Abstract
Amyloidosis is an uncommon disorder in which proteins change conformation, aggregate, and form fibrils that infiltrate tissues, leading to organ failure and death. The most frequent types are light-chain (AL) derived from monoclonal B-cell disorders producing amyloidogenic immunoglobulin light chains, and the hereditary and “senile systemic” (ATTR) variants from mutant and wild-type transthyretin (TTR). Diagnosis requires tissue biopsy. AL is more frequent and causes more organ disease than ATTR. Although both can cause cardiomyopathy and heart failure, AL progresses more quickly, so survival depends on timely diagnosis. Typing is usually based on clinical and laboratory findings with monoclonal gammopathy evaluation and, if indicated, TTR gene testing. Direct tissue typing is required when one patient has 2 potential amyloid-forming proteins. In coming years, widespread use of definitive proteomics will improve typing. New therapies are in testing for ATTR, whereas those for AL have followed multiple myeloma, leading to improved survival. Challenges of diagnosing and caring for patients with amyloidosis include determination of type, counseling, and delivery of prompt therapy often while managing multisystem disease. Recent advances grew from clinical research and advocacy in many countries, and global husbandry of such efforts will reap future benefits for families and patients with amyloidosis.
Introduction
Less than 50 years ago, the composition of AL amyloid fibrils was a matter of controversy.1-3 The connection between a monoclonal gammopathy (itself a powerful concept of that era) and amyloid was not clearly comprehended.4 Investigators were caught in a logical conundrum: amyloid fibrils were composed of an aberrant protein but not always of the same one.5 There were different types of amyloid as in Table 1.6 Since that time, investigators developed a taxonomy based on the several dozen precursor proteins that cause amyloidosis. We now confront the problem of diagnostic confidence in typing amyloid, knowing that different therapies exist for different types.7-9 We also struggle with the core clinical issue: the heart of the matter is that amyloidosis often attacks the heart.10
Several types of systemic amyloidosis
| Amyloid type . | Precursor protein . | Clinical presentations . |
|---|---|---|
| AL | kappa or lambda light chains | Cardiac, renal, hepatic/GI, PNS, soft tissues |
| ATTR | Mutant transthyretin | Cardiac, PNS |
| Senile systemic | Wild-type transthyretin | Cardiac, pulmonary, PNS |
| AA* | Serum amyloid A | Renal |
| A Fib | Mutant fibrinogen A alpha | Renal, hepatic |
| AApo-A1 | Apolipoprotein A1 | Cardiac, renal, hepatic/GI, PNS, skin |
| Amyloid type . | Precursor protein . | Clinical presentations . |
|---|---|---|
| AL | kappa or lambda light chains | Cardiac, renal, hepatic/GI, PNS, soft tissues |
| ATTR | Mutant transthyretin | Cardiac, PNS |
| Senile systemic | Wild-type transthyretin | Cardiac, pulmonary, PNS |
| AA* | Serum amyloid A | Renal |
| A Fib | Mutant fibrinogen A alpha | Renal, hepatic |
| AApo-A1 | Apolipoprotein A1 | Cardiac, renal, hepatic/GI, PNS, skin |
GI indicates gastrointestinal; and PNS, peripheral nervous system.
AA is very rare in the developed world, but cases still occur in association with chronic infection, severe gout, ulcerative colitis, and metastatic renal cancer.
How I treat amyloidosis begins with typing the disease, determining the extent of organ (particularly heart) involvement, and deciding on appropriate therapy, whenever feasible on a clinical trial. Effective therapy requires a comprehensive approach, monitoring both the precursor protein we seek to reduce or eliminate and the markers of organ disease, while providing best supportive care. In this report, I use a case-and-comments approach focused on AL, the type afflicting 10 patients per million person-years, including 10% to 15% of patients with myeloma or Waldenström macroglobulinemia.11
Case 1: a woman with hepatosplenomegaly, proteinuria, and bleeding
A 49-year-old woman presented with early satiety, right upper quadrant fullness, hepatosplenomegaly, alkaline phosphatase 380 U/L (normal, range, 0-115 U/L), thrombocytosis, prolonged prothrombin time (PT)/partial thromboplastin time (PTT), albumin 2.1 g/dL, creatinine 1.9 mg/dL, and 12 530 mg/day of proteinuria. PT/PTT corrected with mixing and factor X was 6%. Marrow contained 12% λ-restricted plasma cells and vascular amyloid. Serum immunofixation showed a monoclonal IgGλ (IgG, 730 mg/dL), and amyloidosis was diagnosed by rectal biopsy. AL type was inferred based on the presentation and factor X deficiency. The biopsy site bled for 6 days despite fresh frozen plasma. Echocardiogram and electrocardiogram were unremarkable. Liver span measured by noncontrast CT was 29 cm. After vaccination, elective splenectomy was performed with recombinant activated human factor VII. Postoperative factor X increased to 26%.
The patient consented to stem cell transplantation (SCT) on protocol with high-dose melphalan (140 mg/m2 because the creatinine clearance was < 51 mL/min). Three months after SCT, she had achieved a complete hematologic response (CR). Three years after SCT, liver span was 12 cm, factor X 60%, and proteinuria 490 mg/day. Hematologic and organ responses have been maintained for more than 10 years. When the free light chain (FLC) assay became available, a baseline frozen serum sample showed λ-FLC 185 mg/L (normal, 5.7-26.3 mg/L) with an abnormal κ-to-λ ratio of 0.07 (normal, 0.26-1.65). Post-SCT samples showed minimally elevated FLC levels with a ratio of 1.25.
Comments
Severe factor X deficiency (< 25%) occurs in 2.5% of patients with AL, usually with hepatosplenic involvement, and is the result of adsorption of calcium-binding vitamin K–dependent factors to amyloid deposits.12-14 Splenectomy can ameliorate the deficiency and, in young SCT-eligible patients, should be considered because the peri-SCT mortality with severe deficiency is 50%.14 Splenic rupture during stem cell mobilization is an additional concern (a rare event in healthy persons during granulocyte colony-stimulating factor mobilization).15 Although splenectomy effectively increased factor X in this patient, it may not work as well in patients without splenomegaly.12
In addition to factor X deficiency, this patient also had renal insufficiency. The proteinuria in amyloidosis is predominantly albuminuria with limited nonselective spillage; hence, the reduced serum albumin.16 Patients with impaired renal function often experience increased toxicity with melphalan at 200 mg/m2; therefore, the dose was attenuated to 140 mg/m2 to minimize occurrence of renal failure requiring dialysis, the risk of which is 5% (14 of 277) in one large series.17-19 Although risk-adapted melphalan probably reduces peri-SCT mortality, it may also reduce efficacy.20,21 However, there has not been a head-to-head comparison of melphalan 200 mg/m2 versus 140 mg/m2, so the relative impact of risk-adapted dosing on survival has not been accurately ascertained.
Response and survival rates with SCT appear to exceed those of oral melphalan and prednisone, a prior standard therapy for AL, although selection bias is obviously a factor.22,23 CR post-SCT is associated with prolonged survival and organ responses.24 The regression of hepatomegaly results from turnover of amyloid and probably hepatic regeneration. Similar significant responses have been noted in patients with autonomic or peripheral neuropathies. In patients with renal amyloidosis who achieve CR, significant improvements occur in proteinuria and serum albumin but not creatinine clearance.25 The kidneys may not effectively turn over amyloid deposits or regenerate, and patients remain at risk of renal failure from other insults, such as dehydration or statin-related rhabdomyolysis. In patients with cardiac amyloidosis, negative effects of amyloid-forming light chains are reversed with CR, as evidenced by reductions in brain natriuretic peptide (BNP) and troponin and improvements in functional status and survival.26 However, only 20% of patients with CR demonstrate reductions in septal or mean left ventricular wall thickness.26 In addition, even with CR, sequelae, such as arrhythmias, progressive left ventricular failure, or sudden death, can occur over time.27
Among newly diagnosed patients with AL, 30% have 3 or more major organ systems involved (heart, kidneys, liver/gastrointestinal tract, peripheral nervous system), whereas the majority have 1 or 2 involved. (Table 1 describes the usual pattern in different types of amyloidosis.) Diagnosis requires Congo red staining of tissue from an involved organ or surrogate site (abdominal fat, gingiva, or rectum) and appreciation of the classic microscopic apple-green birefringence in polarized light (Figure 1A).28 Immunohistochemical staining for typing is unreliable, and routine procurement of an additional sample for immunogold electron microscopy is recommended.7,29 Renal biopsies possess moderate reliability because of immunofluorescent staining of unfixed tissue for typing and the completeness of the pathologic evaluation (staining for fibrinogen, Congo red, electron microscopy).30
Diagnosing and typing amyloidosis. (A) A Congo red stained renal biopsy (left) shows the apple-green birefringence in polarized light (right) indicative of amyloidosis, 200× (images were acquired using an Olympus BX51 microscope equipped with a 20×/0.5 NA objective and mounted DP12 digital camera, and were further processed [cut out and white balanced] with Adobe Photoshop CS2). (B) Characteristic amyloid fibrils extracted from the postmortem spleen of a patient with rapidly progressive κ AL are shown in a transmission electron micrograph, 100 000× (image was obtained at the Sloan-Kettering Institute Core Electron Microscopy Facility). Fibrils are 7 to 10 nM in diameter and variable in length. (C) Exon 4 of TTR shows heterozygosity (left, arrow) in an African American man with amyloidosis due to the Val122Ile variant (codons for aa 121, 122, and 123 are shown). Homozygotes (right, arrow) are rare but can present with severely symptomatic disease in their early 50s. (D) An example of immunogold electron microscopy (IEM) is shown, 50 000× (courtesy of Dr Carl O'Hara, July 2008; the image was obtained at the Boston University School of Medicine Experimental Pathology Laboratory Service Core). Tissue sections on grids were treated overnight with primary polyclonal rabbit anti–human antibodies (for κ and λ light chains and for TTR) and then with goat anti–rabbit gold secondary antibody conjugates. In this instance the patient had a κ light chain paraprotein and mutant TTR. IEM demonstrated ATTR. Note how the gold beads sit along the fibrils. (E) The peptides of the FR1 portion of the κ light chain from panel B are identified by liquid chromatography and mass spectrometry of the extracted amyloid protein (liquid chromatography/tandem mass spectrometry were performed at the Proteomics Resource Center at Rockefeller University).
Diagnosing and typing amyloidosis. (A) A Congo red stained renal biopsy (left) shows the apple-green birefringence in polarized light (right) indicative of amyloidosis, 200× (images were acquired using an Olympus BX51 microscope equipped with a 20×/0.5 NA objective and mounted DP12 digital camera, and were further processed [cut out and white balanced] with Adobe Photoshop CS2). (B) Characteristic amyloid fibrils extracted from the postmortem spleen of a patient with rapidly progressive κ AL are shown in a transmission electron micrograph, 100 000× (image was obtained at the Sloan-Kettering Institute Core Electron Microscopy Facility). Fibrils are 7 to 10 nM in diameter and variable in length. (C) Exon 4 of TTR shows heterozygosity (left, arrow) in an African American man with amyloidosis due to the Val122Ile variant (codons for aa 121, 122, and 123 are shown). Homozygotes (right, arrow) are rare but can present with severely symptomatic disease in their early 50s. (D) An example of immunogold electron microscopy (IEM) is shown, 50 000× (courtesy of Dr Carl O'Hara, July 2008; the image was obtained at the Boston University School of Medicine Experimental Pathology Laboratory Service Core). Tissue sections on grids were treated overnight with primary polyclonal rabbit anti–human antibodies (for κ and λ light chains and for TTR) and then with goat anti–rabbit gold secondary antibody conjugates. In this instance the patient had a κ light chain paraprotein and mutant TTR. IEM demonstrated ATTR. Note how the gold beads sit along the fibrils. (E) The peptides of the FR1 portion of the κ light chain from panel B are identified by liquid chromatography and mass spectrometry of the extracted amyloid protein (liquid chromatography/tandem mass spectrometry were performed at the Proteomics Resource Center at Rockefeller University).
This patient was treated before the FLC assay became available. The serum FLC assay, combined with serum and urine immunofixation, has become essential to the management of patients with AL, identifies the pathologic FLC, and provides the target for therapy.31,32 It reports absolute levels of κ and λ-FLCs and the κ-to-λ ratio. FLCs are metabolized by renal tubular cells, and their measurement is influenced by renal function.31,33 Persons with renal insufficiency without monoclonal gammopathies have κ-to-λ ratios that remain within a broad normal range.34
The clonal plasma cell disease in AL is usually λ (ratio of κ-to-λ clones is 1:4) probably because the 3r and 6a (IGLV6–57) λ variable region germline donors account for 40% of cases.35,36 Pathologic FLCs are widely distributed in body tissues and misfold, forming intermediates possibly injurious to normal cells.37,38 In this view, fibrils may be less toxic than FLC intermediates until the fibrillar burden overwhelms organs and patient.6 These processes remain black boxes, but computational biology and empiric experimental approaches may reveal their mechanisms.39,40
Case 2: an elderly man with heart failure, orthostasis, and proteinuria
Six months after stenting of 2 coronary arteries, a 71-year-old black man reported new-onset dyspnea, lightheadedness, and abdominal cramping. Echocardiogram showed diastolic dysfunction, concentric left ventricular hypertrophy, septal thickness 1.4 cm, and left ventricular ejection fraction 60%. On examination, he was orthostatic by blood pressure but not pulse, had jugular venous distension, reduced aeration at the right base, hepatomegaly, epigastric discomfort, and 2+ edema to the knees. Complete blood count and PT/PTT were normal. Electrocardiogram showed loss of voltage compared with 6 months previous. Endomyocardial biopsy showed amyloidosis. BNP was 3520 pg/mL (normal, 0-100 pg/mL) and troponin I 0.69 ng/mL (normal, < 0.06 ng/mL). Proteinuria was 2550 mg/day and creatinine clearance 74 mL/min. Alkaline phosphatase was elevated at 315 U/L. Marrow showed 23% λ-restricted plasma cells and λ-FLCs were 1260 mg/L with an abnormal ratio. Transthyretin gene sequence was wild-type.
The diagnosis of AL amyloidosis involving heart, autonomic nervous system, kidneys, and liver was inferred based on the extent and tempo of disease. He began 4 consecutive days per month of oral melphalan (0.18 mg/kg per day) and dexamethasone (20 mg/day), and continued diuretics with daily weight and vital sign monitoring, compression stockings, and a bowel regimen to prevent constipation. After 2 months of therapy, λ-FLCs were 115 mg/L, BNP 2650 pg/mL, and troponin I 0.39 ng/mL. He completed 1 year of therapy to a total oral melphalan dose of 544 mg. Orthostasis and constipation resolved, and he became New York Heart Association class IIA. Diuretics (torsemide and eplerenone) were adjusted depending on weight. λ-FLC nadired at 42 mg/L, BNP at 1640 pg/mL, and proteinuria at 570 mg/day. After 2 years, he reported worsening dyspnea. λ-FLCs were 220 mg/L, and BNP and diuretic requirement increased. He was treated with lenalidomide and low-dose dexamethasone but developed fatigue and pneumonia so therapy was changed to low-dose thalidomide and cyclophosphamide without response. Echocardiogram showed worsening left ventricular function. He was then treated with 2 cycles of bortezomib, melphalan, and dexamethasone. λ-FLC decreased to 30 mg/L, but recurrent abdominal bloating and ileus led to discontinuation of therapy. Left ventricular function continued to decline despite the reduction in λ-FLC. He remains New York Heart Association class III surviving nearly 5 years from diagnosis.
Comments
Typing of cardiac amyloidosis in elderly men can be challenging because of the frequency of senile systemic amyloidosis (SSA) resulting from wild-type transthyretin (TTR). In addition, mutant TTR is the most common cause of hereditary amyloidosis, and 4% of blacks carry the gene for an amyloid-forming mutant TTR in which isoleucine replaces valine at position 122 (Val122Ile, Figure 1C).41,42 TTR is produced in the liver and minimally in the choroid plexus, and more than 100 amyloid-forming variants have been identified. Sequencing exons 2, 3, and 4 of TTR from genomic DNA allows rapid identification of wild-type and mutants. Mutations cause substitutions that enable the tetrameric TTR protein to dissociate, increasing the concentration of aberrant amyloidogenic monomers.43 Why wild-type TTR forms amyloid fibrils and why SSA occurs with male gender exclusivity remain unknown. Demonstration of wild-type TTR by gene sequencing is helpful in ruling out hereditary ATTR and in diagnosing SSA.44 The drugs diflunisal and tafamidis stabilize the tetramer in vitro and in vivo and are in clinical testing for ATTR.45,46
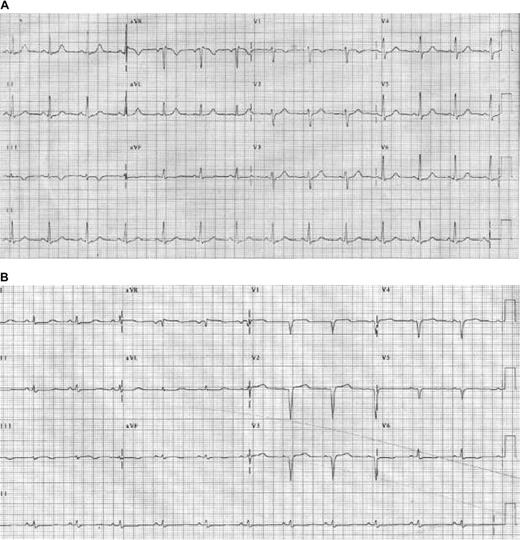
In Portugal and Japan, Val30Met ATTR can present as polyneuropathy in patients 20 to 40 years old, leading to disability and early death unless treated with orthotopic liver transplantation.47 In blacks, Val122Ile ATTR often presents in the sixth or seventh decade with progressive heart failure and/or neuropathy but not with proteinuria, although glomerulosclerosis can mimic AL renal involvement. ATTR variants are inherited as autosomal dominant traits with high penetrance. Because blacks have higher incidences of both Val122Ile and monoclonal gammopathies, a tissue diagnosis of amyloidosis requires testing for 2 potential precursor proteins.48 What happens if the patient has both Val122Ile and a monoclonal gammopathy? In our experience, only one of the precursors causes amyloid disease per patient.8 Tissue typing of amyloid fibrils is then required to determine which is causing disease, using techniques such as immunogold electron microscopy or protein separation and identification by mass spectrometry (Figure 1D-E).49,50 Accurate diagnosis is important for the patient because therapy for AL is marrow toxic, and for the patient's family because Val122Ile ATTR is hereditary. In this case, TTR was wild-type and the extent and tempo of disease allowed the diagnosis of AL (not SSA) to be inferred with confidence, permitting prompt initiation of therapy. The short-term electrocardiographic changes in this case are not unusual (Figure 2).
Electrocardiogram changes in AL amyloidosis. (A) Baseline electrocardiogram of an AL patient with no signs of cardiac involvement. (B) Follow-up study only months later demonstrates changes resulting from cardiac amyloidosis, including pseudo-infarct pattern, and Q waves and loss of voltage in the limb leads. Reprinted from Wright et al60 with permission.
Electrocardiogram changes in AL amyloidosis. (A) Baseline electrocardiogram of an AL patient with no signs of cardiac involvement. (B) Follow-up study only months later demonstrates changes resulting from cardiac amyloidosis, including pseudo-infarct pattern, and Q waves and loss of voltage in the limb leads. Reprinted from Wright et al60 with permission.
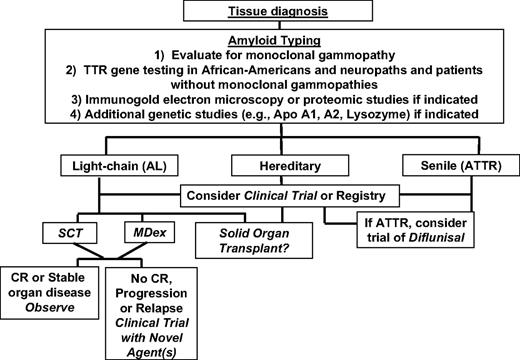
Misdiagnosis of AL is an infrequent but real occurrence. In a series of 375 patients diagnosed with AL in the United Kingdom, 10% were misdiagnosed, many because of misinterpretation of urine immunofixation findings associated with renal damage resulting from the fibrinogen A α hereditary variant.7,51 Renal injury resulting from amyloidosis is associated with spillage of proteins, including Ig-related proteins, in the urine. Urine immunofixation may then show bands difficult to interpret for clonality.52 We have not encountered misdiagnosis of AL for fibrinogen A α in the United States, perhaps because of the frequency of renal biopsies and standard staining for fibrinogen. We have, however, identified black and peripheral neuropathy patients with amyloidosis who have 2 potential precursor proteins, a variant TTR gene and a monoclonal gammopathy. In those instances, the risk of misdiagnosis exists and tissue typing is required.8 Similarly, in elderly men with isolated cardiac involvement and monoclonal gammopathies, tissue typing will distinguish AL from SSA. Within the next decade, as proteomic techniques become more widely available, the risk of misdiagnosis will lessen, provided that the tissue samples tested contain adequate amounts of amyloid.50 Figure 3 contains an algorithm of our current approach to amyloidosis.
Treatment of AL has followed that of multiple myeloma. Oral melphalan and dexamethasone (MDex) and high-dose melphalan with SCT are considered standard therapies.22,27 The hematologic response and survival rates achieved in the initial MDex trial were similar to SCT (Figure 4A).27 The French Myélome Autogreffe Groupe led a multicenter phase 3 trial randomizing AL patients to MDex versus high-dose melphalan with SCT (#NCT00344526).53 The median survival of patients in the MDex group was significantly better (Figure 4B); however, 13 of the 50 patients assigned to SCT never received melphalan because of death or early progression, and 9 died peri-SCT. This high early mortality was the result of inclusion of patients who would not have been considered SCT-eligible in the United States and to the limited experience many centers in the trial had with SCT for AL. Nonetheless, a landmark analysis examining patients surviving 6 months or more showed no advantage for SCT. The results with MDex, a 68% response rate and 57-month median survival, confirmed it as a standard therapy for AL, noting the risks of myelodysplasia and secondary leukemia from oral melphalan.54 Current criteria for SCT eligibility include 1 or 2 major organs involved, left ventricular ejection fraction greater than or equal to 45%, pulmonary diffusion capacity greater than or equal to 50%, and systolic blood pressure greater than or equal to 90 mm Hg. By these criteria, only one-third of newly diagnosed patients are SCT-eligible. The hematologic and organ responses with SCT in historical series are in Table 2.
Survival in systemic AL amyloidosis. (A) Updated survival of the first MDex trial (n = 46) is shown. Median progression-free and overall survivals are 3.8 and 5.1 years, respectively. Data from Palladini et al100 with permission. Treatment with oral melphalan plus dexamethasone produces long-term remissions in AL amyloidosis. (B) The phase 3 comparison of MDex (blue curve) and SCT (red curve) shows improved survival for MDex and dramatic early mortality for SCT (n = 50 in each arm). Reprinted from Jaccard et al53 with permission. (C) Survival by response is shown for AL cardiac patients not eligible for SCT and treated with MDex (n = 37). Variables that negatively influenced survival included baseline troponin I level more than 0.12 ng/mL, interventricular septal thickness more than 1.4 cm, and male sex. Responders had a median survival of 22 months.55 (D) The survival of AL SCT patients by cardiac stage is shown. Data from Dispenzieri et al63 with permission. Staging is defined by NT-proBNP and troponin T thresholds of 332 pg/mL and 0.035 ng/mL: stage I, both NT-proBNP and troponin T under threshold; stage II, either over threshold; and stage III, both equal to or over threshold. (E) Updated survival is shown for AL patients undergoing SCT and then receiving adjuvant thalidomide and dexamethasone therapy if clonal plasma cell disease persisted (n = 45).21 At median follow-up of 52 months, 69% of patients survive.
Survival in systemic AL amyloidosis. (A) Updated survival of the first MDex trial (n = 46) is shown. Median progression-free and overall survivals are 3.8 and 5.1 years, respectively. Data from Palladini et al100 with permission. Treatment with oral melphalan plus dexamethasone produces long-term remissions in AL amyloidosis. (B) The phase 3 comparison of MDex (blue curve) and SCT (red curve) shows improved survival for MDex and dramatic early mortality for SCT (n = 50 in each arm). Reprinted from Jaccard et al53 with permission. (C) Survival by response is shown for AL cardiac patients not eligible for SCT and treated with MDex (n = 37). Variables that negatively influenced survival included baseline troponin I level more than 0.12 ng/mL, interventricular septal thickness more than 1.4 cm, and male sex. Responders had a median survival of 22 months.55 (D) The survival of AL SCT patients by cardiac stage is shown. Data from Dispenzieri et al63 with permission. Staging is defined by NT-proBNP and troponin T thresholds of 332 pg/mL and 0.035 ng/mL: stage I, both NT-proBNP and troponin T under threshold; stage II, either over threshold; and stage III, both equal to or over threshold. (E) Updated survival is shown for AL patients undergoing SCT and then receiving adjuvant thalidomide and dexamethasone therapy if clonal plasma cell disease persisted (n = 45).21 At median follow-up of 52 months, 69% of patients survive.
Outcomes with SCT in large historical patient series
| Reference . | N . | TRM (%) . | RR/CR* (%) . | Organ responses (%) . | Median survival, y . | MEL 200/reduced dose (%) . |
|---|---|---|---|---|---|---|
| 17 | 312 | 16 | NA/27 | 26 | 4.6 | 56/44 |
| 97 | 270 | 11 | 71/33 | NA | NR | 67/33 |
| 98 | 92 | 23 | 37/20 | NA | 5.3 | 69/31 |
| 99 | 107 | 27 | 32/16 | 26 | 3.9 | 46/54 |
| Reference . | N . | TRM (%) . | RR/CR* (%) . | Organ responses (%) . | Median survival, y . | MEL 200/reduced dose (%) . |
|---|---|---|---|---|---|---|
| 17 | 312 | 16 | NA/27 | 26 | 4.6 | 56/44 |
| 97 | 270 | 11 | 71/33 | NA | NR | 67/33 |
| 98 | 92 | 23 | 37/20 | NA | 5.3 | 69/31 |
| 99 | 107 | 27 | 32/16 | 26 | 3.9 | 46/54 |
N indicates number of patients; TRM, treatment-related mortality; NA, not available; RR, overall response rate (partial and complete); NR, not reached; MEL 200, melphalan at 200 mg/m2; reduced dose, MEL 140 or 100 mg/m2.
CR indicates complete hematologic response. It is important to note that the definition of CR has undergone revision since these reports with inclusion of free light chain measurements. Strict CR in AL can now be defined as serum and urine immunofixation with no evidence of the prior monoclonal protein, normalization of pathologic FLC, and κ-to-λ ratio, and marrow aspirate with less than 5% plasma cells without isotype restriction.21
In 37 patients with advanced cardiac AL amyloidosis ineligible for SCT, MDex had a similar overall response rate but a median survival in responders of only 22 months; 1 responder died of secondary leukemia (Figure 4C).55 Similarly, in 22 patients with advanced cardiac involvement treated at a European center, MDex plus thalidomide had a median survival in responders of less than 24 months.56 In contrast, in 347 patients with all stages of cardiac AL amyloidosis, median survival was 70 months in the 51% who responded to therapy.11 These studies highlight the 2 critical determinants of survival in AL: the presence and extent of heart involvement and response to therapy.57
Orthostasis with a drop in systolic blood pressure without concomitant heart-rate response is typical of the presence of both autonomic and cardiac involvement in AL.58 I-123 meta-iodobenzylguanidine myocardial studies have demonstrated the autonomic abnormalities associated with cardiac amyloid.59 Use of diuretics and salt restriction to manage intravascular volume overload, or of β- or calcium-channel blockers for atrial fibrillation (which did not occur in this case), may exacerbate orthostasis. In such instances, midodrine may be useful for blood pressure support and amiodarone for control of atrial fibrillation. Amiodarone has also been used prophylactically in cardiac amyloid patients with documented nonsustained ventricular tachycardia, a practice not yet tested in phase 3 trials.27,60 The excessive vagal stimulation associated with micturition and straining at defecation may precipitate sudden cardiac death, often linked to electromechanical dissociation.61 It is therefore important to offer guidance in these areas to patients and their families.
Given the results in multiple myeloma with novel agents added to standard therapy, efforts are now underway to conduct similar studies in AL.62 The addition of bortezomib to MDex is particularly justified in view of the results of the VISTA trial and a recent phase 1/2 trial of single-agent bortezomib in relapsed AL (see “Case 3, Comments”).62 Phase 3 trials are scheduled to open in 2009 comparing MDex versus MDex plus bortezomib in newly diagnosed patients with AL not eligible for SCT with 200 mg/m2 of melphalan. The availability of a validated staging system for cardiac AL amyloidosis that uses the biomarkers NT-proBNP and troponin and correlates with survival improved the design of these trials, allowing patient stratification to be based objectively on cardiac stage (Figure 4D).63
Case 3: a middle-aged man with edema, hypoalbuminemia, and proteinuria
A 57-year-old man presented with edema, 9330 mg/day proteinuria, albumin 2.4 g/dL, and urine immunofixation showing a clonal λ band. Creatinine clearance was normal and renal biopsy showed amyloidosis, probably λ type. Marrow showed 7% λ-restricted plasma cells and λ-FLCs were 104 mg/L with an abnormal ratio. There was no evidence of other organ involvement. He was treated on a clinical trial with melphalan and SCT, followed by adjuvant therapy with thalidomide and dexamethasone if a strict CR was not achieved 3 months after SCT. Mobilization and SCT were uncomplicated, and post-SCT evaluation showed λ-FLC 67 mg/L with an abnormal ratio and clusters of λ-restricted plasma cells in the marrow. This outcome was neither a complete nor a partial response (> 50% reduction of prior disease), but rather stable disease or no response.
The patient received thalidomide and dexamethasone with aspirin, fluconazole, and trimethoprim-sulfamethoxisole prophylaxis. Maximum tolerable dose of thalidomide was 150 mg nightly. Four-day pulses of dexamethasone 40 mg/day were given twice a month. Notable side effects were constipation, fatigue, and fluid retention causing brawny lower extremity edema. After 6 months, λ-FLCs were 24 mg/L with low-normal ratio 0.27 and proteinuria was stable at 6240 mg/day. At 1 year after SCT, a partial response was achieved. Two years later, the λ-FLC rose to 86 mg/L with an abnormal ratio and BNP of 386 pg/mL. He reported exertional dyspnea and required additional diuretics.
He enrolled in a phase 1 trial of bortezomib in relapsed AL and received 1.6 mg/m2 on the 35-day schedule. After cycle 2, the λ-FLC and ratio had normalized and BNP fell. He received a total of 8 cycles, 6 beyond the point of FLC normalization, and at end-of-study a CR was achieved. Two years later, CR was maintained, proteinuria and BNP were normal, and diuretics were discontinued.
Comments
In the phase 1 portion of the trial of bortezomib for relapsed AL (#NCT00298766), we treated 31 patients in 7 dose cohorts.64 The major side effects were gastrointestinal, neurasthenic, and cardiac, and the hematologic response rate was 50% with 20% CR. Gastrointestinal side effects ranged from limited bouts of diarrhea, unpredictable nausea, and vomiting, to ileus, bloating, and abdominal pain requiring hospital admission and surgical evaluation. With respect to the gastrointestinal side effects, AL patients receiving bortezomib required teaching, prophylaxis, and monitoring.
A maximum tolerated dose was not reached with either the 21-day (days 1, 4, 8, and 11; highest dose level, 1.3 mg/m2 per dose) or a 35-day schedule (days 1, 8, 15, and 22; highest dose level, 1.6 mg/m2). Of particular note, the median time to hematologic response was 1.2 months, creating a dilemma regarding duration of therapy. We currently treat for 4 to 6 cycles beyond maximal response with dose reductions as needed and are studying the role of molecular markers of clonal disease in patients achieving CR. The activity of bortezomib in AL may be related to the dependence of AL clonal plasma cells on proteasome-related protein quality control processes that enable them to tolerate aggregation-prone FLC.65
Better patient selection may improve outcomes with SCT. The staging system for cardiac amyloid identifies patients at high risk for poor outcomes.63 Elevated troponin T or uric acid, excessive fluid retention during mobilization, and the absolute value of the pathologic FLC may predict treatment-related mortality.32,66-68 In addition, expression of calreticulin, the major calcium-binding protein in the endoplasmic reticulum, is significantly higher in the pre-SCT plasma cells of patients achieving a CR compared with those with no response, a prognostic factor that may eventually prove helpful but requires prospective validation.69
Using SCT as a platform and adding more therapy to the schema may also improve outcomes. Tandem autologous SCT, allogeneic stem cell transplantation, and post-SCT adjuvant therapy are examples. In a study of tandem SCT, a melphalan 200 mg/m2 SCT was followed with a melphalan 140 mg/m2 SCT for those not achieving hematologic CR at 6 months (#NCT00075621).70 Enough CD34+ cells for tandem SCT could not be obtained in 11% of patients. Treatment-related mortality was 9%, and 52% of patients achieved CR, including 8% after the second SCT. Median survival was not reached at 43 months of follow-up. Allogeneic SCT has been used rarely in relapsed AL with mixed results and cannot be recommended outside of a clinical trial.71
Thalidomide, lenalidomide, and bortezomib have activity in AL amyloidosis, either alone or in combination with agents, such as dexamethasone and cyclophosphamide.72-74 The first study incorporating a novel agent into SCT was a phase 2 trial using risk-adapted melphalan and 9 months of post-SCT adjuvant thalidomide and dexamethasone for those not achieving strict CR (#NCT00089167).21 Treatment-related mortality was 4%. Among the 69% of patients who received adjuvant therapy, the median dose of thalidomide was 150 mg; 16% discontinued adjuvant therapy because of disease progression and 32% because of toxicity. At 1 year after SCT, 78% responded and 39% had strictly defined CR; 42% of patients who received adjuvant therapy achieved improved hematologic responses. At median follow-up of 52 months, overall survival is 69% (Figure 4E).
A subsequent phase 2 trial used the same design but substituted bortezomib for thalidomide (#NCT00458822).75 Of 22 patients evaluable after SCT, 5 achieved CR and 17 received adjuvant therapy with bortezomib and dexamethasone for persistent clonal plasma cell disease. At 1 year after SCT, 94% responded and 71% had CR; 92% of patients who received adjuvant therapy achieved improved responses. Half of the patients had organ responses. Based on these results, a phase 3 trial comparing SCT versus SCT plus adjuvant bortezomib and dexamethasone would be reasonable.
Case 4: a man with a pulmonary mass and IgA gammopathy
A 62-year-old man presented with macroglossia, cough, numbness of the feet, and fatigue and was found to have firm right cervical nodes and a 5-cm right lower lobe lung mass. Open lung biopsy showed amyloidosis in both lung mass and parenchyma. Serum immunofixation showed an IgAλ monoclonal protein (IgA, 720 mg/dL) and λ-FLCs were 430 mg/L with an abnormal ratio. Marrow biopsy showed CD138+ λ-restricted plasma cells skirting abnormal follicles of small CD20+ cells faintly staining for λ. Lymphoplasmacytic lymphoma (LL) was diagnosed. BNP was 242 pg/mL and proteinuria 820 mg/day. Electrocardiogram showed loss of voltage; echocardiogram and cardiac magnetic resonance imaging (MRI) were unremarkable. Pulmonary diffusing capacity was 65% predicted and positron emission tomography/computed tomography showed widespread adenopathy without 18-fluoro-deoxyglucose avidity. The diagnosis of systemic AL was inferred based on the clinical picture.
He received one month of rituximab, 2 months of MDex, rituximab and cyclophosphamide with granulocyte colony-stimulating factor for stem cell mobilization and then an autologous SCT with BEAM (BCNU, etoposide, cytarabine, and melphalan) without complications. After initial treatment and mobilization, IgA decreased to 340 mg/dL and λ-FLC to 185 mg/L. Three months after SCT, he had achieved a CR, and proteinuria and numbness resolved. At 1 year after SCT, CR was maintained and he was asymptomatic with reduction in the size of the lung mass and other adenopathy.
Comments
The cellular basis of pathologic FLC production in AL is usually a monoclonal plasma cell disorder; however, in 2% of cases, B-cell disorders such as LL or other mature B-cell lymphomas can underlie FLC production and cause AL.76 Although lymphomas frequently produce clonal IgM paraproteins, they can also produce IgG and rarely IgA or only FLC. In all cases of AL, the cellular basis of paraprotein production must be identified. IgM gammopathies cannot be assumed to be Waldenström macroglobulinemia (WM) because they can also occur with monoclonal plasma cell disorders. The identification of the cellular basis of FLC production directly impacts therapeutic decision-making. Recent data regarding the occurrence of AL in patients with WM suggest that a significant fraction of WM patients may develop symptomatic AL.77 The FLC assay is helpful in these patients, providing a target for therapy separate from the M-protein. Standard criteria for AL organ involvement and response are also useful.78
There is no standard therapy for patients with LL with or without AL.77,79,80 The CD138+ fraction is probably the major source of FLC, making treatment decisions difficult given the potential for progressive AL. Nodal involvement with, and nodules of, amyloid are more common in LL patients, but renal and cardiac involvement occurs as well.80 Large masses of amyloid and macroglossia do not occur in hereditary or SSA disease; hence, the AL type was inferred in this patient. Pulmonary involvement in systemic AL is probably underdiagnosed and, as in this case, can cause reduction in diffusing capacity.81 The decision was made to treat with both rituximab and MDex initially and proceed to a standard BEAM SCT after mobilization. High-dose melphalan may have worked as well, although support for the efficacy of BEAM SCT for lymphoma is substantial.82 Although hematologic and organ responses were achieved in this case, multicenter clinical trials are needed to optimize treatment for AL with non-Hodgkin lymphoma.
Localized single-system amyloidosis can occur in the tracheobronchial tree, lungs, small bowel, or bladder. With respect to localized pulmonary amyloidosis, tissue typing is advisable because amyloidosis may be AL associated with monoclonal B cells, AA associated with inflammation, or rarely ATTR.83,84 Patients with Sjögren syndrome can be asymptomatic at presentation but have radiographically striking lung disease resulting from amyloidosis and then remain clinically stable for years with either localized AL or AA.83 However, localized amyloidosis in the aerodigestive tract usually causes symptoms, ranging from vocal changes with laryngeal involvement to obstruction and bleeding with bronchial involvement. Surgical excision and rigid bronchoscopy with focal laser reduction or external beam radiation may be required for palliation.85 Pulmonary amyloidosis can be diffuse and nodular and, in many cases, probably involves local production of FLC by clusters of clonal cells. Progression to systemic AL as well as marrow-based monoclonal disease can rarely occur.
Case 5: a young woman with exertional dyspnea
A 45-year-old social worker began to experience exertional dyspnea. Pulmonary evaluation was unremarkable. Six months later, cardiac catheterization showed normal coronaries, and a cardiologist diagnosed panic attacks. Over the next 6 months, her symptoms worsened. A second cardiologist found mitral valve prolapse and left ventricular hypertrophy on echocardiogram. Baseline systolic blood pressure was 95 mm Hg, and she could not tolerate afterload reduction. Four months later, endomyocardial biopsy showed amyloid. BNP was 975 pg/mL and troponin I was 0.84 ng/mL. There was no evidence of other organ involvement. Serum κ-FLCs were 895 mg/L (normal, 3.3-19.4 mg/L) with an abnormal ratio and marrow studies showed 24% κ-restricted plasma cells containing the t(11;14). She was admitted to hospital emergently with dyspnea and lightheadedness, responded to diuretics and, while being prepared for cardiac transplantation evaluation, had a cardiorespiratory arrest requiring intubation and intensive care. She died 24 hours later with intractable hypotension and pulseless electrical activity. There was no evidence of infection.
Comments
In young patients presenting with exertional dyspnea and no history of hypertension, the presence of a systolic blood pressure of 110 mm Hg or less and left ventricular hypertrophy on echocardiogram should trigger consideration of amyloidosis, leading promptly to cardiac biomarker and FLC testing and endomyocardial biopsy (sent for Congo red staining and electron microscopy). Approximately 5% of AL patients present with isolated cardiac involvement. Young patients with stage I or II cardiac involvement and preserved left ventricular function are usually eligible for SCT, whereas those with more advanced disease may be eligible for cardiac transplantation.86-88 Although few centers in the United States actively list AL cases, one has used “extended donor” grafts and demonstrated prolonged survival in the 40% of AL recipients who survive the wait for a donor.88 As in SCT-ineligible cardiac patients treated with MDex, there is a female sex bias among those who survive the wait. Kidney, and less frequently liver, transplantation can also be significantly useful in the management of young AL patients with organ failure.89,90
The t(11;14) is found in 30% to 40% of AL clones, a higher fraction than in myeloma. It is usually associated with the production of clonal FLC only without an intact immunoglobulin. Although considered indolent, AL clones also have higher expression of cyclin D1 (CCND1).91,92 Given the frequency of this and other cytogenetic abnormalities in AL, the plasma cell disorder causing AL can be considered malignant. How and why such a large fraction of patients achieve and maintain CR are intriguing. How t(11;14) and CCND1 overexpression influences clinical presentation, response to therapy, or likelihood of relapse is currently under investigation.
In patients with a history of hypertension and similar findings, cardiac MRI may help to distinguish amyloidosis from hypertrophic cardiomyopathy. Whether early MRI is an effective screen is under investigation. In patients listed for cardiac transplantation, progression of amyloidosis can occur while waiting. The decision to treat with chemotherapy is not to be made lightly because untimely side effects may interfere with timely organ transplantation. The choice of chemotherapy should take into consideration a probable role for future SCT; hence, avoidance of oral melphalan is reasonable.86-88
If cardiologists considered amyloidosis in their differential as routinely as nephrologists do, and took advantage of new methods for assessing patients, delays in diagnosis would probably be reduced. Early diagnosis of cardiac amyloidosis remains the best strategy for improving outcomes.
In conclusion, in the coming years, although proteomic techniques will make typing amyloid more straightforward, thereby enhancing diagnostic confidence and reducing misdiagnosis, we will still confront unique challenges. AL patients may develop myeloma more frequently, and myeloma patients AL, because both groups are living longer thanks to recent advances.91,93,94 Because new drugs are available for ATTR, affected families and persons have at last become a focus for natural history studies and are encouraged to participate in registries. Because they are now more numerous yet still experience late relapse and progression, long-term survivors with AL have a need for phase 1 and 1/2 clinical trials and are encouraged to participate in them, and clinical researchers are urged to respond to that need and test novel agents, including monoclonal antibodies.95,96 Amyloidosis is a rare global disorder, and national and international collaboration remains the key to continued advances in knowledge and treatment.
Acknowledgments
This work is dedicated to the many colleagues and patients who have contributed over the past several decades to advancing our understanding and treatment of amyloidosis, and to the staffs of the Amyloidosis Foundation and the International Myeloma Foundation whose work is ongoing and so often unheralded.
The author thanks all contributors to the Amyloidosis and Myeloma Research Fund at Tufts, the Tufts Medical Center Division of Hematology/Oncology, the Demarest Lloyd Jr Foundation, and the Werner and Elaine Dannheiser Fund for Research on the Biology of Aging of the Lymphoma Foundation for continued generous research support.
Authorship
Contribution: R.L.C. is the sole author of this manuscript.
Conflict-of-interest disclosure: R.L.C. is on the scientific advisory board of Millennium Pharmaceuticals.
Correspondence: Raymond L. Comenzo, Blood Bank, Stem Cell Processing Laboratory and Neely Cell Therapy Center, Tufts Medical Center, Box 826, 800 Washington St, Boston, MA 02111; e-mail: rcomenzo@tuftsmedicalcenter.org.

![Figure 1. Diagnosing and typing amyloidosis. (A) A Congo red stained renal biopsy (left) shows the apple-green birefringence in polarized light (right) indicative of amyloidosis, 200× (images were acquired using an Olympus BX51 microscope equipped with a 20×/0.5 NA objective and mounted DP12 digital camera, and were further processed [cut out and white balanced] with Adobe Photoshop CS2). (B) Characteristic amyloid fibrils extracted from the postmortem spleen of a patient with rapidly progressive κ AL are shown in a transmission electron micrograph, 100 000× (image was obtained at the Sloan-Kettering Institute Core Electron Microscopy Facility). Fibrils are 7 to 10 nM in diameter and variable in length. (C) Exon 4 of TTR shows heterozygosity (left, arrow) in an African American man with amyloidosis due to the Val122Ile variant (codons for aa 121, 122, and 123 are shown). Homozygotes (right, arrow) are rare but can present with severely symptomatic disease in their early 50s. (D) An example of immunogold electron microscopy (IEM) is shown, 50 000× (courtesy of Dr Carl O'Hara, July 2008; the image was obtained at the Boston University School of Medicine Experimental Pathology Laboratory Service Core). Tissue sections on grids were treated overnight with primary polyclonal rabbit anti–human antibodies (for κ and λ light chains and for TTR) and then with goat anti–rabbit gold secondary antibody conjugates. In this instance the patient had a κ light chain paraprotein and mutant TTR. IEM demonstrated ATTR. Note how the gold beads sit along the fibrils. (E) The peptides of the FR1 portion of the κ light chain from panel B are identified by liquid chromatography and mass spectrometry of the extracted amyloid protein (liquid chromatography/tandem mass spectrometry were performed at the Proteomics Resource Center at Rockefeller University).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/15/10.1182_blood-2009-04-202879/4/m_zh89990941620001.jpeg?Expires=1765891019&Signature=RIvMnHDbAvc9Wav5Fgd89RACvLQXozLRM5QVthOqj1NLeS0JE2d1W3hcuCoQueQpn3NpvL2f5bV1rUaJmS993bOUnSYb5HtsHpsnp5q8J66qXt1AKMJbuyTlVf2-d99NLMOTG12hMrvQy-Hyuzz2cV9ZNnsnETlvIwyfY1HgMO0mu-9KX47AuzUUfRAbrShgNR5Yt1io~7FXLpeqed3zcW4J5GW3mm2nMC4VUKsXB4gjKScN5YXtpv6pSaFt~9WAD~t4BQSmnSYbVpVzNV-APEJycfv4TCj5bgG~-SKuhoO35Zzpp9mOw2srcwIurlilTEJ6BuQUWuzBQ0q1eIp6SQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)