Abstract
In this issue of Blood, Ishii and colleagues shed new, fresh light on molecular mechanisms underlying one form of macrophage activation.
Macrophages are a key element in the orchestration of tissue remodeling and repair and in resistance against pathogens. Macrophages undergo diverse forms of activation in response to cytokines and microbial signals. In particular, IFNγ and IL-4 activate distinct functional programs in mononuclear phagocytes, inducing an M1 (or classic) and M2 (or alternative) form of activation, which mirror the Th1/Th2 dichotomy.1-3 Indeed, polarized M1 and M2 macrophages are extremes of a spectrum in a galaxy of functional state.1,3,4 M1-polarized macrophages mediate resistance to intracellular pathogens, tissue destruction, and antitumor resistance. In contrast, M2-polarized cells come in different flavors and are generally oriented to tissue remodeling and repair, resistance to parasites, immunoregulation, and tumor promotion.
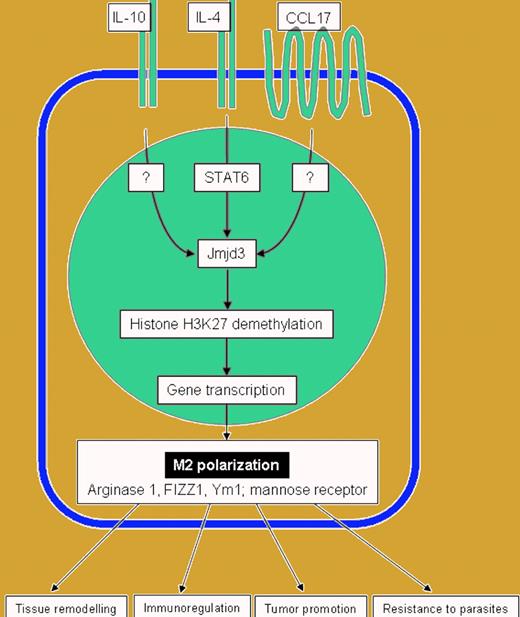
Schematic representation of the role of the histone demethylase Jumonji domain containing 3 (Jmjd3) proteins in macrophage alternative polarization.
Schematic representation of the role of the histone demethylase Jumonji domain containing 3 (Jmjd3) proteins in macrophage alternative polarization.
Molecular mechanisms underlying macrophage polarization remain largely undefined. Tuning of NF-κB activation by p50 homodimers has been associated with M2 differentiation.5 PPARγ agonists have been shown to induce M2-like differentiation,6 and the phosphatase SHIP was shown to play a key role in balancing macrophage polarization, although recent evidence suggests that it may act indirectly through the regulation of IL-4 production by basophils.7
In this issue of Blood, Ishii and colleagues identify a molecular pathway responsible for epigenetic regulation of key M2-associated genes in the mouse (see figure).8 IL-4 up-regulates the histone demethylase Jmjd3 via the transcription factor STAT6. Jmjd3 contributes to demethylation of histone H3 at lysine 27, thus unleashing promoters of M2 marker genes (arginase 1, Ym1, FIZZ1, mannose receptor). As discussed by Ishii and colleagues, these results nicely parallel recent evidence, indicating that chromatin remodeling also plays a role in the control of expression pattern of specific genes in polarized Th lymphocytes.
This report opens new perspectives and raises new questions. Macrophages undergo diverse M2-like activation states in response to different signals,1,2,4 and the role of regulation by Jmjd3, and more in general histone methylation, in these processes remains to be elucidated. Even for the IL-4–induced “alternative” M2 form of activation it is still not known whether the pathway described here accounts for the whole transcription regulation associated to it. Furthermore, FIZZ1 and arginase 1 are not induced by IL-4 in human macrophages.9 It will therefore be important to investigate the importance of regulation of histone methylation in cells of human origin. Finally, Jmjd3 had previously been shown to be induced in a macrophage cell line by lipopolysaccharide,10 indicating that chromatin remodeling might play a role in macrophage biology beyond alternative activation. With these open questions in mind, the new data offer a novel mechanistic insight into macrophage polarization and may pave the way to its targeting and tailoring.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal