Abstract
Expression of vascular endothelial growth factor (VEGF) is tightly regulated to achieve normal angiogenesis. The objective was to examine regulation of VEGF by the activin-like kinase receptors (ALKs) ALK1 and ALK5. Transforming growth factor β1 (TGFβ1) and bone morphogenetic protein-9 (BMP-9) enhanced and suppressed VEGF expression, respectively, in aortic endothelial cells, as determined by real-time polymerase chain reaction, immunoblotting, cell proliferation, and tube formation. The use of small interfering RNA revealed that TGFβ1 stimulated VEGF expression by activating ALK5, TGFβ type II receptor, and SMAD2, whereas BMP-9 suppressed it by activating ALK1, BMP type II receptor, and SMAD1. ALK1 signaling occurred independently of ALK5 activity. Partial ALK1 deficiency in vitro and in vivo resulted in elevated VEGF expression. In vitro, increased BMP-9 levels normalized VEGF expression in cells with partial, but not severe, ALK1 deficiency. Time course experiments revealed that an increase in ALK1 expression induced by BMP-4, an angiogenic stimulus, preceded induction of ALK5 and VEGF in control cells. In ALK1-deficient cells, however, VEGF expression occurred earlier and was abnormally high, even though ALK5 was not induced. Our results suggest that ALK1 and ALK5 are both essential for correct regulation of VEGF, and that disruption of either pathway leads to disease.
Introduction
Vascular endothelial growth factor (VEGF) is a potent angiogenic factor that has been studied extensively as an agent promoting neovascularization in models of tissue ischemia.1,2 It was discovered that increased VEGF expression resulted in the formation of large, dilated, and fragile blood vessels, which resembled arteriovenous malformations (AVM).2-4 Interestingly, elevated plasma levels of VEGF have been previously reported in patients with the disease hereditary hemorrhagic telangiectasia (HHT),3,5 the hallmark of which is the development of AVM. HHT is associated with mutations of endoglin or activin-like kinase receptor-1 (ALK1), both receptors for members of the transforming growth factor β (TGFβ) superfamily.6-8 We and others have previously reported that the ligand TGFβ1 induces the expression of VEGF.9-13 In addition, He and Chen reported the presence in zebrafish of binding elements on VEGF promoter for SMAD proteins, the downstream mediators of TGFβ signaling, supporting that VEGF expression is regulated by the TGFβ family.14
To activate TGFβ signaling, the TGFβ ligand binds to a type II receptor, which becomes activated through autophosphorylation. The activated type II receptor recruits and activates a type I receptor by transphosphorylation and the activated heterodimeric complex, and then activates an intracellular SMAD protein also by transphosphorylation. Activated SMAD binds to a co-SMAD (a SMAD with a nuclear localizing sequence), and the heterodimeric SMAD complex enters the nucleus to regulate target gene expression.15
Both ALK1 and ALK5 are type I receptors that have been previously reported to be activated by TGFβ and use TGFβ type II receptor (TβRII) as their type II receptor.16 However, whereas ALK5 activates SMAD2/3, ALK1 activates SMAD1/5/8, which are known as the bone morphogenetic protein (BMP)–responsive SMADs.16 Furthermore, activation of ALK1 by TGFβ1 was previously reported by Goumans et al to depend on active ALK5 signaling.17 They also reported the activity of ALK1 to be antagonistic to that of ALK5. However, Seki et al compared the knockout phenotypes of ALK1 and ALK5 and reported the expressions of ALK1 and ALK5 to be nonoverlapping in the developing vasculature.18 They later reported different phenotypes between the 2 knockout animals and ALK5 appeared not to play a role in HHT.19 Further complicating this relationship are the recent findings that ALK1 uses BMP-9 and the BMP type II receptor (BMPRII) to signal.20
The ALK1 receptor plays a role in vascular BMP-2 and BMP-4 signaling. We showed that BMP-2 and BMP-4 dose dependently increased ALK1 expression in endothelial and smooth muscle cells.21,22 This induction was in the endothelial cells mediated by the ALK2 receptor.23 Activation of ALK1 led to the induction of matrix Gla protein (MGP), an efficient inhibitor of BMP-2 and BMP-4, and to the induction of VEGF.21 We also showed that BMP-2/4 and MGP existed in an activator-inhibitor relationship, which affected pattern formation of mesenchymal cells in vitro,24 and branching in the pulmonary vascular tree in vivo.25
This study shows that the ALK1 and ALK5 signaling pathways have significantly different effects on VEGF expression. BMP-9 stimulation of the ALK1/BMPRII complex signaling suppresses VEGF expression through SMAD1, whereas TGFβ1 stimulation of the ALK5/TβRII complex enhances VEGF expression through SMAD2. The ALK1 signaling occurs independently of signaling through ALK5 signaling. Depletion of ALK1 in vitro results in a progressive increase of VEGF expression, which is normalized in partial, but not severe, ALK1 deficiency by increased BMP-9 levels. Tissue levels of VEGF were also elevated in ALK1+/− mice with low levels of ALK1. Time course experiments revealed that depletion of ALK1 resulted in changes in the expression and timing of ALK5 and VEGF. Together, our results suggest that ALK1 and ALK5 are critical for a coordinated regulation of VEGF, and that perturbations of this system most likely result in vascular disease.
Methods
Cell culture, transfection, and mice
Human aortic endothelial cells (HAECs) and bovine aortic endothelial cells (BAECs) were obtained and cultured, as previously described.21,26 BAECs and HAECs were cultured in 15% and 20% fetal bovine serum (FBS), respectively. Transient transfections of HAECs with small interfering (si)RNA were performed, as previously described.21 SiRNAs (Silencer Validated siRNA; Ambion) to ALK1, ALK5, BMPRII, TβRII, SMAD1, SMAD2, and scrambled siRNAs with the same nucleotide contents were used. Luciferase assays were performed, as previously described,21 and normalized to Renilla. ALK1+/− mice on C57BL6/J background were obtained from The Jackson Laboratory and cared for in accordance with guidelines from the Institutional Review Board of the University of California.
Vector constructs and growth factors
The ALK1- and BMP-responsive luciferase reporter gene (BRE-lux)27 was provided by Dr Peter ten Dijke (Leiden University Medical Center). The ALK5-responsive luciferase reporter gene (CAGA-lux) constructs were provided by Dr Karen Lyons (University of California). The Renilla luciferase construct was purchased from Promega. BMP-9 (R&D Systems) was inhibited by addition of recombinant human ALK1 extracellular domain/Fc (ALK1/Fc; R&D Systems). Neutralizing antibodies to TGFβ (R&D Systems), the ALK5 inhibitor SB-431542 (Sigma-Aldrich), and the VEGF receptor 2 (VEGFR2) inhibitor SU1498 (Calbiochem) were added 1 hour before treatment with TGFβ1.
Immunoblotting and immunoprecipitation
Immunoblotting was performed, as previously described.28 Blots were incubated with specific antibodies to ALK1 (D-20, 400 ng/mL; Santa Cruz Biotechnology), pSMAD1/5/8 (400 ng/mL; Cell Signaling Technology), and total SMAD (400 ng/mL; Santa Cruz Biotechnology). For optimal detection of VEGF in culture media, VEGF was first immunoprecipitated with anti-VEGF antibodies (Santa Cruz Biotechnology), as previously described,9 and then analyzed by immunoblotting using specific antibodies to VEGF (200 ng/mL; R&D Systems).
Real-time polymerase chain reaction
Real-time polymerase chain reaction (PCR) assays were performed, as previously described.9 The following primers and probes were used: bovine VEGF (bVEGF) forward (5′-CCCACGAAGTGGTGAAGTTCA-3′), bVEGF reverse (5′-CCACCAGGGTCTCGATGG-3′), bVEGF Taqman probe (6-carboxyfluorescein-TCTACCAGCGCAGCTTCTGCCGT-5-(and 6)-carboxytetramethylrhodamine), bovine glyceraldehyde-3-phosphate dehydrogenase (bGAPDH) forward (5′-GGCGCCAAGAGGGTCAT-3′), bGAPDH reverse (5′-GTGGTTCACGCCCATCACA-3′), and bGAPDH Taqman probe (6-carboxyfluorescein-TCTCTGCACCTTCTGCCGATGCC-5-(and 6)-carboxytetramethylrhodamine). The primers and probes for human ALK1, ALK5, BMPRII, TβRII, TGFβ1, and MGP were obtained from Applied Biosystems as part of Taqman Gene Expression Assays.
Proliferation assay
BAECs (transfected and treated, as indicated) were seeded in 24-well plates at a density of 100 000 cells/well, and allowed to attach for 4 to 6 hours. 3H-Thymidine was added at 1 μCi/mL for 4 days, and 3H-thymidine incorporation was determined, as previously described.9
Migration assay
BAECs were plated at 85% confluence in 6-well tissue culture dishes, and treated with the indicated treatments until 100% confluence. Confluent monolayers were “wounded” with a standard 20-μL pipette tip, after which the experiment was continued for the indicated number of hours. Wound width was measured at 2, 5, and 12 hours through the use of the Scion Image Analysis System (Scion Corporation).
Tube formation assay
Matrigel Matrix (BD Biosciences) was diluted 1/3 in medium from treated or transfected BAECs, and 300 μL was added to each well of a 12-well plate and incubated at 37°C for 30 minutes to allow polymerization. BAECs were suspended in the same medium at a density of 5 × 104 cells/well, and 400 μL of the cell suspension was added to each well. Photographs were obtained after 6 hours using a Nikon Eclipse TE2000-S microscope with a Nikon Plan Fluor 4× objective lens (both from Nikon Instruments). The images were captured using an INFINITY2 camera (Lumenera) and the National Institutes of Health ImageJ software (http://rsbweb.nih.gov/ij/).
Statistical analysis
Data were analyzed for statistical significance by 2-way analysis of variance with post hoc Tukey analysis, unless otherwise stated. The analyses were performed using the GraphPad Instat 3.0 software (GraphPad). In all cases, P values less than .05 were considered significant. All experiments were repeated 3 or more times; representative experiments are shown in the figures.
Results
BMP-9 inhibits TGFβ1-induced VEGF expression
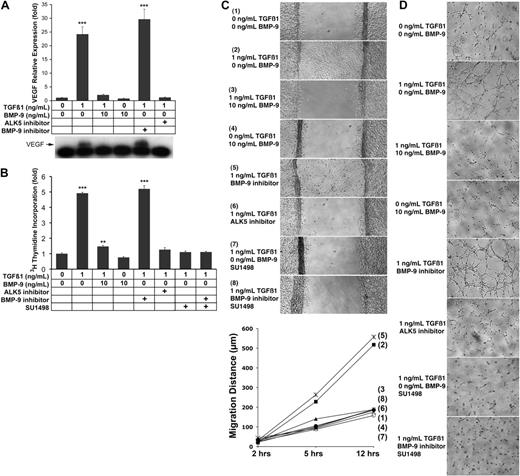
Previously, we demonstrated the induction of VEGF by TGFβ1 in endothelial cells.9,21 Because BMP-9 was recently identified to be a new ligand for ALK1, we determined the effect of BMP-9 alone or in combination with TGFβ1 on the induction of VEGF. We treated BAECs with TGFβ1 (1 ng/mL), BMP-9 (10 ng/mL, which is in the range detected in plasma29 ), or together. As expected, TGFβ1 significantly increased VEGF expression, as determined by real-time PCR and immunoblotting of VEGF in the culture media (Figure 1A). However, BMP-9 and the ALK5 inhibitor (SB-431542; 5 μM), but not the BMP-9 inhibitor (ALK1/Fc; 200 ng/mL), inhibited this effect (Figure 1A). The results suggested that ALK5 mediated the TGFβ1 effect on VEGF expression, and that the effect was antagonized by BMP-9. We then tested the functional significance of the findings in BAECs. The results showed that cell proliferation paralleled the expression of VEGF, in that treatment with TGFβ1 stimulated proliferation, as determined by 3H-thymidine incorporation (Figure 1B). BMP-9 and the ALK5 inhibitor, but not the BMP-9 inhibitor, largely abolished this stimulation (Figure 1B). Furthermore, cell migration and tube formation increased in response to TGFβ1 (Figure 1C-D), but were likewise inhibited by BMP-9 and the ALK5 inhibitor, but not by the BMP-9 inhibitor (Figure 1C-D). We also added the VEGFR2 inhibitor SU1498 (10 μM) together with TGFβ1 to confirm that the TGFβ1 effects were mediated by VEGF signaling. SU1498 abolished any stimulatory effect on proliferation (Figure 1B), migration (Figure 1C), and tube formation (Figure 1D), supporting that VEGF mediated the TGFβ1 effect. Together our results suggested that TGFβ1 was instrumental in promoting VEGF expression, whereas BMP-9 suppressed VEGF expression.
TGFβ1 and BMP-9 have opposite effects on VEGF expression. BAECs were treated with combinations of TGFβ1 (1 ng/mL), BMP-9 (10 ng/mL), the ALK5 inhibitor SB-4311542 (5 μM), the BMP-9 inhibitor ALK1/Fc (200 ng/mL), and the VEGFR2 inhibitor SU1498 (10 μM) for 24 hours. VEGF expression was determined by real-time PCR and immunoblotting of VEGF from the media (A). Cell proliferation was determined by 3H-thymidine incorporation (B). Cell migration was determined by migration assays (C), and tube formation was determined on Matrigel (D); images were obtained 6 hours after plating. Asterisks indicate statistically significant differences compared with control (no treatment). **P < .01; ***P < .001; Tukey test.
TGFβ1 and BMP-9 have opposite effects on VEGF expression. BAECs were treated with combinations of TGFβ1 (1 ng/mL), BMP-9 (10 ng/mL), the ALK5 inhibitor SB-4311542 (5 μM), the BMP-9 inhibitor ALK1/Fc (200 ng/mL), and the VEGFR2 inhibitor SU1498 (10 μM) for 24 hours. VEGF expression was determined by real-time PCR and immunoblotting of VEGF from the media (A). Cell proliferation was determined by 3H-thymidine incorporation (B). Cell migration was determined by migration assays (C), and tube formation was determined on Matrigel (D); images were obtained 6 hours after plating. Asterisks indicate statistically significant differences compared with control (no treatment). **P < .01; ***P < .001; Tukey test.
Because TGFβ signaling can be suppressed through induction of the inhibitory SMAD7 in endothelial cells,30 we determined whether the inhibitory effect of BMP-9 on TGFβ1 activity involved SMAD7. We repeated the experiment presented in Figure 1A in HAECs after transfection of SMAD7 siRNA, which reduced the level of SMAD7 expression to less than 15% of scrambled control. However, no effect was seen from the depletion of SMAD7 (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article), suggesting that BMP-9 does not act through SMAD7.
ALK1 signals independently of ALK5
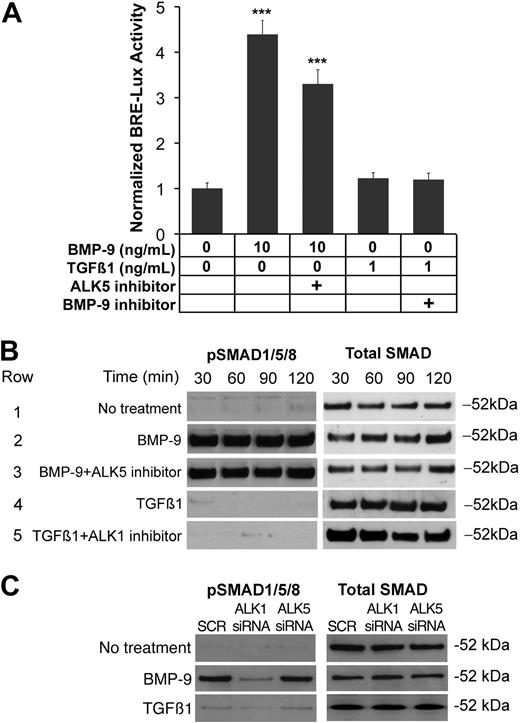
ALK1 was previously reported to be a receptor for both BMP-9 and TGFβ1,16,20 and ALK1 activity was shown to be dependent on active ALK5 signaling.17 However, a recent study demonstrated nonoverlapping expression of ALK1 and ALK5,18 suggesting that the receptors signal independently of each other. Although our results suggested that ALK5 mediated the induction of VEGF, it was unclear whether ALK5 signaling was necessary for ALK1 signaling. We therefore determined the effect of inhibiting ALK5 on ALK1 signaling. We transfected BAECs with BRE-lux (a luciferase reporter gene responsive to ALK1), then treated for 24 hours with combinations of ligands and inhibitors to ALK1 and ALK5. ALK1 was activated with BMP-9 (10 ng/mL) and inhibited with ALK1/Fc (200 ng/mL). ALK5 was activated by TGFβ1 (1 ng/mL) and inhibited with SB-431542 (10 μM). The results showed that inhibiting ALK5 activity did not significantly affect the ALK1 signaling, as determined by luciferase assays (Figure 2A). To confirm these findings, we also determined the effect of inhibiting ALK5 on the phosphorylation of SMAD1/5/8 using the same treatments for up to 2 hours. The results showed again that inhibition of ALK5 did not affect ALK1 signaling, as determined by immunoblotting (Figure 2B). Control experiments showed the inhibitory action of SB-431542 on ALK5 signaling (supplemental Figure 2). To further confirm the independence of ALK1 signaling from ALK5 signaling, HAECs were transfected with siRNA to either ALK1 or ALK5, treated the following day with either BMP-9 (10 ng/mL) or TGFβ1 (1 ng/mL) for 1 hour, and compared with control cells transfected with scrambled siRNA. The siRNA significantly reduced the level of the respective RNA and protein expression to less than 25% of scrambled control23 (supplemental Figure 3). The results showed that depletion of ALK1, but not of ALK5, decreased levels of pSMAD1/5/8 in response to BMP-9, as determined by immunoblotting (Figure 2C). TGFβ1 had no significant effect on phosphorylation of SMAD1/5/8, further supporting the independence of ALK1 signaling from ALK5 activity.
ALK1 signals independently of ALK5 signaling. (A) BAECs were transfected with the BRE-lux luciferase reporter gene, and treated with combinations of BMP-9 (10 ng/mL), TGFβ1 (1 ng/mL), the ALK5 inhibitor SB-431542 (5 μM), and the BMP-9 inhibitor ALK1/Fc (200 ng/mL). After 24 hours, ALK1 signaling was determined by luciferase activity. Asterisks indicate statistically significant differences compared with control (no treatment). ***P < .001; Tukey test. (B) BAECs were treated with the same treatments as in panel A, and samples were collected after the indicated times. Activation of SMAD1/5/8 was determined by pSMAD1/5/8 immunoblotting and compared with total SMAD. (C) HAECs were transfected with scrambled (SCR) siRNA, or siRNA to ALK1 or ALK5. The following day, the cells were left untreated or treated with BMP-9 (10 mg/mL) or TGFβ1 (1 ng/mL) for 1 hour. Activation of SMAD1/5/8 was determined by pSMAD1/5/8 immunoblotting and compared with total SMAD.
ALK1 signals independently of ALK5 signaling. (A) BAECs were transfected with the BRE-lux luciferase reporter gene, and treated with combinations of BMP-9 (10 ng/mL), TGFβ1 (1 ng/mL), the ALK5 inhibitor SB-431542 (5 μM), and the BMP-9 inhibitor ALK1/Fc (200 ng/mL). After 24 hours, ALK1 signaling was determined by luciferase activity. Asterisks indicate statistically significant differences compared with control (no treatment). ***P < .001; Tukey test. (B) BAECs were treated with the same treatments as in panel A, and samples were collected after the indicated times. Activation of SMAD1/5/8 was determined by pSMAD1/5/8 immunoblotting and compared with total SMAD. (C) HAECs were transfected with scrambled (SCR) siRNA, or siRNA to ALK1 or ALK5. The following day, the cells were left untreated or treated with BMP-9 (10 mg/mL) or TGFβ1 (1 ng/mL) for 1 hour. Activation of SMAD1/5/8 was determined by pSMAD1/5/8 immunoblotting and compared with total SMAD.
TGFβ1 and BMP-9 mediate their effects on VEGF expression via ALK5/TβRII/SMAD2 and ALK1/BMPRII/SMAD1, respectively
Because TGFβ1 signaled through ALK5,31 and ALK1 signaled independently of ALK5, we hypothesized that TGFβ1 promoted VEGF expression through ALK5, TβRII, and SMAD2, and BMP-9 suppressed VEGF expression through ALK1, BMPRII, and SMAD1. To test this, we transfected HAECs with siRNA to ALK1, ALK5, BMPRII, TβRII, SMAD1, or SMAD2. The cells were then treated with either TGFβ1 (1 ng/mL) or BMP-9 (10 ng/mL) or control medium for 24 hours starting the day after transfection, and compared with control cells transfected with scrambled siRNA. The siRNA significantly reduced the level of the respective RNA and protein expression to 25% or less of scrambled control23 (supplemental Figure 3). After treatment, VEGF expression, cell proliferation, and tube formation using media from transfected cells were examined. The results showed that depletion of ALK1, BMPRII, or SMAD1 strongly enhanced TGFβ1-induced VEGF expression, whereas depletion of ALK5, TβRII, or SMAD2 diminished the VEGF expression, as determined by real-time PCR (Figure 3A) and immunoblotting of VEGF from the media (Figure 3B). VEGF expression was also increased in control- and BMP-9–treated cells depleted of ALK1, BMPRII, and SMAD1, most likely due to low levels of TGFβ1 normally present in FBS. Similarly to VEGF expression, TGFβ1-induced proliferation was significantly enhanced in cells depleted of ALK1, BMPRII, or SMAD1 (Figure 3C), but inhibited in cells depleted of ALK5, TβRII, or SMAD2, as determined by 3H-thymidine incorporation (Figure 3C). In addition, media from cells depleted of ALK1, BMPRII, or SMAD1 and treated with TGFβ1 strongly enhanced tube formation (Figure 3D), whereas media from cells depleted of ALK5, TβRII, or SMAD2 inhibited tube formation (Figure 3D). Similar to VEGF expression, depletion of ALK1, BMPRII, or SMAD1 increased proliferation and tube formation in control- and BMP-9–treated cells (Figure 3C-D) most likely due to TGFβ1 normally present in serum. Together our results suggested that TGFβ1 promoted VEGF expression through ALK5, TβRII, and SMAD2, and BMP-9 suppressed VEGF expression through ALK1, BMPRII, and SMAD1.
Activation of ALK1 and ALK5 signaling has different effects on VEGF expression, cell proliferation, and tube formation in vitro. HAECs were transfected with scrambled control siRNA (SCR) or siRNA to the indicated receptors and SMADs. The following day, the cells were treated with control medium, TGFβ1 (1 ng/mL), or BMP-9 (10 ng/mL) for 24 hours. VEGF expression was determined by real-time PCR (A), and VEGF in the media was determined by immunoblotting (B). Stimulation of cell proliferation was determined by 3H-thymidine incorporation (C), and the effect on tube formation of media collected from the transfected and treated cells was determined on Matrigel (D); images were obtained 6 hours after plating. Asterisks indicate statistically significant differences compared with control (scrambled siRNA, control treatment). **P < .01; ***P < .001; Tukey test.
Activation of ALK1 and ALK5 signaling has different effects on VEGF expression, cell proliferation, and tube formation in vitro. HAECs were transfected with scrambled control siRNA (SCR) or siRNA to the indicated receptors and SMADs. The following day, the cells were treated with control medium, TGFβ1 (1 ng/mL), or BMP-9 (10 ng/mL) for 24 hours. VEGF expression was determined by real-time PCR (A), and VEGF in the media was determined by immunoblotting (B). Stimulation of cell proliferation was determined by 3H-thymidine incorporation (C), and the effect on tube formation of media collected from the transfected and treated cells was determined on Matrigel (D); images were obtained 6 hours after plating. Asterisks indicate statistically significant differences compared with control (scrambled siRNA, control treatment). **P < .01; ***P < .001; Tukey test.
Suppression of VEGF expression by BMP-9 depends on the level of ALK1 expression
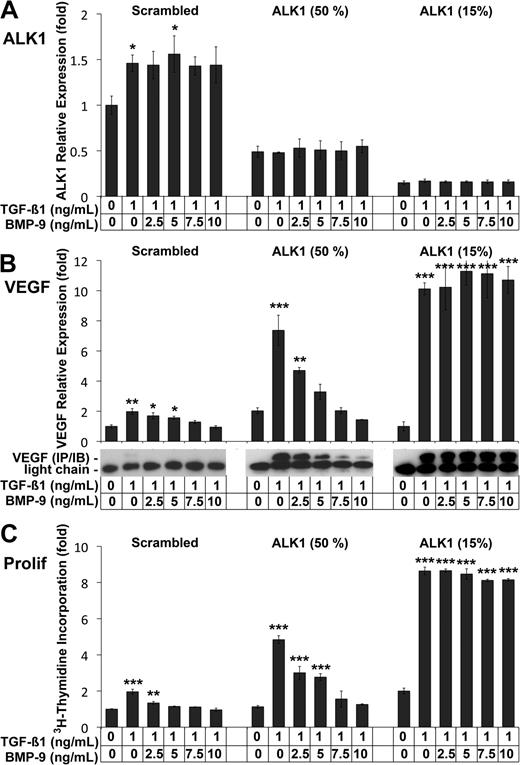
Our results showed that near complete silencing of ALK1 by siRNA resulted in significantly elevated VEGF levels after TGFβ1 treatment (Figure 3A). However, ALK1 deficiency is heterozygous in HHT, and has a highly variable phenotype.32 We therefore compared the effect of different levels of ALK1 expression and BMP-9 on VEGF expression. We transfected HAECs with scrambled control siRNA or with ALK1 siRNA to achieve either 50% or 15% of control levels (Figure 4A), and treated with TGFβ1 (1 ng/mL) and increasing levels of BMP-9 (0-10 ng/mL) on the day after transfection. The results showed that VEGF expression increased significantly in response to TGFβ1 in control cells and in cells with ALK1 levels reduced to 50% and 15% of normal, as determined by real-time PCR and immunoblotting (Figure 4B). Increased levels of BMP-9 progressively suppressed VEGF expression in control cells and in cells with 50% of normal ALK1 levels (Figure 4B), but had no effect in cells with only 15% of control levels (Figure 4B). Consistent with these findings, proliferation increased significantly in response to TGFβ1 in all 3 cases (Figure 4C), as determined by 3H-thymidine incorporation. BMP-9 progressively suppressed it in control cells, and in cells with 50% of normal ALK1 levels. However, BMP-9 was unable to suppress proliferation when ALK1 was only 15% of control levels (Figure 4C). Together the data suggested that the levels of ALK1, BMP-9, as well as TGFβ1 affect the expression of VEGF and cell proliferation, which may explain the variable phenotype seen in HHT.
BMP-9 stimulation of ALK1 suppresses VEGF expression. HAECs were transfected with scrambled control siRNA or 2 concentrations of ALK1 siRNA to reduce ALK1 expression to 50% and 15% of control, respectively. The following day, the cells were treated with TGFβ1 (1 ng/mL) and BMP-9 (0-10 ng/mL) for 24 hours. ALK1 expression was determined by real-time PCR (A). VEGF expression was determined by real-time PCR, and confirmed by immunoblotting of VEGF from the media (B). Cell proliferation was determined by 3H-thymidine incorporation (C). Asterisks indicate statistically significant differences compared with control (no TGFβ1 or BMP-9). *P < .05; **P < .01; ***P < .001; Tukey test.
BMP-9 stimulation of ALK1 suppresses VEGF expression. HAECs were transfected with scrambled control siRNA or 2 concentrations of ALK1 siRNA to reduce ALK1 expression to 50% and 15% of control, respectively. The following day, the cells were treated with TGFβ1 (1 ng/mL) and BMP-9 (0-10 ng/mL) for 24 hours. ALK1 expression was determined by real-time PCR (A). VEGF expression was determined by real-time PCR, and confirmed by immunoblotting of VEGF from the media (B). Cell proliferation was determined by 3H-thymidine incorporation (C). Asterisks indicate statistically significant differences compared with control (no TGFβ1 or BMP-9). *P < .05; **P < .01; ***P < .001; Tukey test.
VEGF levels are elevated in the aorta, lungs, liver, and intestine of ALK1-deficient mice
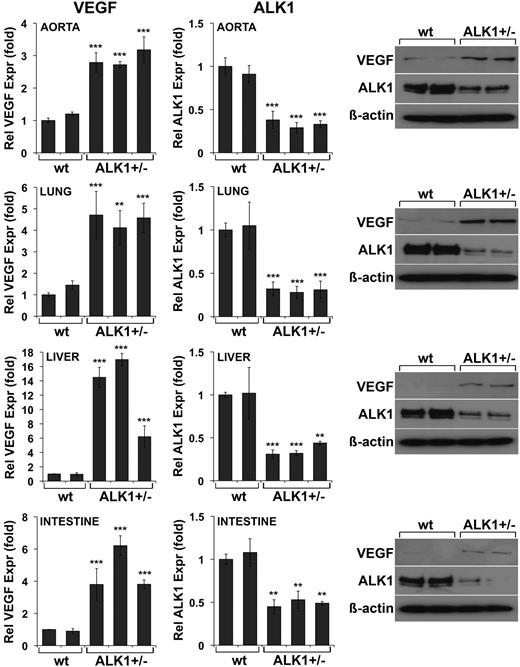
We then hypothesized that tissue levels of VEGF would be elevated in the setting of ALK1 deficiency in vivo. To test this, we determined expression of VEGF and ALK1 in the aorta, lungs, liver, and intestine of ALK1+/− mice. Lungs, liver, and intestine were selected because HHT commonly affects these organs,32,33 and homozygous ALK1 deficiency results in large AVMs involving the aorta.8 Indeed, the results showed that VEGF levels were significantly higher in the aorta, lungs, liver, and intestine of ALK1+/− mice than of control mice, as determined by real-time PCR and immunoblotting (Figure 5). As expected, ALK1 levels were lower in the ALK1+/− mice (Figure 5). This suggested that increased VEGF levels contribute to HHT.
Tissue expression of VEGF is elevated in ALK1+/−mice. RNA and protein were prepared from aorta, lungs, liver, and intestine from wild-type (wt) and ALK1+/− mice. Expression of VEGF and ALK1 was determined by real-time PCR and immunoblotting. Asterisks indicate statistically significant differences compared with control (wt). **P < .01; ***P < .001; Tukey test.
Tissue expression of VEGF is elevated in ALK1+/−mice. RNA and protein were prepared from aorta, lungs, liver, and intestine from wild-type (wt) and ALK1+/− mice. Expression of VEGF and ALK1 was determined by real-time PCR and immunoblotting. Asterisks indicate statistically significant differences compared with control (wt). **P < .01; ***P < .001; Tukey test.
Expression of ALK1 precedes that of ALK5 and VEGF
In the current study, we show that ALK5 signaling enhanced VEGF expression, whereas ALK1 signaling suppressed it. In previous studies, however, we showed that an increase in ALK1 expression induced by BMP-4, an angiogenic factor,34 led to induction of VEGF when TGFβ was present.21 ALK5 was not examined in this study, but we have found that activated ALK1 can induce ALK5 in vascular mesenchymal cells,22 which could explain an increase in VEGF if it also occurs in endothelial cells. Thus, we first hypothesized that there may be a difference between cells with baseline expression of ALK1 and those with high ALK1 expression induced by an angiogenic stimulus. Second, we hypothesized that induction of ALK1 may precede an increase in ALK5 expression.
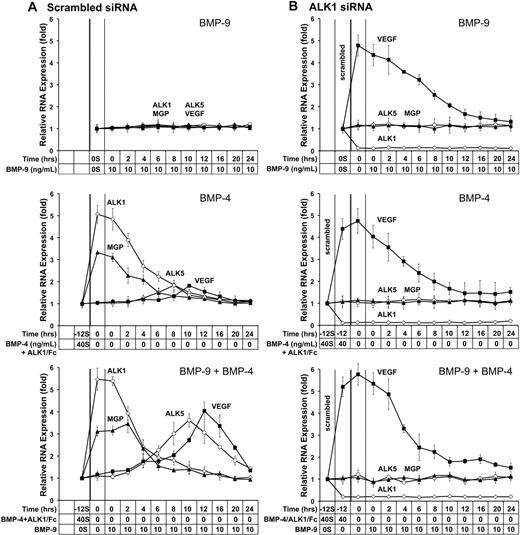
To determine the timing of expression of the 2 receptors and VEGF after stimulation of ALK1, we performed time course experiments in HAECs. To examine the effect of ALK1 depletion, we compared control cells transfected with scrambled siRNA with cells transfected with ALK1 siRNA. We also monitored the expression of matrix Gla protein (MGP), a BMP inhibitor that is induced by activated ALK1 and has been shown to affect VEGF induction.21
In the first time course experiment, we treated HAECs transfected with scrambled siRNA with BMP-9 (10 ng/mL) alone for up to 24 hours. No exogenous TGFβ was added in any of these experiments. Samples were collected at the indicated times (Figure 6), and expression of ALK1, ALK5, MGP, and VEGF was determined. The results showed no difference in expression for any of the proteins (Figure 6A top). We then treated the cells with BMP-4 (40 ng/mL) for 12 hours to mimic an angiogenic stimulus. The BMP-4 treatment was combined with the BMP-9 inhibitor ALK1/Fc (200 ng/mL) to prevent ALK1 signaling from being triggered until BMP-4 and ALK1/Fc were removed. No exogenous BMP-9 was added beyond what was naturally in the 20% FBS culture medium. Samples were subsequently collected for up to 24 hours. The results showed the expected increase in ALK121 (Figure 6A middle) and an increase in MGP, which both preceded small increases in ALK5 and VEGF. In attempt to enhance the increases in ALK5 and VEGF, we next enhanced ALK1 signaling by combining BMP-4 and BMP-9 treatments. Again, BMP-4 in combination with ALK1/Fc was added for 12 hours, and then removed. The cells were subsequently treated with BMP-9 for up to 24 hours. The results showed that expression of ALK1 and MGP preceded that of ALK5 and VEGF (Figure 6A bottom), suggesting that induction and activation of ALK1 after an angiogenic stimulus are followed by an induction of ALK5 and VEGF. In addition, the induction of MGP may limit the extent of the angiogenic stimulus by binding and inhibiting BMP-4.21,35
Depletion of ALK1 in HAECs results in loss of correctly coordinated expression of VEGF. HAECs were transfected with scrambled (A) or ALK1 siRNA (B). The following day, the cells were treated with BMP-9 (10 ng/mL) for up to 24 hours (top); BMP-4 (40 ng/mL) in combination with BMP-9 inhibitor ALK1/Fc (200 ng/mL) for 12 hours, followed by no treatment for up to 24 hours (middle); and BMP-4 and ALK1/Fc for 12 hours, followed by BMP-9 for up to 24 hours (bottom). Cell samples were collected at the indicated time points (0-24 hours), and expression of ALK1, MGP, ALK5, and VEGF was determined by real-time PCR. The starting values from control cells (0S, −12S, and 40S) were used to compare expression also in the ALK1-depleted cells to facilitate comparisons between control and ALK1-deficient cells.
Depletion of ALK1 in HAECs results in loss of correctly coordinated expression of VEGF. HAECs were transfected with scrambled (A) or ALK1 siRNA (B). The following day, the cells were treated with BMP-9 (10 ng/mL) for up to 24 hours (top); BMP-4 (40 ng/mL) in combination with BMP-9 inhibitor ALK1/Fc (200 ng/mL) for 12 hours, followed by no treatment for up to 24 hours (middle); and BMP-4 and ALK1/Fc for 12 hours, followed by BMP-9 for up to 24 hours (bottom). Cell samples were collected at the indicated time points (0-24 hours), and expression of ALK1, MGP, ALK5, and VEGF was determined by real-time PCR. The starting values from control cells (0S, −12S, and 40S) were used to compare expression also in the ALK1-depleted cells to facilitate comparisons between control and ALK1-deficient cells.
To examine the effect of ALK1 deficiency, we repeated the same 3 time courses in HAECs transfected with ALK1 siRNA, which reduced the levels of ALK1 to approximately 15% of scrambled control (Figure 6B). The results showed that ALK1 depletion significantly increased VEGF expression early in the time course in all 3 experiments, and then gradually decreased more than 24 hours (Figure 6B top, middle, and bottom), possibly due to suppressive effect of low levels of remaining ALK1. Note that the starting values from control cells (labeled 0S, −12S, and 40S) were used to compare expression also in the ALK1-depleted cells to facilitate comparisons between control and ALK1-deficient cells. The early VEGF increase did not respond to treatment with BMP-9 and/or BMP-4, and ALK1 deficiency abolished the sequential increases in ALK1, MGP, ALK5, and VEGF expression. Thus, depletion of ALK1 results in abnormally high VEGF expression that does not appear to be affected by BMP-9, BMP-4, MGP, or ALK5, suggesting that VEGF is induced by an alternative mechanism in the absence of ALK1.
The time course experiments suggested that induction of ALK5 was dependent on the ALK1 activation. To verify this, we transfected HAECs with scrambled control or ALK1 siRNA. The following day, the cells were treated with BMP-4 (40 ng/mL) to induce ALK1, and stimulated ALK1 with BMP-9 (0-10 ng/mL) for 24 hours. Expression of ALK1 and ALK5 was determined by real-time PCR and immunoblotting. The results showed that BMP-4 significantly induced ALK1 in control cells, but not in cells transfected with ALK1 siRNA (supplemental Figure 4A,C). BMP-9 stimulation did not affect the expression of ALK1 (supplemental Figure 4A,C), but progressively increased ALK5 expression in control cells (supplemental Figure 4B-C). The ALK5 increase was largely abolished in ALK1-depleted cells (supplemental Figure 4B-C), supporting that BMP-9 activation of ALK1 was required for induction of ALK5.
Discussion
The present study provides evidence that VEGF expression is regulated by the TGFβ superfamily of proteins in a coordinated and sequential fashion. VEGF expression was stimulated by ALK5, TβRII, and SMAD2, and inhibited by BMP-9 via ALK1, BMPRII, and SMAD1. The respective effects on VEGF expression were consistent with proliferation, migration, and tube formation assays, suggesting that TGFβ1/ALK5/TβRII/SMAD2 is responsible for induction of angiogenesis, and BMP-9/ALK1/BMPRII/SMAD1 is responsible for the suppression of angiogenesis. This part of our results is consistent with in vivo studies of vascular development in both mice and zebrafish,36,37 which support that ALK5 is important in activation of angiogenesis and ALK1 suppresses angiogenesis. However, our results also showed that when ALK1 expression was stimulated by BMP-4, an angiogenic factor, a temporary induction of the expression of ALK5 followed, allowing for precisely regulated VEGF expression. This relationship may in part explain difficulties in sorting out the different roles of the receptors.
Overall, our findings are consistent with the work of others. VEGF has been shown to be overproduced in HHT (ALK1 mutations)3,5 and pulmonary arterial hypertension (BMPRII mutations),38 suggesting that both receptors play a role in regulating VEGF expression. Karlsson et al reported that the effects of TGFβ1 were mediated only by signaling complexes containing ALK5,31 supporting our observation that the induction of VEGF expression by TGFβ1 is mediated through ALK5. Scharpfenecker et al showed that BMP-9 was able to inhibit the angiogenesis induced by the administration of exogenous VEGF in vitro,39 which is in accordance with our findings that BMP-9 inhibits TGFβ1-mediated VEGF expression. However, the authors also demonstrated that BMP-9 inhibited basic fibroblast growth factor-stimulated cell proliferation and migration, suggesting that BMP-9 affects multiple steps in the regulation of angiogenesis. Evidence for a direct regulation of VEGF expression by BMP-9/ALK1/BMPRII is derived from a study by He and Chen, in which they identified binding sites on the VEGF promoter for the BMP-SMADs.14 In contrast to our finding that inhibition of SMAD1 promoted VEGF production, their reporter gene assays found that mutating SMAD1 binding sites inhibited VEGF promoter activity, whereas mutating SMAD5 binding sites promoted VEGF promoter activity. However, their results may not be applicable to mammalian cells because zebrafish do not express ALK1; instead, they express violet beauregarde, which although highly homologous to ALK1, may not be functionally identical.
Our results showed that VEGF expression was significantly elevated in the setting of ALK1 deficiency both in vivo and in vitro. However, we were unable to identify the exact mechanism of this VEGF elevation, although it did not appear to be responsive to BMP-9, BMP-9 inhibitor, or BMP-4, or to be associated with induction of ALK5 expression. Further studies will be required to clarify the mechanism of VEGF elevation in ALK1 deficiency.
MGP is an inhibitor of BMP-2 and BMP-4,35,40 which may ultimately cause shutdown of BMP-4–induced expression of ALK1 through negative feedback regulation. This is consistent with our previous findings that high levels of MGP inhibit expression of ALK1 and VEGF in vitro21,22 and in vivo.25 However, low to intermediate levels of MGP appear to have an enhancing effect on TGFβ1 induction of VEGF in vitro,21 which may be due to a positive effect on the induction of ALK5, but remains unclear. In previous studies, we showed that BMP-2/4 and MGP can function in a so-called activator-inhibitor relationship and affect pattern formation in vitro and vascular branching in vivo.24,25 Excessive MGP had an inhibitory effect and formation of side branches in the pulmonary vascular tree.25 The time course experiments in this study showed that BMP-4 and ALK1 induced expression of MGP, thereby setting up the conditions for an activator-inhibitor relationship, which could affect capillary branching and network formation. Thus, BMP and MGP may help define the places in which subsequently VEGF-dependent cell growth and angiogenesis occur. A loss of MGP may contribute to AVMs, which is characterized by a loss of capillary network, and also to an increase in BMP activity in the tissue.
We propose a working model for normal angiogenesis that incorporates our current findings with our past data (Figure 7). At baseline, ALK1 and BMP-9 contribute to the quiescence of the endothelium, as has previously been proposed by David et al.29 However, if an angiogenic factor such as BMP-4 induces ALK1 expression, ALK1 will be activated by circulating BMP-9 in the plasma and induces MGP, which antagonizes BMP-4 and limits the angiogenic stimulus. Activated ALK1 also induces ALK5 expression, and TGFβ in tissue activates ALK5 and stimulates VEGF expression. VEGF then promotes angiogenesis in the location that was determined by the initial ALK1 induction. In the setting of HHT, which could be due to deficiency in ALK1, endoglin, or BMP-9, VEGF expression is abnormally high and untimely, giving rise to large, unorganized vessels and AVMs.
Working model for the roles of ALK1 and ALK5 in angiogenesis. (A) BMP-4 as an angiogenic stimulus causes local induction of ALK1, which is activated by BMP-9 normally circulating in the plasma. (B) ALK1 signaling induces expression of MGP, which prevents excessive angiogenic stimulation by binding to BMP-4, and expression of ALK5. (C-D) ALK5 is activated by local TGFβ, which results in VEGF induction and stimulation of endothelial cell growth and angiogenesis.
Working model for the roles of ALK1 and ALK5 in angiogenesis. (A) BMP-4 as an angiogenic stimulus causes local induction of ALK1, which is activated by BMP-9 normally circulating in the plasma. (B) ALK1 signaling induces expression of MGP, which prevents excessive angiogenic stimulation by binding to BMP-4, and expression of ALK5. (C-D) ALK5 is activated by local TGFβ, which results in VEGF induction and stimulation of endothelial cell growth and angiogenesis.
There are 2 other clinical conditions in addition to HHT in which AVMs are known to develop, congenital heart disease and end-stage liver disease, which may lend further credence to our results. Cavopulmonary anastomosis is an operation performed in patients with congenital heart disease in which the right heart is bypassed by directly connecting blood flow from the upper body to the right lung, and blood flow from the liver is connected to the left lung.41 Within a year, the majority of these patients develop AVM exclusively in the right lung.42 Interestingly, in animal models of cavopulmonary anastomoses, VEGF expression is significantly elevated in the bypassed lung. Any shunts that redirect blood flow from the lower body to the right lung result in complete resolution of AVMs, suggesting there is a hepatic factor that prevents the development of AVMs.43 Indeed, BMP-9 is a circulating factor that, to our current knowledge, is made only in the liver.44 Absence of BMP-9 may ease the ALK1-mediated suppression of VEGF, and explain why patients with end-stage liver disease with cavopulmonary anastomoses develop AVMs.45
In conclusion, the regulation of VEGF through TGFβ1/ALK5 and BMP-9/ALK1 is a highly coordinated process. Disruptions of these pathways are likely to lead to abnormal levels of VEGF and the emergence of vascular disease.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by National Institutes of Health grants HL30568 and HL81397, the American Heart Association (western affiliate), the International High Density Lipoprotein (HDL) Awards Program (Pfizer), and the Ahmanson-University of California Adult Congenital Heart Disease Center.
National Institutes of Health
Authorship
Contribution: E.S.S. designed and performed research, analyzed data, and wrote the paper; L.L. and Y.Y. performed research and analyzed data; and K.I.B. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kristina I. Boström, Division of Cardiology, David Geffen School of Medicine, University of California, Box 951679, Los Angeles, CA 90095-1679; e-mail: kbostrom@mednet.ucla.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal