We thank Dolcetti and colleagues for their interest in our report and for highlighting our observation of lymphocytopenia close in time to diagnosis of HIV-related Hodgkin lymphoma (HL). We agree that this finding makes the timing of CD4+ assessment critical to elucidating the relationship between degree of immune suppression and HL risk in HIV-infected cohorts and that it complicates comparisons between our findings and those based upon CD4+ counts in the year preceding HL diagnosis.1
Dolcetti et al go on to propose that a decline in CD4+ count should be investigated as a marker for HL development. However, it should be noted that the observed decline in cell counts in the proximity of HL diagnosis was not specific to CD4+, but was also seen for CD8+ and total lymphocytes.2
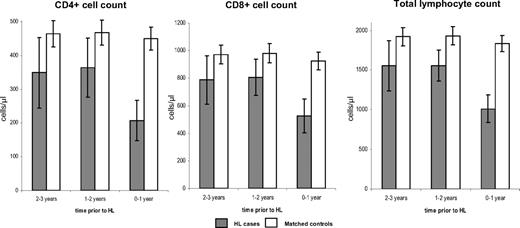
To assess the usefulness of declines in lymphocyte counts to predict future HL development, we reanalyzed CD4+, CD8+, and total lymphocyte counts at an additional time point 2 to 3 years prior to HL diagnosis, using the same case:control pairs as in our paper.2 Complete information across all 3 time points before HL diagnosis, namely 2 to 3 years, 1 to 2 years, and 0 to 1 year, was available for a total of 26 HL cases and 202 matched controls. These data are described in Figure 1. Of note, there was no evidence of a gradual decline in CD4+, CD8+, or total lymphocyte counts across the 3 periods, but only a dramatic drop in cell counts in the year preceding HL diagnosis (Figure 1). As expected, no differences over time were seen in controls. This would suggest that lymphocytopenia does not gradually develop before HL onset, but is rather a consequence of the tumor.
Trends in CD4+ cell, CD8+ cell, total lymphocyte counts and 95% confidence intervals, by time prior to HL diagnosis among 26 HL cases and 202 matched controls.3
Trends in CD4+ cell, CD8+ cell, total lymphocyte counts and 95% confidence intervals, by time prior to HL diagnosis among 26 HL cases and 202 matched controls.3
We agree with Dolcetti et al that other cohorts of closely followed HIV-infected persons, with assessment of CD4+ and CD8+ cell counts at multiple time points with respect to HL diagnosis should be evaluated to clarify these issues. Ideally, EBV-specific CD4+ and CD8+ cell counts might also be assessed.
Authorship
The Swiss HIV Cohort Study (SHCS) has been approved by the ethical committees of all the collaborating clinics and the present analysis was additionally approved by the scientific committee of the SHCS and the ethics committee of the International Agency for Research on Cancer (IARC). Written informed consent was obtained from all SHCS participants in accordance with the Declaration of Helsinki.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Gary Clifford, International Agency for Research on Cancer, 150 cours Albert Thomas, 69372 Lyon cedex 08, France; e-mail: clifford@iarc.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal