Abstract
TNFRSF13B, which encodes TACI (transmembrane activator and calcium-modulator and cyclophilin ligand interactor), is mutated in 10% of patients with common variable immune deficiency (CVID). One of the 2 most common TACI mutations in CVID, A181E, introduces a negative charge into the transmembrane domain. To define the consequence of the A181E mutation on TACI function, we studied the effect of its murine equivalent, mTACI A144E, on TACI signaling in transfected cells and on TACI function in transgenic mice. The mTACI A144E mutant, like its human TACI A181E counterpart, was expressed on the surface of 293T transfectants and was able to bind ligand, but exhibited impaired constitutive and ligand-induced NFκB signaling. In addition, constitutive and ligand-induced clustering of the intracellular domain was deficient for A144E as measured by fluorescence resonance energy transfer. Transgenic mice expressing the A144E mutant on TACI−/− background had low serum IgA levels and significantly impaired antibody responses to the type II T-independent antigen TNP-Ficoll. B cells from A144E transgenic mice were impaired in their capacity to proliferate and secrete IgG1 and IgA in response to TACI ligation. These results suggest that mTACI A144E mutation and its human counterpart, A181E, disrupt TACI signaling and impair TACI-dependent B-cell functions.
Introduction
Common variable immunodeficiency (CVID) is the most common primary immunodeficiency with an estimated prevalence of 1 in 25 000 whites. It is characterized by recurrent bacterial infections, and is complicated by autoimmune manifestations in up to 20% of affected individuals and lymphoproliferation (splenomegaly) in approximately one-third of patients.1,2 CVID is diagnosed on the basis of an impaired ability to produce specific antibodies after vaccination or natural antigen exposure, markedly reduced serum levels of IgG, IgA, and frequently IgM, and exclusion of other causes for antibody deficiency. Most cases of CVID are sporadic, but at least 10% are familial, with a predominance of autosomal dominant over autosomal recessive inheritance.3
TACI (transmembrane activator and calcium-modulator and cyclophilin ligand [CAML] interactor), is a TNF receptor family member that is expressed on B cells. TACI is a receptor for BAFF (B-cell activating factor of the TNF family) and APRIL (a proliferation-inducing ligand),4 which are expressed by multiple cells including dendritic cells, macrophages, and neutrophils.5 We and others have reported that TNFRSF13B, which encodes TACI, is mutated in approximately 10% of patients with CVID.6-8 C104R and A181E are the 2 most common TACI mutations present in CVID. In the majority of cases, only 1 of the 2 TACI alleles is mutated. The C104R mutant protein, which is expressed on the cell surface, is unable to bind BAFF and APRIL.6,7 The A181E mutation is located in the transmembrane domain of TACI, and the mutant protein is expressed on the cell surface and binds BAFF normally.6
TACI has 2 cysteine-rich domains (CRDs) in its extracellular region.9,10 Ligand binding induces the recruitment of signaling proteins that include TNFR-associated factors and CAML to the intracellular (IC) domain of TACI.11 Our studies in mice12 and those of Litinskiy et al13 in humans have shown that TACI mediates isotype switching in naive B cells stimulated with BAFF or APRIL. In particular, TACI is essential for IgA isotype switching by these ligands. TACI knockout mice have significantly decreased serum IgA levels and an impaired antibody response to the type II T-independent (TI) antigens TNP-Ficoll and pneumococcal polysaccharide.14,15 In addition, TACI synergizes with CD40 and TLR9 in driving immunoglobulin production by B cells.16,17
The mechanism by which TACI mutations contribute to B-cell dysfunction and to the development of CVID is not well characterized. Both C104R and A181E homozygous mutations have been described in patients with CVID, but not to date in healthy controls.7 Both mutations are found in the heterozygous state in approximately 1% of the healthy controls; however, their frequency is 10-fold higher in the CVID population.6,7 Homozygous C104R mutation abolishes ligand binding, whereas the A181E mutation has no discernible effect on ligand binding.18 Mouse and human TACI share a similar domain structure, with the exception of an additional N-terminal 20–amino acid–long sequence in human TACI. In addition, they show a high degree of homology in their conserved domains. In particular, the amino acid sequences that surround each of the 2 common mutations associated with CVID (C104R and A181E) are highly conserved in mouse and human TACI. To assess the impact of the A181E mutation on TACI function, we examined the function of the corresponding A144E mutation in murine TACI in vitro and in vivo.
Methods
TACI expression and BAFF binding assays
293T cells were transfected with WT mTACI, WT hTACI, A144E mTACI, A181E hTACI, or with vector DNA alone using FuGENE 6 (Roche). Forty-eight hours after transfection surface expression was determined using PE anti-mTACI antibody (R&D Systems) for mTACI and biotinylated anti–human TACI (Peprotech) followed by streptavidin-PE (BD Pharmingen) for hTACI. To determine murine and human BAFF binding affinity, cells were incubated with FLAG-tagged mBAFF or hBAFF (Alexis Biochemicals), followed by a monoclonal anti-FLAG antibody conjugated to biotin (Sigma-Aldrich) then streptavidin-PE (BD Pharmingen).
NFκB reporter assay
Human 293T cells were transfected with 100 ng full-length TACI expression plasmid along with 100 ng NFκB-luciferase reporter plasmid (gift from Dr Laurie Glimcher, Harvard School of Public Health, Boston, MA) and 10 ng control pRL-TK plasmid (Promega). Four hours after transfection, ZZ-APRIL (50 ng/mL) was added to cells, and then reporter gene activity was determined 20 hours later using a dual-luciferase reporter assay system (Promega). ZZ-APRIL, a trimeric form of human APRIL4 that binds mouse TACI, was provided as a gift from ZymoGenetics and was used as the reagent for all experiments.
Coimmunoprecipitation of TACI monomers in 293T cells
A144E and WT TACI constructs were generated with myc or CFP tags using standard polymerase chain reaction (PCR) cloning techniques. 293T cells were transfected with the constructs using FuGENE 6. Forty-eight hours after transfection, cells were harvested and lysed in buffer containing 0.5% NP-40, 50 mM HEPES, 10 mM iodoacetamide, 250 mM NaCl, 5 mM EDTA, 100 mM PMSF, and complete protease inhibitor cocktail (Sigma-Aldrich). After preclearing with protein G-agarose beads (Upstate), proteins were immunoprecipitated with antimyc agarose beads (Sigma-Aldrich). Immune complexes were washed 3 times with lysis buffer. Proteins were electrophoresed on Tris/glycine gels, transferred to nitrocellulose membranes, and blotted with the indicated antibodies.
Fluorescence resonance energy transfer assay
A144E and WT TACI were generated possessing C-terminal in-frame fusions (at position 194 in place of the intracellular domain) to monomeric CFP or YFP using standard PCR cloning techniques. After confirming sequence and protein expression, 1 μg of each construct was transfected into 293T cells and flow cytometric detection of fluorescence resonance energy transfer (FRET) intensity was performed 48 hours after transfection as previously described19,20 using a CYAN ADP flow cytometer (Dako Cytometer) with 405/488-nm dual-laser excitation. Transfected cells were incubated with ZZ-APRIL (50 ng/mL), recombinant murine BAFF (50 ng/mL; R&D Systems), or recombinant 60-mer BAFF (100 ng/mL; gift from Biogenidec, Inc) for the indicated time points and subsequently analyzed by flow cytometry for FRET.
Generation of TACI−/− mice that express TACI transgenes
PCR-generated TACI WT and mutated gene products were cloned in the XhoI site of the pBSVE6BK vector containing an Eμ enhancer and Ig heavy chain (IgVH) promoter.21,22 Not1-Pvu1 digested fragments of the pBSVE6BK vector containing TACI cloned products were injected into pronuclei of fertilized oocytes from (C57BL/6/SJL) mice. Founders identified by PCR analysis of tail DNA were crossed with TACI−/− mice on a C57BL/6/129Sv background.14 F2 animals that were TACI+/− and transgene positive were crossed again with TACI−/− mice to obtain transgene-positive mice on a TACI−/− background. All mice were bred and housed in a specific pathogen-free animal facility. All experimental procedures performed on the animals were approved by the Animal Care and Use Committee of the Children's Hospital, Boston.
Flow cytometry
Single-cell suspensions were stained with fluorochrome-conjugated antibodies in PBS containing 1% BSA and Fc-block (BD Pharmingen), washed, and analyzed on a FACSCanto cytometer (Becton Dickinson). Conjugated murine mAbs used in these studies were PE anti-mTACI (R&D Systems), FITC anti-B220 (RA3-6B2; BD Pharmingen), and PE anti-CD3 (145-2C11; BD Pharmingen).
Immunizations
Mice 8 to 12 weeks of age were used for immunization experiments. TNP-Ficoll was used for type II T-independent immunizations. Mice received a single intraperitoneal injection of 25 μg TNP-Ficoll in PBS (TNP (83)-AECM-Ficoll; Biosearch Technologies). Serum was collected for analysis at days 0 and 14.
Total serum immunoglobulins and TNP-specific antibody measurements
Total serum immunoglobulins were assayed by enzyme-linked immunosorbent assay (ELISA) as previously described.23 To measure anti-TNP-specific antibody, high-capacity ELISA plates (Maxsorb; Nunc) were coated with 10 μg/mL TNP-conjugated bovine serum albumin (TNP40-BSA; Biosearch Technologies). Dilutions of sera (1:300 and 1:900) in 1% BSA were used for analysis. Alkaline phosphatase–conjugated isotype-specific antibodies (Southern Biotechnology Associates) were used as revealing antibodies.
Purification of naive B cells, proliferation, and immunoglobulin synthesis in vitro
Naive B cells were purified from splenic cell suspensions incubated with biotinylated murine mAbs (anti-IgG1, anti-IgG2a/b, anti-IgG3, anti-IgA, anti-IgE, anti-CD11b, anti-CD43, anti-90.2, and anti-CD138; BD Pharmingen) and then negatively selected using Dynabeads M-280 Streptavidin (Invitrogen). Purified cells (> 95% B220+ by fluorescence-activated cell sorting [FACS] analysis) were suspended in RPMI containing 10% FCS, l-glutamine, and 50 μM β-mercaptoethanol (complete medium). For immunoglobulin synthesis, naive B cells (106 cells/mL) were cultured in complete medium alone, with APRIL (5 nM and 25 nM,), antimouse CD40 (100 ng/mL, HM40-3; Pharmingen) + murine IL-4 (50 ng/mL; R&D systems), or LPS (10 μg/mL; Sigma-Aldrich) + human TGF-β (2 ng/mL; R&D systems). Supernatants were collected after 6 days and assayed for IgA and IgG1 production by ELISA. For proliferation, naive B cells (106 cells/mL) were cultured in complete medium alone, with ZZ-APRIL (5 nM and 25 nM), or in the presence of anti-CD40 (100 ng/mL) with IL-4 (50 ng/mL) for 72 hours, pulsed with 1 μCi (0.037 MBq) [3H]-thymidine for an additional 16 to 20 hours, then harvested and radioactivity was measured with a liquid scintillation counter.
Cell sorting of MZ and FO B cells
Splenic cell suspensions were stained with PE-Cy7 anti-B220 (RA3-6B2; eBioscience), FITC anti-CD21 (8D9; eBioscience), and PE anti-CD21 (B3B4; eBioscience) then resuspended in 0.3% BSA in PBS. Cells were sorted for B220+CD21hi (marginal zone [MZ]) and B220+CD23hi (follicular [FO]) using a FACSAria high-speed sorter SORP system (Becton Dickinson). The purity of both populations was more than 92%.
Data analysis
All flow cytometric data were analyzed by FlowJo software (Version 8.3; Treestar Inc) and fluorescence was plotted on biexponential axes. Statistical analysis was performed using Prism 4.0a (GraphPad Software).
Results
A144E mTACI binds ligand and engages in homotypic interaction, but fails to signal in response to ligand
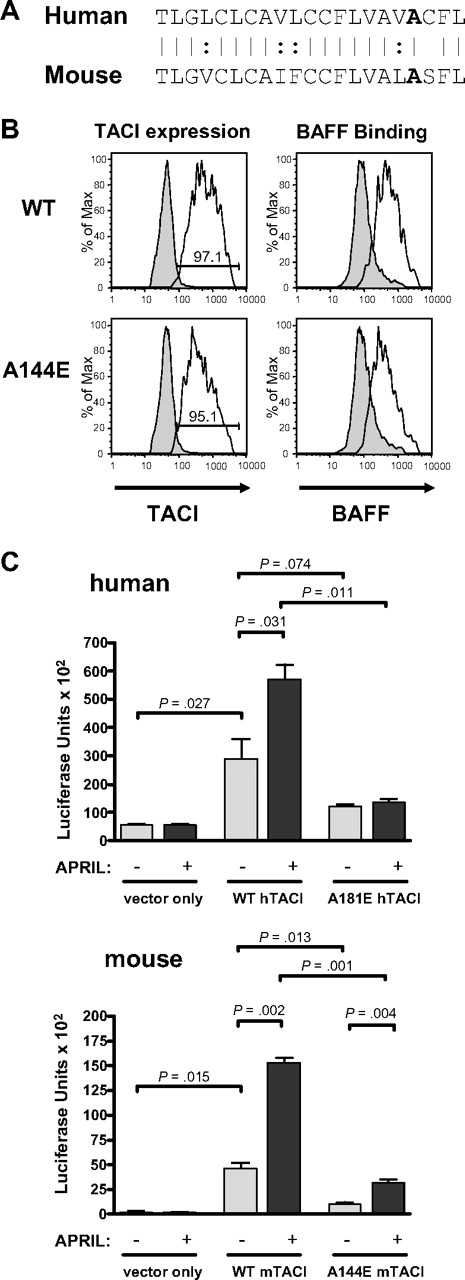
We have previously shown that human A181E TACI is expressed on the surface of 293T transfectants and binds BAFF to an extent comparable with human WT TACI.6 Figure 1A shows that the sequence of the transmembrane domain of TACI is highly conserved between human and mouse and that amino acid residue A181 in human TACI corresponds to residue A144 in mouse TACI. Figure 1B demonstrates that mouse A144E TACI is also expressed on the surface of 293T transfectants and binds BAFF to an extent comparable with murine WT TACI transfectants. To examine the effect of the A181E mutation and its murine counterpart, A144E, on TACI signaling, mutant or WT TACI was transfected into 293T cells together with a NFκB luciferase (LUC) reporter plasmid, and the capacity of APRIL to activate NFκB in the transfectants was examined. Figure 1C shows that in the absence of ligand, NFκB activity was significantly increased in 293T cells transfected with human or mouse WT TACI compared with cells transfected with empty vector, which showed negligible NFκB activity. This is consistent with the known ability of TNF-R–like molecules to signal when overexpressed.24 Baseline signaling in cells transfected with the human or mouse mutants was lower than in cells transfected with the corresponding WT TACI. Addition of APRIL produced a significant increase in NFκB activity in cells transfected with human or murine WT TACI, but no detectable increase in cells transfected with the human mutant, and a small increase in cells transfected with the mouse mutant. These results indicate that the A181E human TACI mutant and its A144E murine counterpart severely impair constitutive, as well as ligand-induced, TACI activation of NFκB. Given the similar behavior of the human and mouse mutants, we subsequently used the mouse A144E mutant to explore the effect of this mutation on TACI structure and function.
A144E TACI is expressed and binds ligand but does not signal in 293T cells. (A) Aligned amino acid sequence of the transmembrane domains of human and mouse TACI. (B) Surface expression and BAFF binding of WT and A144E mTACI in transfected 293T cells. Similar results were obtained in 2 independent experiments. Numbers in the insets represent percentage of cells expressing TACI. Shaded histograms represent isotype controls. (C) NFκB-driven luciferase activity in 293T cells transfected with human A181E and murine A144E TACI mutants and the corresponding WT TACI. Columns and bars represent mean and SD of 3 independent experiments. The Student t test was used to calculate significance.
A144E TACI is expressed and binds ligand but does not signal in 293T cells. (A) Aligned amino acid sequence of the transmembrane domains of human and mouse TACI. (B) Surface expression and BAFF binding of WT and A144E mTACI in transfected 293T cells. Similar results were obtained in 2 independent experiments. Numbers in the insets represent percentage of cells expressing TACI. Shaded histograms represent isotype controls. (C) NFκB-driven luciferase activity in 293T cells transfected with human A181E and murine A144E TACI mutants and the corresponding WT TACI. Columns and bars represent mean and SD of 3 independent experiments. The Student t test was used to calculate significance.
The A144E mutation impairs constitutive association and ligand-induced clustering of the intracellular domain of mTACI
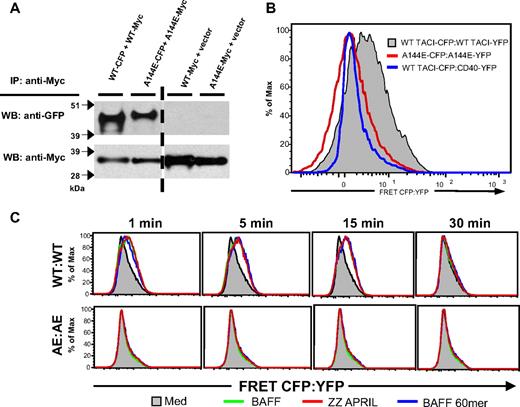
We previously showed that mTACI preassembles in 293T cells and that this assembly is dependent on CRD1.18 We also have used fluorescence resonance energy transfer (FRET) to demonstrate ligand-independent clustering of TACI intracellular (IC) domains.18 Figure 2A shows that A144E TACI, like WT TACI, preassembles in 293T cells as evidenced by coprecipitation of Myc-tagged and CFP-tagged A144E TACI from 293T cells transfected with both constructs. Figure 2B shows that, in contrast to WT TACI, A144E TACI failed to generate a FRET signal in 293T cells transfected with CFP- and YFP-tagged mutant. These data suggest that conformational changes in the structure of preassembled A144E TACI interfere with the ability of its IC domain to associate and generate a FRET signal.
A144E TACI preassembles in 293T cells but the IC domains failed to cluster both at baseline and following ligand stimulation. (A) Self-association of WT and A144E mTACI in 293T transfectants. (B-C) FRET analysis of 293T cells cotransfected with CFP-tagged WT mTACI and YFP-tagged WT mTACI, A144E mTACI, or CD40 control in the absence of ligand (B), and following addition of ZZ-APRIL, BAFF, or BAFF-60mer (C). Similar results were obtained in 3 experiments.
A144E TACI preassembles in 293T cells but the IC domains failed to cluster both at baseline and following ligand stimulation. (A) Self-association of WT and A144E mTACI in 293T transfectants. (B-C) FRET analysis of 293T cells cotransfected with CFP-tagged WT mTACI and YFP-tagged WT mTACI, A144E mTACI, or CD40 control in the absence of ligand (B), and following addition of ZZ-APRIL, BAFF, or BAFF-60mer (C). Similar results were obtained in 3 experiments.
Ligands that belong to the TNF family transduce signals by causing clustering of the IC domain of their receptors.19,25 We examined the effect of binding by ZZ-APRIL, trimeric BAFF, and BAFF-60mer, which is reported to induce stronger signaling via TACI than trimeric BAFF,26 on the clustering of the IC domain of WT and A144E mTACI, as evidenced by an increase in the FRET signal. APRIL, trimeric BAFF, and BAFF-60mer caused a comparable increase in the FRET signal in 293T cells transfected with WT TACI:CFP and WT TACI:YFP (Figure 2C). This increase was evident 1 minute after addition of ligand and was sustained for at least 15 minutes, but was no longer detected at 30 minutes. These ligands caused no detectable increase in the FRET signal in 293T cells transfected with A144E TACI:CFP and A144E TACI:YFP at any of the time points examined (Figure 2C). These results suggest that in addition to altering the pre–ligand receptor complex, the A144E mutation interferes with ligand-induced clustering of the IC domain of TACI.
Generation and phenotypic analysis of lymphocytes in TACI−/− mice reconstituted with A144E TACI transgene
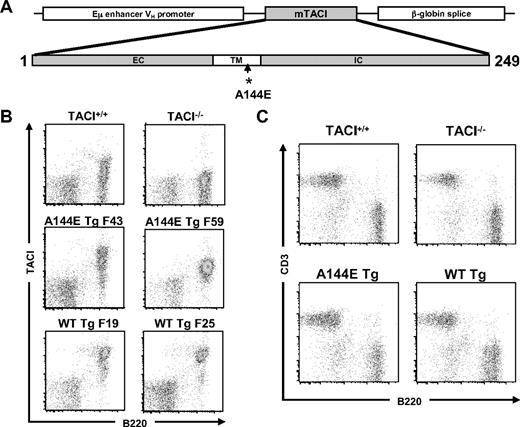
The fact that the sequence surrounding human A181 and murine A144 is highly conserved, and the similar effect of the A181E and A144E mutations on TACI function in transfectants, led us to construct transgenic mice that express the A144E mutant on a TACI-null background, as a model to assess the impact of these mutations on B-cell development and function. We used WT and A144E mutant mTACI constructs driven by the Eμ enhancer VH promoter as illustrated in Figure 3A. The transgenes were placed on the TACI−/− background to generate TACI−/− mice carrying either the WT or mutant TACI transgene, which are designated, respectively, WT Tg mice and A144E Tg mice. For each of the constructs, 2 transgenic lines derived from 2 separate founders were studied. Figure 3B shows that TACI was expressed on B220+ cells from spleens of WT Tg and A144E Tg mice. Almost all splenic B cells from WT Tg mice (90.8% ± 3.1%, n = 8) expressed TACI compared with only a fraction of B cells in TACI+/+ mice (39.9% ± 8.4%, n = 8). The percentage of splenic B cells in A144E Tg mice that expressed TACI was 71.5% ± 6.5%, (n = 7). The mean fluorescence intensity of TACI expression on the surface of TACI+ B cells was higher in WT Tg mice (10 582 ± 457 for WT Tg F25 and 12 974 ± 1024 for WT Tg F19) than in A144E Tg mice (4861 ± 564 for AE Tg F43 and 2029 ± 333 for AE Tg F59) and TACI+/+ mice (mean fluorescence intensity, 2628 ± 245). Similar data were obtained on peripheral blood B cells (data not shown). In all experiments, similar results were obtained with mice derived from separate founder lines carrying the same transgene, therefore we pooled the results from the 2 lines.
Characterization of TACI transgenic mice. (A) Schematic representation of the murine TACI transgenes. (B-C) Representative FACS analysis of TACI surface expression in the 2 WT Tg founder lines (F19 and F25) and the 2 A144E Tg founder lines (F43 and F59) (B), and of CD3+ and B220+cells (C) in splenocytes of TACI+/+, TACI−/−, and TACI transgenic mice.
Characterization of TACI transgenic mice. (A) Schematic representation of the murine TACI transgenes. (B-C) Representative FACS analysis of TACI surface expression in the 2 WT Tg founder lines (F19 and F25) and the 2 A144E Tg founder lines (F43 and F59) (B), and of CD3+ and B220+cells (C) in splenocytes of TACI+/+, TACI−/−, and TACI transgenic mice.
The thymus in both transgenic lines was normal in size, architecture, cellularity, and the distribution of CD4+ and CD8+ cells (data not shown). Bone marrow from both WT Tg and A144E Tg mice lines had normal percentages of B220+CD43+ pro-B cells, B220loIgM− pro- and pre-B cells, and B220loIgM+ immature B cells (data not shown). The total number of splenocytes was reduced in WT Tg and A144E Tg compared with TACI+/+ mice (45.8 ± 3.2 × 106 for WT Tg, 40.5 ± 10.2 × 106 for A144E Tg, and 112.1 ± 10.2 × 106 for TACI+/+ mice, n = 5), whereas this number was increased in TACI−/− mice (168.1 ± 33.9 × 106, n = 5), as previously reported. FACS analysis of splenocytes revealed that the distribution of CD3+ T cells and B220+ cells in WT Tg mice and in A144E Tg mice was comparable with that in TACI+/+ mice (Figure 3C). The percentage of B220+ cells was 42.7% (± 15.9%) for TACI+/+ mice, 38.1% (± 15.1%) for WT Tg mice, and 36.9% (± 14.5%) for A144E Tg mice (n = 3). As previously reported,14 the percentage of splenic B220+ cells (61.3% ± 22.4%, n = 3) was significantly increased in TACI−/− mice compared with TACI+/+ mice. The percentage of transitional T1 and T2 cells, marginal zone (MZ) B cells, and follicular (FO) B cells in the spleen was comparable between WT Tg, A144E Tg, TACI+/+, and TACI−/− mice (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article), with no statistically significant differences between the means of 3 independent experiments. There was no detectable difference in the percentage of apoptotic cells, as evidenced by comparable 7-AAD uptake, between B cells from WT Tg, A144E Tg, and TACI+/+ mice (supplemental Figure 2).
A144E Tg mice exhibit low serum IgA level and impaired response to type II TI antigen
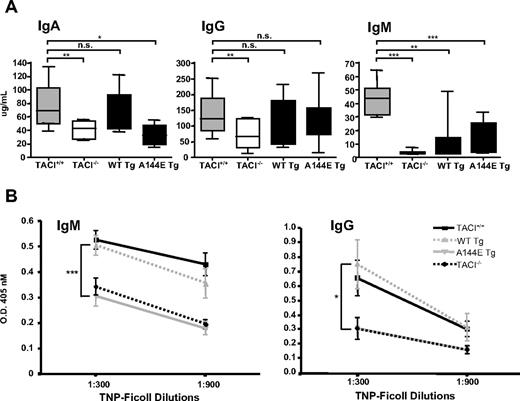
TACI−/− mice have been reported to have a selective decrease in serum IgA and IgM levels, with normal levels of serum IgG.14,15 In our hands, TACI−/− mice had significantly decreased serum IgG levels (Figure 4A). We confirmed this finding in TACI−/− mice bred on C57BL/6 background (supplemental Figure 3). Introduction of WT Tg onto the TACI−/− background resulted in normalization of serum IgA levels (Figure 4A). In contrast, reconstitution of TACI−/− mice with the A144E Tg failed to restore serum IgA levels. Serum IgM levels were only partially restored in WT Tg and A144E Tg mice and remained significantly lower than those in TACI+/+ mice. Introduction of WT Tg and A144E Tg increased serum IgG levels compared with TACI−/− mice but these levels remained lower than those of TACI+/+ mice, although the differences were not significant.
Serum immunoglobulin levels and antibody responses to TNP-Ficoll in TACI transgenic mice. (A) Serum IgA, IgG, and IgM levels from nonimmunized 8- to 12-week-old TACI+/+, TACI−/−, and TACI transgenic mice. The median (center line), 25th to 75th percentiles (box), and lowest and highest values (bars) are shown for each (n = 10-12 mice per group). (B) IgM and IgG anti-TNP antibody responses 14 days after immunization. Bars represent the standard error (n = 10-12 mice per group). For IgG anti-TNP, the lines representing A144E and TACI−/− groups are overlapping. The Mann-Whitney test was used in panel A, and 2-way ANOVA was used in panel B to calculate significance. (*P < .05, **P < .01, ***P < .005.)
Serum immunoglobulin levels and antibody responses to TNP-Ficoll in TACI transgenic mice. (A) Serum IgA, IgG, and IgM levels from nonimmunized 8- to 12-week-old TACI+/+, TACI−/−, and TACI transgenic mice. The median (center line), 25th to 75th percentiles (box), and lowest and highest values (bars) are shown for each (n = 10-12 mice per group). (B) IgM and IgG anti-TNP antibody responses 14 days after immunization. Bars represent the standard error (n = 10-12 mice per group). For IgG anti-TNP, the lines representing A144E and TACI−/− groups are overlapping. The Mann-Whitney test was used in panel A, and 2-way ANOVA was used in panel B to calculate significance. (*P < .05, **P < .01, ***P < .005.)
TACI−/− mice have deficient response to immunization with type II TI antigens.14,15 We examined the ability of A144E Tg mice and WT Tg controls to mount a specific antibody response to immunization with the type II TI antigen TNP-Ficoll. WT Tg mice mounted robust IgM and IgG anti-TNP responses that were comparable with those of TACI+/+ mice (Figure 4B). In contrast, A144E Tg mice had very poor IgM and IgG responses to TNP-Ficoll that were not significantly different from those of TACI−/− mice. The impaired antibody response to TNP-Ficoll was not due to a global B-cell dysfunction, because A144E Tg mice had a normal IgM and IgG responses to the T-dependent antigen KLH (supplemental Figure 4). Taken together, these results suggest that the A144E mutation impairs TACI-dependent B-cell function in vivo.
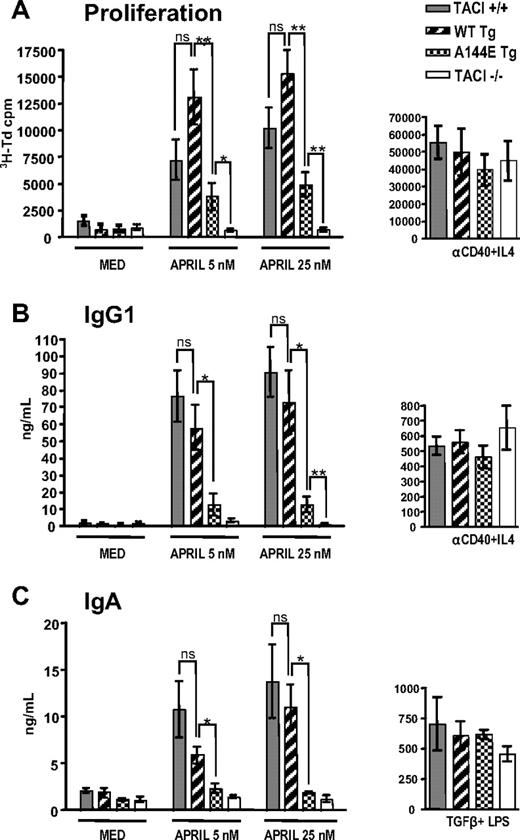
Proliferation and in vitro immunoglobulin production in response to APRIL are impaired in B cells from A144E Tg mice
APRIL causes proliferation and production of IgG1 and IgA in mouse splenic B cells.12,27 This is likely mediated via TACI, because freshly isolated splenic B cells express TACI but virtually no detectable BCMA.28 More importantly, proliferation and production of IgG1 and IgA in response to APRIL is preserved in B cells from BCMA−/− mice, but lost in B cells from TACI−/− mice.12 We compared the capacity of negatively selected naive B cells from WT Tg and A144E Tg to proliferate and secrete IgG1 and IgA in response to stimulation with 2 concentrations of APRIL (5 nM and 25 nM). Figure 5A shows that B cells from WT Tg mice proliferated vigorously in response to APRIL, and higher than B cells from TACI+/+ controls, but the difference was not significant. In contrast, B cells from A144E Tg mice had significantly decreased proliferation compared with B cells from TACI+/+ and WT Tg mice. However, they proliferated significantly more than B cells from TACI−/− mice, which as expected, completely failed to proliferate to APRIL. B cells from all 4 strains of mice proliferated comparably in response to anti-CD40+IL-4 (Figure 5A).
B-cell proliferation and immunoglobulin synthesis in vitro. Naive B cells were examined for (A) proliferation, (B) IgG1, and (C) IgA synthesis in response to APRIL, 5 and 25 nM. For controls, B cells were stimulated with anti-CD40+IL-4 for proliferation and IgG1 production and with TGF-β+LPS for IgA production (n = 5-9 mice per group). Mann-Whitney test was used to calculate significance. P values as in Figure 4.
B-cell proliferation and immunoglobulin synthesis in vitro. Naive B cells were examined for (A) proliferation, (B) IgG1, and (C) IgA synthesis in response to APRIL, 5 and 25 nM. For controls, B cells were stimulated with anti-CD40+IL-4 for proliferation and IgG1 production and with TGF-β+LPS for IgA production (n = 5-9 mice per group). Mann-Whitney test was used to calculate significance. P values as in Figure 4.
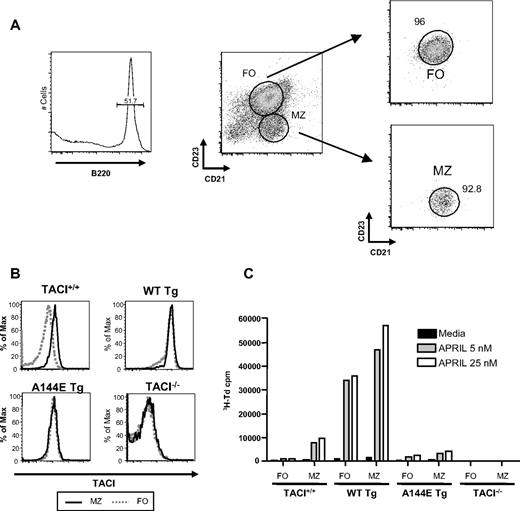
We compared the proliferative response to APRIL of purified MZ and FO B cells isolated by sorting (Figure 6A). As previously reported, TACI expression was higher in MZ B cells than FO B cells in WT mice.29 MZ B cells from WT Tg mice, but not A144E Tg mice, expressed higher levels of TACI than their counterparts from TACI+/+ mice. FO B cells from WT Tg and A144E Tg mice expressed TACI at similar levels compared with their MZ B cells (Figure 6B). MZ B cells from TACI+/+ mice proliferated substantially to APRIL, whereas FO B cells from these mice showed minimal proliferation (Figure 6C). MZ B cells from WT Tg mice had a more vigorous response to APRIL compared with their counterparts from TACI+/+ mice, consistent with their higher expression of TACI. More importantly, MZ B cells from A144E Tg mice proliferated poorly to APRIL compared with their counterparts from TACI+/+ mice, although they expressed comparable amounts of TACI on their surface. Unlike FO B cells from TACI+/+ mice and A144E Tg mice, FO B cells from WT Tg mice proliferated vigorously to APRIL. There was no detectable proliferation of either MZ B cells or FO B cells from TACI−/− mice. These results suggest that MZ B cells are the major B-cell population that proliferates in response to APRIL and that this proliferation is mediated by TACI and is abolished by the A144E mutation.
Proliferation of splenic MZ and FO B cells in response to APRIL. (A) Strategy of sorting and representative FACS analysis of sorted MZ and FO B cells. (B) TACI expression on sorted MZ and FO B cells. (C) Proliferation of sorted MZ and FO B cells in response to APRIL. Results represent mean of 2 experiments.
Proliferation of splenic MZ and FO B cells in response to APRIL. (A) Strategy of sorting and representative FACS analysis of sorted MZ and FO B cells. (B) TACI expression on sorted MZ and FO B cells. (C) Proliferation of sorted MZ and FO B cells in response to APRIL. Results represent mean of 2 experiments.
Stimulation with APRIL caused naive B cells from WT Tg mice to secrete IgG1 and IgA in amounts that were not significantly different from those secreted by B cells from TACI+/+ mice. (Figure 5B-C). At both concentrations of APRIL, B cells from A144E Tg mice secreted significantly less IgG1 and IgA than B cells from WT Tg mice and TACI+/+ controls. At the lower concentration of APRIL (5 nM), there was no significant increase in IgG1 or IgA production by A144E Tg B cells compared with TACI−/− B cells. At the higher concentration of APRIL (25 nM), there was a small but significant production of IgG1, but not IgA, by A144E Tg B cells compared with TACI−/− B cells. As expected, naive B cells from TACI−/− mice failed to secrete IgG1 or IgA in response to APRIL.
The decreased APRIL-driven proliferation and IgG1 and IgA production of A144E Tg B cells was not due to increased apoptosis, as there were no differences in apoptosis between B cells from TACI+/+, WT Tg, and A144E Tg mice that were freshly isolated or cultured for 4 days in the presence of absence of APRIL or anti-CD40+IL-4 (supplemental Figure 2). Impaired production of IgG1 and IgA by A144E Tg B cells in response to APRIL was not due to a general impairment in the ability of the B cells to produce these 2 immunoglobulin isotypes, because A144E Tg B cells secreted normal amounts of IgG1 and IgA in response to stimulation with anti–CD40+IL-4 and LPS+TGFβ, respectively. Taken together, these results strongly suggest that the A144E mutation impairs the TACI-mediated in vitro response of B cells to APRIL.
Discussion
Our data demonstrate that the murine A144E TACI mutation, which corresponds to the human A181E mutation associated with CVID, does not interfere with TACI expression, preassociation, or ligand binding, but severely impairs TACI signaling in vitro and TACI function in vivo.
Data from both 293T transfectants and B cells from A144E Tg mice demonstrated that murine A144E TACI, like human A181E TACI, is expressed on the cell surface (Figures 1B,3B). More importantly, A144E TACI, like the human mutant A181E TACI,6 retained the ability to bind ligand, as evidenced by comparable binding of BAFF by 293T cells transfected with A144E mTACI or WT TACI (Figure 1B). This is not surprising because CRD2, which is responsible for ligand binding,10 is intact in the mutant. In contrast, both A144E mTACI and A181E hTACI were severely impaired in their ability to activate NFκB in 293T transfectants (Figure 1C). This could not have been due to altered levels of expression of the mutant and WT TACI, because the levels of expression of WT and mutant TACI in the transfectants were comparable (Figure 1B and Castigli et al6 ). The modest increase in NFκB activation upon addition of APRIL to cells transfected with the A144E mutant suggests that residual signaling might still occur.
Signaling via receptors of the TNFR family, to which TACI belongs, is thought to be contingent on their ability to oligomerize and recruit signaling molecules.24 Impaired signaling by A144E TACI was not due to its failure to self-associate because A144E TACI monomers differentially labeled with Myc and CFP tags that were transfected into 293T cells coprecipitated to a degree comparable with that observed with similarly tagged WT TACI monomers (Figure 2A). Similar data were obtained with human A181E TACI (data not shown). This is expected since CRD1, which mediates preassociation of mTACI monomers,18 is intact in the mutant. However, ligand-independent association of the TACI IC domains was altered by the A144E mutation, as evidenced by the reduced FRET between fluorescent proteins fused to the C-terminal end of the receptor (Figure 2B). Taken together, these data suggest that the introduction of a negative charge in the transmembrane domain of TACI interferes with formation of the proper quaternary structure of the receptor chains, even if the preligand association domain is intact. The failure of A144E TACI to increase FRET and thus IC domain clustering, upon addition of ligand, compared with WT TACI supports this conclusion (Figure 2C), and likely underlies the reduced signaling capacity of this mutant. Reduced receptor interaction resulting from a transmembrane mutation is a novel mechanism of impairing receptor signaling in the TNF-R superfamily.
To validate our in vitro findings with transfected cells, and to examine the impact of the A144E mutation on B-cell development and function, we created transgenic mice that express the A144E Tg in their B cells on a TACI−/− background. Although TACI+/+ mice expressed TACI on a fraction of their B cells, B cells from Tg mice expressed TACI on the majority of their B cells (Figure 3B). This is most likely because expression of the transgene is driven by the Eμ enhancer VH promoter, which is active in all stages of B-cell development. Detailed analysis of B-cell subpopulations in bone marrow and spleen revealed no detectable effect of the transgenes on B-cell maturation, as the distribution of B-cell subsets was normal. However, the total number of splenocytes was reduced by approximately 2.5-fold in both WT Tg and A144E Tg mice. This suggests that expression of the transgenes may have interfered with the homeostasis of splenic B cells, although there was no detectable increase in apoptosis of freshly isolated splenic B cells (supplemental Figure 2). It is of interest that B-cell percentages in the spleen, which are significantly increased in TACI−/− mice,14,15 were normal not only in WT Tg mice but also in A144E Tg mice, despite impaired signaling by the mutant. It has been suggested that the inhibitory effect of TACI on B-cell hyperproliferation may result from a proapoptotic signal delivered by TACI,30 or from competition for BAFF binding with BAFF-R, which delivers a strong survival signal to B cells.28
Lack of increased apoptosis in B cells of WT Tg mice that express higher levels of TACI as well as normal percentages of B cells in spleens of A144E Tg mice, which have impaired TACI signaling, support the latter hypothesis. Furthermore, unlike what has been reported in TACI−/− mice, A144E Tg mice did not develop lymphoproliferation or high levels of anti-DNA antibodies compared with TACI+/+ mice when followed up to 20 months of age (data not shown).
In our hands, TACI−/− mice, on both mixed and C57BL6 background, had decreased serum IgG (supplemental Figure 3) in addition to decreased serum IgA and IgM (Figure 4A). Differences in environmental exposure in different animal facilities may have contributed to the differences in IgG levels between our study and prior studies. Decreased serum IgG levels in TACI−/− mice suggest that the serum Ig level abnormalities in these mice parallel more closely than previously thought the serum Ig levels abnormalities in CVID. Reconstitution of B cells from TACI−/− mice with WT TACI transgene, but not with A144E transgene, significantly increased serum IgA levels and the antibody response to the type II TI antigen TNP-Ficoll to the normal range (Figure 4). These results provide strong evidence that the A144E mutation severely impairs TACI function in vivo. Both transgenes resulted in increases in serum IgG and IgM levels compared with TACI−/− mice. The reason why the WT TACI Tg did not fully restore serum IgM and IgG levels could be related to effects that the artificially high level of expression of the transgene exerted in vivo. Introduction of the WT TACI transgene, however, had no effect on the normal response of TACI−/− mice to the T cell–independent antigen TNP-Ficoll or T cell–dependent antigen KLH (Figure 4B and supplemental Figure 3).
Analysis of B-cell responses to TACI in vitro revealed that MZ B cells are the major B-cell population that proliferates in response to APRIL and that this proliferation is mediated by TACI. More importantly, WT Tg restored the ability of TACI−/− B cells to proliferate and secrete IgG1 and IgA in response to APRIL (Figure 5). In fact, naive B cells and MZ B cells from WT Tg mice proliferated more vigorously to APRIL than B cells from TACI+/+ mice, likely due to their higher surface expression of TACI. The A144E Tg only partially restored B-cell proliferation to APRIL and completely failed to restore the capacity of TACI−/− B cells to secrete IgG1 and IgA in response to low concentrations of APRIL. At higher APRIL concentrations, there was a modest but significant production of IgG1, but not IgA, by A144E Tg B cells. This finding is consistent with the increase in IgG serum levels observed following introduction of the A144E Tg in TACI−/− mice and the residual NFκB signaling observed in ligand-stimulated 293T cells transfected with the A144E mutants.
In summary, our results demonstrate that the A144E mutation severely impairs TACI function in vitro and in vivo. We have found that A181E assembles with WT TACI (R.S.G., unpublished observations, June 2008) and thus has the potential of being dominant negative. Mutations in the transmembrane domain of other tyrosine kinase–containing receptors, such as HER2/neu or FGF-R3, also cause disease through a dominant mechanism.31,32 The study of A144E Tg mice on a TACI+/− background has given us equivocal results as to whether this mutation exerts a dominant negative effect on TACI function (R.S.G., unpublished observations, June 2008). We are currently constructing knockin mice expressing A144E TACI in the endogenous locus to generate a stable model of this mutation, which better mimics human A181E heterozygotes.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Chris Carmon for the modified CFP and YFP vectors; Yen-Ming Hsu from Biogenidec Inc for BAFF 60mer; members of the Geha laboratory for useful discussions; and Drs H. Oettgen, J. Manis, L. Notarangelo, and M. Massaad for reading the paper.
This work was supported by NIH grants P01-AI-031541 and T32-AI-007512; American Academy of Allergy, Asthma, and Immunology (AAAAI) GlaxoSmithKline (GSK) Award; and Austrian Science Fund J2744-B12.
National Institutes of Health
Authorship
Contribution: J.J.L. performed all of the studies on transgenic mice, analyzed the data, and prepared the paper; L.G. created the constructs used to establish the transgenic mouse lines and participated in the initial analysis of the mice; I.R. performed the TACI expression and BAFF binding studies and NFκB assay; E.O. participated in initial screen and in vitro studies of mice; T.S. immunized and bled mice for the studies; R.B. provided the TACI knockout mice; A.C.C. and R.M.S. performed FRET analysis; S.R.D. provided the ZZAPRIL used in the studies; H.J. helped plan and perform the sorting experiments for MZ and FO B cells and edited the paper; and R.S.G. planned the experiments and extensively edited the paper.
Conflict-of-interest disclosure: R.B. is a co-inventor on patents related to TNFRSF13B gene that is held by St Jude Children's Research Hospital (Memphis, TN). S.R.D. is an employee and stockholder of ZymoGenetics Inc. The remaining authors declare no competing financial interests.
Correspondence: Raif S. Geha, Division of Immunology, Children's Hospital, Karp 10, 300 Longwood Ave, Boston, MA 02115; e-mail: raif.geha@childrens.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal