Abstract
Allogeneic hematopoietic stem cell transplantation (HSCT) is the only curative treatment for Griscelli syndrome type 2, an inherited immune disorder causing fatal hemophagocytic lymphohistiocytosis (HLH). Optimal therapeutic modalities are not yet well known. We retrospectively analyzed the outcome for 10 patients who underwent HSCT in a single center between 1996 and 2008. Seven patients (70%) were cured of the primary immune defect (mean follow-up, 5.2 years; range, 0.8-12.0 years), 4 of them without neurologic sequelae. In the 3 deceased patients, death occurred within 110 days of HSCT and was probably due to adverse reaction to HSCT in 2 patients and to HLH relapse in one patient. One patient received 2 transplants because of graft failure. Clinical events included veno-occlusive disease (n = 5), acute (n = 7) or chronic (n = 1) graft-versus-host disease II-III, and Epstein-Barr virus–induced lymphoproliferative disease (n = 2). Of the 7 patients with neurologic involvement before HSCT, 4 survived and 2 presented sequelae. Furthermore, 1 patient lacking neurologic involvement before HSCT developed long-term sequelae. These results demonstrate the efficacy of HSCT in curing the immune disorder but also show that neurologic HLH before HSCT is a major factor, given the neurologic sequelae after otherwise successful HSCT. Additional studies are required to improve treatment.
Introduction
Griscelli syndrome type 2 (GS2; Online Mendelian Inheritance in Man [OMIM] no. 607624) is an autosomal, recessively inherited disease. Phenotypically, it is associated with hypopigmentation (characterized by the silvery sheen of a patient's hair and eyelashes) and hemophagocytic lymphohistiocytosis (HLH), which is fatal if not treated.1 Characteristic hypopigmentation is a common feature among individuals with GS1, GS2, and GS3. GS1, caused by a myosin 5a-defect, mostly associates the hypopigmentation with a primary neurologic impairment presenting with muscular hypotonia at onset, mental retardation, or regressive neurologic processes,2,3 while GS3, caused by a melanophilin-defect is restricted to hypopigmentation.4 GS2 is caused by mutations in the gene encoding the small GTPase Rab27a.5 Rab27a-deficiency causes defects in the exocytosis of cytotoxic granules from T cells and natural killer (NK) cells (accounting for an impaired cytotoxicity6 ) and melanosome exocytosis.4 A natural mouse model of HLH in GS2 (the ashen mouse) has also been reported.7
Hemophagocytic lymphohistiocytosis is characterized by overwhelming T cell and macrophage activation that leads to fever, splenomegaly, cytopenia, hypofibrinogenemia, and/or hypertriglyceridemia and hyperferritinemia.8 HLH in GS2, in Chédiak-Higashi syndrome, and in most familial HLH syndromes, such as perforin- and munc13-4-deficiencies, share common pathogenic mechanisms. In these patients with impaired cytotoxicity, pathogenesis of HLH is most probably based on the effector cells' inability to kill antigen-presenting cells.9,10 Although infected cells can be recognized (leading to cytotoxic cell activation and expansion of the T-cell pool), the resulting cytotoxic cell population fails to kill infected cells and thus to remove the infectious source of stimulation.
If not treated, HLH in GS2 patients has a poor prognosis. Removal of the HLH trigger and chemotherapy- or immunotherapy-based treatment can achieve remission from HLH.8,11 However, allogeneic hematopoietic stem cell transplantation (HSCT) is the only currently available curative treatment for GS2. The first successful HSCT in a GS2 patient was reported by Schneider et al in 1990.12 Few cases of successful HSCT from compatible donors have been reported in children with GS2 thereafter.13-17 A recent study emphasizes the neurologic sequelae in 5 of 5 GS2 patients who received HSCT after treatment according to the HLH-94/HLH-2004 protocol.18 The present study shows and discusses the results and complications of HSCT performed in 10 GS2 patients in a single center between 1996 and 2008.
Methods
Patients
Between spring 1996 and fall 2008, 10 GS2 patients underwent HSCT (with a related or unrelated donor) at Necker Children's Hospital (Paris, France). One additional patient was not considered for transplantation due to severe neurologic involvement and another additional patient's family refused HSCT. The Histiocyte Society's criteria were used for HLH diagnosis.8 Central nervous system involvement was determined via clinical examination, cerebrospinal fluid (CSF; for pleocytosis), and neuroradiologic studies. We performed a retrospective analysis of the HSCT outcomes. The end point for analysis was February 6, 2009. First-line treatment of HLH consisted of an immunotherapy regimen with corticosteroids and cyclosporin A (CSA), combined (if required) with antithymoglobulin (ATG; IMTIX-Sangstat)19 (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article), and, in one case, etoposide (VP-16; according to the treatment protocol detailed by Henter et al8 ). Patients with neurologic involvement received intrathecal injections of a methotrexate (MTX)–hydrocortisone combination. Patient 7 (P7), suffering from peripheral neuropathy, received intrathecal treatment as well. From 2001 onwards, patients with Epstein-Barr virus (EBV)–triggered HLH were treated with an anti-CD20 antibody (rituximab). Complete remission20 before HSCT was defined by the complete disappearance of the clinical and biologic criteria of HLH; partial remission (PR) was defined as a significant improvement but with persistent clinical and/or biologic manifestations. The retrospective data collection was performed in accordance with the ethical committee of the Hôpital Necker-Enfants Malades.
Transplantation procedures
In the absence of a human leukocyte antigen (HLA)-identical sibling donor, the decision to perform haploidentical HSCT was made when matched, unrelated donors (URDs) were not found within a 3-month search period. Bone marrow was used as the stem cell source. The conditioning regimen for HSCT consisted in rabbit ATG (25-50 mg/kg), busulfan (16 or 20 mg/kg according to weight, adapted if necessary according to the measured busulfan plasma concentration, and administered intravenously in all patients other than P1, P2, and P3, who received oral busulfan), and cyclophosphamide (200 mg/kg). The conditioning regimen in P1 additionally consisted of anti–lymphocyte function–associated antigen-1 (anti–LFA-1) and anti-CD2 antibodies. Transplants from URDs or haploidentical donors were T cell–depleted by positive CD34+ selection using the Clinimacs system (Miltenyi Biotec). All patients received appropriate anti-infectious therapy; antimicrobial prophylaxis during the transplantation period consisted of gut decontamination with nonabsorbable antibiotics, itraconazole during aplasia, and acyclovir (750 mg/m2 or 1500 mg/m2 for herpes simplex or cytomegalovirus [CMV] prophylaxis, respectively) from day −1 to day 60. All patients received intravenous immunoglobulin and were placed in a sterile isolator or laminar flow room. They received parental nutrition through a central venous catheter, if necessary. One patient received a second HSCT because of primary graft failure; before undergoing the second HSCT, this patient received immunosuppressive treatment with corticosteroids and CSA to improve the chances of sustained remission. Myeloid engraftment was confirmed by chimerism analysis and an absolute neutrophil count greater than 0.5 × 109/L for 3 consecutive days. Chimerism was assessed on whole blood and, in cases of mixed chimerism, on positively selected CD3+ T cells and negatively selected CD3− mononuclear cells by using variable nucleotide tandem repeat marker analysis or X and Y chromosome-specific probes in fluorescence in situ hybridization in cases of gender mismatch. Standard prophylaxis for graft-versus-host disease (GVHD) included the use of CSA and short-course MTX for non–T cell–depleted HSCT. The GVHD was graded as described elsewhere21 and, whenever possible, confirmed by appropriate histologic studies. The diagnosis of hepatic veno-occlusive disease (VOD) was made according to the Baltimore criteria.22 After HSCT, patients were investigated annually in the outpatient department via a clinical examination and laboratory tests including chimerism analysis. Neuroradiologic studies were repeated at least once after HSCT and as often as was appropriate. Neuropsychologic performance and school attendance were recorded.
Results
Patient and disease characteristics before HSCT
RAB27A sequencing revealed biallelic nonsense mutations in 8 of the 10 patients (Table 1). Six of these were homozygous. Missense mutations were found in one patient (P5). In another patient (P9), no RAB27A mutations could be found in either the genomic DNA or the cDNA. In this patient (P9), diagnosis of GS2 was based on the combination of HLH, decreased CD3+ T cell–mediated cytotoxicity, and typical microscopic hair shaft features. P4 and P7 were siblings and had an unusual anomaly of the RAB27A gene, since they had an alternative duplication of exon 2 (in the absence of protein expression, as assessed by Western blot analysis). In P1 and P2, diagnosis was made before 2000 based on the combination of HLH, decreased CD3+ T cell–mediated cytotoxicity, and typical microscopic hair shaft features; genetic analyses in these 2 patients were performed retrospectively on preserved material. The mean age at the first episode of HLH in patients with nonsense RAB27A mutations was 3.6 years (median, 3.0 years; range, 0.0-10.7 years) and 6.9 years in P5 with missense mutations. Disease onset in the oldest patient (P4, age 10.7 years) probably occurred earlier than had been documented, since putative HLH had been described as fever of unknown origin. The diagnostic criteria for HLH were fulfilled in 6 of the 10 patients during their first episode of HLH and partially fulfilled in 4 patients (P10, P8, P7, and P9). In fact, in P10, sequencing of RAB27A and the diagnosis of GS2 were made before any HLH because of the silvery sheen of the patient's hair and a prophylactic treatment with CSA was initiated at the age of 4 months. The first episode of HLH was associated with an EBV infection in 4 patients. In all, 6 patients experienced EBV-associated HLH before undergoing HSCT. Anti-CD20 antibody treatment was successfully applied in 3 patients with EBV-triggered HLH (P5, P7, and P10). Two patients (P5, P8) had concomitant neurologic HLH at first presentation, and 3 patients (P3, P4, P7) developed neurologic symptoms during the pre-HSCT period (P3: facial paralysis, somnolence, vomiting, bilateral papillary edema, signs of cerebral edema in brain computer tomography; P4: ascending areflexic paralysis progressing to respiratory failure requiring mechanical ventilation during 10 days; P7: peripheral neuropathy located in the lower legs). Histopathologic examination of a sural nerve biopsy from P4 revealed interstitial and perivascular cell infiltrates mainly composed of lymphocytes and, rarely, macrophages in the vicinity of the sural nerve. In P2 and P6, neurologic HLH was, respectively detected by pleocytosis in the CSF and abnormal lesions on brain magnetic resonance imaging (MRI) without any clinical correlate. Abnormal brain MRI findings were present in 4 patients before HSCT: white matter lesions were seen in P3, P6, and P8, and rare, small-sized intracerebral hemorrhages were observed in P5. The longest total duration of HLH was observed in the 2 patients with the longest delay in the onset of immunosuppressive treatment (P3, P4) and in 1 patient with early-onset HLH (P1). Before HSCT, complete remission from HLH was achieved by P5, P7, and P8, and complete clinical remission with persistence of some abnormal laboratory results was achieved by P4, P6, and P9. One patient (P2) had active disease at the time of HSCT.
Patient characteristics before HSCT
| Patient . | Sex . | RAB27A mutation . | Effect of mutation . | Age at HLH onset, y . | Time until onset of HLH treatment . | Number of HLH relapses . | Neurologic disease . | Infectious trigger . | Treatment . | Total duration of HLH, wks . | HLH status prior HSCT . | Reference . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 (UPN 389) | F | del154-343 × 2 | deletion exon 3 + 4 | 0.0 | 2 wks | 1 | CS, CSA, ATG | 44 | PR (HM) | P1(5) | ||
| 2 (UPN 483) | F | del154-343 × 2 | deletion exon 3 + 4 | 2.0 | <1 wk | 0 | Isolated CSF pleocytosis | EBV | CS, CSA, ATG, MTX i.t., VP16 | 16 | active disease (fever, anemia, hyperferritinemia, hypertriglyceridemia) | P18(5) |
| 3 (UPN 533) | M | del154-343 × 2 | deletion exon 3 + 4 | 5.1 | 12 mos | 5 | Facial paralysis, intracranial hypertension, CSF pleocytosis | EBV CMV | CS, CSA, MTX i.t. | 76 | PR (HSM, bicytopenia) | |
| 4 (UPN 556) | F | duplication of exon 2 × 2 | duplication of exon 2 | 10.7 | 8 mos | 1 | Peripheral neuropathy | EBV | CS, CSA | 27 | PR (pleocytosis in CSF) | |
| 5 (UPN 643) | F | T209C and C227T | L70P and A76V | 6.9 | 2 wks | 0 | Coma | EBV | CS, CSA, ATG, MTX i.t., anti-CD20 | 10 | CR | |
| 6 (UPN 635) | M | del154-343 × 2 | deletion exon 3 + 4 | 0.8 | 1 wk | 0 | Abnormal MRI | CS, CSA, ATG, MTX i.t. | 14 | PR (anemia) | ||
| 7 (UPN 688) | M | duplication of exon 2 × 2 | duplication of exon 2 | 5.4 | <1 wk | 2 | Peripheral neuropathy | EBV | CS, CSA, ATG, MTX i.t., anti-CD20 | 5 | CR | |
| 8 (UPN 716) | M | del510AAGCC and C550T | frameshift and R184X | 2.2 | 4 wks | 0 | Seizures | CS, CSA, MTX i.t. | 8 | CR | ||
| 9 (UPN728) | F | No RAB27A mutation | 3.0 | <1 wk | 1 | CS, CSA | 3 | PR (hyperferritinemia) | ||||
| 10 (UPN 744) | F | C352T x2 | Q118X | 3.1 | <1 wk | 0 | EBV | CS, CSA, anti-CD20 | 4 | PR (HM, anemia) |
| Patient . | Sex . | RAB27A mutation . | Effect of mutation . | Age at HLH onset, y . | Time until onset of HLH treatment . | Number of HLH relapses . | Neurologic disease . | Infectious trigger . | Treatment . | Total duration of HLH, wks . | HLH status prior HSCT . | Reference . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 (UPN 389) | F | del154-343 × 2 | deletion exon 3 + 4 | 0.0 | 2 wks | 1 | CS, CSA, ATG | 44 | PR (HM) | P1(5) | ||
| 2 (UPN 483) | F | del154-343 × 2 | deletion exon 3 + 4 | 2.0 | <1 wk | 0 | Isolated CSF pleocytosis | EBV | CS, CSA, ATG, MTX i.t., VP16 | 16 | active disease (fever, anemia, hyperferritinemia, hypertriglyceridemia) | P18(5) |
| 3 (UPN 533) | M | del154-343 × 2 | deletion exon 3 + 4 | 5.1 | 12 mos | 5 | Facial paralysis, intracranial hypertension, CSF pleocytosis | EBV CMV | CS, CSA, MTX i.t. | 76 | PR (HSM, bicytopenia) | |
| 4 (UPN 556) | F | duplication of exon 2 × 2 | duplication of exon 2 | 10.7 | 8 mos | 1 | Peripheral neuropathy | EBV | CS, CSA | 27 | PR (pleocytosis in CSF) | |
| 5 (UPN 643) | F | T209C and C227T | L70P and A76V | 6.9 | 2 wks | 0 | Coma | EBV | CS, CSA, ATG, MTX i.t., anti-CD20 | 10 | CR | |
| 6 (UPN 635) | M | del154-343 × 2 | deletion exon 3 + 4 | 0.8 | 1 wk | 0 | Abnormal MRI | CS, CSA, ATG, MTX i.t. | 14 | PR (anemia) | ||
| 7 (UPN 688) | M | duplication of exon 2 × 2 | duplication of exon 2 | 5.4 | <1 wk | 2 | Peripheral neuropathy | EBV | CS, CSA, ATG, MTX i.t., anti-CD20 | 5 | CR | |
| 8 (UPN 716) | M | del510AAGCC and C550T | frameshift and R184X | 2.2 | 4 wks | 0 | Seizures | CS, CSA, MTX i.t. | 8 | CR | ||
| 9 (UPN728) | F | No RAB27A mutation | 3.0 | <1 wk | 1 | CS, CSA | 3 | PR (hyperferritinemia) | ||||
| 10 (UPN 744) | F | C352T x2 | Q118X | 3.1 | <1 wk | 0 | EBV | CS, CSA, anti-CD20 | 4 | PR (HM, anemia) |
Anti-CD20 indicates rituximab; ATG, anti-thymoglobulin; CR, complete remission; CS, corticosteroids; CSA, cyclosporin A; EBV, Epstein-Barr virus; HLH, hemophagocytic lymphohistiocytosis; H(S)M, hepato(spleno)megaly; MTX i.t., intrathecal methotrexate with steroids; and PR, partial remission.
Engraftment and survival
Ten patients received a total of 11 transplants (Table 2). The mean age at time of HSCT was 4.5 years (range, 0.5-11.5 years). Engraftment occurred in 10 of the attempts. Primary engraftment failure occurred in one patient (P1). One patient (P6) had secondary engraftment failure with progressive loss of donor chimerism (from 82% of lymphocytes in the blood on day 20 to 10% of CD3+ cells on day 92 post-HSCT). Three patients (P4, P6, and P7) died within 110 days of HSCT (on days 99, 105, and 110, respectively). One of these patients (P6) died during treatment of EBV-induced lymphoproliferative disease and HLH. The other 2 patients died of acute respiratory distress, most likely resulting from capillary leak syndrome in P4 and alveolar hemorrhage in P7 who was suffering from grade 3 GVHD and multiple post-HSCT infections. Seven patients have survived with a mean follow-up after HSCT of 5.2 years (range, 0.8-12.0 years) and a mean age at last follow-up of 9.8 years (range, 4.8-14.8 years).
HSCT and outcomes
| Patient . | Age at HSCT, y . | Conditioning regimen . | Donor . | Amount of CD34+ cells, × 106 cells/kg . | T-cell depletion . | Engraftment . | Acute GVHD . | Chronic GVHD . | Other complications . | Infections . | Follow-up after HSCT, days . | Outcome/clinical status . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 (UPN 389) | 0.5 (1st HSCT) | Bu (by mouth), Cy, anti-LFA1, anti-CD2 | URD 10/10 | nd | + | No | 0 | HLH | ||||
| 1 (UPN 389) | 0.8 (2nd HSCT) | ATG, Bu (by mouth), Cy anti-LFA1, anti-CD2 | URD 10/10 | 10.4 | + | Yes | III | Extensive | 4343 (12 years) | Alive, impaired cognitive abilities | ||
| 2 (UPN 483) | 2.3 | ATG, Bu (by mouth), Cy | HLA id | 2.5 | − | Yes | 0 | 0 | 3414 (9 years) | Alive, well | ||
| 3 (UPN 533) | 6.8 | ATG, Bu (by mouth), Cy | HLA id | 3.4 | − | Yes | II | 0 | VOD, AH, DIC | Adenovirus, CMV, bacterial sepsis | 2894 (8 years) | Alive, impaired cognitive abilities, necrosis of femoral head |
| 4 (UPN 556) | 11.5 | ATG, Bu, Cy | HLA id | 5.4 | − | Yes | I | NA | VOD, CLS | 99 | Death (ARDS) | |
| 5 (UPN 643) | 7.2 | ATG, Bu, Cy | URD 10/10 | 5.4 | − | Yes | II | 0 | LPD, pancreatitis | VZV, EBV, Aspergillus nidulans pneumonia | 1585 (4 years) | Alive, well |
| 6 (UPN 635) | 1.0 | ATG, Bu, Cy | Phenoid parent | 1.5 | − | Yes | 0 | NA | VOD, LPD, HLH | EBV | 105 | Death (LPD, HLH) |
| 7 (UPN 688) | 7.4 | ATG, Bu, Cy | URD 9/10 | 2.5 | − | Yes | III | NA | HC (BK-virus), AH | Adenovirus, CMV, Aspergillus, HSV, BK-virus | 110 | Death (ARDS) |
| 8 (UPN 716) | 2.9 | ATG, Bu, Cy | URD 9/10 | 5.8 | + | Yes | II | 0 | VOD, CLS | Adenovirus | 651 (20 months) | Alive, epilepsy |
| 9 (UPN728) | 5.3 | ATG, Bu, Cy | Haploid | 3.0 | + | Yes | II | 0 | VOD | Parvovirus B19, VZV, Bacterial sepsis | 519 (17 months) | Alive, well |
| 10 (UPN 744) | 4.3 | ATG, Bu, Cy | Haploid | 7.3 | + | Yes | II | 0 | Severe mucositis | Bacterial sepsis | 309 (10 months) | Alive, well |
| Patient . | Age at HSCT, y . | Conditioning regimen . | Donor . | Amount of CD34+ cells, × 106 cells/kg . | T-cell depletion . | Engraftment . | Acute GVHD . | Chronic GVHD . | Other complications . | Infections . | Follow-up after HSCT, days . | Outcome/clinical status . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 (UPN 389) | 0.5 (1st HSCT) | Bu (by mouth), Cy, anti-LFA1, anti-CD2 | URD 10/10 | nd | + | No | 0 | HLH | ||||
| 1 (UPN 389) | 0.8 (2nd HSCT) | ATG, Bu (by mouth), Cy anti-LFA1, anti-CD2 | URD 10/10 | 10.4 | + | Yes | III | Extensive | 4343 (12 years) | Alive, impaired cognitive abilities | ||
| 2 (UPN 483) | 2.3 | ATG, Bu (by mouth), Cy | HLA id | 2.5 | − | Yes | 0 | 0 | 3414 (9 years) | Alive, well | ||
| 3 (UPN 533) | 6.8 | ATG, Bu (by mouth), Cy | HLA id | 3.4 | − | Yes | II | 0 | VOD, AH, DIC | Adenovirus, CMV, bacterial sepsis | 2894 (8 years) | Alive, impaired cognitive abilities, necrosis of femoral head |
| 4 (UPN 556) | 11.5 | ATG, Bu, Cy | HLA id | 5.4 | − | Yes | I | NA | VOD, CLS | 99 | Death (ARDS) | |
| 5 (UPN 643) | 7.2 | ATG, Bu, Cy | URD 10/10 | 5.4 | − | Yes | II | 0 | LPD, pancreatitis | VZV, EBV, Aspergillus nidulans pneumonia | 1585 (4 years) | Alive, well |
| 6 (UPN 635) | 1.0 | ATG, Bu, Cy | Phenoid parent | 1.5 | − | Yes | 0 | NA | VOD, LPD, HLH | EBV | 105 | Death (LPD, HLH) |
| 7 (UPN 688) | 7.4 | ATG, Bu, Cy | URD 9/10 | 2.5 | − | Yes | III | NA | HC (BK-virus), AH | Adenovirus, CMV, Aspergillus, HSV, BK-virus | 110 | Death (ARDS) |
| 8 (UPN 716) | 2.9 | ATG, Bu, Cy | URD 9/10 | 5.8 | + | Yes | II | 0 | VOD, CLS | Adenovirus | 651 (20 months) | Alive, epilepsy |
| 9 (UPN728) | 5.3 | ATG, Bu, Cy | Haploid | 3.0 | + | Yes | II | 0 | VOD | Parvovirus B19, VZV, Bacterial sepsis | 519 (17 months) | Alive, well |
| 10 (UPN 744) | 4.3 | ATG, Bu, Cy | Haploid | 7.3 | + | Yes | II | 0 | Severe mucositis | Bacterial sepsis | 309 (10 months) | Alive, well |
AH indicates alveolar hemorrhage; ARDS, acute respiratory stress syndrome; ATG, anti-thymoglobulin; Bu, busulfan; CLS, capillary leak syndrome; CMV, cytomegalovirus; Cy, cyclophosphamide; DIC, disseminated intravascular coagulation; EBV, Epstein-Barr virus; GVHD, graft-versus-host disease; HC, hemorrhagic cystitis; HSV, herpes simplex virus; LPD, lymphoproliferative disease; NA, not applicable; nd, not determined; URD, matched unrelated donor; VOD, veno-occlusive disease; and VZV, varicella zoster virus.
Toxicity, GVHD, and infections
VOD was observed in 5 patients. Apparently, VOD might have occurred less frequently if ATG-containing immunosuppression was used for HLH treatment before HSCT (1 case of VOD of 5 in ATG-treated patients vs 4 of 5 in non–ATG-treated patients), suggesting that HLH could be a predisposing factor for VOD, but the difference does not reach significance (P = .067). Other factors related to disease activity could also play a role. Busulfan levels did not correlate with the occurrence of VOD. The total duration of HLH before HSCT was slightly but not significantly longer in the patients who developed VOD, compared with VOD-free patients (mean duration ± SD, 26 ± 13 vs 16 ± 7 weeks, respectively). Of the 3 patients who died after HSCT, 2 had developed VOD. Patients having developed VOD did not significantly differ from those without VOD in terms of age at HSCT or graft type. Grade II-III acute (n = 7) and chronic (n = 1) GVHD was successfully controlled in all cases with the exception of P7. Post-HSCT adenovirus, CMV, and EBV infections occurred in 3, 2, and 2 cases, respectively. In view of the small number of patients, we were not able to establish any correlations between infections, GVHD, and pre- or post-HSCT immunosuppression status. Alveolar hemorrhage occurred in 2 patients, capillary leak syndrome occurred in another 2 patients, and pancreatitis was observed in 1 patient.
EBV-induced lymphoproliferative disease and HLH recurrence
Two patients (P5 and P6) developed EBV-induced lymphoproliferative disease on days 20 and 67 after HSCT, respectively. A laryngeal location in P5 led to respiratory distress and required mechanical ventilation from day 20 to day 24 after HSCT. A local resection was performed, and the patient was treated with anti-CD20 antibody; no relapse occurred. In P6, donor chimerism on day 20 after HSCT revealed 82% of donor lymphocytes. On day 67, EBV-induced lymphoproliferative disease was diagnosed, together with massive hepatosplenomegaly and an increase in the EBV load and B-cell count (832 × 109/L CD19+ cells; 64% of the blood lymphocytes were CD19+). Despite treatment with anti-CD20 antibody (6 courses), cyclophosphamide, vincristine, and prednisone (on day 98), the tumoral mass grew. Donor lymphocyte reinjections were performed on days 78 and 85. By day 88 after HSCT, the patient had clear manifestations of HLH, and autologous T cells were detected in the blood. Despite further treatment with corticoids (including a bolus on day 94), anti-CD52 antibodies (day 95), and etoposide (day 102), the patient developed severe neurologic involvement and died on day 105 after HSCT.
Neurologic outcome
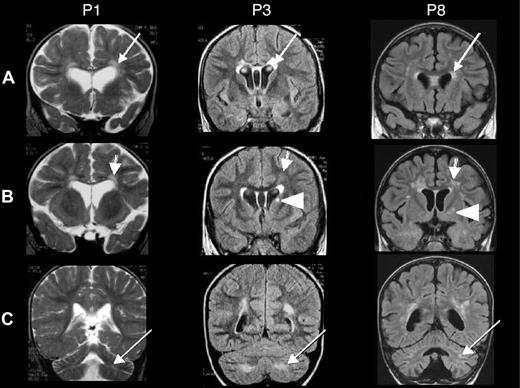
Of the 7 patients with neurologic HLH before HSCT, 3 patients (P4, P6, and P7) died shortly after HSCT. Two of the 7 patients (P2 and P5) have not shown any neurologic sequelae 9 and 4 years after HSCT, respectively. In contrast, 2 patients (P3 and P8) exhibited the following neurologic sequelae after HSCT: P3 showed impaired cognitive abilities with a clinically estimated developmental age of 5 years for a chronologic age of 7. Brain MRI showed abnormal hyperintensities in the periventricular white matter and signal abnormality in the caudate nucleus (Figure 1 middle panel). P8 continues to suffer from pharmacologically resistant epilepsy and also presents ataxia. Brain MRI showed cerebellar and periventricular white matter lesions and signal abnormality in the putamen (Figure 1 right panel). Furthermore, P1 (having suffered from neonatal-onset HLH, but who had not presented any neurologic involvement before HSCT) developed cognitive difficulties after HSCT and showed a clinically estimated developmental age of 3 years for a chronologic age of 6.5. Here again, brain MRI showed white matter abnormalities in the periventricular and cerebellar areas (Figure 1 left panel). Taken together, of the 7 patients who survived, 4 patients (P2, P5, P9, P10) were cured without neurologic sequelae with a mean follow-up after HSCT of 4 years (range, 10 months to 9 years).
Brain MRIs of patients 1, 3, and 8. Coronal brain MRIs of patients P1 (taken at age 20 months, 11 months after HSCT), P3 (age 6 years, 2 months before HSCT), and P8 (age 3 years, 3 months after HSCT). T2-weighted images are shown for P1, and fluid attenuation inversion recovery (FLAIR) images are shown for P3 and P8. (A) Bilateral, abnormal hyperintensities in the periventricular white matter (arrows) and the anterior part of the corpus callosum. (B) Abnormal hyperintensities in the white matter, the periventricular area but not of the U fibers (arrows), and a unilateral signal abnormality in the basal ganglia (the caudate nucleus in P3 and the putamen in P8; arrowheads). (C) White matter abnormalities in the cerebellum (arrows).
Brain MRIs of patients 1, 3, and 8. Coronal brain MRIs of patients P1 (taken at age 20 months, 11 months after HSCT), P3 (age 6 years, 2 months before HSCT), and P8 (age 3 years, 3 months after HSCT). T2-weighted images are shown for P1, and fluid attenuation inversion recovery (FLAIR) images are shown for P3 and P8. (A) Bilateral, abnormal hyperintensities in the periventricular white matter (arrows) and the anterior part of the corpus callosum. (B) Abnormal hyperintensities in the white matter, the periventricular area but not of the U fibers (arrows), and a unilateral signal abnormality in the basal ganglia (the caudate nucleus in P3 and the putamen in P8; arrowheads). (C) White matter abnormalities in the cerebellum (arrows).
Other late-onset complications
Iatrogenic sequelae consisted of necrosis of the head of the femur in one patient (P3). Two patients (P1 and P8) had “leopard-like” areas of skin hyper- or hypopigmentation—a feature that is related to the inborn defect in melanosome exocytosis.
Discussion
This is the largest single-center, retrospective analysis to have documented detailed HSCT procedures and outcomes in GS2 patients. Of the 10 patients, 7 were cured of the otherwise fatal immune disorder HLH. One patient suffered from fatal HLH relapse after HSCT. Death in 2 other patients was most probably related to treatment toxicity. In the case of delayed or absent treatment, GS2 has a poor prognosis, due to the immune disorder that leads to HLH.23-27 The present study demonstrates the efficacy of HSCT in curing the immune disorder in GS2. HLH occurred in all 10 GS2 patients. Three showed spontaneous control of HLH several weeks after onset and survived the HLH episode without treatment. However, all 3 patients had HLH relapse thereafter. Spontaneous recovery from an HLH episode has been reported in another GS2 patient, who experienced recurrent episodes with a fatal outcome several years later.28 In contrast, spontaneous survival of HLH without treatment is very rare in patients with complete perforin deficiency. Our present observations also correspond to those made in murine models, since Rab27a-deficient mice had spontaneous control and better survival rates of HLH than perforin-deficient mice.7 Moreover, post-HSCT survival in the cohort reported on here is comparable with previous reports on HSCT for other HLH syndromes,8,16,29 even though our patients were older at time of HSCT—a feature that could have influenced the outcome. Furthermore, HLH onset occurred later in the GS2 patients reported here, in comparison with patients with complete perforin deficiency.30,31 This is also consistent with previous reports on GS2 patients.24,27 All these various observations could be associated with a residual cytotoxic function of Rab27a-deficient lymphocytes, as suggested by studies showing a residual cytotoxic function of Rab27a-deficient T cells and NK cells5,32,33 under some conditions. The presence of residual cytotoxic activity might be enough to allow clearance of some infectious agents and, to some extent, the spontaneous regression of HLH episodes.
We confirmed previous observations whereby neurologic involvement is common in GS2 patients,34 as also observed by Trottestam et al.18 Indeed, neurologic involvement is a major issue concerning the indication for HSCT and long-term sequelae. Of the 7 patients with neurologic involvement before HSCT, 4 survived and 2 had persistent, neurologic, long-term sequelae. Furthermore, one patient without apparent pre-HSCT neurologic involvement developed long-term neurologic sequelae. Undetected neurologic HLH before HSCT or brain infection could be an explanation, although all the clinical, neuroradiologic, and CSF examinations were normal. It is noteworthy that this patient also suffered from severe, multiorgan, chronic GVHD, and thus one cannot rule out central nervous GVHD.35 There is currently a debate as to whether neurologic affection is a manifestation of previously undetected central nervous HLH or whether noninflammatory degenerative neurologic affections occur in Griscelli syndrome. The favorable long-term outcome observed here in 3 patients argues against the second hypothesis. In addition, Rab27a seems not to be expressed in the brain.36 Hence, in contrast to what has been shown in Chediak-Higashi syndrome with central nervous HLH as well as progressive neurologic disease,37 which, at least in the mouse model, seems to be correlated with a degenerative loss of Purkinje cells,38 neurologic symptoms in GS2 patients probably result uniquely from HLH-related sequelae that predate the HSCT and thus underline the benefit of early transplantation in GS2. Two of our patients (siblings P4 and P7) suffered from pre-HSCT neuropathy that involved the peripheral nerves and (in one of them) the spinal nerve roots. This represents a new sign of HLH in GS2 that, to the best of our knowledge, has not been reported previously. Our results show that pre-HSCT therapy with ATG, corticosteroids, and cyclosporin A cannot always prevent occurrence of central nervous system disease as also observed in patients with VP-16, corticosteroid, and cyclosporin A treatment according to the HLH-94/HLH-2004 protocol.18 The data further stress that, in general, the occurrence of HLH-related neurologic disease carries a high risk for sequelae.39
Three patients died within 110 days of HSCT. Two of these were the oldest patients at the time of HSCT, and the other was the second youngest patient. The oldest patient's pre-HSCT clinical status was not good and featured a severe neurologic affection. The second-oldest patient had suffered from less severe but longer-lasting disease before HSCT. Furthermore, these 2 patients were siblings and had an unusual RAB27A abnormality, with duplication of exon 2. One can only speculate as to whether the poor outcome was associated with the genotype or age at HSCT (ie, advanced disease) in these 2 patients. All 3 deceased patients had neurologic involvement before HSCT. In murine models, neurologic involvement develops late in the course of the disease.40,41 Thus, the presence of neurologic involvement further supports the idea of advanced, late-stage disease. Early immunosuppressive treatment of HLH and early HSCT in a patient in good clinical condition therefore appear to be critical for a positive outcome. Ideally, bone marrow transplantation in GS2 patients should be performed before the first episode of HLH.12,17
Primary engraftment failure occurred in one patient (who was not in complete remission from HLH at time of transplantation). This fits with previous reports of HSCT failure in patients with active HLH at the time of HSCT29,31,42 and could be explained by a negative effect of circulating cytokines during the active disease phase; for example, interferon-γ (IFN-γ) has been shown to have a negative effect on hematopoiesis in murine models.43 However, most of the patients were not in complete remission at the time of HSCT, and only one of them experienced primary HSCT engraftment failure. Although one should aim at achieving complete remission of active HLH before performing HSCT, transplantation should not be postponed because of only PR, unless definitive evidence can be found in favor of this type of treatment strategy. The use of ATG in the conditioning regimen appears to be required to achieve remission and perhaps to avoid HLH-related post-HSCT complications such as graft rejection, HLH relapse, and VOD.
Two patients had EBV-associated lymphoproliferative disease that was diagnosed soon after HSCT. Therapy-associated immunosuppression might have contributed to the emergence of the lymphoproliferative disease. Alternatively, the inborn defect in cytotoxicity may have led to impaired clearance of infected cells as well as neoplastic cells. Only a few cases of Griscelli syndrome have been described in the literature. Interestingly, EBV-associated lymphoproliferation seems to have induced HLH relapse in a patient with predominantly donor chimerism who experienced secondary graft loss thereafter. A selective advantage of the cells with deficient cytotoxicity would seem to be the most likely explanation. Anti-CD20 antibody was useful in the treatment of EBV-triggered HLH before HSCT (as has been suggested for X-linked lymphoproliferative syndrome44 ) and could be a valuable, nontoxic approach for inducing remission from HLH and preventing post-HSCT EBV replication. Given the high observed toxicity of HSCT so far in GS2, reduced intensity conditioning might be considered—at least in fragile patients with HLA-matched donors—on the basis of the results in patients with familial HLH.45 In conclusion, HSCT is an efficient treatment for curing the immune disorder of GS2. However, improvement of the transplantation procedure is needed to reduce its toxic effects, and diagnosis should be made as early as possible to reduce long-term, posttransplant (mainly neurologic) sequelae.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients and their families for cooperation, the nurses and the clinicians for the care of the patients, and Nathalie Lambert and Magali Salmon for their assistance.
This work was supported by grants from Inserm, the Agence Nationale de la Recherche (ANR-05-MIM-010 and BLAN06-3_145379), the Fondation pour la Recherche Médicale (Equipe labélisée FRM 2007), and through Coordination Theme 1 of the European Community's FP7, grant agreement no. HEALTH-F2-2008-201461. J.P.S. received grants from the Fondazione Ettore e Valeria Rossi and the Walter und Gertrud Siegenthaler Stiftung.
Authorship
Contribution: J.P.S. collected and analyzed the data, participated in writing of the report, and contributed to patients' care; D.M. contributed to data collection, writing of the report, and patients' care; N.B. evaluated neuroimaging; S.B. contributed to study design, data analysis, writing of the report, and patients' care; M.C.C. and L.D.C. contributed to the processing and quality control of stem-cell transplantation; B.N. and M.T. contributed to patients' care; G.S.B. performed genetic analyses and participated in data analysis and writing of the report; and A.F. contributed to study design, data analysis, writing of the report, and patients' care.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alain Fischer, Professor of Pediatrics, Director of the Pediatric Immunology and Hematology Department, Hôpital Necker Enfants-Malades, 149 rue de Sèvres, Paris, F-75015, France; e-mail: alain.fischer@nck.ap-hop-paris.fr.