Abstract
This phase 3 prospective randomized trial evaluated the efficacy and long-term safety of erythropoietin (EPO) with or without granulocyte colony-stimulating factor plus supportive care (SC; n = 53) versus SC alone (n = 57) for the treatment of anemic patients with lower-risk myelodysplastic syndromes. The response rates in the EPO versus SC alone arms were 36% versus 9.6%, respectively, at the initial treatment step, 47% in the EPO arm, including subsequent steps. Responding patients had significantly lower serum EPO levels (45% vs 5% responses for levels < 200 mU/mL vs ≥ 200 mU/mL) and improvement in multiple quality-of-life domains. With prolonged follow-up (median, 5.8 years), no differences were found in overall survival of patients in the EPO versus SC arms (median, 3.1 vs 2.6 years) or in the incidence of transformation to acute myeloid leukemia (7.5% and 10.5% patients, respectively). Increased survival was demonstrated for erythroid responders versus nonresponders (median, 5.5 vs 2.3 years). Flow cytometric analysis showed that the percentage of P-glycoprotein+ CD34+ marrow blasts was positively correlated with longer overall survival. In comparison with SC alone, patients receiving EPO with or without granulocyte colony-stimulating factor plus SC had improved erythroid responses, similar survival, and incidence of acute myeloid leukemia transformation.
Introduction
The myelodysplastic syndromes (MDSs) are a heterogeneous group of clonal myeloid disorders characterized by abnormal bone marrow proliferation and differentiation of hematopoietic stem cells. MDS primarily affects patients older than 60 years and is characterized by symptomatic cytopenias, predominantly anemia, and a propensity to evolve into acute myeloid leukemia (AML).1 The International Prognostic Scoring System (IPSS) for MDS is widely used to characterize the patient's prognostic risk status.2 The IPSS lower-risk MDS group (low and intermediate-1) includes the majority of patients and is associated with relatively longer survival and lower incidence of progression to AML compared with higher-risk (intermediate-2 and high) patients. Treatment options reviewed and recommended by the US National Comprehensive Cancer Network MDS Guidelines Panel3 include managing anemia with erythroid-stimulating agents (ESAs; eg, recombinant human erythropoietin, erythropoietin [EPO], and darbepoetin), based on data indicating effective erythroid responses and decreased red blood cell (RBC) transfusion requirements in prior studies in this patient population.4-9 Therapy with granulocyte-stimulating factor (G-CSF) synergizes with EPO and improve erythroid responses in a portion of MDS patients not responding to EPO alone.10-13 Several studies demonstrated improvement in quality of life (QOL) in MDS patients responding to ESAs,12,14-17 whereas one study showed no such improvement.13 Although therapy with ESAs in certain non-MDS cancer patients was associated with an increased risk of cardiovascular and thrombotic events and shortened survival,18,19 nonrandomized trials with EPO treatment of MDS patients have not demonstrated such adverse events.20,21
To address the issues of clinical efficacy, long-term safety and clinical outcomes, and QOL related to use of these growth factors in MDS, we performed a randomized prospective controlled trial of EPO with or without G-CSF plus RBC transfusion support versus transfusion supportive care (SC) alone for symptomatically anemic lower-risk MDS patients. To determine whether the patients' clinical outcomes were related to the immunophenotype of their marrow blasts, flow cytometric analysis was used to characterize the antigenic phenotype of these cells.
Methods
Eligibility criteria
Patients with the following MDS subtypes were eligible for enrollment: refractory anemia, refractory anemia with ring sideroblasts (RARS), refractory anemia with excess blasts (RAEB), or nonproliferative chronic myelomonocytic leukemia according to the French-American-British (FAB) group criteria.22 Patients were required to have pretreatment bone marrow blasts less than 10% and peripheral blasts less than 5%. Thus, eligible persons included RAEB-1 but not RAEB-2 patients, as classified by World Health Organization (WHO) criteria.23 A hematocrit of less than 30 vol% or hemoglobin less than 9.5 g/dL, a platelet count of more than 30 000/cmm, and documentation of adequate iron stores, were required at the time of enrollment and at each treatment step. Central pathology (J.M.B.) and cytogenetic review (G.D.) were performed. Cytogenetic findings were categorized according to the IPSS classification.2 Patients were stratified at the time of the initial randomization by FAB morphologic subtype, RBC transfusion requirement to maintain a hematocrit more than 25 vol%, and serum EPO level of at least 200 mU/mL or less than 200 mU/mL. Patients were older than 18 years of age and provided signed institutional Investigational Review Board informed consent in accordance with the Declaration of Helsinki. All studies received approval from the individual sites' institutional review boards. Patients who had received more than 30 000 U of EPO/week for 4 weeks or EPO or other recombinant growth factors within 1 month before registration were ineligible. Additional ineligibility criteria were active bleeding, uncontrolled hypertension, bilirubin more than 3 mg/dL, or creatinine more than 2 mg/dL. EPO for the study was provided by Ortho Biotech. G-CSF (filgrastim; Amgen) was obtained commercially.
Objectives
The primary objectives of the study were to compare the relative benefits of treatment with EPO versus SC for correcting the patients' anemia or decreasing RBC transfusion requirements, and to evaluate whether adding G-CSF or increasing the EPO dose enhanced the erythroid response in patients whose disease initially failed to respond to lower doses of EPO. The secondary objectives were to compare the toxicity, incidence of AML, overall survival, and QOL in patients between the study arms.
Response criteria
Responses were defined as complete response (CR), good response (GR), partial response (PR), stable disease, and progressive disease (for erythroid response, ie, lack of erythroid response).10-13 Responses needed to be maintained for at least 4 months. Transformation to AML required the presence of more than 30% marrow blasts (FAB criteria). Our CR and GR = major response, PR = minor response in the International Working Group (IWG) 2000 MDS response criteria,24 except that we required the erythroid response to be at least 4 months rather than IWG requirement of at least 2 months.
Study design
Patients were randomly assigned to receive SC alone (arm A) or EPO 150 U/kg daily subcutaneously plus SC (arm B) for 4 months (step 1). Patients crossed over from arm A to arm B and moved to step 2 after the initial 4-month period of observation if there was absence of erythroid response. For nonresponders at steps 1 or 2, G-CSF (1 μg/kg per day) was added (step 3). Step 3 responders continued this treatment, and nonresponders received increased EPO doses (300 U/kg per day) plus G-CSF (step 4). Erythroid responders were scheduled to remain on their effective treatment for 1 year. For hematocrit increases to more than 40 vol% (hemoglobin 13 g/dL), EPO was withheld and restarted at decreased frequency when the hematocrit decreased to less than 32 vol%. G-CSF doses were incrementally adjusted based on the absolute neutrophil count.10
Flow cytometry
CD34+ precursor cells were assessed for their antigen profile by multiparameter 3-color flow cytometry using a FACSCalibur flow cytometer (BD Biosciences) and CellQuest software. Mononuclear cells (MNCs) were isolated from heparinized bone marrows by Ficoll-Hypaque density gradient centrifugations. Antibodies tested included anti-CD117, CD33, CD13, CD15, CD19, CD7, and anti-HLA-DR antibody, all from BD Biosciences, CD65s (Immunotech), and TdT, using monoclonal antibody HT-6 (Supertechs) and Fix&Perm for fixation and permeabilization (Invitrogen). To assess P-glycoprotein (Pgp) expression, the unconjugated anti-Pgp antibody, MRK-16, from Kamiya, was used. MRK-16 recognizes a surface epitope of Pgp and was recommended for Pgp measurements.25 Eastern Cooperative Oncology Group (ECOG) has used this antibody in other multidrug resistance studies.26 Data are given as percentage of CD34+ cells staining with a given antibody with fluorescence intensity at least 2% beyond that of the matched isotype control, using methodology previously described.26
Whereas CD33 and CD13 are myeloid-lineage associated antigens, expressed by early myeloid precursor cells, granulocyte-monocyte colony forming cells, and myeloblasts, in normal bone marrow, CD65s has been classified as a more mature antigen, not found on normal myeloblasts.27,28 Similarly, CD15 is considered a more mature myeloid antigen and not expressed by normal CD34+ myeloid bone marrow precursors.29 ECOG has used CD65s expression to differentiate between undifferentiated (≤ 30% CD65+ myeloblasts) from differentiated AML (> 30% CD65+ myeloblasts).30
QOL assessment
QOL assessments were performed at the time of randomization and at the 4-month time point. QOL was measured by the General version of the Functional Assessment of Cancer Therapy (FACT-G; Version 3),31 a self-administered questionnaire assessing 5 QOL domains: physical, social/family, relationship with doctor, emotional, and functional well-being. Fatigue was measured with the FACT Fatigue Scale.32
Statistics
Patient demographic factors and disease characteristics, incidence of toxicities, and response rates were compared using Fisher exact tests. Overall survival was defined as time from randomization to death from any cause, with follow-up censored at the date of last contact. Kaplan-Meier estimates were used for event-time distribution. Stratified log-rank tests were used to detect treatment differences for overall survival and time to leukemic transformation. The stratification factors were FAB morphologic subtype, RBC transfusion requirement before entering the study, and the serum EPO level at randomization. The univariate Cox model, stratified on the same factors, including only treatment effect, was used to estimate hazard ratios and tests for significance of overall survival.
Multivariate Cox models were used to compare overall survival and time to leukemic transformation between treatment arms, adjusted for known potential risk factors. In addition to the cause-specific hazard analysis (stratified log-rank test), the treatment effect on time to leukemic transformation was assessed by cumulative incidence analysis with death as the competing event. Landmark analysis at 4 months (when patients had their erythroid response evaluated) was used to compare the overall survival between responders and nonresponders.
Results
Demographics
Between December 1997 and June 2004, 118 patients with MDS enrolled into the study. However, information on 8 patients was unevaluable because of insufficient data. Thus, 110 patients enrolled though ECOG institutions were included in this study. Data entry continued until August 2008, with a median follow-up period of 5.8 years (range, 0.8-9.6 years). After central pathology review, 7 patients, on step 1 (4 on arm A, 3 on arm B) either withdrew or died before the initial 4-month response evaluation time point and were determined to be ineligible. One patient on step 1 (arm A) never started treatment. Three patients at step 2, 4 at step 3, and 1 at step 4 were ineligible and one did not receive treatment at step 3. These patients were included for evaluation of survival and leukemic transformation but not for erythroid response. The demographic factors and disease characteristics of the patients show no significant differences between treatment arms (Table 1). The median age was 73 years, and 61% of the patients had required RBC transfusion support.
Baseline demographic characteristics of study patients
| Variable . | Arm A: SC, % (n = 57) . | Arm B: EPO, % (n = 53) . | Total, % (n = 110) . |
|---|---|---|---|
| Age ≥ 65 y | 88 | 81 | 85 |
| Male sex | 63 | 62 | 63 |
| MDS subtype | |||
| RA | 39 | 38 | 38 |
| RARS | 30 | 38 | 34 |
| RAEB | 30 | 23 | 26 |
| CMML | 2 | 1 | 2 |
| Platelets < 100 000/cmm | 30 | 25 | 27 |
| Ferritin > 500 ng/mL | 58 | 53 | 56 |
| EPO < 200 mU/mL | 67 | 72 | 69 |
| Cytogenetics* | |||
| Good | 70 | 71 | 71 |
| Intermediate | 16 | 15 | 15 |
| Poor | 14 | 14 | 14 |
| IPSS category | |||
| Low/intermediate-1 | 84 | 83 | 83 |
| Intermediate-2/high | 16 | 17 | 17 |
| Prior transfusion support | 61 | 60 | 61 |
| Variable . | Arm A: SC, % (n = 57) . | Arm B: EPO, % (n = 53) . | Total, % (n = 110) . |
|---|---|---|---|
| Age ≥ 65 y | 88 | 81 | 85 |
| Male sex | 63 | 62 | 63 |
| MDS subtype | |||
| RA | 39 | 38 | 38 |
| RARS | 30 | 38 | 34 |
| RAEB | 30 | 23 | 26 |
| CMML | 2 | 1 | 2 |
| Platelets < 100 000/cmm | 30 | 25 | 27 |
| Ferritin > 500 ng/mL | 58 | 53 | 56 |
| EPO < 200 mU/mL | 67 | 72 | 69 |
| Cytogenetics* | |||
| Good | 70 | 71 | 71 |
| Intermediate | 16 | 15 | 15 |
| Poor | 14 | 14 | 14 |
| IPSS category | |||
| Low/intermediate-1 | 84 | 83 | 83 |
| Intermediate-2/high | 16 | 17 | 17 |
| Prior transfusion support | 61 | 60 | 61 |
International Prognostic Scoring System risk category.2
Responses
At the 4-month evaluation point (step 1), a significantly improved erythroid response rate (CR + GR + PR) of 36% (18 of 50 patients) occurred in the EPO arm compared with 9.6% (5 of 52 patients) in the SC arm (P = .002). Using more stringent IWG 2006 erythroid response criteria (ie, excluding PRs, minor responses in IWG 2000),33 the response rates were 34% versus 5.8%, respectively (P = .001). At this time point, 29% (EPO arm) versus 51% (SC arm) patients were still receiving RBC transfusions. Seven of the 23 eligible patients (30%) who crossed over from the SC arm (arm A) to the EPO arm responded at this step (GR + PR). Thus, for all patients at steps 1 or 2 who received EPO, the response rate was 34.2% (25 of 73 patients).
Six of 27 patients (22%) responded to step 3 therapy (EPO + G-CSF); 5 patients were new responders, the other had responded transiently to EPO in step 1. Six of 12 patients (50%) who proceeded to step 4 (EPO at higher dose + G-CSF) had erythroid responses, 4 of whom were new responders. Thus, 9 more individual patients subsequently responded at steps 3 and 4, generating an overall erythroid response rate to EPO plus or minus G-CSF of 46.6% (34 of 73 patients). Of the 27 eligible patients entering step 3 (EPO 150 U/kg per day + G-CSF), 13 were RARS patients. Among them, 5 (39%) patients responded to the step 3 therapy. For the remaining 14 non-RARS patients, only 1 (7%) patient responded to the therapy. Although the RARS patients tended to respond better to the addition of G-CSF, the difference was not statistically significant (P = .08). At step 4, both types of patients had similar responses to therapy. Treatment duration was similar for patients on both study arms.
By univariate analysis, only lower baseline EPO levels (< 200 mU/mL vs ≥ 200 mU/mL) had predictive value for response at step 1 (data not shown). A total of 73 patients (66%) received EPO on study, 50 of whom were initially randomized to EPO and 23 who crossed over from SC after not responding or having (erythroid) progression. For these patients, correlation of step 1/2 responses with clinical factors showed that the MDS subtype (P = .006) and pretreatment serum EPO levels (P = .002) were significant predictors of erythroid response (Table 2). Baseline serum EPO levels for EPO responders versus nonresponders were 40 mU/mL (range, 9-638 mU/mL) versus 142 mU/mL (range, 22-5466 mU/mL).
Clinical predictors of erythroid responses for patients receiving EPO (Step 1/2)
| Variable . | Total patients (n = 73) . | Responders, percentage (n = 25) . | P . |
|---|---|---|---|
| Age | |||
| < 65 y | 10 | 20 | |
| ≥ 65 y | 63 | 37 | .48 |
| Sex | |||
| Male | 48 | 40 | |
| Female | 25 | 24 | .21 |
| FAB subtype | |||
| RA, RARS | 51 | 45 | |
| RAEB | 21 | 10 | .006 |
| EPO level | |||
| < 200 mU/mL | 53 | 45 | |
| > 200 mU/mL | 19 | 5 | .002 |
| Cytogenetics* | |||
| Good, intermediate | 57 | 42 | |
| Poor | 8 | 13 | .14 |
| IPSS score† | |||
| Low/intermediate-1 | 61 | 34 | |
| Intermediate-2/high | 11 | 27 | .74 |
| Prior transfusion support | |||
| Yes | 29 | 31 | |
| No | 43 | 37 | .62 |
| Variable . | Total patients (n = 73) . | Responders, percentage (n = 25) . | P . |
|---|---|---|---|
| Age | |||
| < 65 y | 10 | 20 | |
| ≥ 65 y | 63 | 37 | .48 |
| Sex | |||
| Male | 48 | 40 | |
| Female | 25 | 24 | .21 |
| FAB subtype | |||
| RA, RARS | 51 | 45 | |
| RAEB | 21 | 10 | .006 |
| EPO level | |||
| < 200 mU/mL | 53 | 45 | |
| > 200 mU/mL | 19 | 5 | .002 |
| Cytogenetics* | |||
| Good, intermediate | 57 | 42 | |
| Poor | 8 | 13 | .14 |
| IPSS score† | |||
| Low/intermediate-1 | 61 | 34 | |
| Intermediate-2/high | 11 | 27 | .74 |
| Prior transfusion support | |||
| Yes | 29 | 31 | |
| No | 43 | 37 | .62 |
RA indicates refractory anemia.
IPSS risk category.2
Eight patients lacking central cytogenetic review had institutional cytogenetic reports reviewed.
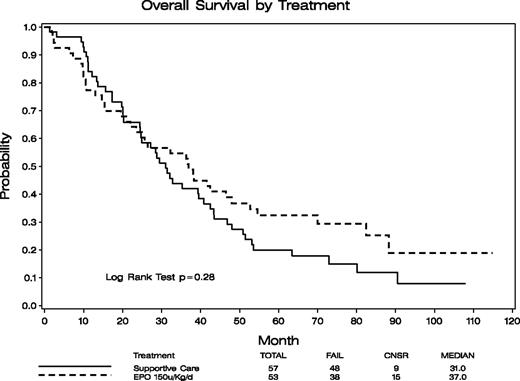
Survival
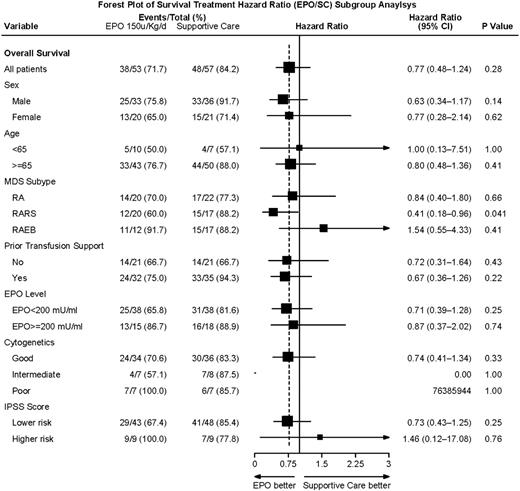
Overall survival was similar for arms A and B: 2.6 versus 3.1 years median, respectively (P = .28; Figure 1). Although survival appears to be slightly worse in the EPO-treated group at the time of the crossover and throughout the first year (treatment and time interaction, P = .01), the survival rates at 6 months (SC, 96%; EPO, 92%) and at 1 year (SC, 84%; EPO, 77%) indicated lack of significant difference between the 2 arms (P = .43, P = .47, respectively) even during this time period. We also separately compared survival for (1) those assigned to arm A who did not cross over versus arm B, and (2) those in arm A who did not cross over versus patients initially in arm B plus those who crossed over from arm A. In these comparisons, survival between the compared groups also did not differ (data not shown). In univariate analysis, the results were consistent for most of the factors examined despite the relatively small sample sizes of each subgroup, with an overall survival hazard ratio (EPO/SC) for survival of 0.77 (95% confidence interval, 0.48-1.24), indicating a trend toward EPO treatment being better in most subgroups (Forest plot, Figure 2). These subgroup analyses were exploratory and ad hoc. Without adjusting for multiplicity, patients with RARS had significantly improved survival on arm B (HR, EPO/SC: 0.41, P = .04). Morphologic review of the marrows of these patients indicated that 36 of the 37 RARS patients were classified as refractory cytopenia with multilineage dysplasia and ring sideroblasts by WHO criteria.23
Overall survival of patients randomized to the supportive care alone (arm A, n = 57) and EPO/G-CSF (arm B, n = 53) arms. No statistically significant difference in overall survival between these 2 groups of patients was demonstrated (P = .28).
Overall survival of patients randomized to the supportive care alone (arm A, n = 57) and EPO/G-CSF (arm B, n = 53) arms. No statistically significant difference in overall survival between these 2 groups of patients was demonstrated (P = .28).
Forest plot of hazard ratios (EPO/SC) for overall survival related to treatment for clinical subgroups. A univariate Cox proportional hazard model, stratified on randomization features, estimated hazard ratios, and significance for overall survival. The horizontal lines provide the 95% confidence interval for the ratios. The dotted vertical line represents the overall hazard ratio (0.77).
Forest plot of hazard ratios (EPO/SC) for overall survival related to treatment for clinical subgroups. A univariate Cox proportional hazard model, stratified on randomization features, estimated hazard ratios, and significance for overall survival. The horizontal lines provide the 95% confidence interval for the ratios. The dotted vertical line represents the overall hazard ratio (0.77).
For all patients, erythroid response versus nonresponse at step 1 was significantly associated with improved survival: 5.5 versus 2.3 years, median (P = .004), assessed from the 4-month time point, when step 1 response was determined. Similarly, for only those patients receiving EPO, overall survival was better in responders versus nonresponders: 3.7 versus 1.7 years, median (P = .018).
A multivariate Cox proportional hazards model, stratified on morphologic subtype, transfusion requirement, and EPO level at randomization, was used to examine treatment effect on overall survival, adjusting for relevant risk factors. Overall survival was similar for arm A and arm B (P = .60), consistent with the results from the univariate model. With 110 patients and 86 events, the study had 80% power to detect 46% reduction in hazard rate in overall survival in the EPO arm, using a one-sided log-rank test at the significance level of 0.025.
AML transformation
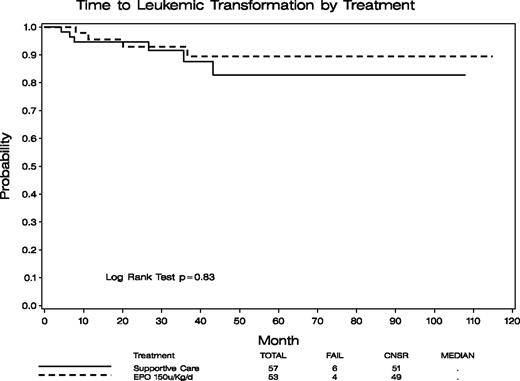
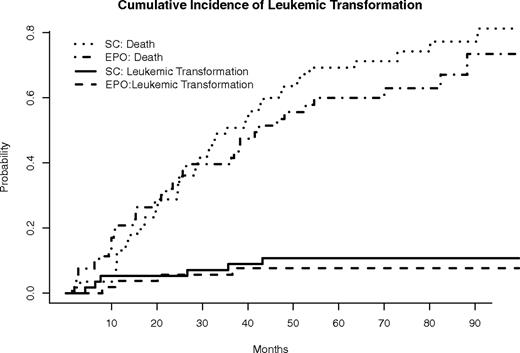
Transformation to AML occurred in 4 (7.5%) and 6 (10.5%) patients randomized to the EPO/arm B and SC/arm A arms, respectively (P = .74; no patients in arm B and 4 patients in arm A during the treatment period, the remainder transformed during follow-up). Figure 3 provides Kaplan-Meier plots of time to leukemic transformation. Figure 4 provides cumulative incidence plots with death as competing risk events. From both analyses, no statistical differences in leukemic transformation between the 2 arms were observed. In addition, as performed for the survival analysis, we separately compared AML transformation for (1) those assigned to arm A who did not cross over versus arm B, and (2) for those in arm A who did not cross over versus patients initially in arm B plus those who crossed over from arm A. In these comparisons, AML transformation between the compared groups also did not differ (data not shown).
Time to leukemic transformation of patients randomized to the SC alone (arm A, n = 57) and EPO/G-CSF (arm B, n = 53) arms. No statistically significant difference in time to transformation between these 2 groups of patients was demonstrated (P = .83).
Time to leukemic transformation of patients randomized to the SC alone (arm A, n = 57) and EPO/G-CSF (arm B, n = 53) arms. No statistically significant difference in time to transformation between these 2 groups of patients was demonstrated (P = .83).
Cumulative incidence of leukemic transformation, with death as a competing risk event. No statistically significant difference in time to transformation between these 2 groups of patients was demonstrated when the competing risk of death was included (P = .34, cumulative incidence analysis).
Cumulative incidence of leukemic transformation, with death as a competing risk event. No statistically significant difference in time to transformation between these 2 groups of patients was demonstrated when the competing risk of death was included (P = .34, cumulative incidence analysis).
A multivariate Cox proportional hazards model, stratified on morphologic subtype, transfusion requirement, and EPO level at randomization, was used to examine treatment effect on AML transformation, adjusting for relevant risk factors. Consistent with the univariate model, no difference in leukemic transformation was observed between treatment arms (P = .58). With 110 patients and 10 events, the study had 80% power to detect 83% reduction in hazard rate in time to AML transformation in the EPO arm, using a one-sided log-rank test at the significance level of 0.025.
Marrow CD34 cell immunophenotyping
Mononuclear cells were isolated from the bone marrows of 70 patients, on whom bone marrow specimens were submitted to ECOG's Leukemia Translational Studies Laboratory. In 3 patients, the MNC yield was too low to allow for testing. In mononuclear cell fractions from 9 patients, CD34+ precursor cells were undetectable. In the remaining 58 marrow samples, between 0.4% and 15% of MNCs were characterized as CD34+ precursor cells (median, 1% of MNCs). In each case, CD34+ precursors expressed CD117, CD33, and CD13 and lacked CD19, identifying them as belonging to the myeloid lineage.
Using a threshold of 30% or less for CD65s, 17 of 58 patients demonstrated differentiated and 39 undifferentiated features of their CD34+ myeloblasts (Table 3). After adjusting for multiple comparisons, only CD15 differed significantly between the 2 groups (P = .001). That the expression of CD65s and CD15 was highly correlated is not surprising given their sequence of expression in normal myeloid hematopoiesis.29 Similar proportions of differentiated versus undifferentiated patients were evaluated in Arms A and B (46% vs 54%). Based on univariate Cox model analysis, no difference was observed in erythroid response rates (P = .35), overall survival (P = .62), or rate of leukemic transformation (P = .16) between differentiated and undifferentiated patients, or for antigen expression profiles of CD34+ myeloblasts (with one exception) within either treatment group. Notably, irrespective of the level of CD34 cell maturation, the percentage of Pgp+ CD34+ blasts was positively correlated with longer overall survival (P = .004).
Association of marrow blast antigenic expression with CD65s status
| Variable . | Differentiated,* percentage (median) (n = 17) . | Undifferentiated, percentage (median) (n = 39) . |
|---|---|---|
| CD65s | 52† | 10† |
| CD15 | 34† | 16† |
| Pgp | 83 | 66 |
| CD34 | 99 | 99 |
| CD117 | 99 | 99 |
| CD33 | 99 | 99 |
| HLA-DR | 99 | 99 |
| CD13 | 99 | 99 |
| CD7 | 10 | 8 |
| TdT | 0 | 8 |
| Percentage blasts | 2 | 1 |
| Variable . | Differentiated,* percentage (median) (n = 17) . | Undifferentiated, percentage (median) (n = 39) . |
|---|---|---|
| CD65s | 52† | 10† |
| CD15 | 34† | 16† |
| Pgp | 83 | 66 |
| CD34 | 99 | 99 |
| CD117 | 99 | 99 |
| CD33 | 99 | 99 |
| HLA-DR | 99 | 99 |
| CD13 | 99 | 99 |
| CD7 | 10 | 8 |
| TdT | 0 | 8 |
| Percentage blasts | 2 | 1 |
CD65s more than 30% expression.
Nominal P < .001.
Cytogenetic information was available for all but 2 of the patients analyzed for blast cell expression. All of the 7 patients with del(5q) who had been evaluated for CD65s were found in the differentiated group. The 3 patients with monosomy 7 as sole or part of a complex karyotype were in the undifferentiated cohort.
QOL analyses
QOL assessments were completed by 102 patients (53 SC, 49 EPO) at baseline and by 84 patients at 4 months (42 SC, 42 EPO) after initiating therapy. There were no significant differences in FACT subscale scores and fatigue scores between those assigned to EPO and those assigned to SC, both at baseline and 4 months later (2-sample t tests). However, those patients who had an erythroid response at 4 months reported significant improvement from baseline in physical (P = .007), emotional (P = .02), and functional (P = .005) well-being, as well as fatigue (P = .02) and overall QOL (P = .02; 2-way analysis of variance).
Toxicity
The overall drug-related toxicities were comparably low and not significantly different between arms A and B (step 1), with the exception of generally transient grade 3 or 4 thrombocytopenia (P < .001) and transient hyperbilirubinemia (P = .002). The grade 3 or 4 thrombocytopenia occurred in 13 patients on arm B, 7 of whom had initial platelet levels less than 70 000/mm3. On arm B, 1 patient developed a grade 3 cardiac adverse event (congestive heart failure) and 1 patient developed a deep venous thrombosis.
Discussion
In our randomized study treating anemic MDS patients, the data demonstrated substantially improved erythroid responses in the EPO treatment arm (arm B) compared with the SC alone arm (arm A): 36% versus 9.6% initially with EPO alone, 46.6% overall with EPO plus or minus G-CSF. These responses were particularly evident for patients with lower serum EPO levels and for patients with lower marrow blast percentages (ie, refractory anemia and RARS vs RAEB patients), as also reported in previous studies.5,6,11,12 In addition, our study indicated that the combination of EPO plus G-CSF was synergistic for a proportion of patients who either did not respond initially to EPO or whose response was transient. Higher doses of EPO further enhanced the erythroid responses in a proportion of patients who initially failed to respond to treatment. The total weekly doses of EPO and G-CSF administered in our study are similar to and reflect the National Comprehensive Cancer Network–recommended dosing guidelines3 and those in prior studies,11-13 although current approaches provide the drug once or twice weekly rather than daily as in our trial. These findings support the use of this cytokine combination to help optimize erythroid responses for MDS patients. These data confirm and extend those of earlier studies, which also demonstrated the efficacy of adding G-CSF to EPO and higher EPO doses for enhancing erythroid responses in MDS.10-13
Our study also demonstrated that the overall survival and incidence of AML transformation were not different between the 2 treatment arms, indicating that the use of these cytokines was safe in this patient population. These findings were also noted when considering the effects of the crossover from arm A to arm B for progressing arm A patients. In a subset analysis, the erythroid responders had significantly improved survival compared with nonresponders (median, 5.5 vs 2.3 years). Of note, on morphologic review, all but one of the RARS patients were also classified as refractory cytopenia with multilineage dysplasia and ring sideroblasts by WHO criteria and had improved survival on the EPO treatment arm. This differs from previous findings in which improved survival and responses to cytokines were mainly seen in the “pure” RARS patients.34,35
Although there have been other reports from nonrandomized trials addressing the lack of negative impact of chronic EPO plus or minus G-CSF treatment on survival and AML evolution in MDS,20,21 we believe these long-term findings from our prospective randomized study (median follow-up, 5.8 years) provide valuable new information. Our study demonstrated no negative effects of EPO plus or minus G-CSF treatment on morbidity, survival, or AML evolution when anemic lower-risk MDS patients were treated with EPO with or without G-CSF. Although the number of untreated patients in our control group was somewhat small for these comparisons, the findings of a low incidence of AML transformation and of thrombotic events in both study arms are noteworthy. The recent nonrandomized studies in lower-risk MDS patients treated with these agents using historical controls indicated either improved survival21 or both improved survival and decreased AML progression compared with historical or concurrent controls (reviewed in Greenberg et al3 ).
These data are particularly germane given the recent Food and Drug Administration alerts regarding the use of ESAs.36 The Food and Drug Administration noted that increased mortality, possible tumor promotion, and thromboembolic events were observed in some studies of non-MDS patients (ie, with advanced solid tumors) receiving ESAs without curative intent therapy.18,19,36 However, other studies in solid tumor patients receiving chemotherapy did not demonstrate an adverse effect of ESAs on survival.37-40
Findings from our study also demonstrated the relative safety of G-CSF in MDS and reflect similar results in the aforementioned nonrandomized studies20,21 using G-CSF plus EPO for treating anemic lower-risk MDS patients. In addition, an earlier preliminary report of a randomized international phase 3 trial of G-CSF versus SC in higher-risk MDS patients (RAEB and RAEBT) did not demonstrate G-CSF usage to have an increased potential for AML progression.41 These findings in MDS differ from other nonrandomized studies with long-term G-CSF exposure for patients with other disorders (eg, aplastic anemia, postchemotherapy for a variety of other malignancies), which indicated AML evolution in a portion of these patients.42,43 These data suggest that the negative effects of the cytokines demonstrated in some patients with the other diseases may relate to biologic and clinical features or the specific treatments associated with the differing disorders studied.
It has previously been demonstrated that CD34+ cells in the bone marrows of patients with MDS correlate with morphologically detectable blast cells44 and are associated with a poor prognosis.45 Prior studies found antigen expression profiles of MDS bone marrow CD34+ cells generally differed from that of normal myeloblasts, that is, expressed increased levels of CD15, CD56, CD11b, and Pgp.46-48 Our study adds CD65s to this list of aberrantly expressed antigens in CD34+ MDS precursors as almost one-third of the patients had substantial expression of this antigen. In normal myeloid differentiation, the sialylated carbohydrate antigen CD65s appears at a time when CD34 expression is lost.28 Thus, normal CD34+ myeloblasts lack CD65s expression. In our patients, expression of CD65s was also highly correlated with that of CD15, another carbohydrate antigen associated with the myeloid lineage.29 Other than prolonged survival in patients with Pgp+CD34+ blasts, we did not find a significant difference in outcome between patients with differentiated versus undifferentiated CD34+ myeloblasts. It is noteworthy that all patients with del(5q) were in the differentiated group. The relatively good prognosis and responsiveness to lenalidomide therapy of del(5q) MDS patients49 are in agreement with the superior outcome of differentiated, CD65+ AML.30
Accumulating data in MDS indicate that fatigue and transfusion dependence negatively impact patients' QOL.12,17,50 In our study, we demonstrated no differences in fatigue and QOL subscale scores between treatment groups (EPO vs SC) at 4 months. However, patients who had erythroid responses endorsed significantly improved physical, emotional, and functional well-being plus improved fatigue and overall QOL, as previously reported.8,9,14-16 Thus, albeit this was a subset analysis, improving anemia appears to be an important intervention to maintain or improve QOL in patients with MDS.
Another finding in our study was good long-term drug tolerance; and with the exception of transient thrombocytopenia in patients with low baseline platelet levels, a low incidence in adverse events in patients treated with the cytokines (arm B) compared with those on SC alone (arm A). Specifically, no significant treatment-related increased incidence of either cardiovascular or thrombotic events or transformation to AML occurred in the patients who received EPO alone or with G-CSF compared with those in the SC arm.
Our data demonstrated that a major portion of patients with MDS and symptomatic anemia had substantial clinical benefit from treatment with these hematopoietic cytokines. This was observed without evidence of enhanced adverse consequences compared with SC alone control subjects, which have been described in a number of other clinical settings. These findings should provide useful information for aiding management of these patients.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
Presented in part at the 46th annual meeting of the American Society of Hematology, San Diego, CA, December 2004.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was conducted by the Eastern Cooperative Oncology Group (Robert L. Comis, Chair) and the Canadian Leukemia Studies Group (R.v.d.J., Chair), supported in part by Public Health Service grants (CA23318, CA66636, CA21115, CA80775, CA07190, CA11083, CA17145, CA13650) from the National Cancer Institute, National Institutes of Health, and the Department of Health and Human Services.
Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute or of the Department of Veterans Affairs.
National Institutes of Health
Authorship
Contribution: P.L.G. designed the research, analyzed the data, conducted the trial, and wrote the paper; Z.S. performed the statistical data analysis; K.B.M. designed the research, conducted the trial, and provided study patients; J.M.B. performed morphologic reviews; M.S.T. helped with study design and provided study patients; G.D. performed cytogenetic reviews; E.P. performed the flow cytometry studies; R.v.d.J. reviewed the manuscript; J.H. provided study patients; M.L.T. developed the quality-of-life component of the study and performed the quality-of-life analyses; D.C. performed the quality-of-life analyses; and J.M.R. helped with study design and provided study patients. All authors reviewed and approved the manuscript.
Conflict-of-interest disclosure: P.L.G. has received research support from Amgen, Novartis, Celgene, and Johnson & Johnson and is a consultant for Novartis and Celgene. The remaining authors declare no competing financial interests.
A complete list of participants in the ECOG 1996 trial appears in the supplemental Appendix, available on the Blood website (see the Supplemental Materials link at the top of the online article).
Correspondence: Peter L. Greenberg, Hematology Division, Stanford University Medical Center, 875 Blake Wilbur Dr, Rm 2335, Stanford, CA 94305; e-mail: peterg@stanford.edu.