Abstract
Complement is a major innate immune defense against pathogens, tightly regulated to prevent host tissue damage. Atypical hemolytic uremic syndrome (aHUS) is characterized by endothelial damage leading to renal failure and is highly associated with abnormal alternative pathway regulation. We characterized the functional consequences of 2 aHUS-associated mutations (D254G and K325N) in factor B, a key participant in the alternative C3 convertase. Mutant proteins formed high-affinity C3-binding site, leading to a hyperfunctional C3 convertase, resistant to decay by factor H. This led to enhanced complement deposition on the surface of alternative pathway activator cells. In contrast to native factor B, the 2 mutants bound to inactivated C3 and induced formation of functional C3-convertase on iC3b-coated surface. We demonstrated for the first time that factor B mutations lead to enhanced C3-fragment deposition on quiescent and adherent human glomerular cells (GEnCs) and human umbilical vein endothelial cells (HUVECs), together with the formation of sC5b-9 complexes. These results could explain the occurrence of the disease, since excessive complement deposition on endothelial cells is a central event in the pathogenesis of aHUS. Therefore, risk factors for aHUS are not only mutations leading to loss of regulation, but also mutations, resulting in hyperactive C3 convertase.
Introduction
The alternative pathway of the complement system is one of the major defense mechanisms against pathogens.1 It is initiated by the spontaneous hydrolysis of C3, the C3 “tickover,” which occurs through the cleavage of a thioester bond in C3 to form C3(H2O) to yield an initial fluid-phase C3 convertase. This convertase cleaves C3 molecules into C3a and C3b. C3b binds covalently to microorganisms or to any surface. C3b interacts with FB to form the C3 convertase (C3bBb) of the alternative pathway amplifying loop. FB carries the catalytic site of the C3 convertase C3bBb, a highly specific and tightly regulated serine protease. The C3 convertase is stabilized by the binding of properdin, which also serves as a platform for the assembly of C3bBb on the surface of microorganisms, apoptotic cells, or malignant cells.2 Negative regulators—factor H, membrane cofactor protein (MCP, CD46), and decay accelerating factor (DAF, CD55)—prevent C3 deposition and C3 convertase formation on healthy host cells. Factor H prevents C3b nonspecific binding to surfaces and, if this binding occurs, allows factor I–mediated degradation of C3b into the nonfunctional fragment iC3b. In addition factor H competes with FB for C3b binding and is able to dissociate the C3 convertase. Altering the proteins' regulatory capacity can lead to inappropriate C3 convertase formation and can damage host tissues.3
Atypical hemolytic uremic syndrome (aHUS) is a rare microangiopathic disease with a poor renal prognosis.4,5 Its association with defective alternative pathway regulatory mechanisms is now established.6-8 Thus, it is generally accepted that aHUS is a disease of excessive complement activation on renal glomerular and arteriolar cells.9 After still not well-identified triggering events, endothelial cells lose their integrity, undergo activation, and become a target for an uncontrolled complement attack. The release of C5a and of the cytolytically inactive form of the membrane attack complex induces tissue factor expression and transition to a procoagulant phenotype.10 This deleterious triggering of the complement and the coagulation cascades leads to development of the thrombotic microangiopathy of the aHUS. Genetic abnormalities involving either factor H, MCP, or factor I have been identified in approximately 50% of aHUS patients.4,5 Recent studies showed that mutations in C3 and FB, 2 pivotal components of the alternative pathway C3 convertase C3bBb, also predispose to the disease.7,8 The effect of these gain-of-function mutations in the protein-protein interactions has been investigated. However their contribution to the cell damage has not been elucidated.
We screened the FB gene of 210 patients with atypical HUS and identified 2 novel mutations: D254G and K325N. By forming a high-affinity C3- and C3b-binding site, these FB mutations resulted in a hyperactive C3 convertase that was resistant to decay by factor H. Moreover, FB mutants acquired a binding capacity for a novel ligand—iC3b—and thus the ability to form a C3-convertase from the inactivated form of C3b. The D254G and K325N FB led to C3b deposition on quiescent glomerular cells (GEnCs) and human umbilical vein endothelial cells (HUVECs). This allowed the terminal C5b-9 complexes formation despite the intact complement regulation. Currently, aHUS is generally considered to be related to a lack of complement control. We here demonstrated for the first time that a hyperfunctional C3 convertase is capable of inducing endothelial damage, leading to aHUS.
Methods
Patients
The French aHUS cohort consists of 210 patients (age at the onset: 1 month to 85 years; median, 20 years; 133 females and 77 males). A diagnosis of aHUS was defined by the coexistence of hemolytic mechanical anemia (hemoglobin < 100 g/L [10 g/dL], lactate dehydrogenase level > 600 IU/L, and presence of schizocytes on blood smear), thrombocytopenia (platelet count < 150 × 109/L), and acute renal failure. The ADAMTS13 activity was normal. We selected for this study 3 patients with low C3 levels and genetic abnormalities in factor B. All screened individuals signed with informed consent in accordance with the Declaration of Helsinki and the study was approved by the local ethics committee at Inserm.
Family I (patients 1 and 2).
In this family, both father and daughter were affected. The father (patient 1) had 2 episodes of aHUS leading to end-stage renal disease 6 months after onset at 53 years of age. The patient received 2 cadaver kidney transplants, which were lost within a few weeks after transplantation with diagnosis of vascular rejection or recurrence of HUS. His daughter (patient 2) had one episode of aHUS requiring dialysis and plasma therapy at 33 years of age. Under continuous plasma therapy, the renal function recovered 18 months after the onset.
Family II (patient 3).
This patient developed aHUS at 1 month of age without recovery of the renal function. At the age of 19 months, she received a cadaver kidney transplant. HUS recurrence occurred at day 15 after transplantation. Plasma exchanges with fresh frozen plasma appeared inefficient. Nevertheless, the renal function was maintained under intravenous immunoglobulin until the child returned to dialysis at age 6 years 4 months. There was no family history of renal disease.
Enzyme-linked immunosorbent assay for FB binding to C3 or iC3b
Microtiter wells were coated with purified human C3(H2O) (Calbiochem) or purified iC3b,11 at 0.5 μg/well in PBS, pH 7.4, for 1 hour at 37°C; any residual binding sites were blocked with 0.4% wt/vol Tween 20 in PBS for 1 hour at 37°C and washed with PBS, pH 7.4, containing 0.05% Tween 20. Serial dilutions of the recombinant proteins in a low ionic strength (75 mM NaCl) 10 mM HEPES buffer, containing 10 mM Mg2+, pH 7.4, were incubated for 45 minutes at 37°C. After washing with the same HEPES buffer, wells were incubated with a biotinylated sheep polyclonal anti–human FB antibody (1:1000; Abcam) for 1 hour at 37°C. The amount of bound recombinant protein was detected by incubation with streptavidin-HRP (1:1000) conjugate. Color was developed using OPD substrate and OD490 nm was measured. The background binding was subtracted. For iC3b binding, microtiter wells were prepared as with C3b, but 25 mM NaCl was used in the FB incubation step.
Surface plasmon resonance
The interaction of wild-type and mutant FB with C3 was analyzed using surface plasmon resonance technology with Biacore 2000 equipment. C3(H2O) and iC3b were coupled to the CM5 biosensor chip, using standard amide-coupling technology, according to the manufacturer. For coupling of C3b to the chip, C3 convertase was performed on the surface, applying Jokiranta et al's approach.12 The recombinant wild-type and mutant FB were used as an analyte at concentrations 25, 12.5, 6.25, 3.125, 1.56, 0.78, and 0.39 nM and were flowed at 10 μL/min in Mg2+- or Ni2+-containing HEPES buffer (10 mM HEPES, 50 mM NaCl, 1 mM MgCl2 or NiCl2, pH 7.4) over the C3-, C3b-, or iC3b-containing surfaces and an empty, activated/deactivated flow cell as a control. Data were analyzed using BIAevaluation software and the data from the blank flow cell were subtracted. Kinetic parameters were calculated by fitting the obtained sensorograms into 1:1 interaction with drifting baseline algorithm, which gave the lowest χ2.
Hemolysis assays
Classical pathway C3 convertase was assembled on sheep erythrocytes. It was used to coat the cell surface with C3b, as previously described.13 Samples made from 100 μL 108 C3b-coated sheep erythrocytes/mL, 40 ng purified human factor D (Calbiochem), and FB proteins (serial dilutions starting from 15 ng, recombinant WT or mutant forms/tube) in DGVB2+ were incubated at 30°C for 30 minutes. The negative control did not contain FB. Alternative pathway C3 convertase sites were developed with 300 μL of a 1:40 dilution of rat serum in GVB-EDTA buffer at 37°C for 45 minutes. Hemolysis was detected at OD 414 nm. Hemolytic activity levels for the FB mutants were expressed as Z values, which is equivalent to the average number of lytic sites per cell.13 Control experiments were performed in DGVB buffer, containing Ni2+.
To assess the ability of recombinant FB proteins to form a C3-convertase on iC3b bound to the cell surface, erythrocyte-bound C3b was inactivated to iC3b by incubation with C6-deficient serum or purified factor H (100 μg/mL) and factor I (20 μg/mL) for 1 hour at 37°C. These cells were incubated with FB proteins (serial dilutions starting from 120 ng/tube in DGVB buffer, containing Ca2+ and Ni2+) at 30°C for 30 minutes. The formation of lytic sites on the membrane was assessed as in the previous paragraph.
Measurement of convertase decay by factor H
AP convertases were assembled on sheep erythrocytes using FB (1 < Z < 2) as for the hemolytic assay. After a 30-minute incubation of C3b-coated cell samples with FB and factor D, 100 μL GVB-EDTA buffer-diluted pooled human plasma (serial dilutions starting from 1:50) were added to prevent the assembly of new convertases, and incubation was continued at 30°C to allow decay by factor H, present in the plasma. After 30 minutes, 300 μL of 1:40 rat serum diluted in GVB-EDTA buffer was added, and samples were incubated at 37°C for 45 minutes. Hemolysis obtained during this period was measured and Z values were calculated. Results for the factor H–induced convertase decay are presented as percentage of the C3-convertase (Z equivalent for all recombinant proteins), incubated in GVB-EDTA only, where only spontaneous decay occurred. Control experiments were performed to measure the spontaneous decay of the C3-convertases, formed in the presence of Ni2+ instead of Mg2+.
Endothelial cells assay
Conditionally immortalized human GEnCs have been descried in detail by Satchell et al.14 Briefly, the GEnCs were grown in 24-well plates, allowed to proliferate at 33°C (permissive temperature) until reaching confluence, and then were transferred at 37°C (nonpermissive temperature) for 7 days. The cells were cultured in EGM-2/bullet kit medium (Lonza), containing VEGF.
HUVECs were isolated from human umbilical cord veins as described15 and cultured in M199 medium (Gibco, Paisley, GB) with 20% fetal calf serum. Third-passage quiescent cells were grown to confluence into 24-well plates. The overnight detached HUVECs were collected and shown to be necrotic cells (annexin V, propidium iodide, and trypan blue positive). Adherent quiescent cells (annexin V, propidium iodide, and trypan blue negative), as well as the staurosporine-induced (supplemental methods, available on the Blood website; see the Supplemental Materials link at the top of the online article) apoptotic and necrotic cells, were incubated with 125 μL AB group normal human serum or FB-depleted serum (Calbiochem or CompTech), supplemented with recombinant WT FB or supernatants, containing 4 μg mutant FB protein, to a final volume of 500 μL M199 medium. The FB-depleted serum still contained 32 μg/mL residual FB (Calbiochem lot D00045605) or 38 μg/mL (CompTech lot 15) and had normal C3 levels (1140 μg/mL for Calbiochem and 1250 μg/mL for CompTech). Therefore, approximately 4 μg native and 4 μg recombinant FB were present in the final volume. Supernatant from HEK293T cells transfected with the vector alone (SN0) was used as a negative control. In some experiments, EGTA-Mg was added to block the classical and lectin pathways. After 30-minute incubation at 37°C, the supernatant was recovered for determination of soluble C5b-9 (sC5b-9), (Quidel, enzyme-linked immunosorbent assay [ELISA] kit). Cold PBS was added to the cells to stop the complement reaction. Adherent cells were detached with PBS–5 mM EDTA on ice for 30 minutes. The quiescent, apoptotic, and necrotic cells were labeled with anti-C3c, a monoclonal antibody (Quidel) or a control mouse IgG1, followed by phycoerythrin (PE)–labeled secondary antibody (Beckman Coulter). Quiescent HUVECs and GEnCs were probed for expression of complement regulators MCP (CD46), DAF (CD55), CD59, and CR1 (CD35) using PE-labeled antibodies (Becton Dickinson). Cells were analyzed by flow cytometry on a Becton Dickinson FACSCalibur, using CellQuest software.
Results
Characterization of the molecular abnormalities in complement genes
Patients 1 and 2 presented with normal FB concentration (Table 1). Their FB had a heterozygous mutation in exon 6 (c.837A>C; pAsp279Gly), encoding a D254 residue within the VWF-A domain (Figure 1B-C), which is exactly the in silico predicted mutation D254G reported by Hourcade et al.13 Bidirectional DNA sequencing of factor H, MCP, and factor I did not reveal any mutations in either of these genes. Patient 2 carried one copy of CFHR1/3.
Levels of complement proteins and found mutations for the 2 discussed families
| . | Family 1 . | Family 2 . | Normal ranges . | ||||
|---|---|---|---|---|---|---|---|
| P1 . | P2 . | I.1 . | I.2 . | P3 . | |||
| I.1 . | II.1 . | II.2 . | II.2 . | ||||
| C3, mg/L | 256 | 985 | 287 | 833 | 725 | 172 | 660-1250 |
| C4, mg/L | 372 | 205 | 305 | 174 | 111 | 207 | 93-380 |
| FB, mg/L | 110 | 132 | 109 | 128 | 99 | 55 | 90-320 |
| H, mg/L | 555 | 545 | 474 | 566 | 520 | 499 | 338-682 |
| I, mg/L | 68 | 63 | 63 | 67 | 61 | 69 | 42-78 |
| CD46 | nd | 850 | 904 | 909 | 917 | 818 | 660-1400 |
| Factor B *(protein) | p.Asp279Gly | p.Asp279 | p.Asp279Gly | p.Lys350 | p.Lys350 | p.Lys350Asn | |
| Factor B† (structure) | Asp254Gly (D254G) | Asp254 | Asp254Gly (D254G) | Lys325 | Lys325 | Lys325Asn (K325G) | |
| . | Family 1 . | Family 2 . | Normal ranges . | ||||
|---|---|---|---|---|---|---|---|
| P1 . | P2 . | I.1 . | I.2 . | P3 . | |||
| I.1 . | II.1 . | II.2 . | II.2 . | ||||
| C3, mg/L | 256 | 985 | 287 | 833 | 725 | 172 | 660-1250 |
| C4, mg/L | 372 | 205 | 305 | 174 | 111 | 207 | 93-380 |
| FB, mg/L | 110 | 132 | 109 | 128 | 99 | 55 | 90-320 |
| H, mg/L | 555 | 545 | 474 | 566 | 520 | 499 | 338-682 |
| I, mg/L | 68 | 63 | 63 | 67 | 61 | 69 | 42-78 |
| CD46 | nd | 850 | 904 | 909 | 917 | 818 | 660-1400 |
| Factor B *(protein) | p.Asp279Gly | p.Asp279 | p.Asp279Gly | p.Lys350 | p.Lys350 | p.Lys350Asn | |
| Factor B† (structure) | Asp254Gly (D254G) | Asp254 | Asp254Gly (D254G) | Lys325 | Lys325 | Lys325Asn (K325G) | |
Bold indicates values below the normal range.
Numbering with the peptide signal.
Numbering without the peptide signal.
Genetic analysis of the described patients. (A) Pedigree of the 2 families. (B) Histograms from the patients sequencing with indication of found mutations. (C) Localization of mutations within the factor B gene (rectangles represent exons; line represents introns) and within the protein sequence (the 3 domain types—CCP [complement control protein], VWFA [von Willebrand type A], and serine protease are indicated). Numbering is made with the first methionine referred as 1 (as described in Hourcade et al13 ).
Genetic analysis of the described patients. (A) Pedigree of the 2 families. (B) Histograms from the patients sequencing with indication of found mutations. (C) Localization of mutations within the factor B gene (rectangles represent exons; line represents introns) and within the protein sequence (the 3 domain types—CCP [complement control protein], VWFA [von Willebrand type A], and serine protease are indicated). Numbering is made with the first methionine referred as 1 (as described in Hourcade et al13 ).
Patient 3 had low FB plasma levels (Table 1). We identified a heterozygous substitution c.1050G>C in exon 8 (p.Lys350Asn) of the FB gene, which resulted in missense mutation K325N, located in the VWF-A domain (Figure 1B-C). Her unaffected father and mother did not carry this genetic abnormality. DNA sequencing of factor H, MCP, and C3 did not reveal a mutation in either of these genes. Patient 3 and her unaffected mother presented a heterozygous mutation located in factor I gene (IVS12 + 5) with normal FI levels and unknown functional consequence. Patient 3 is homozygous for the aHUS risk haplotype in FH (FH GTGT) as well as in MCP (MCP GGGAC) and carries 2 copies of CFHR1/3 gene.
The nucleotide changes in FB gene (p.Lys350Asn and pAsp279Gly) were not identified in 100 healthy white control individuals.
aHUS mutations cluster in regions of the VWF domain, which undergo structural changes upon FB activation
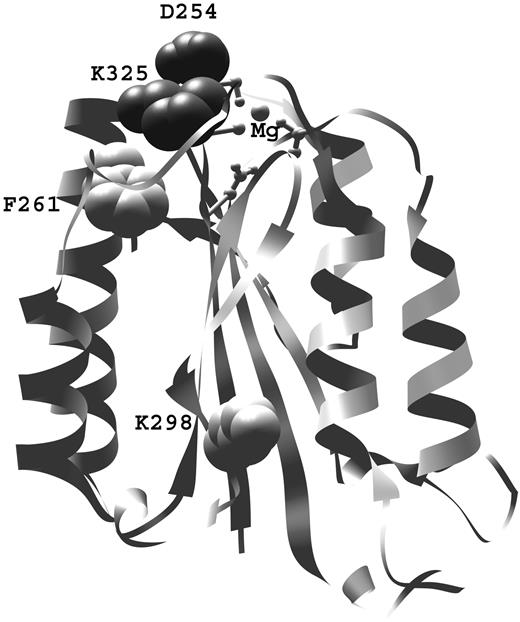
Mapping of D254- and K325-mutated residues revealed that both are located in the Bb fragment, at the apex of the VWF-A domain, in close proximity to the Mg2+ adhesion site (MIDAS; Figure 2). These 2 residues form a salt bridge after transition from the nonactive to the active form. The F261L mutation is also located in close proximity to the MIDAS site.8 Both novel residues could be accommodated without clashes in the active form (1RRK and 1Q05).
Structural analysis. Positions of aHUS-associated mutations found in this study (D254 and K325), in relation to the MIDAS site (in ball and stick representation) and previously found mutations of F261 and K2988 on the VWF-A domain of factor B. The aHUS-associated mutations described by Goicoechea de Jorge et al have been originally named according to the protein numbering with the leader peptide.8 For clarity in this work, the number of both previously described residues is given according to the protein structure: F286 is F261 here and K323 is K298 here.
Structural analysis. Positions of aHUS-associated mutations found in this study (D254 and K325), in relation to the MIDAS site (in ball and stick representation) and previously found mutations of F261 and K2988 on the VWF-A domain of factor B. The aHUS-associated mutations described by Goicoechea de Jorge et al have been originally named according to the protein numbering with the leader peptide.8 For clarity in this work, the number of both previously described residues is given according to the protein structure: F286 is F261 here and K323 is K298 here.
Gain of function of D254G and K325N binding to the natural FB ligands C3 and C3b
The interactions between recombinant FB proteins and C3(H2O) or C3b were specific and dose dependent (Figure 3, supplemental Figures 2A,C-D and 3). An ELISA was performed to assess whether recombinant proteins were able to bind to C3 (Figure 3Ai) in the presence of Mg2+ or Ni2+ (supplemental Figure 4). All mutant proteins bound C3 up to 2 times more efficiently compared with the wild type. A similar effect was observed by inhibitory ELISAs (supplemental Figure 3). To further investigate these differences, the binding of WT, D254G, and K325N FB to the natural ligands C3(H2O) and C3b was analyzed by surface plasmon resonance (SPR)–based assay (Figure 3Aii,Bii) in the presence of Mg2+ or Ni2+. Increased binding of the 2 mutants was observed in all cases. The kinetic parameters of the binding to C3 were determined in the presence of Mg2+ (Table 2). D254G and K325N binding compared with the WT was characterized by approximately to 26 and 16 times faster association, respectively. The dissociation rate was approximately 3.6 and 3 times slower for D254G and K325N, respectively. These accounted for the mutants' higher apparent affinity, compared with the WT. FB mutants were examined for their capacity to form a membrane-bound C3 convertase with a hemolytic assay. In this test, the formation of lytic sites is proportional to the number of C3 convertases formed on the erythrocyte surface. This assay (Figure 3Bi) demonstrated a 6- plus or minus 2-fold and 10- plus or minus 2.4-fold stronger lytic efficiency for K325N and D254G, respectively, compared with the WT in the same concentration (0.125 ng/tube). The same trend was observed in the presence of Ni2+, but approximately 10 times lower concentrations were sufficient to obtain Z = 2. The 2 mutants formed a convertase, resistant to spontaneous decay (data not shown). Factor H was capable of completely dissociating the convertase formed by the WT (Figure 3C). In contrast, the same concentrations of factor H resulted in only slight dissociation of the C3 convertase formed by K325N and D254G.
Interaction of the WT and mutant factor B proteins with factor B natural ligands—C3 and C3b. (A) ELISA (Ai) or SPR (Aii) assay with C3 coated to the plate or to the biosensor chip, respectively. In panel Aii, the kinetic fit for each sensorogram was given in gray. (B) Hemolytic assay (Bi) or SPR analysis (Bii) with C3b-coated erythrocytes or C3b-coated biosensor chip, respectively. Native C3b was generated on the 2 surfaces by C3 convertase. Erythrocytes lysis was induced only in the presence of C3b, factor D, and recombinant WT or mutant BF together. Removing one component from the system abrogated the lysis (data not shown). (C) Hemolytic test for the dissociation of the C3 convertase (C3bBb), formed by the WT or mutant factor B on the surface of the erythrocytes. The results are presented as percentage of the lysis, obtained in absence of plasma (Z∼2), that is, only spontaneous dissociation of the formed C3 convertase. Supernatant from cells transfected with the vector only was used as a control (SN0) for all experiments. For the SPR and the hemolytic tests, 1 representative experiment of 3 to 5 performed is given. For the ELISA average ± SD, n = 3.
Interaction of the WT and mutant factor B proteins with factor B natural ligands—C3 and C3b. (A) ELISA (Ai) or SPR (Aii) assay with C3 coated to the plate or to the biosensor chip, respectively. In panel Aii, the kinetic fit for each sensorogram was given in gray. (B) Hemolytic assay (Bi) or SPR analysis (Bii) with C3b-coated erythrocytes or C3b-coated biosensor chip, respectively. Native C3b was generated on the 2 surfaces by C3 convertase. Erythrocytes lysis was induced only in the presence of C3b, factor D, and recombinant WT or mutant BF together. Removing one component from the system abrogated the lysis (data not shown). (C) Hemolytic test for the dissociation of the C3 convertase (C3bBb), formed by the WT or mutant factor B on the surface of the erythrocytes. The results are presented as percentage of the lysis, obtained in absence of plasma (Z∼2), that is, only spontaneous dissociation of the formed C3 convertase. Supernatant from cells transfected with the vector only was used as a control (SN0) for all experiments. For the SPR and the hemolytic tests, 1 representative experiment of 3 to 5 performed is given. For the ELISA average ± SD, n = 3.
Kinetic constants, obtained by SPR
| . | Factor B binding to C3(H2O) . | ||
|---|---|---|---|
| WT . | K325N . | D254G . | |
| Kassociation | 0.18 × 105 | 2.91 × 105 | 4.69 × 105 |
| Kdissociation | 18.7 × 10−4 | 5.20 × 10−4 | 6.40 × 10−4 |
| Ka | 9.56 × 106 | 5.60 × 108 | 7.33 × 108 |
| KD | 1.05 × 107 | 1.79 × 109 | 1.36 × 109 |
| . | Factor B binding to C3(H2O) . | ||
|---|---|---|---|
| WT . | K325N . | D254G . | |
| Kassociation | 0.18 × 105 | 2.91 × 105 | 4.69 × 105 |
| Kdissociation | 18.7 × 10−4 | 5.20 × 10−4 | 6.40 × 10−4 |
| Ka | 9.56 × 106 | 5.60 × 108 | 7.33 × 108 |
| KD | 1.05 × 107 | 1.79 × 109 | 1.36 × 109 |
Appearance of a binding site for a novel ligand: iC3b on D254G and K325N FB
Significant and dose-dependent binding to iC3b was observed by ELISA for D254G and K325N, whereas the binding of the WT FB was negligible (Figure 4A, supplemental Figure 2A-B). When analyzed by SPR (Figure 4B), K325N and D254G binding to iC3b was weak but detectable, in contrast to the WT FB, which did not bind to iC3b. Moreover, the 2 aHUS-associated mutants were able to form a C3-convertase on iC3b-coated erythrocytes. This new enzymatic complex iC3bBbmutant was functionally active, since it led to efficient hemolysis (Figure 4C). No lysis was observed for the WT FB, even at more than 10 times higher concentration than the amount of mutants giving 50% lysis.
Interaction of the WT and mutant factor B proteins with the novel ligand iC3b. (A) ELISA assay with iC3b-coated plates (average ± SD, n = 3). (B) SPR measurement of factor B proteins binding to iC3b coupled to CM5 chip. (C) Hemolytic assay for the C3-convertase forming capacity of WT and mutant factor B proteins on iC3b-coated erythrocytes. One representative experiment of 3 performed is given.
Interaction of the WT and mutant factor B proteins with the novel ligand iC3b. (A) ELISA assay with iC3b-coated plates (average ± SD, n = 3). (B) SPR measurement of factor B proteins binding to iC3b coupled to CM5 chip. (C) Hemolytic assay for the C3-convertase forming capacity of WT and mutant factor B proteins on iC3b-coated erythrocytes. One representative experiment of 3 performed is given.
The D254G and K325N mutations induced complement activation on endothelial cell surface
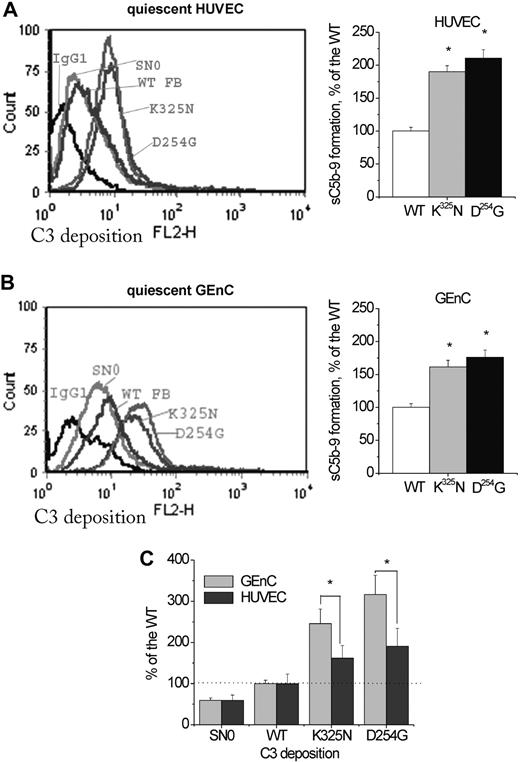
To study complement activation on endothelial cells, we used HUVECs, an accepted model, previously applied to the analysis of functional consequences of factor H mutations,16-18 as well as GEnCs, which are a more relevant model for studying the glomerular endothelial damage.19 We developed a novel experimental setting to analyze different endothelial cell populations (supplemental Figure 5): adherent, quiescent cells (Figure 5) and detached necrotic (Figure 6) and apoptotic (supplemental Figure 6) cells. They were treated with FB-depleted serum, reconstituted with recombinant FB. In our experimental setting, we used half native and half mutant FB, similar to the situation of the patients, where both proteins are secreted. Quiescent HUVECs and GEnCs expressed the complement regulators MCP, DAF, and CD59, but no CR1. Incubation of these quiescent cells with 30% serum from a healthy donor resulted in minimal deposition of C3, detectable at similar MFI (8 ± 3, n = 20 for the HUVECs and 5 ± 3, n = 10 for the GEnCs) by the anti-C3c mAb. C3 deposition on necrotic cells was much stronger, reaching 349 plus or minus 33 MFI, respectively (n = 6). C3 deposition on quiescent GEnCs and HUVECs incubated with the FB-depleted serum, reconstituted with K325N or D254G, was significantly higher, compared with the WT (Figure 5Ai,Bi). The levels of deposition on GEnCs appeared higher compared with the HUVECs (Figure 5C). Mutants led to increased C3 deposition on a complement activator surface, such as the membrane of necrotic (Figure 6A) or staurosporine-induced apoptotic (supplemental Figure 6) HUVECs.
Complement activation on quiescent endothelial cells. Adherent, quiescent, and confluent HUVECs (Ai) or GEnCs (Bi) were incubated with 125 μL FB-depleted serum, supplemented with recombinant WT FB or supernatants, containing 4 μg mutant FB protein, to a final volume of 500 μL. C3-fragment deposition was assessed by flow cytometry with anti-C3c mouse monoclonal antibodies, followed by goat anti–mouse IgG-PE. Irrelevant mouse IgG1 was used as a control. The figure shows a representative histogram from 10 independent experiments with HUVECs and 3 with GEnCs. (B) Levels of sC5b-9 in the serum after incubation with HUVECs (i) or GEnCs (ii). Supernatants from 3 independent experiments, were tested by the Quidel ELISA kit sC5b-9 Plus. The level of sC5b-9 for the wild type in each experiment was taken as 100%. Results are presented as the mean of the independent experiments obtained for each mutation. The 2 mutations gave significantly higher C5b-9 formation compared with the WT (t test, P < .05). (C) Comparison between the levels of C3 deposition on quiescent, adherent GEnCs and HUVECs. The anti-C3c mean fluorescence intensity obtained with each mutant (each value being normalized with the isotype control) was presented as percentage of the WT. The fluorescence intensities for the WT and SN0 were comparable for HUVECs and GEnCs. Differences between mutants and WT, for each particular cell type, as well as differences between the 2 different cell types, for a given mutant, were statistically significant (n = 3, P < .05, t test). Potential artifacts due to endotoxin contamination were excluded by addition of endotoxin inhibitor polymyxin B or intentional contamination with LPS. These treatments did not change the levels of C3 deposition (data not shown).
Complement activation on quiescent endothelial cells. Adherent, quiescent, and confluent HUVECs (Ai) or GEnCs (Bi) were incubated with 125 μL FB-depleted serum, supplemented with recombinant WT FB or supernatants, containing 4 μg mutant FB protein, to a final volume of 500 μL. C3-fragment deposition was assessed by flow cytometry with anti-C3c mouse monoclonal antibodies, followed by goat anti–mouse IgG-PE. Irrelevant mouse IgG1 was used as a control. The figure shows a representative histogram from 10 independent experiments with HUVECs and 3 with GEnCs. (B) Levels of sC5b-9 in the serum after incubation with HUVECs (i) or GEnCs (ii). Supernatants from 3 independent experiments, were tested by the Quidel ELISA kit sC5b-9 Plus. The level of sC5b-9 for the wild type in each experiment was taken as 100%. Results are presented as the mean of the independent experiments obtained for each mutation. The 2 mutations gave significantly higher C5b-9 formation compared with the WT (t test, P < .05). (C) Comparison between the levels of C3 deposition on quiescent, adherent GEnCs and HUVECs. The anti-C3c mean fluorescence intensity obtained with each mutant (each value being normalized with the isotype control) was presented as percentage of the WT. The fluorescence intensities for the WT and SN0 were comparable for HUVECs and GEnCs. Differences between mutants and WT, for each particular cell type, as well as differences between the 2 different cell types, for a given mutant, were statistically significant (n = 3, P < .05, t test). Potential artifacts due to endotoxin contamination were excluded by addition of endotoxin inhibitor polymyxin B or intentional contamination with LPS. These treatments did not change the levels of C3 deposition (data not shown).
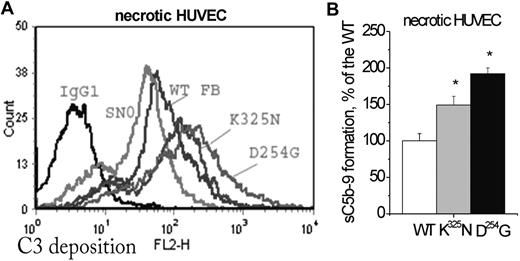
Complement activation on necrotic endothelial cells. (A) Necrotic HUVECs (spontaneously detached during a 24-hour culture) were incubated with one-fourth diluted factor B–depleted human serum, reconstituted with recombinant WT or mutant factor B, and analyzed as in Figure 5A. The figure presents a typical flow cytometry histogram. The anti-C3c mean fluorescence intensity for the 2 mutations (n = 4 independent experiments) was significantly higher than the WT (t test, P < .05). (B) Levels of sC5b-9 in the serum after incubation with necrotic HUVECs. Results represent the mean ± SD of the results obtained from samples from 2 independent experiments tested in duplicate.
Complement activation on necrotic endothelial cells. (A) Necrotic HUVECs (spontaneously detached during a 24-hour culture) were incubated with one-fourth diluted factor B–depleted human serum, reconstituted with recombinant WT or mutant factor B, and analyzed as in Figure 5A. The figure presents a typical flow cytometry histogram. The anti-C3c mean fluorescence intensity for the 2 mutations (n = 4 independent experiments) was significantly higher than the WT (t test, P < .05). (B) Levels of sC5b-9 in the serum after incubation with necrotic HUVECs. Results represent the mean ± SD of the results obtained from samples from 2 independent experiments tested in duplicate.
The levels of sC5b-9 formation in the supernatant from the same cells were higher with the 2 mutants, compared with the WT (Figures 5Aii,Bi and 6B). On quiescent HUVECs incubated with FB-depleted serum, supplemented with empty supernatant (SN0), little anti-C3 mAb binding was observed, nonsignificantly different from the isotype control. Apoptotic and necrotic cells deposited C3 in FB-depleted serum, supplemented with SN0. This deposition was diminished in the presence of EGTA-Mg, but still present presumably due to the residual FB.
Discussion
Here we identified 2 new mutations in FB (D254G and K325N) in patients with aHUS. These, together with the FB mutations described in 2 Spanish pedigrees,8 establish mutations in FB as a susceptibility factor for aHUS. In this study, our aim was to delineate the mechanism by which D254G and K325N predispose to aHUS.
FB is composed of 3 complement control protein domains (CCP1-3), a von Willebrand type A domain (VWF-A domain), and a serine protease domain.20,21 The 2 mutated residues were located on the VWF-A domain apex, near the Mg2+ adhesion site (MIDAS). They, together with previously described mutation F261L,8 lie in regions undergoing structural changes during FB activation. Because this is the case of 3 of 4 known aHUS-related FB mutations, we could predict that these regions could be potential hot spots for disease-associated genetic abnormalities.
Direct evidence for the involvement of the VWF-A domain and the MIDAS site in C3-binding and formation of the C3-convertase was first reported by Hourcade et al.13 The C3-convertase 3-dimensional structure was recently determined by single-particle electron microscopy.22 To stabilize the convertase, the authors used a residue substitution in the MIDAS region D254G, described previously by Hourcade at al,13 to result in stronger C3b binding and increased convertase stability.13 Strikingly, this is exactly one of the mutations found in this study in a familial form of aHUS. Recently, Goicoechea de Jorge et al reported the 2 first cases of aHUS associated with FB mutations.8 One of those mutations (F261L) was in the vicinity of the MIDAS and led to enhanced C3 convertase, when studied by SPR. The second mutation, K298E, was near the DAF interaction site23 and resulted in increased resistance to inactivation by complement regulators factor H and DAF.8 In the present study, we characterized in detail the binding mechanism of the FB recombinant proteins D254G and K325N by SPR and ELISA and analyzed the consequences of these mutations by different functional tests. The kinetic of the interaction of the 2 mutants with C3 and C3b was marked by much faster association rate (26- and 16-fold increase for D254G and K325N, respectively, compared with WT). Faster association phase has also been observed for the F261L mutation, which is located beneath the C3-contact interface. All these mutants seem thus to require fewer structural changes than WT FB for the initial encounter complex formation with C3 and for the rearrangements in the VWF-A domain, needed for the MIDAS formation. Indeed, D254 and K325 (as well as F261L) belong to regions that alter their conformation during the transition from disordered (2OK5) to ordered (1RRK, 1Q05) MIDAS. The FB mutations could affect the binding of the Mg2+ ion, which is critical for C3/FB interactions. This, together with the higher affinity binding site resulting from the mutations, could explain the gain of function of the D254G and K325N mutants toward C3 and C3b. The mutant C3-convertase was more stable also on the cell surface, where mutants were approximately 10 times more efficient in lytic site formation, compared with the same concentration of WT FB. Moreover, mutant C3-convertases were resistant to spontaneous and factor H–induced decay. This lack of control is another aspect of the gain of function, contributing to the tissue-damaging potential.
Our results allow us to suggest that the D254G and K325N mutations lead to the appearance of a new function apart from the high-affinity binding site. Mutants FB were able to bind to the inactive form of C3b, iC3b (measurable by ELISA and SPR), and to form a C3 convertase from this iC3b on an activator surface. Although we cannot exclude the presence of residual C3b on the activator surface, our results highly suggest that the erythrocytes lysis observed with the mutants was due to iC3b binding. This phenomenon, if it occurs in vivo, could lead to complement-induced damage of the cells lacking CR1, where factor I cleaves C3b into iC3b, but cannot further degrade it into C3c and C3d,g. Such examples are the endothelial cells, which express MCP, DAF, and CD59, but not CR1.
To better understand the relationship between this gain of function of FB mutations and the pathogenesis of the aHUS, we tested the ability of mutants and WT FB to deposit complement on the endothelial cell surface. We used HUVECs as a classical model to study aHUS mutations as well as GEnC line,14 which is a more reliable model for the fenestrated glomerular endothelium. Host cells are protected from autologous complement activation by surface expressed regulators, mainly MCP and DAF and by the membrane composition.3 We hypothesized that D254G and K325N would lead to C3 deposition on quiescent endothelial cells, despite the normal surface protection. As expected, minimal deposition of C3b/iC3b was detected after incubation of quiescent endothelial cells with normal human serum. This C3b/iC3b deposition was strongly increased on the apoptotic or necrotic cells. These results are in accordance with previous findings showing complement activation on apoptotic Jurkat and THP1 cells.24 The same trends of C3 deposition were observed on endothelial cells incubated with FB-depleted serum reconstituted with recombinant WT FB. The deposited C3b was inactivated by factor I into iC3b by factor H and MCP, but the cleavage did not proceed further due to the lack of CR1.
Significantly increased deposition of C3b/iC3b on quiescent endothelial cells, and even more on dead (apoptotic or necrotic) cells, was observed when the FB-depleted serum was reconstituted with D254G and K325N. The level of C3 deposition in the presence of the 2 mutants was significantly higher on GEnCs than on HUVECs. This might be related to the endothelial membrane composition and/or to the presence of the fenestrae. If occurring in vivo, the increased susceptibility of glomerular endothelial cells to complement attack from the hyperfunctional C3-convertase could, at least in part, explain the particular localization of aHUS thrombotic microangiopathy in glomerular microvessels. Moreover, in vivo the basal membrane is exposed in the glomerular fenestrae and it could be an additional target for the super-C3 convertase attack. The gain of function is readily observable at heterozygous-like conditions and at low levels of mutant FB present, similar to the case in some patients, where the mutant protein is rapidly consumed. The mutants' ability to form a hyperactive C3 convertase and to consume C3 both on the surface and in the fluid phase (due to enhanced C3(H2O) binding) explains the low C3 levels observed in all patients with FB gain-of-function mutations.
Our data allow us to propose at least 3 mechanisms that contribute to this enhanced C3 deposition endothelial cells. Mutants FB form a C3 convertase with increased half-life, due to (1) the higher affinity between FB mutants and C3; (2) the reduced dissociation rate by factor H; and (3) the mutants' ability to bind to iC3b and potentially to form an active iC3bBb convertase, which amplifies the C3b deposition. It is thus conceivable that in individuals carrying such FB mutations (even in heterozygous form), constant excessive complement deposition might occur on the endothelium. In the presence of a weak trigger, complement hyperactivation might overwhelm otherwise properly functional complement regulatory systems. A proposed mechanism is summarized at Figure 7.
Schematic representation of the proposed mechanism of endothelial damage by the hyperfunctional C3-convertase, associated with the FB mutations D254G and K325N. The mutant FB binds to C3(H2O) and C3b with increased affinity. These C3-convertases are resistant to FH-assisted decay. The increased half-life and the loss of regulation of the fluid phase and surface C3-convertases led to increased C3 deposition on quiescent and damaged endothelium. Potentially mutant FB could bind to iC3b and form a C3-convertase on the inactivated C3 fragment. The formation of sC5b-9 and liberation of C5a could be associated with a procoagulant phenotype and aHUS.
Schematic representation of the proposed mechanism of endothelial damage by the hyperfunctional C3-convertase, associated with the FB mutations D254G and K325N. The mutant FB binds to C3(H2O) and C3b with increased affinity. These C3-convertases are resistant to FH-assisted decay. The increased half-life and the loss of regulation of the fluid phase and surface C3-convertases led to increased C3 deposition on quiescent and damaged endothelium. Potentially mutant FB could bind to iC3b and form a C3-convertase on the inactivated C3 fragment. The formation of sC5b-9 and liberation of C5a could be associated with a procoagulant phenotype and aHUS.
Complement activation in the presence of D254G and K325N resulted in increased formation of terminal complement complex sC5b-9. Formation of C5b-9 and C5a is known to induce in vivo an endothelial procoagulant phenotype.10 This could be responsible for one of the major manifestations of the aHUS, that is, the formation of microthrombi in the renal microvasculature.
Altogether, 9 cases of FB mutation–associated HUS have now been reported.8 As for aHUS–factor H patients, long-term outcome is overall severe. The age of onset is variable, between 1 month and 48 years. Five of the 8 well-described patients had an evolution to end-stage renal disease within the year after the first episode. Of note, 2 patients (patient 2 in our cohort and patient H85 in the Spanish cohort) recovered renal function under plasma infusions. We did not identify healthy carrier in the French families, but incomplete penetrance of the disease has been illustrated in one of the Spanish families, in which 4 unaffected carriers have been found. According to our results for the French family 1, we presume that unaffected carriers may develop the disease at elder ages, as suggested by Caprioli et al.25 Interestingly, among the 4 pedigrees identified with an FB mutation, 2 had sporadic aHUS form with de novo mutations (BRA in the Spanish cohort and P3 in the French cohort) and 2 are familial HUS form (PER in Spanish cohort and family 1 in the French cohort). A high risk of aHUS recurrence after renal transplantation is common, both in adults and in children. Recurrence of HUS usually occurs within a few months after transplantation of patients with genetic abnormalities in factor H or factor I—plasma proteins produced by the liver. In our series, 2 patients received kidney transplant and recurrence of HUS occurred within the 2 following months.
Gain-of-function mutations are rare, compared with loss of function. Currently, more than 100 loss-of-function mutations, leading to abnormal C3 convertase regulation, have been identified in patients with aHUS.3,26 It is generally admitted that uncontrolled C3 convertase formation results in vascular endothelium damage in patients with defective factor H, factor I, or MCP.6 Our report emphasizes the critical role of C3 convertase itself in the pathogenesis of the disease. We provide evidence, for the first time, for a link between the observed FB genetic abnormality, the formation of a hyperactive C3-convertase, endothelial cell C3-depositon, and the manifestation of aHUS. We showed that not only insufficient regulation, but also hyperfunctional C3 convertase (due to gain of function mutations in FB and C3), which overwhelms the normal complement regulation, predispose to aHUS. A logical option for patients carrying mutations leading to excessive complement activation could be a therapeutic agent able to prevent the abnormal convertase formation, by blocking FB or its consequences, by blocking C5.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Prof Marc Fontaine for providing polyclonal anti-C3 Ab.
This work was supported by grants from AP-HP (Program Hospitalier de Recherche Clinique [AOM05130/P051065] 2005 and [AOM 08198]2008 to V.F.B.), by AIRG France, and by Inserm. L.T.R. is a recipient of an EMBO Long Term Fellowship ALTF 444-2007.
Authorship
Contribution: L.T.R., M.J., and V.F.-B. designed the research; L.R. M.J., C.H., M.C., and J.B. performed the research; L.T.R., V.F.-B., L.H.-M., J.D.D., M.-A.D.-D., C.S.-F., W.H.F., and C.H.R. analyzed the data; L.T.R. and V.F.-B. wrote the paper; S.C.S. and P.W.M. contributed vital new reagents and reviewed the paper; and M.-A.M., D.R., L.M., L.R., and C.L. provided clinical information and were in charge of the patients.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: V. Fremeaux-Bacchi, Service d'Immunologie Biologique, Hôpital Européen Georges Pompidou, 20-40 rue Leblanc, 75908 Paris cedex 15, France; e-mail: veronique.fremeaux-bacchi@egp.aphp.fr.

![Figure 1. Genetic analysis of the described patients. (A) Pedigree of the 2 families. (B) Histograms from the patients sequencing with indication of found mutations. (C) Localization of mutations within the factor B gene (rectangles represent exons; line represents introns) and within the protein sequence (the 3 domain types—CCP [complement control protein], VWFA [von Willebrand type A], and serine protease are indicated). Numbering is made with the first methionine referred as 1 (as described in Hourcade et al13).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/13/10.1182_blood-2009-01-197640/4/m_zh89990941170001.jpeg?Expires=1765887619&Signature=QSBrc3QwNH8k8t0Jb7tVmG064OYP5EgY69s50pVFv9DZXMrx6Kixtze9fwXbqjzrqN274nKTJSoTDlJJPjqnlgM-SgK8UgX2c4jhoPPjpW4ICX-lk2MTcgg2WQQwCeRT0L-HaOi8rnBb8XVyCzMjc94XkdqGRruP4p7YJJxiQ-G3UwexFmCkTg2ASiZiTCJLtZAI-Ju4clCwltgjamm66oH5kGDfb~wpjusdxXjauS9dbUeYNe94sdvGnJYrcCDAcvlRuASv7FbfJZaZN5nWqrdIojW1bp5Uz56LLcozpZrvk~fRmqWrWreFjUEEAjyemT4JyfrW8OfKr297rICBCg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal