Abstract
Waldenström macroglobulinemia (WM) is a distinct B-cell disorder resulting from the accumulation, predominantly in the bone marrow, of clonally related IgM-secreting lymphoplasmacytic cells. Genetic factors play an important role, with 20% of patients demonstrating a familial predisposition. Asymptomatic patients should be observed. Patients with a disease-related hemoglobin level less than 10 g/L, platelet count less than 100 × 109/L, bulky adenopathy or organomegaly, symptomatic hyperviscosity, peripheral neuropathy, amyloidosis, cryoglobulinemia, cold-agglutinin disease, or evidence of disease transformation should be considered for therapy. Plasmapheresis should be considered for symptomatic hyperviscosity and for prophylaxis in patients in whom rituximab therapy is contemplated. The use of rituximab as monotherapy or in combination with cyclophosphamide, nucleoside analog, bortezomib, or thalidomide-based regimens can be considered for the first-line therapy of WM and should take into account specific treatment goals, future autologous stem cell transplantation eligibility, and long-term risks of secondary malignancies. In the salvage setting, the reuse or use of an alternative frontline regimen can be considered as well as bortezomib, alemtuzumab, and stem cell transplantation. Newer agents, such as bendamustine and everolimus, can also be considered in the treatment of WM.
Introduction
Waldenström macroglobulinemia (WM) is a distinct B-cell disorder resulting from the accumulation, predominantly in the bone marrow, of clonally related lymphoplasmacytic cells, which secrete a monoclonal IgM protein.1 This condition is considered to correspond to the lymphoplasmacytic lymphoma (LPL) as defined by the Revised European American Lymphoma and World Health Organization classification systems.2,3 Most cases of LPL are WM, with less than 5% of cases made up of IgA, IgG, and nonsecreting LPL.
Clinical features
The clinical and laboratory findings for 356 newly diagnosed patients who presented to our institution are depicted in Table 1. Unlike most indolent lymphomas, splenomegaly and lymphadenopathy are present in only a minority of patients (< 15%). The morbidity associated with WM is typically mediated by tissue infiltration by neoplastic cells, the physicochemical and immunologic properties of the monoclonal IgM, or both. As shown in Table 2, the monoclonal IgM can produce clinical manifestations through several distinct mechanisms, including an effect on serum viscosity, mediation of autoantibody activity, interactions with other proteins, precipitation on cooling, and tissue deposition.4-6
Clinical and laboratory findings for 356 newly diagnosed patients with the consensus panel diagnosis of WM presenting to the Dana-Farber Cancer Institute
| . | Median . | Range . | Normal reference range . |
|---|---|---|---|
| Age, y | 58 | 32-91 | NA |
| Sex, male/female | 215/141 | NA | |
| Bone marrow involvement, percentage | 30 | 5-95 | NA |
| Adenopathy, percentage | 15 | NA | |
| Splenomegaly, percentage | 10 | NA | |
| IgM, mg/dL | 2620 | 270-12 400 | 40-230 |
| IgG, mg/dL | 674 | 80-2770 | 700-1600 |
| IgA, mg/dL | 58 | 6-438 | 70-400 |
| Serum viscosity, cp | 2.0 | 1.1-7.2 | 1.4-1.9 |
| Hematocrit, percentage | 35.4 | 17.2-45.4 | 34.8-43.6 |
| Platelets, ×109/L | 275 | 42-675 | 155-410 |
| White blood cells, ×109/L | 6.4 | 1.7-22 | 3.8-9.2 |
| β-2 microglobulin, mg/dL | 2.5 | 0.9-13.7 | 0-2.7 |
| LDH, U/mL | 313 | 61-1701 | 313-618 |
| . | Median . | Range . | Normal reference range . |
|---|---|---|---|
| Age, y | 58 | 32-91 | NA |
| Sex, male/female | 215/141 | NA | |
| Bone marrow involvement, percentage | 30 | 5-95 | NA |
| Adenopathy, percentage | 15 | NA | |
| Splenomegaly, percentage | 10 | NA | |
| IgM, mg/dL | 2620 | 270-12 400 | 40-230 |
| IgG, mg/dL | 674 | 80-2770 | 700-1600 |
| IgA, mg/dL | 58 | 6-438 | 70-400 |
| Serum viscosity, cp | 2.0 | 1.1-7.2 | 1.4-1.9 |
| Hematocrit, percentage | 35.4 | 17.2-45.4 | 34.8-43.6 |
| Platelets, ×109/L | 275 | 42-675 | 155-410 |
| White blood cells, ×109/L | 6.4 | 1.7-22 | 3.8-9.2 |
| β-2 microglobulin, mg/dL | 2.5 | 0.9-13.7 | 0-2.7 |
| LDH, U/mL | 313 | 61-1701 | 313-618 |
NA indicates not applicable.
Morbidities mediated by the monoclonal IgM protein in WM
| Property of IgM monoclonal protein . | Diagnostic condition . | Clinical manifestations . |
|---|---|---|
| Pentameric structure | Hyperviscosity | Headaches, blurred vision, epistaxis, retinal hemorrhages, leg cramps, impaired mentation, intracranial hemorrhage |
| Precipitation on cooling | Cryoglobulinemia (type I) | Raynaud phenomenon, acrocyanosis, ulcers, purpura, cold urticaria |
| Autoantibody activity to myelin-associated glycoprotein (MAG), ganglioside M1 (GM1), sulfatide moieties on peripheral nerve sheaths | Peripheral neuropathies | Sensorimotor neuropathies, painful neuropathies, ataxic gait, bilateral foot drop |
| Autoantibody activity to IgG | Cryoglobulinemia (type II) | Purpura, arthralgias, renal failure, sensorimotor neuropathies |
| Autoantibody activity to red blood cell antigens | Cold agglutinins | Hemolytic anemia, Raynaud phenomenon, acrocyanosis, livedo reticularis |
| Tissue deposition as amorphous aggregates | Organ dysfunction | Skin: bullous skin disease, papules, Schnitzler syndrome; GI: diarrhea, malabsorption, bleeding; kidney: proteinuria, renal failure (light chain component) |
| Tissue deposition as amyloid fibrils (light chain component most commonly) | Organ dysfunction | Fatigue, weight loss, edema, hepatomegaly, macroglossia, organ dysfunction of involved organs: heart, kidney, liver, and peripheral sensory and autonomic nerves |
| Property of IgM monoclonal protein . | Diagnostic condition . | Clinical manifestations . |
|---|---|---|
| Pentameric structure | Hyperviscosity | Headaches, blurred vision, epistaxis, retinal hemorrhages, leg cramps, impaired mentation, intracranial hemorrhage |
| Precipitation on cooling | Cryoglobulinemia (type I) | Raynaud phenomenon, acrocyanosis, ulcers, purpura, cold urticaria |
| Autoantibody activity to myelin-associated glycoprotein (MAG), ganglioside M1 (GM1), sulfatide moieties on peripheral nerve sheaths | Peripheral neuropathies | Sensorimotor neuropathies, painful neuropathies, ataxic gait, bilateral foot drop |
| Autoantibody activity to IgG | Cryoglobulinemia (type II) | Purpura, arthralgias, renal failure, sensorimotor neuropathies |
| Autoantibody activity to red blood cell antigens | Cold agglutinins | Hemolytic anemia, Raynaud phenomenon, acrocyanosis, livedo reticularis |
| Tissue deposition as amorphous aggregates | Organ dysfunction | Skin: bullous skin disease, papules, Schnitzler syndrome; GI: diarrhea, malabsorption, bleeding; kidney: proteinuria, renal failure (light chain component) |
| Tissue deposition as amyloid fibrils (light chain component most commonly) | Organ dysfunction | Fatigue, weight loss, edema, hepatomegaly, macroglossia, organ dysfunction of involved organs: heart, kidney, liver, and peripheral sensory and autonomic nerves |
Diagnostic workup
History taking
There is a strong familial predisposition in WM7-9 ; therefore, a good family history is important. Although the identification of such familiarity does not at this time influence treatment decisions, it may spawn a discussion in families with multiple cases of WM or related B-cell disorders to participate in familial studies aimed at identifying genetic predispositions to WM, which are currently under way at the Dana-Farber Cancer Institute and the National Institutes of Health. Exposure to hepatitis C has been implicated in some, but not all, studies, and evaluation of risks/exposure is important, particularly among patients who have type II (mixed) cryoglobulinemia.10-12 A thorough review of systems is very important in the workup of WM patients, given the vast array of presenting symptoms, and may well impact on treatment considerations. A review of systems checklist, which we use at our institution, along with their implications for the care of WM patients is presented in Table 3 and can be used in the workup of WM patients.
A review of systems and their implications in the workup of patients with WM
| Symptom . | Implications . | Action . |
|---|---|---|
| Energy level/changes in activities of daily life | Anemia, fatigue without anemia | Evaluate for anemia, underlying etiology, including iron deficiency, hemolytic anemia (warm and cold antibodies); consider amyloidosis; exclude other medical causes of anemia |
| Constitutional symptoms | Tumor-related fever, chills, night sweats | |
| Recurrent sinus and bronchial infections | Chronic sinusitis, usually on the basis of IgA and IgG hypogammaglobulinemia | Antibiotic support, if refractory to multiple antibiotic courses, hospitalizations, or life-threatening, strongly consider intravenous immunoglobulin replacement |
| Headaches, blurry vision or visual loss, confusional episodes, epistaxis | Hyperviscosity | Funduscopic examination for hyperviscosity-related changes, obtain serum IgM and viscosity levels; consider emergency plasmapheresis for symptomatic hyperviscosity; strongly consider in patients with serum viscosity > 4.0 cp given high risk of hyperviscosity-related events |
| Easy bruising, bleeding diathesis | Thrombocytopenia, acquired von Willebrand disorder | Complete blood count, evaluate for immune thrombocytopenia or hypersplenism if indicated; consider evaluation for von Willebrand disorder; consider amyloidosis |
| Progressive symmetric numbness, tingling, burning, pain in feet and hands; unsteady gait, deficits in motor function | IgM-related neuropathy or myopathy; amyloidosis | Obtain anti-MAG, anti-GM1, anti-sulfatidyl IgM antibody studies; if myopathy is present, consider obtaining antidecorin antibodies; consider obtaining fat pad biopsy and stain for amyloid; consider EMG studies |
| Raynaud-like symptoms, acrocyanosis, ulcerations on extremities | Cryoglobulinemia, cold agglutinemia | Obtain cryoglobulins, cold agglutinins, in patients suspected of having cryoglobulins, all studies, including quantitative immunoglobulins, should be obtained in a warm bath to avoid cryoprecipitation and false lowering of serum IgM levels |
| Diarrhea, gastrointestinal cramping | Malabsorption, secondary to amyloidosis, IgM deposition, tumor involvement; rarely, autonomic neuropathy on basis of autoantibody or amyloidosis | Endoscopy to evaluate small bowel, biopsy to evaluate for amyloidosis, IgM deposition, tumor involvement |
| Hearing loss | Hyperviscosity, sensorineural hearing loss, amyloid or tumor deposition, thrombus formation | Consider evaluation for anti-Hu and anti-hsp 70 antibodies, MRI to assess for amyloidoma, tumor deposition; evaluate for hyperviscosity syndrome (as earlier in Table 3); assess for IgM antiphospholipid antibodies |
| Thrombotic events | Antiphospholipid antibody syndrome | Assess for IgM antiphospholipid antibodies |
| Urticaria, papules, dermatitis | Schnitzler syndrome (nonpruritic urticaria), IgM or tumor cell infiltration, amyloid deposition | Skin biopsy, histologic examination for tumor cell infiltration, stain for IgM, Congo red for amyloid |
| Symptom . | Implications . | Action . |
|---|---|---|
| Energy level/changes in activities of daily life | Anemia, fatigue without anemia | Evaluate for anemia, underlying etiology, including iron deficiency, hemolytic anemia (warm and cold antibodies); consider amyloidosis; exclude other medical causes of anemia |
| Constitutional symptoms | Tumor-related fever, chills, night sweats | |
| Recurrent sinus and bronchial infections | Chronic sinusitis, usually on the basis of IgA and IgG hypogammaglobulinemia | Antibiotic support, if refractory to multiple antibiotic courses, hospitalizations, or life-threatening, strongly consider intravenous immunoglobulin replacement |
| Headaches, blurry vision or visual loss, confusional episodes, epistaxis | Hyperviscosity | Funduscopic examination for hyperviscosity-related changes, obtain serum IgM and viscosity levels; consider emergency plasmapheresis for symptomatic hyperviscosity; strongly consider in patients with serum viscosity > 4.0 cp given high risk of hyperviscosity-related events |
| Easy bruising, bleeding diathesis | Thrombocytopenia, acquired von Willebrand disorder | Complete blood count, evaluate for immune thrombocytopenia or hypersplenism if indicated; consider evaluation for von Willebrand disorder; consider amyloidosis |
| Progressive symmetric numbness, tingling, burning, pain in feet and hands; unsteady gait, deficits in motor function | IgM-related neuropathy or myopathy; amyloidosis | Obtain anti-MAG, anti-GM1, anti-sulfatidyl IgM antibody studies; if myopathy is present, consider obtaining antidecorin antibodies; consider obtaining fat pad biopsy and stain for amyloid; consider EMG studies |
| Raynaud-like symptoms, acrocyanosis, ulcerations on extremities | Cryoglobulinemia, cold agglutinemia | Obtain cryoglobulins, cold agglutinins, in patients suspected of having cryoglobulins, all studies, including quantitative immunoglobulins, should be obtained in a warm bath to avoid cryoprecipitation and false lowering of serum IgM levels |
| Diarrhea, gastrointestinal cramping | Malabsorption, secondary to amyloidosis, IgM deposition, tumor involvement; rarely, autonomic neuropathy on basis of autoantibody or amyloidosis | Endoscopy to evaluate small bowel, biopsy to evaluate for amyloidosis, IgM deposition, tumor involvement |
| Hearing loss | Hyperviscosity, sensorineural hearing loss, amyloid or tumor deposition, thrombus formation | Consider evaluation for anti-Hu and anti-hsp 70 antibodies, MRI to assess for amyloidoma, tumor deposition; evaluate for hyperviscosity syndrome (as earlier in Table 3); assess for IgM antiphospholipid antibodies |
| Thrombotic events | Antiphospholipid antibody syndrome | Assess for IgM antiphospholipid antibodies |
| Urticaria, papules, dermatitis | Schnitzler syndrome (nonpruritic urticaria), IgM or tumor cell infiltration, amyloid deposition | Skin biopsy, histologic examination for tumor cell infiltration, stain for IgM, Congo red for amyloid |
Laboratory studies
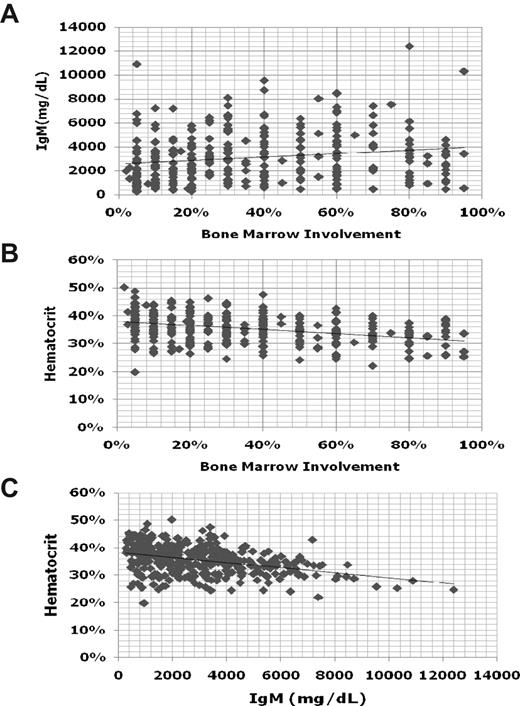
To establish the diagnosis of WM, it is necessary to demonstrate an IgM monoclonal protein, along with histologic evidence of infiltration of the bone marrow by lymphoplasmacytic cells.1 There is no minimal serum IgM level, nor a minimal percentage of bone marrow infiltration to establish the diagnosis of WM because patients can be symptomatic and in need of treatment even at low levels of IgM (< 1000 mg/dL) or bone marrow involvement. Indeed, there can be great heterogeneity among patients between their respective serum IgM levels and bone marrow involvement (Figure 1). In general, for an individual patient, serum IgM levels tend to show good correlation with disease burden. There are some exceptions to this point, which usually emerge in patients with cryoglobulinemia, as well as those patients undergoing treatment with certain biologic agents (ie, rituximab, bortezomib). The assessment of response for patients undergoing treatment with biologic agents is further discussed in “Response assessment.”
Comparisons of serum IgM, hematocrit, and bone marrow disease involvement for 356 newly diagnosed patients with WM.
Comparisons of serum IgM, hematocrit, and bone marrow disease involvement for 356 newly diagnosed patients with WM.
Peripheral blood testing
Baseline serum protein electrophoresis, quantitative immunoglobulins, complete blood counts, liver function tests, blood urea nitrogen, and creatinine should be obtained. A warm bath collection should be considered for those patients suspected of having cryoglobulinemia to avoid underestimation of the serum IgM levels. It is important to note that serum immunoglobulin levels can vary considerably between institutions; hence, for comparative purposes, serum immunoglobulin levels are best followed by the same laboratory so as to avoid misinterpretation. Because the value of serum-free light chain testing remains to be clarified in the management of WM patients, its inclusion in routine testing of WM patients is not advised at this time.
Whereas IgM levels will be elevated in almost all WM patients, IgA and IgG levels are subnormal and may contribute to recurring sinus and bronchial infections.13 Serum IgA and IgG levels seldom return to normal, even after good remissions (including attainment of complete response) and may reflect a constitutional defect in plasma cell development. Although liver function tests should be evaluated, these seldom are abnormal on the basis of disease. Azotemia can often present in WM on the basis of light chain or amyloid deposition, as well as parenchymal involvement by lymphoplasmacytic cells. Therefore, renal function should carefully be evaluated. Complete blood counts should also be carefully evaluated and expanded up by evaluation of the mean corpuscular volume and reticulocyte count for evidence of underlying autoimmune hemolysis. Lastly, β-2 microglobulin and albumin levels can obtained for purposes of prognostication, although their use in making treatment-related decisions remains to be clarified.14,15
Selective blood testing
There can be considerable heterogeneity between bone marrow disease involvement and anemia in patients with WM (Figure 1). As such, the underlying basis for anemia may need to be better delineated, particularly in patients whose anemia appears to be of proportion to the level of their disease involvement. Autoimmune hemolytic anemia can commonly occur either on the basis of cold or warm antibodies in WM patients. The examination of reticulocyte counts, lactic dehydrogenase, and haptoglobin levels can be useful, although most often hemolysis is extravascular.16,17 Testing for cold agglutinins, and thereafter direct and indirect Coombs antibody testing if cold agglutinins are negative, is advised as part of the workup of patients presenting with autoimmune hemolytic anemia. Iron deficiency–related anemia is also commonly encountered in WM and often is refractory to oral but not intravenous iron repletion. As such, iron studies can be useful in patients presenting with microcytic anemia. For some patients, correction of the iron deficiency with intravenous iron leads to improvements in anemia and may defer the necessity for immediate chemotherapeutic intervention. At higher serum IgM levels, anemia may also be more pronounced because of a hemodilutional effect (Figure 1).
Obtaining a serum viscosity (SV) level is helpful in patients in whom hyperviscosity is suspected. Whereas most WM patients will exhibit an elevated SV level, that is, more than 1.8 centipoise (cp), patients typically become symptomatic at SV levels of more than 4.0 cp. However, there can be great variability in the SV level at which patients become symptomatic. At SV levels as low as 3.0 cp, patients can exhibit retinal changes, including hemorrhages, thereby warranting intervention at lower SV levels.18 Conversely, patients with SV levels greater than 4.0 cp can often be asymptomatic, suggesting that variables besides the level of serum IgM alone play a role in producing symptomatic hyperviscosity, such as the presence of cryoglobulinemia.19 As such, testing for cryoglobulins should be considered in patients with symptomatic hyperviscosity who display relatively low serum IgM and SV levels.
Peripheral neuropathy is an important morbidity in patients with WM, with up to 20% to 25% of patients demonstrating disease-related peripheral neuropathy, which most often is sensory in nature.20,21 In patients suspected of having IgM-related peripheral neuropathy, the evaluation of anti–myelin-associated glycoprotein, anti-ganglioside M1, and anti-sulfatide IgM antibodies is appropriate. Although the presence of one of these antibodies may support the diagnosis of IgM-related neuropathy, their absence should not exclude the diagnosis because other myelin-associated antigens may be targeted that are not clinically evaluable at this time. Amyloidosis should also be considered in patients presenting with a peripheral neuropathy, and a fat pad biopsy with Congo red staining obtained.22 Electromyography may be helpful and often shows a demyelinating neuropathy. A sural nerve biopsy should be avoided because of frequent neuropathic complications. In rare circumstances where a myopathy may be suspected on the basis of WM, the investigation for antidecorin IgM antibodies can be considered.23
Bone marrow evaluation
The bone marrow is almost always involved in WM; and as such, a bone marrow biopsy and aspiration should be obtained. Central to the diagnosis of WM is the demonstration of bone marrow infiltration by a lymphoplasmacytic cell population manifested by small lymphocytes with evidence of plasmacytoid/plasma cell differentiation.1 The pattern of bone marrow infiltration may be diffuse, interstitial, or nodular and is usually intertrabecular. A solely paratrabecular pattern of infiltration is unusual and should raise the possibility of follicular lymphoma.1 The bone marrow infiltration should be supported by immunophenotypic studies (flow cytometry and/or immunohistochemistry) showing the following profile: sIgM+CD19+CD20+CD22+CD79+.24,25 Up to 20% of cases may express CD5, CD10, or CD23.26 In such cases, care should be taken to satisfactorily exclude chronic lymphocytic leukemia and mantle cell lymphoma.1 An increased number of mast cells, usually in association with the lymphoid aggregates, is commonly found in WM, and their presence may help in differentiating WM from other B-cell lymphomas.2,3
Cytogenetic studies
Multiple studies have been published on cytogenetic findings in WM and have demonstrated a great variety of numerical and structural chromosomal abnormalities. Chromosome 6q deletions encompassing 6q21-25 have been observed in up to half of WM patients, and at a comparable frequency among patients with and without a familial history.7,27,28 Several candidate tumor suppressor genes in this region are under study, including BLIMP-1.29 Despite an earlier study suggesting prognostic significance to 6q deletions in WM, a more recent study did not confirm such significance.27,30 As such, routine cytogenetic testing is not advised at this time. An exception, however, is the use of cytogenetics to clarify the diagnosis of WM from suspected cases of IgM myeloma. In the latter, IgH switch region rearrangements (14q32 translocations) are a predominant feature, whereas these are typically absent in WM.31
Imaging studies
CT scans of the chest, abdomen, and pelvis should be obtained at time of diagnosis to properly stage the patient.32 Up to 20% of WM patients may have extramedullary disease, and CT scans offer an opportunity to assess for adenopathy, splenomegaly, and for other extramedullary disease sites. Follow-up CT scans are necessary only for those patients with baseline extramedullary disease (or who are later suspected of having extramedullary disease), and may be used to assess disease progression, as well as response. There is no role for routine magnetic resonance imaging; as well, there is not a routine role for positron emission tomography scanning unless disease transformation is suspected.
Ophthalmologic examination
Patients with WM often exhibit retinal changes resulting from hyperviscosity-related changes that occur as a consequence of elevated IgM levels. Retinal findings associated with hyperviscosity can include peripheral dot-and-blot-like hemorrhages, dilated retinal veins, central hemorrhages, tortuous blood vessels, venous “sausaging,” and/or optic disc edema. In one study, retinal changes were observed at serum IgM and viscosity levels as low as 3000 mg/dL and 2.4 cp, respectively.18 Importantly, plasmapheresis can lead to prompt resolution of hyperviscosity-related retinal changes.33 Examination of the retina may therefore be useful in identifying the symptomatic threshold of serum viscosity levels in patients with WM and may be used as an important gauge for the effectiveness of both plasmapheresis and chemotherapy. In our practice, we typically recommend a baseline ophthalmologic examination in those WM patients whose serum IgM levels are more than 3000 mg/dL.
Treatment approaches to WM
Management of the asymptomatic or smoldering WM patient
Patients with a disease-related hemoglobin level less than 10 g/dL, platelet count less than 100 × 109/L, bulky adenopathy or organomegaly, symptomatic hyperviscosity, moderate to severe or advancing peripheral neuropathy on the basis of disease, symptomatic amyloidosis, cryoglobulinemia, or cold-agglutinin disease should be considered for therapy.14 Initiation of therapy should not be based on serum monoclonal protein levels per se, and asymptomatic patients should be observed. Asymptomatic patients with a low β-2 microglobulin (< 3 g/dL) and a hemoglobin level of more than 12 g/dL may have an indolent course and not require therapy for a long period of time, even when their monoclonal protein exceeds 3000 mg/dL. As such, the identification of the asymptomatic patient is important, and close observation (ie, every few months) rather than therapy is appropriate for these patients.
Management of the symptomatic WM
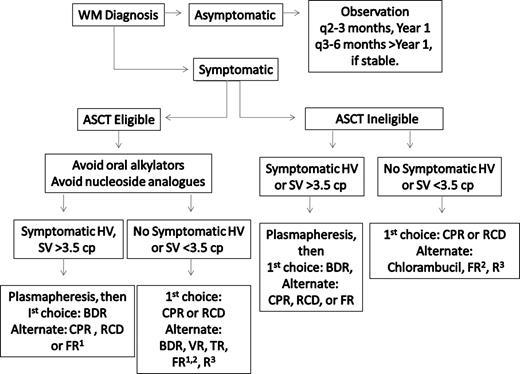
Frontline treatment options for WM include oral alkylators (eg, chlorambucil), nucleoside analogs (cladribine or fludarabine), the monoclonal antibody rituximab, as well as combinations of these agents.34,35 Individual patient considerations, including the presence of cytopenias, need for more rapid disease control, age, and candidacy for autologous transplantation therapy, should be taken into account in making the appropriate choice of a first-line agent. A suggested algorithm for the primary therapy of WM is provided in Figure 2. For patients who are candidates for autologous transplantation therapy, exposure to continuous oral alkylator therapy (such as chlorambucil) or nucleoside analogs should be limited. The use of nucleoside analogs in particular should be approached cautiously in patients with WM because difficulties with stem cell collection, as well as increased risk of disease transformation, myelodysplasia, and acute myelogenous leukemia have been reported.36-39
Guide to the primary therapy of WM. HV indicates hyperviscosity; cp, centipoise; BDR, bortezomib, dexamethasone, rituximab; CPR, cyclophosphamide, prednisone, rituximab; RCD, rituximab, cyclophosphamide, dexamethasone; VR, bortezomib, rituximab; FR, fludarabine, rituximab; and R, rituximab. (1) Because of potential risk of stem cell damage and/or secondary malignancies, may consider as an alternative option if other treatment choices are either unavailable or inappropriate for a particular patient. (2) Consider an attenuated schedule for fludarabine administration in patients with more indolent disease presentation. (3) Avoid as monotherapy in patients with hyperviscosity and with FcγRIIIA-158 F/F polymorphism. For rituximab-based therapies, consider maintenance rituximab in responding patients. Clinical trials should be considered for patients whenever possible.
Guide to the primary therapy of WM. HV indicates hyperviscosity; cp, centipoise; BDR, bortezomib, dexamethasone, rituximab; CPR, cyclophosphamide, prednisone, rituximab; RCD, rituximab, cyclophosphamide, dexamethasone; VR, bortezomib, rituximab; FR, fludarabine, rituximab; and R, rituximab. (1) Because of potential risk of stem cell damage and/or secondary malignancies, may consider as an alternative option if other treatment choices are either unavailable or inappropriate for a particular patient. (2) Consider an attenuated schedule for fludarabine administration in patients with more indolent disease presentation. (3) Avoid as monotherapy in patients with hyperviscosity and with FcγRIIIA-158 F/F polymorphism. For rituximab-based therapies, consider maintenance rituximab in responding patients. Clinical trials should be considered for patients whenever possible.
Treatment of the WM patient requiring immediate disease control
For WM patients presenting with circumstances requiring immediate disease control, such as symptomatic hyperviscosity, cryoglobulinemia, or moderate to severe cytopenias resulting from cold agglutinemia or immune-related thrombocytopenia, emphasis should be placed on achieving rapid paraprotein reduction. In these circumstances, plasmapheresis can be initially performed.19 Typically, 2 to 3 sessions of plasmapheresis are required to reduce serum IgM levels by 30% to 60%. For each plasmapheresis, we usually perform one complete plasma volume exchange and use albumin for replacement product. Great care should be exercised in the timing of red blood cell transfusions in patients presenting with symptomatic hyperviscosity and ideally should follow plasmapheresis so as to not aggravate whole blood viscosity levels.19 After plasmapheresis, treatment should be initiated as soon as possible, as IgM levels will steadily begin to rise and return to baseline levels in 4 to 5 weeks.19 For patients requiring immediate disease control, the use of bortezomib-based therapy, such as bortezomib, dexamethasone, and rituximab (BDR) is preferable so as to achieve more rapid disease control.40 The time to at least a minimum response in WM patients treated with BDR was 1.1 months in a Waldenstrom's Macroglobulinemia Clinical Trials Group (WMCTG) study, whereas the overall response rate with BDR was 96%, with 22% of patients achieving a complete response. With a median follow-up of 2 years, 80% of patients remained free of disease progression, including all patients achieving a very good partial response or better in this study. Herpes zoster prophylaxis should be instituted with BDR therapy using an oral antiviral agent, such as acyclovir, famvir, or valcyclovir, and maintained throughout the course of BDR, and thereafter for at least 6 months. A close watch for the development of bortezomib-related neuropathy should be maintained on BDR. Treatment-related peripheral neuropathy, which occurred at a grade 3 level in 30% of patients, was reversible in most patients, who benefited by interim support with pregabalin. A rituximab-related flare with BDR was observed in only 9% of patients, which may reflect the ability of bortezomib to suppress IgM production independent of tumor cell killing.41,42 As an alternative to the twice-a-week schedule of bortezomib used with BDR, the use of once-a-week bortezomib at a higher dose (ie, 1.6 mg/m2) may be considered with rituximab and appears in one study to be associated with lower risk of neuropathy.43,44 Disease control may lag, and the incidence of rituximab-related IgM flare may be higher (20%) with once-a-week versus twice-a-week administration of bortezomib.
As an alternative to bortezomib-based therapy, a cyclophosphamide-based rituximab-containing regimen can be considered in patients younger than 70 years45-48 ; a nucleoside analog in combination with rituximab can also be considered in patients 70 years of age or older or in younger patients where the use of bortezomib- or cyclophosphamide-based therapy may not be an option.49-51 In a recent update of the Southwest Oncology Group–directed Intergroup Trial S9003, the 10-year event-free survival to single-agent fludarabine was 20%.52 By multivariate analysis, patients with lower levels of β-2 microglobulin (< 3 mg/L) demonstrated significantly better event-free survival in this series. It is unclear whether the inclusion of cyclophosphamide to a nucleoside analog–containing rituximab regimen (such as fludarabine, cyclosphosphamide, rituximab) in WM extends response, and its addition may contribute to additional toxicity.53 The overall response rates with cyclophosphamide or nucleoside analog-based rituximab-containing regimens is 70% to 80%, with complete response attainment in approximately 10% of patients. Cytokine support following American Society of Clinical Oncology guidelines should be considered with either cyclophosphamide or nucleoside analog-based therapy.
There has been considerable debate on the value of including doxorubicin and vincristine to cyclophosphamide-based therapy in WM. Dimopoulos et al46 reported that the combination of rituximab, cyclophosphamide, and dexamethasone (R-CD) led to overall and complete responses in 78% and 7% of WM patients, respectively, and a 2-year progression-free survival of 80%, which appear on par with those results achieved in a comparable population of untreated WM who received cyclophosphamide, doxorubicin, vincristine, and prednisone with rituximab (CHOP-R).45,47 Ioakimidis et al48 compared the activity and toxicity associated with CHOP-R, cyclophosphamide, vincristine, and prednisone with rituximab (CVP-R), and cyclophosphamide and prednisone with rituximab (CP-R) in patients with WM. The use of CP-R was associated with analogous response rates to CVP-R and CHOP-R in the frontline treatment of WM, whereas treatment-related complications, including febrile neutropenia, hospitalizations, and vincristine-related neuropathy, were less. As such, CP-R or R-CD may be preferable to CVP-R or CHOP-R in the management of WM.
An important consideration in the treatment of WM patients, particularly those with high IgM levels, is the potential for a rituximab-mediated IgM flare, which may lead to symptomatic hyperviscosity, as well as worsening of IgM-related neuropathy, cryoglobulinemia, and other IgM-related complications.54-60 The occurrence of an IgM flare is quite common after rituximab therapy in WM patients, with a 40% to 50% occurrence rate when rituximab is used as monotherapy.54,55 In combination therapy, the occurrence of the rituximab-mediated IgM flare can vary considerably and appear dependent on both the regimen used as well as the sequencing of rituximab administration.40,43,48,61-63
Because of concern over a rituximab-related IgM flare aggravating serum viscosity levels or IgM-related morbidity, the omission of rituximab can also be considered for the first 1 or 2 cycles of treatment. Serum IgM levels should be closely monitored (at least weekly) during the time patients are receiving rituximab-based therapy. The IgM flare may last for several weeks, and even months, and does not per se herald treatment failure.54,62 The use of rituximab is best avoided as single-agent therapy in patients with high IgM levels because in 2 studies considerably lower response rates were observed in those patients with higher serum IgM levels (> 4000 mg/dL).64,65
Treatment of the WM patient requiring nonimmediate disease control
For those WM patients presenting with disease not requiring immediate disease control, several options can be considered and should ideally take into account specific disease-controlling objectives. The use of CP-R or R-CD may be considered and may be particularly preferable in younger transplantation-eligible patients. The overall reported response rates with CP-R or R-CD are 70% to 80%, and the 2-year progression-free survival rates with these regimens is 70%.46,48 In patients older than 70 years, nucleoside analog-based therapy may also be considered.49-51 In a recent study by the WMCTG, the overall response rate to fludarabine with rituximab was 96%, and the median progression-free survival was 51.2 months; in patients achieving a very good partial response, the median progression-free survival in this series was in excess of 88 months.50 Because of the significant and prolonged myelosuppression observed in the WMCTG study, which used 6 cycles of fludarabine, each with 5-day courses at 25 mg/m2, a more condensed course (ie, 4 days of fludarabine at 25 mg/m2 per cycle for 4 cycles) may be preferable in more indolent patients.50 The use of thalidomide in combination with rituximab (TR) also represents an alternative choice in the management of WM patients not requiring immediate disease control, and is associated with an overall response rate of 70%, and a median progression-free survival of 3 years.61 TR may be particularly applicable to those WM patients presenting with significant myelosuppression. Peripheral neuropathy (> grade 2) was seen in 40% of WM patients treated with TR, in whom doses of 200 to 400 mg daily were used.62 Lower doses of thalidomide (ie, 100 mg daily) may be more appropriate in WM patients given the increased propensity for WM patients to develop treatment-related neuropathy. The use of lenalidomide has been explored in WM and was associated in one study with an acute drop in hematocrit and hospitalizations of several patients resulting from aggravated anemia and related complications.63 Despite dose reductions, lenalidomide-related anemia persisted in many patients. As such, the use of lenalidomide should be avoided in WM patients. The use of rituximab as a single agent can also be considered in select patients with WM, such as those presenting with a low tumor burden, mild to moderate cytopenias resulting from bone marrow involvement or autoimmune-related destruction (ie, cold agglutinemia, immune mediated thrombocytopenia), or IgM-related neuropathy (see “Treatment of peripheral neuropathy”). Overall response rates with 4 weekly infusions of rituximab are 20% to 30%,64-66 whereas the use of extended-dose rituximab (ie, 4 weekly infusions followed by 4 more weekly infusions at week 12) has been associated with higher overall response rates (40%–50%).67,68 Polymorphisms in position 158 of the CD16 (FcγRIIIA-158) receptor have been shown in WM and related indolent lymphomas to predict response.69 In WM patients, a 4-fold higher rate of response has been reported among WM patients bearing the V/V or V/F polymorphism versus F/F. Testing for the FcγRIIIA-158 polymorphism was recently cleared by the US Food and Drug Administration and may help in predicting response to single-agent rituximab. In WM patients with the FcγRIIIA-158 F/F polymorphism, clinicians can consider alternatives to single-agent rituximab treatment, such as the use of rituximab in combination therapy or the use of a non–rituximab-based therapy.
Treatment of peripheral neuropathy
The treatment of IgM-related neuropathy is usually rituximab based, with improvements in sensory function accompanying reduction in antineuronal antibody titers observed in several studies, including a recent placebo-controlled trial.70-72 The use of single-agent rituximab can be considered in patients with mild, progressive neuropathy. In patients with moderate to severe IgM-related neuropathy, or where the course of the IgM neuropathy appears aggressive, the use of CP-R or R-CD may be preferable to achieve more robust paraprotein reductions. There is debate on the role of novel agents, such as bortezomib or thalidomide, in combination with rituximab for the treatment of IgM-related neuropathy because these agents are associated with treatment-related neuropathy. Despite these concerns, improvements in IgM-related neuropathy have been observed in patients receiving rituximab with either bortezomib or thalidomide.40,62 The risk/benefit of treatment-related neuropathy versus control of IgM-related neuropathy needs to be considered, and the use of either bortezomib or thalidomide is better considered as a salvage measure for those patients not responding to CP-R or R-CD. In such cases, an attenuated schedule or dosing of these agents should be considered to minimize treatment-related neuropathy.
Maintenance therapy in WM
There is considerable debate on the use of maintenance rituximab in WM patients. In our practice, we typically use maintenance rituximab in those patients who have responded to rituximab-containing regimens. Although there have been no prospective, randomized trials addressing the role of maintenance rituximab in WM patients per se, the lessons learned in related indolent lymphomas appear applicable to the care of WM patients whose response characteristics to rituximab therapy closely parallel those attained in patients with other indolent B-cell lymphomas. Moreover, in 2 studies examining the role of extended rituximab in WM patients, improvements in the overall response rate and possibly progression-free survival were observed versus earlier studies examining standard 4 weekly rituximab infusions.67,68 As with other indolent B-cell lymphomas, the exact schedule and length of maintenance therapy with rituximab in WM patients remain to be clarified. In our practice, we typically administer one single infusion of rituximab (at 375 mg/m2) every 3 months for 2 years based on the schedule reported by van Oers et al.73 In some patients, the IgM flare can occur in the maintenance phase of rituximab administration and can be mistaken for progression. For this reason, a bone marrow biopsy should be obtained after induction therapy and can be repeated if there is ambiguity about whether the patient is experiencing an IgM flare related to rituximab or whether disease progression is occurring during maintenance therapy.
Salvage therapy in WM
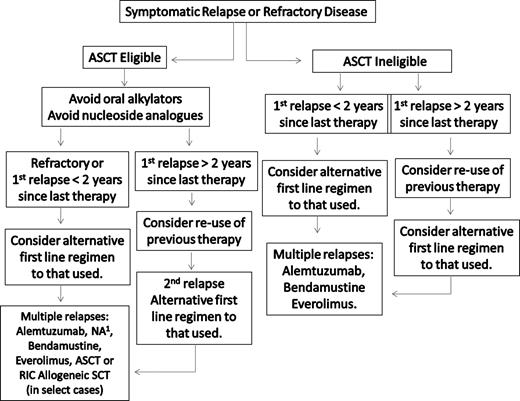
A suggested algorithm for the salvage therapy of WM patients is provided in Figure 3. For patients in relapse or who have refractory disease, the use of an alternative first-line agent as presented in Figure 2 can be considered, with the caveat that in patients for whom autologous transplantation is seriously being considered, exposure to stem cell damaging agents, such as chlorambucil or nucleoside analogs, should be avoided, and a non–stem cell–toxic approach considered if stem cells have not previously been harvested.34,35 In addition to the use of an alternative frontline treatment regimen, bortezomib-based therapy can be considered given overall reported response rates of 60% to 80%.41-44,74,75 In our clinic, we typically administer bortezomib at 1.3 mg/m2 on days 1, 4, 8, and 11 as part of a 3-week cycle, along with 40 mg intravenous dexamethasone with each bortezomib dosing given potential synergism of these agents.76 Prophylaxis against herpes zoster should be strongly considered with bortezomib and steroid combinations,40 and patients should be carefully evaluated for the development of bortezomib-related peripheral or autonomic neuropathy. If disease control is established after a few cycles of twice-a-week bortezomib therapy, once-a-week therapy with bortezomib can be considered at 1.6 mg/m2 per week to attenuate further risk of neuropathy.43,44
Guide to the salvage therapy of WM. ASCT indicates autologous stem cell transplantation; RIC, reduced intensity allogeneic stem cell transplantation; and NA, nucleoside analog-based therapy. (1) In patients being considered for an ASCT, stem cell collection should be undertaken before exposure to a nucleoside analog. Patients should be considered for clinical trials whenever possible.
Guide to the salvage therapy of WM. ASCT indicates autologous stem cell transplantation; RIC, reduced intensity allogeneic stem cell transplantation; and NA, nucleoside analog-based therapy. (1) In patients being considered for an ASCT, stem cell collection should be undertaken before exposure to a nucleoside analog. Patients should be considered for clinical trials whenever possible.
As a third-line approach to the salvage therapy of WM, alemtuzumab can be also be considered.34,35 Alemtuzumab targets CD52, which is widely expressed on both bone marrow WM cells as well as on mast cells, which provide growth and survival signals to WM cells.77,78 As part of a WMCTG effort,79 28 patients with LPL (27 with WM) were treated with alemtuzumab. Twenty-three of these patients were previously treated, and all had rituximab previously. All patients received herpes zoster and Pneumoncystis carinii pneumonia prophylaxis. The overall response rate in this study was 76%, with major responses in 32% of patients. Hematologic toxicities, as well as cytomegalovirus reactivation and infection, were common among previously treated patients, with the latter possibly related to one death. With a median follow-up exceeding 9 months, 11 of 19 responding patients were free of progression. High response rates with alemtuzumab were also reported in another series of heavily pretreated WM patients.80 Infectious complications were common, and cytomegalovirus reactivation occurred in 3 of 7 patients requiring ganciclovir therapy; 3 patients were also hospitalized for bacterial infections. Opportunistic infections occurred in 2 patients and were responsible for their deaths. Alemtuzumab should therefore be used with caution, and patients closely monitored for hematologic and infectious complications.
Stem cell transplantation (SCT) remains an option for the salvage therapy of WM, particularly among younger patients who have had multiple relapses, or for those patients with primary refractory disease. The European Bone Marrow Transplant Registry recently reported the largest experience for both autologous as well as allogeneic SCT in WM.81 Among 202 WM patients, most of whom had relapsed or refractory disease, the 5-year progression-free and overall survival rates after autologous SCT were 33% and 61%, respectively. Chemosensitive disease at time of the autologous SCT was the most important prognostic factor for nonrelapse mortality, response rate, progression-free, and overall survival in this series. The outcome of allogeneic SCT transplantation in 106 WM patients was also included in this study. Included in this series were 44 patients who received a conventional myeloablative allogeneic SCT and 62 patients who received reduced-intensity conditioning (RIC) allogeneic SCT. Most of the patients had advanced WM. The 3-year nonrelapse mortality rate for all patients was 33%. The 5-year progression-free and overall survival rates in this series were 48% and 63%, respectively. Forty-eight patients in this series developed acute, and 16 and 11 patients developed limited and extensive chronic graft versus host disease, respectively. The potential role for RIC allogeneic SCT to induce responses, including complete responses, among patients with very advanced WM has also been reported by Maloney,82 who observed 6 complete, 1 near complete, and 4 partial responses among 13 patients. The median number of prior therapies was 5, and the overall and progression-free survival was 60% in this series. Day 100 nonrelapse mortality was 8%, with 54% of patients experiencing at least grade 2 GVHD.
Autologous as well as RIC allogeneic SCT may therefore be considered as appropriate salvage modalities for relapsed or refractory WM patients, although the risks and benefits of these modalities should be carefully weighed against other available treatment options.34,35 Conversely, myeloablative allogeneic SCT represents a high-risk option given the reported transplantation-related mortality, and should only be considered in the context of a clinical trial. Although, in general, we opt in our clinic to defer autologous stem cell transplantation (ASCT) or RIC allogeneic SCT as a salvage modality for WM patients who have had multiple relapses, or refractory disease, the use of ASCT can be considered as a consolidation strategy for patients presenting with amyloid-related organ dysfunction.83
Additional options for therapy of WM
The Study Group for Lymphomas recently examined the activity of bendamustine plus rituximab (BR) versus CHOP-R in a large cohort of previously untreated patients with indolent non-Hodgkin lymphoma.84 Included in this study were 42 patients with WM, 40 of whom were available for response assessment.85 The overall response rate with BR in this study was similar to CHOP-R (96% vs 94%, respectively). With a median follow-up of 26 months, progressive disease was documented in 2 of 23 patients treated with BR, whereas 7 of 17 patients treated with CHOP-R progressed. BR was associated with a lower incidence of grade 3 or 4 neutropenia, infectious complications, and alopecia in this study. These results suggest that BR may be a preferable option to CHOP-R in the frontline therapy of WM. Studies addressing the role of bendamustine in combination with other active agents, and as a salvage therapy for indolent NHL patients are currently under way.
Everolimus (RAD001) is an oral inhibitor of the mTOR pathway, which recently was approved by the US Food and Drug Administration for the treatment of renal cell carcinoma. Gene expression profiling and quantitative reverse-transcribed polymerase chain reaction analysis of WM tumor cells shows activation of the Akt-mTOR-p70 pathway, and inhibition of this pathway leads to apoptosis in primary WM cells, as well as WM cell lines.86,87 A phase 2 study of everolimus was recently reported in 50 patients with relapsed/refractory WM, who had a median number of prior therapies of 3.88 Patients in this study received everolimus at 10 mg daily, with dose reduction permitted to 5 mg per day for toxicity. The overall response rate in this study was 70%, with 44% of patients achieving a major response and 28% of patients achieved a minor response. At 1 year, 67% of patients remain progression free. Tolerance to therapy in this series was good, and a clinical trial examining the activity of everolimus in previously untreated patients with WM has recently been initiated by the WMCTG.
Finally, as has been emphasized by the Consensus Panels on the Treatment of WM,34,35 patients should be considered whenever possible for participation in a clinical trial given the paucity of reported clinical trials in WM. Several novel clinical trials, including novel combination strategies with rituximab, bortezomib, bendamustine, as well as novel signal inhibitors, proteasome inhibitors, epigenetic modifiers, and immunomodulators have been initiated or are contemplated. Details on several these studies as well as other WM-related clinical trials can be found at www.clinicaltrials.gov.
Response assessment
Consensus-based uniform response criteria for WM were developed as part of the International Workshops on WM.32,34,89 The category of minor response was adopted at the Third International Workshop on WM, given that clinically meaningful responses have been observed with newer biologic agents, such as rituximab, and is based on more than 25% to less than 50% decrease in serum IgM level, which is used as a surrogate marked of disease in WM.32,34 The term major response is used to denote a response of more than 50% in serum IgM levels and includes partial and complete responses.32 Response categories and criteria for progressive disease in WM are summarized in Table 4. Although IgM is commonly used as a surrogate marker of WM disease burden, it can fluctuate with biologic agents, such as rituximab and bortezomib.41,42,54,55 Rituximab can induce a flare in serum IgM levels, which can occur when used as monotherapy and in combination therapy, and may last for weeks to months.40,48,54,61-63 Conversely, bortezomib can suppress IgM levels in some patients independent of tumor cell killing.41,42 Moreover, Varghese et al90 recently showed that residual IgM-producing plasma cells are spared in patients treated with selective B cell–depleting agents, such as rituximab and alemtuzumab, and may therefore skew response assessment. Therefore, in circumstances where serum IgM levels may appear out of clinical context, a bone marrow biopsy should be considered to clarify the patient's underlying disease burden. Soluble CD27 may serve as an alternative surrogate marker in WM and appears to remain a faithful marker of disease in patients experiencing a rituximab-related IgM flare, as well as plasmapheresis.91,92
Summary of updated response criteria from the Third International Workshop on Waldenstrom's Macroglobulinemia
| Response . | Criteria . |
|---|---|
| Complete response (CR) | Disappearance of monoclonal protein by immunofixation; no histologic evidence of bone marrow involvement, and resolution of any adenopathy/organomegaly (confirmed by CT scan), along with no signs or symptoms attributable to WM; reconfirmation of the CR status is required at least 6 weeks apart with a second immunofixation |
| Partial response (PR) | A > 50% reduction of serum monoclonal IgM concentration on protein electrophoresis and > 50% decrease in adenopathy/organomegaly on physical examination or on CT scan; no new symptoms or signs of active disease |
| Minor response (MR) | A > 25% but < 50% reduction of serum monoclonal IgM by protein electrophoresis; no new symptoms or signs of active disease |
| Stable disease (SD) | A < 25% reduction and < 25% increase of serum monoclonal IgM by electrophoresis without progression of adenopathy/organomegaly, cytopenias, or clinically significant symptoms resulting from disease and/or signs of WM |
| Progressive disease (PD) | A > 25% increase in serum monoclonal IgM by protein electrophoresis confirmed by a second measurement or progression of clinically significant findings resulting from disease (ie, anemia, thrombocytopenia, leukopenia, bulky adenopathy/organomegaly) or symptoms (unexplained recurrent fever > 38.4°C, drenching night sweats, > 10% body weight loss, or hyperviscosity, neuropathy, symptomatic cryoglobulinemia, or amyloidosis) attributable to WM |
| Response . | Criteria . |
|---|---|
| Complete response (CR) | Disappearance of monoclonal protein by immunofixation; no histologic evidence of bone marrow involvement, and resolution of any adenopathy/organomegaly (confirmed by CT scan), along with no signs or symptoms attributable to WM; reconfirmation of the CR status is required at least 6 weeks apart with a second immunofixation |
| Partial response (PR) | A > 50% reduction of serum monoclonal IgM concentration on protein electrophoresis and > 50% decrease in adenopathy/organomegaly on physical examination or on CT scan; no new symptoms or signs of active disease |
| Minor response (MR) | A > 25% but < 50% reduction of serum monoclonal IgM by protein electrophoresis; no new symptoms or signs of active disease |
| Stable disease (SD) | A < 25% reduction and < 25% increase of serum monoclonal IgM by electrophoresis without progression of adenopathy/organomegaly, cytopenias, or clinically significant symptoms resulting from disease and/or signs of WM |
| Progressive disease (PD) | A > 25% increase in serum monoclonal IgM by protein electrophoresis confirmed by a second measurement or progression of clinically significant findings resulting from disease (ie, anemia, thrombocytopenia, leukopenia, bulky adenopathy/organomegaly) or symptoms (unexplained recurrent fever > 38.4°C, drenching night sweats, > 10% body weight loss, or hyperviscosity, neuropathy, symptomatic cryoglobulinemia, or amyloidosis) attributable to WM |
Data from Kimby et al.32
Acknowledgments
The author thanks the staff of the Bing Center for Waldenstrom Macroglobulinemia and, in particular, Robert J. Manning, Christopher J. Patterson, and Lefkothea Ioakimidis for the data collection used in this manuscript.
This work was supported by the Peter and Helen Bing Fund for Waldenstrom Macroglobulinemia, the Linda and Edward Nelson Fund, the Bailey Family Fund for Waldenstrom Macroglobulinemia, and the International Waldenstrom Macroglobulinemia Foundation.
Authorship
Contribution: S.P.T. reviewed data and prepared the article.
Conflict-of-interest disclosure: The author has received research support, honoraria and/or consultation fees in connection with products discussed in this article from Berlex Oncology Inc, Biogen IDEC Inc, Celgene Corporation, Genentech BioOncology Inc, Millenium Pharmaceuticals Inc, the Takeda Company, and PGX Health Inc.
Correspondence: Steven P. Treon, Bing Center for Waldenström's Macroglobulinemia, Dana-Farber Cancer Institute, M548, 44 Binney St, Boston, MA 02115; e-mail: steven_treon@dfci.harvard.edu.