Abstract
Plasmacytoid dendritic cells (pDCs) represent a major cellular component of our front-line defense against viruses because of their capacity to rapidly secrete type I interferon (IFN)–α and -β after infection. Constant immunosurveillance of the host requires that lymphocytes traffic through lymph nodes (LNs) to sample antigen, yet little is known about the dynamics of pDC accumulation within the secondary lymphoid organs. Here we show that pDCs readily accumulate within the secondary lymphoid organs of mice after virus infection. Interestingly, retention of pDC within LNs is enhanced in the presence of the sphingoshine-1-phosphate receptor agonist FTY720 in a manner similar to that observed for B and T lymphocytes. Ex vivo comparison of mouse pDCs with lymphocytes revealed that pDCs express sphingoshine-1-phosphate 4 and also constitutively express CD69, which is further up-regulated upon virus infection. In IFN-β−/− mice, accumulation of pDC and lymphocytes within LNs is reduced both during viral infection and under steady state conditions, and these defects can be reversed by adding recombinant IFN-β in vivo. These data suggest that pDC and lymphocytes use similar mechanisms for retention within LNs and that these processes are influenced by IFN-β even in the absence of viral infection.
Introduction
To patrol the host for evidence of infection, lymphocytes constantly circulate between the blood and the secondary lymphoid organs. Lymphocytes enter lymph nodes (LNs) from the blood via high endothelial venules (HEVs), guided by the expression of selectins and integrins as well as chemokine gradients. Lymphocytes then percolate through LNs searching for antigen, a process that takes anywhere from 8 to 24 hours. After their passage through the LNs, lymphocytes egress into lymph that is collected in the thoracic and right lymphatic ducts and are subsequently returned to the blood.1 The transit time through a LN is regulated by gradients of the lysophospholipid sphingoshine-1-phosphate (S1P). S1P binds to G-protein–coupled S1P receptors resulting in S1P-receptor down-modulation and provoking lymphocyte egress from the LN into the lymph.2 This signal is attenuated in the presence of inflammation such as viral infection, thus encouraging a longer transit time for lymphocytes within the LNs when lymphocyte interactions with antigen-presenting dendritic cells (DCs) are most critically required.3,4
Plasmacytoid DCs (pDCs) express both TLR7 and TLR9 and quickly respond to DNA and RNA viruses by producing large amounts of interferon-α (IFN-α) and interferon-β (IFN-β).5 Their secretion of IFN-α and IFN-β positions them as a link between the innate and adaptive immune system.6 Indeed, there is significant evidence implicating pDCs in the activation of other immune cells: IFN-α secreted by human pDCs can activate natural killer (NK) cell cytolytic function and IFN-γ production,7 and IFN-α/β can induce immunoglobulin G (IgG) secretion from B cells.8,9 Through their secretion of IFN-α and IFN-β, mouse pDCs have been shown to induce up-regulation of CD694 and production of IFN-γ10,11 from CD4+ T cells. pDCs can also prime CD8 T cells in the context of influenza virus infection12 and can cross-present HIV antigens.13 Therefore, the profile of both cytokine and chemokine secretion by pDC positions these cells as important intermediates that influence the adaptive immune response.
During infection, naive lymphocytes are primed within the draining LNs. Therefore, it is critical to understand the dynamics of pDC accumulation at these anatomical sites during inflammation because pDCs can shape the subsequent adaptive immune response. pDCs use selectins to enter LNs from the blood via HEVs,14-16 a situation analogous to that of naive lymphocytes,17 but different from conventional DCs (cDCs), which enter inflamed LNs through the lymphatics.18 However, in the context of inflammation, pDCs also can enter LNs via the afferent lymphatics, as evidenced in 2 large mammal models (sheep, pig).19 pDCs constitutively express CXCR314,20 and may also express CXCR420,21 and CCR7.22 The ability of pDCs to respond to the constitutive chemokine CXCR4 ligand CXCL12/SDF-1 is greatly enhanced in the presence of CXCR3 ligands (CXCL9/CXCL1), which are produced during inflammation such as viral infection.20 In the case of migration of pDCs to the spleen, the splenic stroma has been shown to provide survival signals, chemoattractant signals (CXCL12), and negative regulatory signals (TGFβ) to virus-activated pDCs.23 The fate of pDCs within the lymphoid tissues both during homeostatic circulation and in the context of inflammation as the result of viral infection has not been examined.
The type I IFN family of cytokines is composed of multiple IFN-α subtypes, as well as single IFN-β, -ω, -κ, -τ, and -δ subtypes.24 In addition to their role in antiviral responses, type I IFNs have been shown to influence pDCs themselves, the main producers of IFN-α/β upon TLR7/9 triggering.25 pDCs from IFNAR−/− mice treated with Toll-like receptor (TLR) ligands fail to optimally up-regulate CD40 and CD86 and do not migrate to form clusters in the T-cell zones.25 IFN-α and IFN-β are markedly induced during viral infection, with IFN-β expression preceding that of IFN-α in some situations.26 Notably, however, IFN-β expression is also important in maintaining the architecture of lymphoid tissues.27 Viewed altogether, IFN-β may be a good candidate for regulating the accumulation of lymphocytes, or perhaps pDCs themselves, in the LNs under noninflamed homeostatic conditions as well as during the early antiviral response.
Here we have asked some basic questions about the dynamics of pDC accumulation in the LNs under both inflamed and noninflamed conditions. During influenza infection of mice, we observed pDC accumulation in the spleen and LNs (depending on the route of infection). In addition, we found that, like lymphocytes, pDCs are retained in the LNs after FTY720 treatment, a potent agonist for S1P1, S1P3, S1P4, and S1P5. Our data reveal a novel mechanism for immunosurveillance whereby pDCs can accumulate in the LN tissues with their transit time regulated by IFN-β both under homeostatic conditions and during viral infection.
Methods
Mice
C57Bl/6 mice were purchased from The Jackson Laboratory. IFNβ−/− mice on the C57Bl/6 background (backcrossed > 10 generations) were bred in-house. All mice were used at 6 to 10 weeks of age. All animals were housed in specific pathogen-free conditions, and experiments were performed according to the University of Toronto and University Health Network–approved animal use protocols.
Reagents and antibodies
FTY720 and SEW2871 were purchased from Cayman Chemical Company. Anti–mPDCA-1-FITC was purchased from Miltenyi Biotec Inc. Anti–mCD11c-PE, anti–mTCRβ-PE-Cy5, anti–mCD19-PE-Cy5, anti–mB220-APC, anti–mCD69-Biotin, and Streptavidin-APC-Cy7 were purchased from eBiosciences Inc. Anti–LYVE-1 and goat anti–rabbit IgG conjugated with Alexa Fluor 594 were purchased from Upstate Biotechnology Inc and Molecular Probes, respectively.
Treatments and influenza infections
C57Bl/6 mice were pretreated with FTY720 (1 μg/g body weight) or vehicle control intravenously. For SEW2871 treatments, mice received 10 μg/g by gavage administration and were euthanized 10 hours after treatment. In the case of FTY720 and SEW2871 treatments, controls received diluent alone. FTY720 was dissolved in dimethyl sulfoxide and subsequently diluted in phosphate-buffered saline (PBS), and SEW2871 was dissolved in ethanol followed by dilution in PBS plus 20% Tween-20. For influenza infections, C57Bl/6 mice or IFN-β−/− were infected with 2 HAU of influenza virus intraperitoneally or by intranasal instillation. After 18 hours, peripheral blood, LNs (mediastinal), and spleen were harvested. For treatment with recombinant IFN-β or IFN-α, 100000 units of either cytokine were injected intraperitoneally at 0 and 8 hours relative to intranasal challenge with 2 HAU of influenza virus, or in naive uninfected mice, as indicated. Mice were harvested at 18 hours after treatment (both for naive mice and infected mice). Details on cell preparation can be found in supplemental data (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Immunohistochemistry
Immunohistochemistry was performed as described.28 In brief, brachial and inguinal LNs were collected and embedded in OCT compound (Tissue-Tek; Sakura Finetek) and flash-frozen in an ice-cold bath of 2-methylbutane. Cryostat sections (5 μm) were obtained by the use of a Leica 1035S cryostat, fixed in acetone for 7 minutes, and stained with anti–LYVE-1, anti–mB220-APC, and anti–mPDCA1-FITC. Sections were then stained with secondary anti–rabbit IgG conjugated with Alexa Fluor 594. Images of stained sections were obtained by the use of a Leica DM-R digital fluorescence microscope.
Quantitative real-time PCR for S1P receptor mRNA expression
T cells, B cells, and pDCs were sorted from LNs and spleens of wild-type (WT) C57Bl/6 mice, and total RNA was isolated by the use of the RNeasy kit (Canada-QIAGEN Inc). Complementary DNA (cDNA) was synthesized with AffinityScript Multi Temperature cDNA Synthesis Kit (Stratagene) by the use of random hexamers. Real-time polymerase chain reaction (PCR) was performed with an Applied Biosystems 7300 Real-Time PCR System by the use of SYBR Green JumpStart Taq ReadyMix for Quantitative PCR (Sigma-Aldrich Canada Ltd). Levels of S1P receptors were normalized to hypoxanthine guanine phosphoribosyl transferase levels and then compared with each other as described in the figure legend (Figure 2). Conditions and primer sequences can be found in supplemental data.
Statistical analysis
Statistical significance of differences was determined by the unpaired 2-tailed Student t test. Differences were considered statistically significant when P was less than .05, P was less than .01, and P was less than .005.
Results
pDCs accumulate in secondary lymphoid organs after infection with influenza virus via 2 different routes
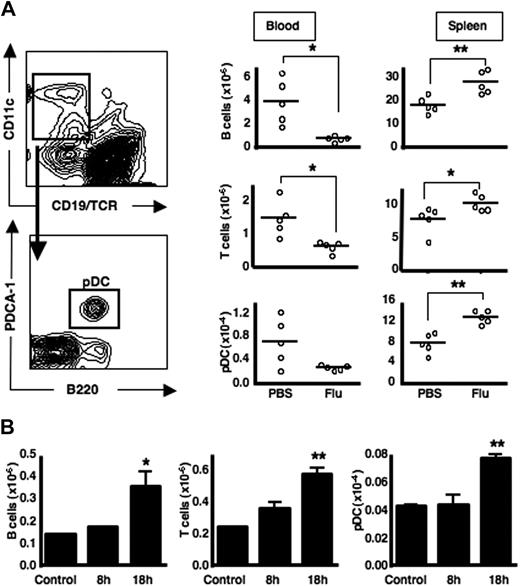
During viral infection, transient lymphopenia is observed.3,4 Because pDCs also can recirculate from blood to LNs via HEVs, we asked whether pDCs would similarly be reduced in the peripheral-blood compartment after viral infection. Upon intraperitoneal infection with influenza virus, we observed a decrease in T and B lymphocytes from the blood and a corresponding increase in the spleen; this pattern also was observed for CD19−TCRαβ−B220+PDCA-1+ pDC (similar gating strategies were used throughout the study; Figure 1A). Upon intranasal administration of influenza virus, the natural route of infection, T and B lymphocytes, as well as pDCs also accumulate in the lung-draining mediastinal LNs (Figure 1B). A corresponding decrease in T and B cells as well as pDCs is observed in the spleen and in the blood (supplemental Figure 1). Therefore, during systemic virus infection (intraperitoneal immunization) a paucity of pDCs is observed in the blood, with a concomitant accumulation of these cells within the spleen. In the case of a localized infection (intranasal immunization) pDCs accumulate in the mediastinal LNs rather than the spleen, in a manner similar to what was observed for T and B lymphocytes.
pDCs accumulate in secondary lymphoid tissues after infection with influenza virus via 2 different routes. (A) C57Bl/6 mice were either mock-infected (PBS) or infected with influenza virus intraperitoneally. After 18 hours, peripheral blood and spleens were harvested, stained, and analyzed by flow cytometry. A representative FACS plot for pDC gating is shown here derived from a spleen from a resting mouse. (B) C57BL/6 mice were either mock-infected or infected with influenza virus by intranasal instillation. After 8 or 18 hours, MLNs were analyzed by flow cytometry. Each group contained 5 mice, and the experiment was performed 3 times with similar results. Lymphocyte and pDC numbers in mediastinal nodes are shown here as the mean ± SEM of 5 animals. *P < .05; **P < .005
pDCs accumulate in secondary lymphoid tissues after infection with influenza virus via 2 different routes. (A) C57Bl/6 mice were either mock-infected (PBS) or infected with influenza virus intraperitoneally. After 18 hours, peripheral blood and spleens were harvested, stained, and analyzed by flow cytometry. A representative FACS plot for pDC gating is shown here derived from a spleen from a resting mouse. (B) C57BL/6 mice were either mock-infected or infected with influenza virus by intranasal instillation. After 8 or 18 hours, MLNs were analyzed by flow cytometry. Each group contained 5 mice, and the experiment was performed 3 times with similar results. Lymphocyte and pDC numbers in mediastinal nodes are shown here as the mean ± SEM of 5 animals. *P < .05; **P < .005
pDCs express S1P4, and FTY720 treatment causes accumulation of pDCs in LNs with a corresponding decrease of pDCs in the blood
In the steady state, lymphocytes traffic to LNs, and their retention in these secondary lymphoid organs is governed in part by S1P responsiveness.1 Indeed during viral infection, the observed increase of lymphocytes within LNs is not only caused by enhanced entry into the LNs but also by a protracted resident time within the organ caused by decreased responsiveness to S1P.4 It is possible that a similar mechanism could contribute toward the observed accumulation of pDC within the mediastinal LN (MLN) as late as 18 hours after infection (Figure 1B). Therefore, we first measured the levels of S1P receptors on pDC and found that S1P4 was abundantly expressed (Figure 2A). Because S1P4 is responsive to FTY720-mediated agonism29,30 we next asked whether the pDC numbers in the blood and the LNs were affected by FTY720 administration. As expected, FTY720 treatment resulted in a reduction in B and T lymphocytes in the blood and a corresponding increase in the LNs. Interestingly, the same pattern was observed for pDCs (Figure 2B). The sensitivity to FTY720 was statistically significant when evaluating both lymphocyte and pDC subsets; however, pDCs were less sensitive to FTY720 treatment compared with T and B lymphocytes. Specifically, a 2.7-fold (± 0.03) decrease of lymphocytes in the blood was observed compared with a 1.7-fold (± 0.59) decrease for pDCs (measurements calculated per 1 mL blood). Likewise, there was a 1.9-fold (± 0.19) increase in LN-resident lymphocytes compared with a 1.5-fold (± 0.27) increase in LN-resident pDCs. We did not observe a decrease in T cells, B cells, or pDCs in the spleen (supplemental Figure 2), which is consistent with other published observations that demonstrated little or no effect of FTY720 on egress of splenocytes.29,31
pDCs express S1P receptors, and FTY720 treatment causes accumulation of pDCs in LNs with a corresponding decrease of pDCs in the blood. (A) T cells, B cells, and pDCs were sorted from LNs and spleens of C57Bl/6 mice. The expression of S1Ps1-5 was determined by quantitative real-time PCR. For the first 3 graphs, the amount of S1P1 mRNA was set as 1, and the value of S1P4 mRNA expression was expressed as relative amount compared with the S1P1 value. For the fourth graph, the amount of S1P4 mRNA in T cells was set as 1, and the value of S1P4 mRNA expression in other cell subsets was expressed as relative amount compared with T cells. Data from 1 of 3 independent experiments are shown. (B) C57Bl/6 mice were treated with FTY720 intravenously. After 18 hours, blood and LNs were assessed for B cells, T cells, and pDCs by flow cytometry. Lymphocyte and pDC numbers calculated per 1 mL blood and in 6 harvested LNs (2 inguinal, 2 axillary, 2 brachial) from 3 independent experiments are shown. *P < .05; **P < .005.
pDCs express S1P receptors, and FTY720 treatment causes accumulation of pDCs in LNs with a corresponding decrease of pDCs in the blood. (A) T cells, B cells, and pDCs were sorted from LNs and spleens of C57Bl/6 mice. The expression of S1Ps1-5 was determined by quantitative real-time PCR. For the first 3 graphs, the amount of S1P1 mRNA was set as 1, and the value of S1P4 mRNA expression was expressed as relative amount compared with the S1P1 value. For the fourth graph, the amount of S1P4 mRNA in T cells was set as 1, and the value of S1P4 mRNA expression in other cell subsets was expressed as relative amount compared with T cells. Data from 1 of 3 independent experiments are shown. (B) C57Bl/6 mice were treated with FTY720 intravenously. After 18 hours, blood and LNs were assessed for B cells, T cells, and pDCs by flow cytometry. Lymphocyte and pDC numbers calculated per 1 mL blood and in 6 harvested LNs (2 inguinal, 2 axillary, 2 brachial) from 3 independent experiments are shown. *P < .05; **P < .005.
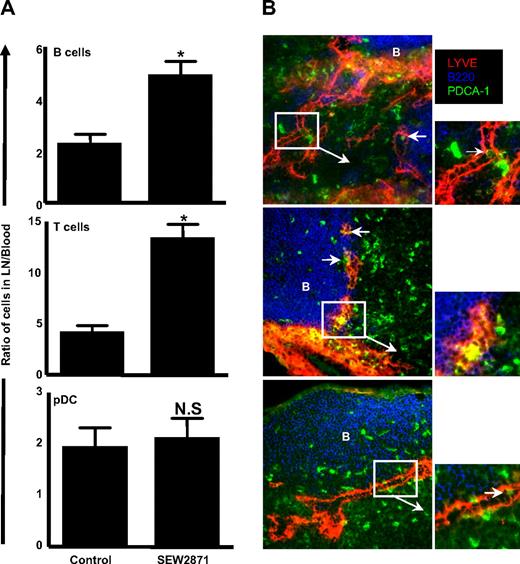
Given the selective expression of S1P4 on pDC, we asked whether a specific modulator of S1P1 would have an effect on pDC trafficking indirectly because of its effects on lymphocyte trafficking. We examined the effect of SEW2871, a selective S1P1 receptor agonist in both human and mouse that does not activate the S1P2-5 receptors,32 on pDC accumulation in LNs. By indexing the LNs versus blood lymphocyte counts for each individual mouse, an internally controlled ratio was derived representing the relative retention of lymphocytes in the LNs. Treatment with SEW2871 resulted in a significant increase in the LN to blood ratio of both T and B cells; however, pDCs were not affected by the SEW2871 treatment (Figure 3A). Thus, these results confirm that S1P1 is inactive on pDCs and that the effects of FTY720 on pDC trafficking are likely caused by direct effects of S1P4 on pDCs rather than a redistribution of pDCs attributed to the retention of lymphocytes in the LNs.
SEW2871 treatment results in B and T lymphocyte but not pDC accumulation in the LNs, and pDCs are found in the cortical sinusoids of SEW2871-treated mice. (A) C57Bl/6 mice were treated with SEW2871 to prevent S1P1-mediated egress. B cells, T cells, and pDCs were quantified in 6 pooled LNs versus 1 mL blood, and the numbers were used to generate a ratio of cells in LN/blood. The experiment was performed 3 times with similar results. *P < .05. (B) LNs from SEW2871-treated mice were frozen, sectioned, and stained for immunofluorescence microscopy. Images were obtained using a ×20 objective. LNs from 3 representative mice are shown from a collection of 6 different mice. Arrows indicate close associations between PDCA-1–positive cells and LYVE-1 cortical sinusoids.
SEW2871 treatment results in B and T lymphocyte but not pDC accumulation in the LNs, and pDCs are found in the cortical sinusoids of SEW2871-treated mice. (A) C57Bl/6 mice were treated with SEW2871 to prevent S1P1-mediated egress. B cells, T cells, and pDCs were quantified in 6 pooled LNs versus 1 mL blood, and the numbers were used to generate a ratio of cells in LN/blood. The experiment was performed 3 times with similar results. *P < .05. (B) LNs from SEW2871-treated mice were frozen, sectioned, and stained for immunofluorescence microscopy. Images were obtained using a ×20 objective. LNs from 3 representative mice are shown from a collection of 6 different mice. Arrows indicate close associations between PDCA-1–positive cells and LYVE-1 cortical sinusoids.
In addition, we took advantage of the effects of SEW2871, which “empties” the LN sinuses of lymphocytes, to obtain evidence that pDCs enter these structures in the LNs to egress into the lymphatics. We used an Ab to the lymphatic marker (LYVE-1), which stains hyaluronan receptors on lymphatic (but not vascular) endothelium. Although this Ab readily demarcates the medullary sinuses, in agreement with others,33 we observed LYVE-1 staining in the paracortex adjacent to the B-cell follicle (Figure 3B). Within these LYVE-1+ cortical sinusoids, we observed occasional PDCA-1+ cells within or directly adjacent to these structures, suggesting that pDCs are actively leaving the LNs (Figure 3B). In agreement with its inhibitory effect on S1P1-mediated cell trafficking, we failed to observe B cells within the cortical sinusoids of SEW2871-treated mice. Taken together, pDC express S1P4 and accumulate in the LNs upon FTY720 treatment.
CD69 is expressed on pDCs and is further increased upon influenza infection
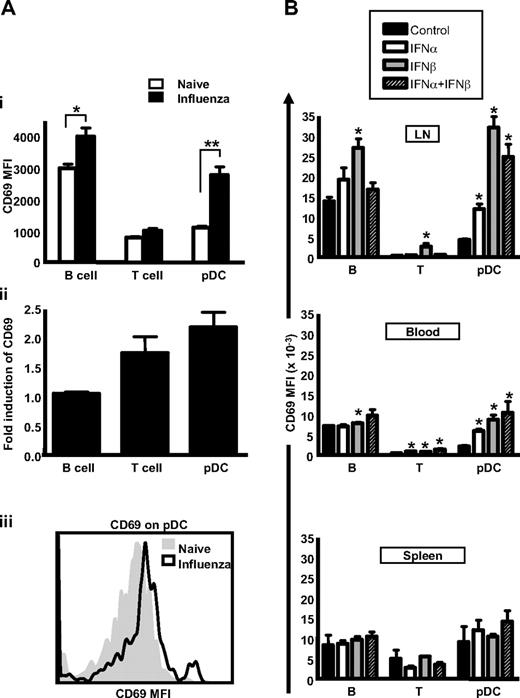
Decreased responsiveness to S1P in lymphocytes has been shown to depend on CD69 up-regulation and corresponding desensitization of the S1P1 receptor.4 Indeed, resting lymphocytes express low levels of CD69 in naive animals, and this low level is likely required to optimize their transit time through LNs, thus achieving a balance between S1P1 activation and S1P1 desensitization. If pDCs are similarly controlled by an S1P-dependent pathway for their transit time in LNs, these cells should express CD69. Indeed, we detected CD69 expression on pDCs, and this level was further increased upon systemic influenza infection (Figure 4Ai, with a representative histogram shown in Figure 4Aii). Indeed, the induction of CD69 on pDCs was even greater, when expressed as a fold-increase over background, then what was observed on B and T cells (Figure 4Aii). Therefore, the expression of CD69 on pDCs and its up-regulation upon virus infection provides further evidence that these cells are using an S1P-sensitive pathway for LN egress.
CD69 is expressed on pDCs and is further increased upon influenza infection. (A) C57Bl/6 mice were infected with influenza virus or PBS intraperitoneally. After 18 hours, CD69 expression on the surface of lymphocytes and pDCs was determined by flow cytometry. The value of CD69 was expressed as the average mean fluorescence intensity on cells from 5 mice (i) or as the fold-induction of CD69 on different cell types induced by influenza infection (ii). A representative histogram of CD69 expression on pDC is shown (iii). The experiment is a representative example of at least 5 independent experiments. (B) C57Bl/6 mice were injected with 105 U IFN-α, IFN-β, or a combination of 105 U of each IFN. After 18 hours, CD69 expression on the surface of lymphocytes and pDCs were determined by flow cytometry. The value of CD69 was expressed as the average mean fluorescence intensity on cells from 5 mice. *P < .05.
CD69 is expressed on pDCs and is further increased upon influenza infection. (A) C57Bl/6 mice were infected with influenza virus or PBS intraperitoneally. After 18 hours, CD69 expression on the surface of lymphocytes and pDCs was determined by flow cytometry. The value of CD69 was expressed as the average mean fluorescence intensity on cells from 5 mice (i) or as the fold-induction of CD69 on different cell types induced by influenza infection (ii). A representative histogram of CD69 expression on pDC is shown (iii). The experiment is a representative example of at least 5 independent experiments. (B) C57Bl/6 mice were injected with 105 U IFN-α, IFN-β, or a combination of 105 U of each IFN. After 18 hours, CD69 expression on the surface of lymphocytes and pDCs were determined by flow cytometry. The value of CD69 was expressed as the average mean fluorescence intensity on cells from 5 mice. *P < .05.
Recombinant IFN-α and IFN-β treatment results in pDC accumulation in the LNs
The up-regulation of CD69 on lymphocytes during virus infection is influenced by IFN-α/β.4 Indeed, IFNs α and β are particularly important for the loss of B cells from the peripheral-blood compartment during virus-induced lymphopenia,34 and direct injection of IFN-α/β similarly causes lymphopenia.3,35 We therefore tested whether IFN-α, IFN-β, or a combination of both cytokines also induce CD69 expression on pDCs and whether these treatments have a similar effect on accumulation of pDCs in the LNs. Indeed, both IFN-α and IFN-β promote the expression of CD69, particularly on B cells and pDCs, and most notably in response to IFN-β (Figure 4B). We also noted that the effects on CD69 expression were most pronounced in LNs compared with the blood or the spleen. Consistent with a role for CD69 in the down-regulation of S1P1 mRNA on lymphocytes,4 we also observed a reduction in S1P4 mRNA on pDC after IFN-β treatment (supplemental Figure 3).
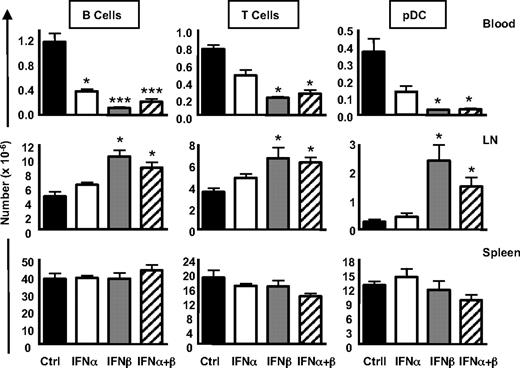
To evaluate whether IFN-α/β–induced expression of CD69 has a consequence on the retention of pDCs in the LNs, we next evaluated the numbers of lymphocytes and pDCs in the spleen, blood, and LNs. We found that IFN-β treatment resulted in a significant decrease in B cells, T cells, and pDCs in the blood, whereas IFN-α treatment only had a significant effect on B cells in the blood, with T cells and pDCs being moderately but not significantly reduced (Figure 5 Blood). Furthermore, IFN-β treatment induced a statistically significant accumulation of B cells, T cells, and pDCs in the LNs, whereas IFN-α induced a trend toward increases in these cells in the LNs that was not statistically significant over control treated mice (Figure 5 LN). Finally, the combination of IFN-α and IFN-β did not exacerbate decreases of B cells, T cells, and pDCs in the blood (or the accumulation of these cells in the LN) beyond what was observed for IFN-β treatment alone. No statistically significant changes were observed in the spleen for any of these cell types after cytokine treatment (Figure 5 Spleen). Therefore, in agreement with other studies demonstrating that type I IFNs induce lympho-penia (B and T cells),3,35 we also found this to be true for pDCs, particularly upon exposure to IFN-β.
Effects of recombinant IFN-α/β on lymphocyte and pDC accumulation in lymphoid tissues. C57Bl/6 mice were injected with 105 U IFN-α, IFN-β, or a combination of 105 U of each IFN. After 18 hours, numbers of lymphocytes and pDCs in the blood, LNs, and spleen were determined by flow cytometry. Averages represent pooled data from 5 mice. *P < .05; ***P < .005.
Effects of recombinant IFN-α/β on lymphocyte and pDC accumulation in lymphoid tissues. C57Bl/6 mice were injected with 105 U IFN-α, IFN-β, or a combination of 105 U of each IFN. After 18 hours, numbers of lymphocytes and pDCs in the blood, LNs, and spleen were determined by flow cytometry. Averages represent pooled data from 5 mice. *P < .05; ***P < .005.
IFN-β influences pDC accumulation in LNs during inflammation and in naive mice
Because IFN-β is an important IFN subtype that is rapidly produced by pDCs before IFN-α during viral infection26 and because the addition of recombinant IFN-β has a particularly marked effect on pDC accumulation in the LNs compared with IFN-α, we asked whether the absence of IFN-β alone would influence the dynamics of pDC accumulation during an infection. Indeed, when we examined the cellularity of the mediastinal LNs of WT versus IFN-β−/− mice after intranasal influenza infection, the accumulation of B and T lymphocytes within the LNs was found to be dependent on IFN-β. Furthermore, the accumulation of pDCs within the LNs was also found to depend on IFN-β (Figure 6A). Despite the impairment of pDC accumulation in the MLNs in IFN-β−/− mice, we were still able to detect IFN-α produced within the lung after influenza infection (supplemental Figure 4). Therefore, pDCs as well as lymphocytes accumulate in the inflamed draining LNs 18 hours after influenza infection in an IFN-β-dependent manner, and IFNα production is not sufficient for the retention of pDCs in the LNs during inflammation.
IFN-β influences pDC migration during inflammation and under homeostatic conditions. (A) C57Bl/6 mice or IFN-β−/− mice were either mock-infected (PBS) or infected with influenza virus intranasally. After 18 hours, MLNs were collected, and B cells, T cells, and pDCs were analyzed by flow cytometry. Numbers of lymphocytes or pDCs in the MLNs of influenza-infected mice were compared with the number in MLNs of uninfected mice to obtain a LN expansion ratio. The data are representative of 2 independent experiments. (B) IFN-β influences pDC migration under homeostatic conditions. LNs (left column) and spleens (right column) were harvested from WT C57BL/6 mice or IFN-β−/− mice and B cells, T cells. and pDCs in LNs and spleen of WT versus IFN-β−/− mice were assessed by flow cytometry. (C) CD69 levels on B cells, T cells, and pDCs from WT versus IFN-β−/− mice were evaluated by flow cytometry. Data are from 3 independent experiments. *P < .05, **P < .005.
IFN-β influences pDC migration during inflammation and under homeostatic conditions. (A) C57Bl/6 mice or IFN-β−/− mice were either mock-infected (PBS) or infected with influenza virus intranasally. After 18 hours, MLNs were collected, and B cells, T cells, and pDCs were analyzed by flow cytometry. Numbers of lymphocytes or pDCs in the MLNs of influenza-infected mice were compared with the number in MLNs of uninfected mice to obtain a LN expansion ratio. The data are representative of 2 independent experiments. (B) IFN-β influences pDC migration under homeostatic conditions. LNs (left column) and spleens (right column) were harvested from WT C57BL/6 mice or IFN-β−/− mice and B cells, T cells. and pDCs in LNs and spleen of WT versus IFN-β−/− mice were assessed by flow cytometry. (C) CD69 levels on B cells, T cells, and pDCs from WT versus IFN-β−/− mice were evaluated by flow cytometry. Data are from 3 independent experiments. *P < .05, **P < .005.
IFN-β is also required to sustain homeostasis of certain immune cells in a naive animal and is needed to optimize lymphocyte organization within the secondary lymphoid organs.27 Because pDCs are found in the blood and can enter LNs through the HEVs, we asked whether the homeostatic trafficking of these cells was also influenced by IFN-β. Indeed, both lymphocytes and pDCs were found to be reduced in numbers in the LNs but not the spleen in IFN-β−/− mice (Figure 6B). Because IFN-α/β affects CD69 expression, we measured CD69 levels on lymphocytes and pDCs from WT versus IFN-β−/− mice. Under resting conditions, we did not observe a difference in baseline levels of CD69 on lymphocytes, however IFN-β−/− pDC exhibited a significant reduction in CD69 expression (Figure 6C). Therefore, IFN-β is required for the maximal accumulation of pDC and lymphocytes both during virus infection and in the naive animal, and IFN-β is required for the optimal expression of CD69 on pDC but not lymphocytes.
Addition of IFN-β restores the balance of lymphocytes and pDC in the blood versus LNs in naive and influenza-infected IFN-β−/− mice
To confirm a role for IFN-β in regulating the migratory dynamics of lymphocytes and pDC in naive and influenza-infected mice, recombinant IFN-β was injected into IFN-β−/− mice to examine its effect on restoration of the balance of lymphocytes and pDC in the LNs versus the blood. Compared with vehicle control treated mice, both WT and IFN-β−/− mice exhibited an elevated ratio of B cells, T cells, and pDCs in the peripheral LNs versus the blood compartment (statistically significant for all groups, Figure 7A). Upon intranasal infection with influenza virus, the ratio of pDCs, B, and T cells in the mediastinal LNs versus the blood compartment was also elevated in IFN-β–treated WT and IFN-β−/− mice, with IFN-β−/− pDC proving especially sensitive to IFN-β treatment. The absolute numbers of pDCs in IFN-β−/− influenza-infected mice increased from 2.1 × 103 to 4.7 × 103 cells/MLNs in IFN-β–treated mice (P < .01). Furthermore, although IFN-β−/− pDCs do not appreciably up-regulate CD69 in response to influenza infection, we observed a substantial increase in CD69 levels on IFN-β−/− pDC when these mice were treated with IFN-β (Figure 7C). This increase was most notable in the MLNs, the LN draining the site of infection (Figure 7C). In conclusion, IFN-β treatment restores the lymphocyte and pDC balance in the blood versus LN in both naive and virus-infected mice.
Administration of recombinant IFN-β restores balance of pDCs and lymphocytes in the LNs of IFN-β−/− mice. Naive (A) and influenza-infected (B) WT C57Bl/6 mice or IFN-β−/− mice were treated with 105 U recombinant IFN-β by intraperitoneal injection. After 18 hours, blood and LNs were harvested, and ratios of lymphocyte or pDC numbers in LNs/blood were obtained by flow cytometry. A total of n = 5-7 mice per group per experiment was examined, and the experiment was performed twice. *P < .05, **P < .005, ***P < .0005. (C) CD69 levels on pDCs from IFN-β−/− mice were determined 18 hours after influenza infection, with and without the addition of recombinant IFN-β. A representative example of CD69 staining on pDC is shown from a total of 5 mice. Shaded histogram indicates pDCs from uninfected mice. Broken line histogram, pDCs from influenza infected mice. Solid line histogram, pDCs from influenza-infected mice treated with recombinant IFN-β.
Administration of recombinant IFN-β restores balance of pDCs and lymphocytes in the LNs of IFN-β−/− mice. Naive (A) and influenza-infected (B) WT C57Bl/6 mice or IFN-β−/− mice were treated with 105 U recombinant IFN-β by intraperitoneal injection. After 18 hours, blood and LNs were harvested, and ratios of lymphocyte or pDC numbers in LNs/blood were obtained by flow cytometry. A total of n = 5-7 mice per group per experiment was examined, and the experiment was performed twice. *P < .05, **P < .005, ***P < .0005. (C) CD69 levels on pDCs from IFN-β−/− mice were determined 18 hours after influenza infection, with and without the addition of recombinant IFN-β. A representative example of CD69 staining on pDC is shown from a total of 5 mice. Shaded histogram indicates pDCs from uninfected mice. Broken line histogram, pDCs from influenza infected mice. Solid line histogram, pDCs from influenza-infected mice treated with recombinant IFN-β.
Discussion
pDCs are an important cellular bridge between the innate and adaptive immune system, yet relatively little is known about the dynamics of their accumulation in LNs, where they may exert immunomodulatory effects on lymphocytes. Indeed, given that pDCs use HEVs to enter LNs and are responsive to homeostatic chemokines, it is likely that these cells use similar mechanisms for optimizing their transit time in the LNs to maximize encounters with components of the adaptive immune system. In addition, other innate immune cells, such as NK cells, use a S1P-dependent mechanism for migration,36 and because NK cells can be activated by IFN-α secreted by pDCs,7 the use of similar trafficking mechanisms would enhance their likelihood of encountering one another. Because pDCs respond to influenza virus via TLR7 engagement, we have used in vivo influenza infection as a means to follow the dynamic accumulation of pDC in the spleen and LNs. Indeed, within the same time period that has been described for virus-induced lymphopenia (6-18 hours),4 pDCs also were found to accumulate in the spleen (intraperitoneal infection) and mediastinal LNs (intranasal infection) in concert with T and B cells, corresponding to a decrease in pDC numbers in the blood.
During inflammation, pDCs have been shown to use lymphatics for entry into LNs in pigs and sheep,19 although under resting conditions, pDCs require E- and L-selectin for entry via HEVs.14-16 In the case of cDCs, it is generally accepted that these cells die in the LNs after antigen presentation to T cells, but that in some cases cDCs can exit LNs through efferent lymphatics.37 Indeed, CD11c+ DCs have been detected in thoracic duct preparations in mice,38 sheep,39 and rats,40,41 and technical limitations could arguably underestimate the numbers of DCs in efferent lymph. Because the retention of lymphocytes in LNs is dependent on engagement of S1P receptors, in particular S1P1 for lymphocytes,2 we reasoned that pDCs may also be sensitive to retention signals to optimize their transit time through the LNs. To explore this hypothesis, we treated mice with the S1P agonist FTY720 and asked whether pDC numbers decreased in the blood. Indeed, correlating with a decrease in lymphocytes in the blood 24 hours after FTY720 treatment, pDCs likewise decreased in the blood, with a corresponding increase in the LNs. Interestingly, unlike cDCs, pDCs can activate lymphoid-specific genetic programs,42 and their migration into LNs via the HEV is the same as the migration pathway for naive lymphocytes but markedly different from cDC.14-16 In this study, we highlight yet another similarity between pDC and lymphocytes: their retention in LNs upon either IFN-β or FTY720 treatment.
pDCs also were detected in proximity and occasionally within the LYVE-1+ cortical lymphatic sinuses, providing indirect evidence that pDC, like lymphocytes, may use these structures for egress from LNs. The authors of a previous study33 did not observe any CD11c+ cells in LYVE-1+ sinuses in the paracortex, although lymphocytes in these structures were abundant. However, the level of expression of CD11c on pDC is significantly lower than what is observed on cDC; therefore, it is possible that the authors did not detect pDCs with this method. In addition, we noted that the PDCA-1 staining on cells within the LYVE-1+ structures appeared dimmer and more particulate than what was observed on pDC in the adjacent parenchyma (Figure 3). It is possible that pDCs alter their surface phenotype and/or function upon entry into these structures and may be difficult to detect in efferent lymph using conventional flow cytometry methods or using IFN-α/β secretion as a surrogate readout for pDC. This explanation could possibly account for the differing results in the identification of putative pDCs in lymph samples from rat43 versus sheep/pig.19 Given their reduced sensitivity to FTY720 treatment compared with lymphocytes (Figure 2) and the possibility that their surface phenotype and capacity to secrete IFN-α/β may be altered within the lymph, future work will be required to definitively test whether pDCs egress from LNs.
The sensitivity of pDCs to FTY720 was statistically significant although not as marked as that observed for lymphocytes. Lymphocytes primarily use S1P1 for migration toward S1P, however, S1P4 is also expressed.2 In contrast, we found that pDCs express predominantly S1P4 and not S1P1. Although S1P4 has been shown to provoke migration toward S1P in some systems,44,45 this finding has not been clear in other scenarios.46 We postulate that in the absence of S1P1 on pDC, S1P4 can promote pDC migration but with reduced efficiency. Alternatively, it is possible that a portion of pDCs do not egress from the LNs but die in this location, as has been suggested for cDCs. The net overall affect of FTY720 on pDC retention would therefore be less obvious than for lymphocytes.
IFN-α/β induction by virus34 or treatment with recombinant IFN-α or IFN-β3,35 can mediate transient lymphopenia. IFN-α and IFN-β induce the expression of CD69 on lymphocytes in the context of virus infection, and IFNAR−/− B cells fail to up-regulate CD69.4 A role for IFN-β in the naive animal in controlling pDC/lymphocyte accumulation in the LNs is curious. IFN-β is required for lymphoid tissue organization.27 Furthermore, depending on the specific TLR activated by virus, IFN-β is the first type I IFN induced upon viral infection.26 In this study, we have shown that recombinant IFN-β can induce the accumulation of pDCs and lymphocytes in the LNs, and this seems to be more potent than a similar administration of IFN-α. Furthermore, we were able to detect the presence of IFN-α in the lungs of influenza-infected IFN-β−/− mice, suggesting that IFN-α on its own is not sufficient for the accumulation of pDC in the inflamed LNs (supplemental Figure 4). It is unclear why IFN-α causes less lymphopenia than IFN-β, although there are reports of differences between these 2 cytokines at the level of biophysical binding to IFNAR1.47
Given our observations that IFN-β has a particularly potent effect on pDC accumulation in the LNs, we assessed the consequence of blunting the early IFN-β response to virus without affecting subsequent IFN-α production and viral clearance using IFN-β−/− mice as our model system. We first asked whether CD69 levels on pDC and lymphocytes in naive animals were altered in IFN-β−/− mice. Indeed, CD69 was decreased on IFN-β−/− pDC, and this could be markedly restored in the presence of recombinant IFN-β (Figure 7C). In contrast, CD69 remained at normal levels on IFN-β−/− lymphocytes, suggesting that CD69 expression cannot fully account for the reduced numbers of lymphocytes observed in IFN-β−/− LNs. Alternatively, because CD69 levels on resting lymphocytes is quite low, it is possible that CD69 plays a more relevant role in retaining lymphocytes once they become activated. For example, inflammation-induced CD69 up-regulation results in a down-regulation of S1P1 on lymphocytes,4 and we have found that under conditions where CD69 is increased on pDC, such as during IFN-β treatment, S1P4 levels are also reduced on pDC (supplemental Figure 3). Finally, it is possible that IFN-β treatment may also induce up-regulation of homeostatic chemokines such as CCL19/CCL21 within the LNs themselves to further encourage pDC retention within the LNs.
The dominant role of IFN-β in the retention of lymphocytes and pDCs in the LNs in naive mice prompted us to examine whether recombinant IFN-β could restore pDC accumulation in the MLNs in IFN-β−/− mice during influenza infection. Interestingly, in the case of WT B and T cells, exogenous IFN-β further encourages the retention of these cells in the MLNs of influenza infected mice, but IFN-β treatment has less of an effect on WT pDC retention in the MLNs. In contrast, IFN-β treatment has a dramatic effect on retention of both pDC and B cells in the MLNs of influenza-infected IFN-β−/− mice. We hypothesize that IFNAR may be chronically down-regulated in WT pDCs as a consequence of continual IFNAR-mediated signaling for homeostatic maintenance of pDC numbers in the LNs. Indeed, in multiple sclerosis (MS) patients receiving IFN-β treatment, this chronic IFNAR down-regulation has been documented.48 Therefore, taken together, our data suggest a dominant function for IFNβ on the accumulation of pDC in the LNs both in naive mice, and in the context of infection.
IFN-β therapy is currently used in MS patients, and the therapeutic benefits of FTY720 treatment is being examined in this autoimmune disease.49 In rodent models of MS, pDC infiltration into the central nervous system (CNS) has been shown to be associated with disease remission because depletion of pDC increased the severity of the disease.50 It is possible that CNS-infiltrating pDCs may ameliorate MS by virtue of their secretion of IFN-α/β. Future work will test whether FTY720 treatment modulates pDC trafficking to the CNS and whether selective inhibition of S1P1 to exclusively limit lymphocyte and not pDC entry into the CNS is a preferable therapeutic approach.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We acknowledge Ms Dionne White for management of the Faculty of Medicine Flow Cytometry facility and Dr Darren Baker for recombinant IFN-β. We also acknowledge Dr Tania Watts, Dr Dana Philpott, and Dr James Carlyle for critical reading of the manuscript.
This research was supported by an operating grant from the Canadian Institutes of Health Research (CIHR/IRSC 15 094) to E.N.F., an operating grant to J.L.G. from the CIHRS/IRSC (67 157) for the initiation of the project, and an MS Society of Canada operating grant to J.L.G. for its completion. Y.G. holds an MS Society of Canada Post-Doctoral Fellowship, E.N.F. is a Canada Research Chair, and J.L.G. holds a CIHR/IRSC New Investigator award.
Authorship
Contribution: Y.G. and J.L.G. planned, designed, and performed the experiments and wrote the paper; B.M.-K. contributed to the design, planning, and execution of the influenza experiments and performed real-time quantitative PCR analysis; and E.N.F. contributed to the design, planning, and execution of the influenza experiments, contributed the IFN-β−/− mice, and performed real-time quantitative PCR analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jennifer L. Gommerman, Department of Immunology, University of Toronto, 1 King's College Cir, Toronto, ON M5R 3C5 Canada; e-mail: jen.gommerman@utoronto.ca.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal