraffin, and sectioned. All images were shot at 40×, and the sections were stained with H&E (i,iii) or immunohistochemistry was performed (ii,iv) to localize rat PMNs (red) and to demonstrate the pulmonary parenchyma by staining the membranes green (WGA linked to Cy-3) and the nuclei blue (bis-benzamide). Rat lungs from animal injected with NS and then infused with OX27 demonstrated normal pulmonary histology without evidence of ALI (i) with a few PMNs visible in the lung (arrow, ii). In contrast, in rats injected with LPS and then infused with OX27 there are obvious signs of ALI with pulmonary edema, hyaline membrane formation, and arcuate inflammation with PMNs (iii). The immunohistochemistry demonstrated widespread PMN infiltration throughout the lung parenchyma as shown by the red color further demarcated by the white arrows (iv; n = 2).
) and infused with mouse IgG did not demonstrate EBD leak, compared with the LPS/NS controls, and neither did LPS-treated rats infused with 0.15 mg/kg OX18 or 0.075 mg/kg OX27. However, rats injected with LPS then infused with 0.30 to 0.45 mg/kg OX18 and 0.15 to 0.30 mg/kg OX27 had a significant amount of lung injury compared with LPS/NS and LPS/mouse IgG (*P < .05 from LPS/NS; †P < .05 from LPS/mouse IgG, n = 4). (B) Rats were injected with saline (NS) or LPS 2 hours prior to the infusion of 0.15 mg/kg OX27. After completion of the experimental protocol, the rats were humanely killed, and the lungs were snap-frozen and fixed, embedded in paraffin, and sectioned. All images were shot at 40×, and the sections were stained with H&E (i,iii) or immunohistochemistry was performed (ii,iv) to localize rat PMNs (red) and to demonstrate the pulmonary parenchyma by staining the membranes green (WGA linked to Cy-3) and the nuclei blue (bis-benzamide). Rat lungs from animal injected with NS and then infused with OX27 demonstrated normal pulmonary histology without evidence of ALI (i) with a few PMNs visible in the lung (arrow, ii). In contrast, in rats injected with LPS and then infused with OX27 there are obvious signs of ALI with pulmonary edema, hyaline membrane formation, and arcuate inflammation with PMNs (iii). The immunohistochemistry demonstrated widespread PMN infiltration throughout the lung parenchyma as shown by the red color further demarcated by the white arrows (iv; n = 2).
The total protein in the BALF also significantly increased in those animals that had antibody-induced ALI, EBD leak, compared with LPS/IgG isotypic controls: (LPS/mouse IgG [0.3 mg/kg]: 9.0 ± 0.5 mg, LPS/OX18 [0.3 mg/kg]: 31.5 ± 6.1 mg,* LPS/OX27 [0.15 mg/kg]: 19.8 ± 5.1 mg,* and LPS/OX27 [0.3 mg/kg]: 16.8 ± 1.6 mg*; *P < .05). Furthermore, the amount of interstitial EBD in the lungs increased significantly in those animals that demonstrated ALI compared with the LPS/isotypic IgG controls (LPS/OX18 [0.3 mg/kg]: 218.2 ± 15.4 μg* and LPS/OX27 [0.15 mg/kg]: 285.0 ± 7.8 μg* compared with LPS/mouse isotypic IgG [0.30 mg/kg]: 166.9 ± 20.1 μg; *P < .05).
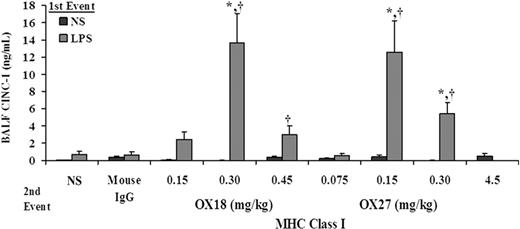
ALI was further confirmed by the lung histology and measurement of CINC-1 in the BALF. Rats treated with NS and infused with 0.15 mg/kg OX27 did not evidence ALI (Figure 4Bi) with a few PMNs present in these lungs (Figure 4Bii). However, treatment of rats with LPS followed by the same concentration of OX27 resulted in significant ALI as demonstrated by PMN infiltration, inflammation with PMN-mediated destruction of the alveoli and capillary endothelium, and edema (Figure 4Biii) with an abundant number of PMNs in the alveoli (Figure 4Biv). The histology from rats treated with LPS/OX18 (0.3 mg/kg) demonstrated identical PMN infiltration and ALI similar to LPS/OX27 (0.15 mg/kg) (results not shown). In addition, CINC-1 levels in the BALF were significantly higher in the rats injected with LPS and infused with both OX18 (0.3-0.45 mg/kg) and OX27 (0.15-0.3 mg/kg) compared with the LPS/mouse IgG, LPS/NS, NS/OX18, or NS/OX27 (P < .05), the latter 2 at all concentrations used (Figure 5).
CINC-1 levels increase in the BALF from rats transfused with MHC class I abs. CINC-1 levels (ng/mL) were measured in the BAL fluid (BALF) via commercial ELISA and are shown as a function of treatment group: control rats injected with NS (■) or LPS ( ) and infused with murine IgG isotype controls (0.30 mg/kg), OX18 (0.15-0.45 mg/kg), or OX27 (0.075-4.5 mg/kg). Rats injected with NS did not evidence increases in CINC-1 in the BALF nor did animals injected with LPS and then infused with NS, 0.15 mg/kg OX18 or 0.075 mg/kg OX18. However, in those groups in which ALI was seen, specifically rats injected with LPS and transfused with 0.30 to 0.45 mg/kg OX18 and 0.15 to 0.30 mg/kg OX27 had a significant increase in CINC-1 levels from LPS/NS and LPS/mouse IgG. (*P < .05 from LPS/NS; †P < .05 from LPS/mouse IgG, n = 4 for each group.)
) and infused with murine IgG isotype controls (0.30 mg/kg), OX18 (0.15-0.45 mg/kg), or OX27 (0.075-4.5 mg/kg). Rats injected with NS did not evidence increases in CINC-1 in the BALF nor did animals injected with LPS and then infused with NS, 0.15 mg/kg OX18 or 0.075 mg/kg OX18. However, in those groups in which ALI was seen, specifically rats injected with LPS and transfused with 0.30 to 0.45 mg/kg OX18 and 0.15 to 0.30 mg/kg OX27 had a significant increase in CINC-1 levels from LPS/NS and LPS/mouse IgG. (*P < .05 from LPS/NS; †P < .05 from LPS/mouse IgG, n = 4 for each group.)
CINC-1 levels increase in the BALF from rats transfused with MHC class I abs. CINC-1 levels (ng/mL) were measured in the BAL fluid (BALF) via commercial ELISA and are shown as a function of treatment group: control rats injected with NS (■) or LPS ( ) and infused with murine IgG isotype controls (0.30 mg/kg), OX18 (0.15-0.45 mg/kg), or OX27 (0.075-4.5 mg/kg). Rats injected with NS did not evidence increases in CINC-1 in the BALF nor did animals injected with LPS and then infused with NS, 0.15 mg/kg OX18 or 0.075 mg/kg OX18. However, in those groups in which ALI was seen, specifically rats injected with LPS and transfused with 0.30 to 0.45 mg/kg OX18 and 0.15 to 0.30 mg/kg OX27 had a significant increase in CINC-1 levels from LPS/NS and LPS/mouse IgG. (*P < .05 from LPS/NS; †P < .05 from LPS/mouse IgG, n = 4 for each group.)
) and infused with murine IgG isotype controls (0.30 mg/kg), OX18 (0.15-0.45 mg/kg), or OX27 (0.075-4.5 mg/kg). Rats injected with NS did not evidence increases in CINC-1 in the BALF nor did animals injected with LPS and then infused with NS, 0.15 mg/kg OX18 or 0.075 mg/kg OX18. However, in those groups in which ALI was seen, specifically rats injected with LPS and transfused with 0.30 to 0.45 mg/kg OX18 and 0.15 to 0.30 mg/kg OX27 had a significant increase in CINC-1 levels from LPS/NS and LPS/mouse IgG. (*P < .05 from LPS/NS; †P < .05 from LPS/mouse IgG, n = 4 for each group.)
PMN depletion prevents ALI
To determine the role of PMNs in this model, rats were infused with 200 μL rat PMN antiserum or isotypic control and returned to their cages for 24 hours. Following incubation, a heparinized sample was taken from the femoral line to assess the effectiveness of antibody depletion, and the rats were then pretreated with LPS, and 2 hours later were infused with either 10% D42 PRBCs or 0.15 mg/kg OX27. The rats that received the isotypic IgG evidenced ALI by EBD leak into the BALF, which was abrogated by immunodepletion of PMNs (Figure 6). Leukocyte differentials on blood obtained 24 hours after the infusion of the antibodies to rat granulocytes demonstrated effective (99% ± 0.5%) PMN depletion, whereas rats injected with isotypic control antibodies had 15% to 20% PMNs. Lastly, to assess whether PMN-specific antibodies caused ALI in this model, the rats were pretreated with NS or LPS and then 0.15 to 0.3 mg/kg granulocyte-specific antibody was infused. This antibody did not cause ALI as the second event in this 2-event model compared with controls (NS/mouse IgG: 0.13 ± 0.11, LPS/mouse IgG: 0.14 ± 0.12, NS/rat PMN antibody: 0.19 ± 0.02, LPS/rat PMN antibody: 0.25% ± .09% plasma EBD leak into the BAL).
Neutrophil depletion abrogates lung injury in a 2-event model. Rats were pretreated with 200 μL NS or rabbit anti–rat PMN antiserum transfused over a 30-minute period (4 mL/hour), and the rats treated with the anti-PMN antibodies became neutropenic over a 24-hour period, as evidenced by a leukocyte differential obtained from the femoral vein prior to initiating the ALI protocol. The rats then received either NS or LPS intraperitoneally, and 2 hours later were infused with NS, 10% D42 PRBCs, or 0.15 mg/kg OX27. The figure depicts ALI (% BAL EBD/% plasma EBD) as a function of treatment group. Rats injected with NS and infused with NS (■) or injected with LPS and infused with NS ( ) did not evidence ALI. Rats that were infused with isotypic antibody controls, treated with LPS, and infused with either 10% D42 PRBCs or 0.15 mg/kg OX27 had a significant amount of ALI (
) did not evidence ALI. Rats that were infused with isotypic antibody controls, treated with LPS, and infused with either 10% D42 PRBCs or 0.15 mg/kg OX27 had a significant amount of ALI ( ). However, rats given the PMN antisera 24 hours prior to induce neutropenia, as confirmed by leukocyte differentials, did not evidence ALI (▧), similar to the controls. (*P < .05 from LPS/NS; n = 3.)
). However, rats given the PMN antisera 24 hours prior to induce neutropenia, as confirmed by leukocyte differentials, did not evidence ALI (▧), similar to the controls. (*P < .05 from LPS/NS; n = 3.)
Neutrophil depletion abrogates lung injury in a 2-event model. Rats were pretreated with 200 μL NS or rabbit anti–rat PMN antiserum transfused over a 30-minute period (4 mL/hour), and the rats treated with the anti-PMN antibodies became neutropenic over a 24-hour period, as evidenced by a leukocyte differential obtained from the femoral vein prior to initiating the ALI protocol. The rats then received either NS or LPS intraperitoneally, and 2 hours later were infused with NS, 10% D42 PRBCs, or 0.15 mg/kg OX27. The figure depicts ALI (% BAL EBD/% plasma EBD) as a function of treatment group. Rats injected with NS and infused with NS (■) or injected with LPS and infused with NS ( ) did not evidence ALI. Rats that were infused with isotypic antibody controls, treated with LPS, and infused with either 10% D42 PRBCs or 0.15 mg/kg OX27 had a significant amount of ALI (
) did not evidence ALI. Rats that were infused with isotypic antibody controls, treated with LPS, and infused with either 10% D42 PRBCs or 0.15 mg/kg OX27 had a significant amount of ALI ( ). However, rats given the PMN antisera 24 hours prior to induce neutropenia, as confirmed by leukocyte differentials, did not evidence ALI (▧), similar to the controls. (*P < .05 from LPS/NS; n = 3.)
). However, rats given the PMN antisera 24 hours prior to induce neutropenia, as confirmed by leukocyte differentials, did not evidence ALI (▧), similar to the controls. (*P < .05 from LPS/NS; n = 3.)
MHC class I antibodies localize to PMNs in vivo
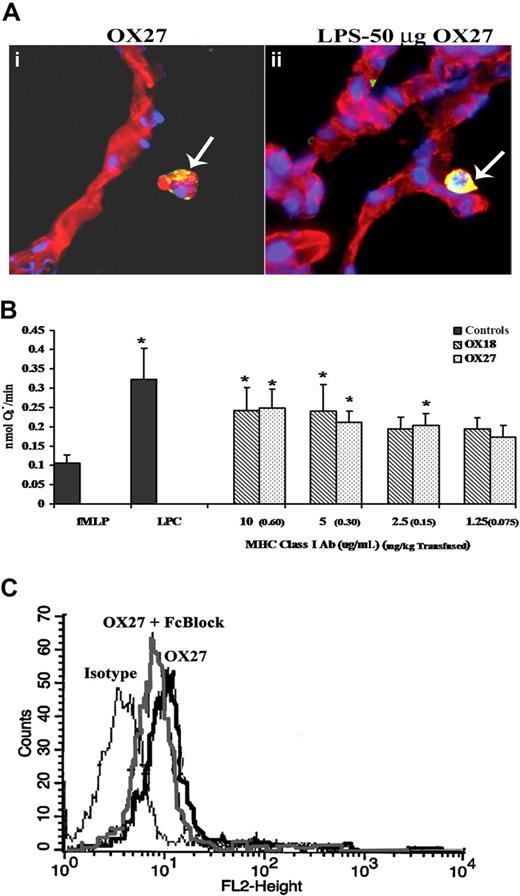
To visualize the cellular locality of the antibodies to the rat MHC class I antigens, lung sections from rats pretreated with NS or LPS followed by 0.15 mg/kg OX27 were stained with bis-benzamide (blue, a nuclear stain) and WGA linked to Cy-3 (red, a membrane specific stain) and probed with donkey anti–mouse antibody labeled with Alexa 488 (green). Multiple lung sections from 3 different animals were visualized, and there were significant amounts of murine antibody (yellow) localized to the surface of the PMNs but not to the surface of the capillary endothelium or to the airway epithelial cells of the alveoli from rats treated with NS/OX27 (Figure 7A). LPS/OX27 (0.15 mg/kg) demonstrated increased yellow colocalization compared with NS/OX27 (Figure 7A). Identical experiments with OX18 yielded similar data (results not shown).
MHC class I Abs bind to the PMN, not the endothelium, and interact with antigens on the PMN surface. (A) Lungs were taken from rats injected with either NS (i) or LPS and infused with 0.15 mg/kg OX27 (ii) and snap frozen, fixed, and sectioned. The lung sections were stained with bis-benzamide (blue, a nuclear stain), WGA linked to Cy-3 (red, a membrane-specific stain) and probed with donkey anti–mouse antibody labeled with Alexa 488 (green). Lungs from rats injected with NS and infused with OX27 had OX27 on the PMN membrane as demonstrated by the colocalization (yellow) of the red membrane WGA and the green Alexa 488 antibody (i, arrow), with no colocalization on the endothelium. Moreover, when the rat was pretreated with LPS and transfused with OX27, which caused ALI, there is more intense colocalization of OX27 with the PMN membrane (arrow) with little to no antibody present on the endothelium or on the lung parenchyma (ii; n = 3). (B) Rat PMNs were isolated from whole blood and 3.5 × 105 PMNs used in each group. PMNs were incubated with 1.25 to 10 μg/mL (equal to 0.075-0.60 mg/kg antibody transfused; in parentheses next to in vitro dose) of either OX18 (▧) or OX27 ( ) for 5 minutes and stimulated with fMLP, and the release of superoxide was measured (nmol O2−/minute). Both OX18 and OX27 caused significant priming (P < .05 from fMLP alone) at the higher concentrations (5-10 μg/mL), although only OX27 primed at 2.5 μg/mL; whereas neither Ab primed at 1.25 μg/mL (*P < .05 from fMLP alone, n = 5). (C) Rat PMNs were isolated and incubated for 10 minutes at 4°C with either buffer or Fc block (Fcγ receptor blocker; Accurate Chemical) followed by a 30-minute incubation at 4°C with PE-labeled OX27 and fixed with paraformaldehyde. PMNs incubated with fluorescent OX27 antibodies demonstrated an increase in mean fluorescent intensity compared with the PMNs incubated with the isotypic controls. The Fc block had little effect on the OX27-mediated fluorescent shift (n = 3).
) for 5 minutes and stimulated with fMLP, and the release of superoxide was measured (nmol O2−/minute). Both OX18 and OX27 caused significant priming (P < .05 from fMLP alone) at the higher concentrations (5-10 μg/mL), although only OX27 primed at 2.5 μg/mL; whereas neither Ab primed at 1.25 μg/mL (*P < .05 from fMLP alone, n = 5). (C) Rat PMNs were isolated and incubated for 10 minutes at 4°C with either buffer or Fc block (Fcγ receptor blocker; Accurate Chemical) followed by a 30-minute incubation at 4°C with PE-labeled OX27 and fixed with paraformaldehyde. PMNs incubated with fluorescent OX27 antibodies demonstrated an increase in mean fluorescent intensity compared with the PMNs incubated with the isotypic controls. The Fc block had little effect on the OX27-mediated fluorescent shift (n = 3).
MHC class I Abs bind to the PMN, not the endothelium, and interact with antigens on the PMN surface. (A) Lungs were taken from rats injected with either NS (i) or LPS and infused with 0.15 mg/kg OX27 (ii) and snap frozen, fixed, and sectioned. The lung sections were stained with bis-benzamide (blue, a nuclear stain), WGA linked to Cy-3 (red, a membrane-specific stain) and probed with donkey anti–mouse antibody labeled with Alexa 488 (green). Lungs from rats injected with NS and infused with OX27 had OX27 on the PMN membrane as demonstrated by the colocalization (yellow) of the red membrane WGA and the green Alexa 488 antibody (i, arrow), with no colocalization on the endothelium. Moreover, when the rat was pretreated with LPS and transfused with OX27, which caused ALI, there is more intense colocalization of OX27 with the PMN membrane (arrow) with little to no antibody present on the endothelium or on the lung parenchyma (ii; n = 3). (B) Rat PMNs were isolated from whole blood and 3.5 × 105 PMNs used in each group. PMNs were incubated with 1.25 to 10 μg/mL (equal to 0.075-0.60 mg/kg antibody transfused; in parentheses next to in vitro dose) of either OX18 (▧) or OX27 ( ) for 5 minutes and stimulated with fMLP, and the release of superoxide was measured (nmol O2−/minute). Both OX18 and OX27 caused significant priming (P < .05 from fMLP alone) at the higher concentrations (5-10 μg/mL), although only OX27 primed at 2.5 μg/mL; whereas neither Ab primed at 1.25 μg/mL (*P < .05 from fMLP alone, n = 5). (C) Rat PMNs were isolated and incubated for 10 minutes at 4°C with either buffer or Fc block (Fcγ receptor blocker; Accurate Chemical) followed by a 30-minute incubation at 4°C with PE-labeled OX27 and fixed with paraformaldehyde. PMNs incubated with fluorescent OX27 antibodies demonstrated an increase in mean fluorescent intensity compared with the PMNs incubated with the isotypic controls. The Fc block had little effect on the OX27-mediated fluorescent shift (n = 3).
) for 5 minutes and stimulated with fMLP, and the release of superoxide was measured (nmol O2−/minute). Both OX18 and OX27 caused significant priming (P < .05 from fMLP alone) at the higher concentrations (5-10 μg/mL), although only OX27 primed at 2.5 μg/mL; whereas neither Ab primed at 1.25 μg/mL (*P < .05 from fMLP alone, n = 5). (C) Rat PMNs were isolated and incubated for 10 minutes at 4°C with either buffer or Fc block (Fcγ receptor blocker; Accurate Chemical) followed by a 30-minute incubation at 4°C with PE-labeled OX27 and fixed with paraformaldehyde. PMNs incubated with fluorescent OX27 antibodies demonstrated an increase in mean fluorescent intensity compared with the PMNs incubated with the isotypic controls. The Fc block had little effect on the OX27-mediated fluorescent shift (n = 3).
MHC class I antibodies prime PMNs
Because BRMs (lipids, sCD40L, and HNA-3A antibodies) implicated in TRALI all prime human PMNs, we tested the priming activity of OX18 and OX27 on rat PMNs.19,20,22,32 Identical to in vivo concentrations, OX27 primed the fMLP-activated respiratory burst at an in vitro concentration of 2.5 μg/mL (0.15 mg/kg), whereas both OX18 and OX27 primed at 5 to 10 μg/mL (0.30-0.60 mg/kg) (Figure 7B). Furthermore, as a positive control, LPCs (4.5 μM) caused significant priming of the oxidase in the same concentrations found in the transfused host (Figure 7B).13,19,32,35 In addition, the priming activity of OX27 and OX18 was unaffected by Fcγ receptor blockade (buffer/fMLP: 0.05 ± 0.02, OX27 [10 μg/mL]/fMLP: 0.147 ± 0.044*; OX27 [10 μg/mL] + Fc block/fMLP: 0.159 ± 0.014*; OX27 [10 μg/mL] + CD32/fMLP: 0.114 ± 0.028*; *P < .05 compared with buffer/fMLP). Importantly, the rat granulocyte antibodies did not prime the oxidase (fMLP: 0.02 ± 0.01; rat PMN antiserum/fMLP: 0.03 ± 0.01).
As further controls, PMNs were first treated with monoclonal antibodies against rat CD32, a commercial Fc blocking reagent (Fc receptor block), or isotypic IgG followed by incubation with either fluorescently labeled OX18 or OX27. Antibody blockade of the Fc receptors had little effect on the gain in mean fluorescence intensity demonstrated by the binding of OX27 to its cognate antigens with similar data found with OX18 (Figure 7C, data not shown).
Discussion
The plasma from stored PRBCs, both prestorage leukoreduced and unmodified (10% D28 and 5%-10% D42)FINAL, and antibodies directed against MHC class I antigens caused ALI as the second event in this 2-event in vivo model. ALI was demonstrated in a concentration-dependent manner in multiple ways including EBD leak, lung histology, increases in total protein and CINC-1 in the BAL, and antibody-mediated TRALI increases in EBD in the pulmonary interstitium. Importantly, in NS-injected rats neither the plasma from stored PRBCs nor the antibodies caused ALI, even when the concentration of OX27 was increased to 4.5 mg/kg, a level at which a monoclonal antibody induced ALI alone in vivo.9 In addition, LPS was not lethal and did not cause (1) EBD leak into the BAL or the pulmonary interstitium or (2) increases in total protein or CINC-1 in the BAL or histologic evidence of ALI, but did induce pulmonary sequestration of PMNs.19 LPS was used as the first event because acute, active infection (bacterial or viral) was implicated as a predisposing clinical event in TRALI.18 Bacterial and viral infections induce proinflammatory activation of the vascular endothelium, which leads to adherence/sequestration of PMNs.21,36,,–39 Other first events have been implicated, including cytokine administration, massive transfusion, recent surgery (especially cardiovascular surgery), and induction chemotherapy, but only infections have been studied to date in vivo.2,18,27,40,,,–44 PMNs were also required because granulocyte depletion inhibited ALI. Furthermore, the OX18 and OX27 antibodies demonstrated both antigen recognition and the ability to prime the fMLP-activated respiratory burst of rat PMNs, which were not affected by Fc receptor blockade, implying that these interactions were specific to antibody:antigen binding. Lastly, granulocyte (PMN)–specific antibodies, which caused immunodepletion of PMNs from rats, did not elicit ALI nor did they prime the rat PMN oxidase.
Both clinical TRALI and this model are similar, with the onset of ALI within 6 hours, mortality of 5% to 10%, identical histologic evidence of ALI, and a dose-response relationship of antibodies or plasma from stored PRBCs to elicit TRALI as the second event such that lower concentrations did not cause TRALI or elicited milder ALI.5,45,46 Furthermore, ALI in this model was determined by EBD leak into the BAL because EBD complexes with albumin and albumin leak into the alveolar spaces are synonymous with the noncardiogenic pulmonary edema (eg, TRALI)5,45,–47 ; ALI was also determined by increases in total protein and CINC-1 in the BAL, known markers of ALI.48,–50 None of these animals were volume overloaded as determined by the measurement of rat BNP-32 from post-ALI plasma from all animals in Figure 1.51,52 The observed ALI was also PMN dependent as postulated for clinical TRALI and demonstrated in a murine model.9 Furthermore, antibody-induced PMN depletion did not cause ALI in the rats, although such granulocyte antibodies have been implicated in TRALI.6,10,14 These data are to be expected if one examines human alloimmune neutropenia in which maternal antibodies cross the placenta and immunodeplete the PMNs in the fetus or following transfusion of donor antibodies that bind to the PMNs of the recipient and cause immunodepletion, termed transfusion-related alloimmune neutropenia (TRAIN).53,–55 Alloimmune neutropenia is dependent upon the type of the antibody or the antigen recognized. Antibodies that prime PMNs through antigen recognition (eg, induce a proinflammatory change) may lead to TRALI and not antibody-mediated clearance, as shown in the presented model.
Antibodies directed against MHC class I antigens could also produce ALI as the second event, congruous with several donor look-back studies that demonstrated that transfusion of a donor antibody to a recipient that expressed the cognate antigen did not always cause TRALI, and the clinical condition of the patient appeared relevant for its genesis.15,–17 In addition, these antibodies were found on the surface of PMNs that were sequestered in the lung, but not on the surface of pulmonary vascular endothelium as demonstrated in the murine model.9 Both OX18 and OX27 primed the rat PMN oxidase in vitro, identical to previous data that demonstrated that antibodies to HNA-3a could prime PMNs that expressed HNA-3a on their surface; moreover, Fc receptor blockade did not affect priming activity or cognate antigen immunoreactivity, implying specificity for these antigen:antibody interactions.20 Therefore, several specific prerequisites appear to be required for antibody-induced TRALI that may explain its relatively low prevalence: (1) the recipient has an underlying clinical condition that predisposes to TRALI, (2) the donor antibody must recognize the cognate antigen on the host's leukocytes that are sequestered in the lung, and (3) the antigen:antibody interaction must cause a proinflammatory change in the PMN.
With regard to antigen specificity, OX18 recognizes the RT1A region of the rat MHC class I locus.56,,,–60 Treatment of rats with OX18 or its F(ab′)2 fragments enhances natural killer (NK)–cell cytolysis of syngeneic target cells, independent of antibody-dependent cellular cytotoxicity.60 Furthermore, OX18 is a monomorphic determinant of the entire MHC class I locus and recognizes loci in rats that are homologues to identical regions in mice.56,61,,,,–66 These RT1A regions are similar to the HLA-A, HLA-B, and HLA-C loci in humans, although there is no direct correlation.56,61,,,,–66 In contrast, OX27 is a polymorphic determinant that specifically recognizes the RT1Ac locus and has been used to differentiate myelogenous cells from cells of neurogenic origin.67,,,,,–73 Importantly, with regard to precipitating TRALI, OX27 caused ALI in the 2-event model at lower concentrations than OX18, which may be due to its cognate antigen being more numerous on the PMN membrane, although these differences may also be related to the function of the specific antigens recognized on the PMN surface or the relative strength of antibody binding.73 Furthermore, both antibodies also produced less ALI at higher doses, a phenomenon already observed by other groups: the first used murine antibodies against rat platelets in vivo in which administering increasing antibody concentrations did not cause more thrombocytopenia, and the second was the administration of human antibodies to bovine serum albumin (BSA), and BSA to rats did not result in the production of more immune complexes.74,75 The exact nature of this phenomenon is not well understood, but could be due to a protective effect of the Fc-γ receptor increasing the clearance of the antibody. Further work is required to elucidate the differences in these antibodies with regard to their ability to cause ALI. In addition, the ability of the plasma from stored PRBCs, both LR-PRBCs and unmodified, to cause ALI as the second event in this 2-event model is compatible with previous clinical data and has provided in vivo evidence that the presence of biologically active lipids present in LR-PRBC that are structurally dissimilar to LPCs (data not shown) may explain why prestorage leukoreduction has not decreased the incidence of TRALI or the incidence of ARDS and multiple organ failure in trauma patients in a randomized clinical trial.76,–78
The presented model is superior to other in vivo modeling because it better approximates clinical TRALI: (1) the first event, LPS, approximates acute active infection, a proposed risk factor for TRALI, and causes proinflammatory activation of the pulmonary capillary bed without causing death or other organ injury.13,19,79,,,–83 LPS has been used in many animal models and even injected into human volunteers and mimics infection.13,19,79,,,,,–85 (2) ALI was induced by 2 separate second events, like clinical TRALI, and the second events demonstrated a concentration:response relationship in ALI using multiple parameters, unlike other in vivo models.9 (3) The MHC class I antibodies localized to the antigen on the PMN membrane, identical to Popovsky's proposed antibody pathophysiology, and did not implicate Fc-mediated indirect activation, reminiscent of immune complex disease, not currently implicated in clinical TRALI.5,9,10 (4) The novel pathophysiology of antibody- and BRM-mediated TRALI in this model is due to direct proinflammatory changes (priming) of PMNs induced by the second events, synonymous with in vitro studies using human pulmonary endothelium, PMNs and granulocyte antibodies, sCD40L, or lipids.19,,–22,32,35 This proinflammatory requirement may explain why not all antibodies may cause TRALI and induce TRAIN. (5) Disparate from the murine model, this model used antibody concentrations 15- to 30-fold less and did not require supraphysiologic concentrations of a specific antibody. If one calculates the amount of this specific antibody needed to cause clinical TRALI, then this antibody must comprise 10% of all IgG in 200 mL transfused FFP.9,20
In conclusion, the presented model is congruous with human TRALI and demonstrates that both plasma from stored PRBCs and antibodies against MHC class I antigens may precipitate TRALI as a second event in a 2-event in vivo model. To avoid TRALI, many blood providers have initiated the use of male-only plasma, to decrease the number of donor HLA antibodies infused into patients who may express the cognate antigen. However, antibody:antigen recognition alone does not precipitate TRALI and the underlying clinical condition of the patient is important.15,–17 In addition, in a recent review of published TRALI cases, 48% of the implicated donors were male.86 Washing of products may decrease patient exposure to plasma containing antibodies or BRMs. Importantly, TRALI is heterogeneous and has been reported in neutropenic patients, and in these patients permeability agents (eg, VEGF) have been implicated, indicating that further work is required to understand TRALI and to make transfusions safer.87
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This research was supported in part by Bonfils Blood Center and grants HL-59355 and GM-4922 from the National Heart, Lung and Blood Institute (NHLBI, Bethesda, MD) and National Institute of General Medical Sciences (NIGMS, Bethesda, MD), respectively.
National Institutes of Health
Authorship
Contribution: M.R.K., T.M., S.D., X.M., X.L., J.N., S.S.G., S.Y.K., Y.S., and F.G.-R. performed experiments; M.R.K and C.C.S wrote the paper; and E.E.M. and C.C.S. designed the experiments.
Conflict-of-interest disclosure: C.C.S. received a research grant from Navigant and an honorarium from Pall Corporation. The remaining authors declare no competing financial interests.
Correspondence: Christopher C. Silliman, Research Department, Bonfils Blood Center, 717 Yosemite Street, Denver, CO 80230; e-mail: christopher.silliman@uchsc.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal