In this issue of Blood, Calado and colleagues discover that androgens via conversion into estradiol enhance TERT expression in hematopoietic cells, potentially identifying the mechanism by which androgens exert therapeutic effects in patients with aplastic anemia and other marrow failure states.
Androgens have been used to treat patients with genetic and acquired bone marrow failure. Initial clinical reports showing that androgen therapy enhanced hematopoietic recovery in patients with aplastic anemia were reported nearly 50 years ago.1 However, the mechanism responsible for enhanced hematopoeisis remained elusive. The importance of understanding the molecular mechanism of androgen therapy cannot be emphasized enough, because improved approaches could be developed to enhance current treatment strategies and concurrently eliminate unwanted side effects.
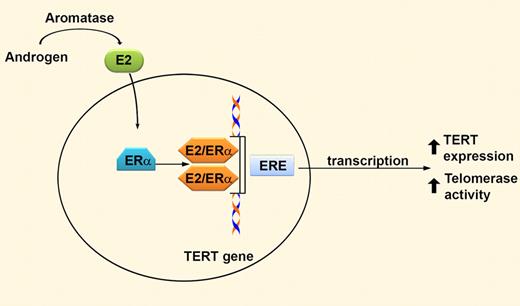
Androgens are enzymatically converted into estradiol (E2) via aromatase. E2 passively diffuses into cells and binds the α isoform of the estrogen receptor (ERα), which acts as a transcriptional activator by binding to estrogen response elements (ERE) in genomic DNA. The telomerase reverse transcriptase (TERT) promoter contains 2 putative EREs. Therefore, both androgens and estrogens increase TERT expression, ultimately resulting in increased telomerase activity in hematopoietic cells.
Androgens are enzymatically converted into estradiol (E2) via aromatase. E2 passively diffuses into cells and binds the α isoform of the estrogen receptor (ERα), which acts as a transcriptional activator by binding to estrogen response elements (ERE) in genomic DNA. The telomerase reverse transcriptase (TERT) promoter contains 2 putative EREs. Therefore, both androgens and estrogens increase TERT expression, ultimately resulting in increased telomerase activity in hematopoietic cells.
In a variety of normal and malignant cells from reproductive tissues, sex steroid hormones, including androgens and estrogens, induce TERT transcription resulting in increased telomerase activity.2 TERT is a core component of telomerase, which diminishes telomere attrition due to incomplete DNA replication at the ends of chromosomes. Intact telomerase function is essential for telomere length maintenance, with impaired telomerase activity being associated with progressive telomere shortening, cellular senescence and apoptosis, and increased chromosomal instability. Therefore, it is not surprising that increased telomerase activity is commonly observed in malignant cells and is capable of immortalizing cells in tissue culture. Mutations in several telomere maintenance proteins, including TERT, result in a genetic marrow failure syndrome, dyskeratosis congenita, and have been shown to increase the risk of developing acquired bone marrow failure and acute myeloid leukemia.3,4 In sum, these observations highlight a critical role for telomere maintenance and intact TERT function in the regulation of hematopoiesis.
Given these previous data, Calado et al question whether androgen therapy may increase telomerase activity in primary hematopoietic cells. Their novel studies demonstrate that androgens increased telomerase activity via a transcriptional mechanism in normal peripheral blood lymphocytes, bone marrow CD34+ cells, and lymphocytes from patients harboring heterozygous telomerase mutations. Interestingly, estradiol had a similar effect. Further investigation of the molecular basis for increased telomerase activity demonstrated that androgens undergo aromatization to estradiol, which then binds to the estrogen receptor-α to increase TERT expression. This is likely through estrogen response elements in the TERT promoter. Collectively, these data provide the first potential mechanism for how androgen therapy improves hematologic function during the treatment of inherited and acquired bone marrow failure. Importantly, these findings have significant implications regarding the selection of hormone therapy used to treat marrow failure (ie, androgens that efficiently undergo aromatization or alternatively estradiol). Furthermore, if estradiol is as efficacious as androgens in enhancing TERT activity, and therefore hematopoietic function, then it may be more rational to treat females with estradiol to obviate the masculogenic effects of androgen therapy. However, before significant changes in practice occur, additional preclinical and clinical studies will need to be conducted to address important questions that arise as a result of these findings.
Most importantly, no hematopoietic stem/progenitor cell functional assays were included in the studies by Calado et al. Although it is logical that increasing TERT activity would increase hematopoietic stem/progenitor cell function, no direct studies addressed this critical question. Understanding whether androgen/estradiol-induced TERT activation also occurs in hematopoietic stem/progenitors is crucial because it is loss of these cells that is at the crux of genetic and acquired bone marrow failure conditions. Whereas the investigators used bone marrow CD34+ cells, which are enriched for stem/progenitors, this cell population is very heterogeneous. They contain a low frequency of stem/progenitor cells. In addition, pharmacologic doses of hormones were used in their studies. It is unclear whether the concentrations of the hormones used are achieved clinically. Therefore, future clinical studies assessing TERT activity in peripheral blood cells from patients before and after androgen therapy would be very interesting. Moreover, comparing increased TERT activity in patients who have a hematologic response after androgen therapy with patients who are androgen nonresponders and have no change in TERT activity would provide considerable evidence that the novel mechanism identified by these authors is evident in vivo.
Given the data presented by Calado et al, it is intriguing to speculate whether other tissue-specific stem/progenitor cells may enhance TERT activity in response to endogenously produced hormones. As one example, it is well established that premenopausal women have lower rates of cardiovascular disease compared with men, although those rates increase after menopause when estrogen levels are lower, coinciding with lower telomere lengths in hematopoietic cells.5,6 Could stem/progenitor cells of the vascular system also be dependent on estrogen to activate TERT, thereby enhancing cell lifespan, survival, and proliferation? Many similar provocative questions regarding aging and cancer arise from the studies by Calado et al, an attribute ascribed to groundbreaking scientific investigation.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal