Abstract
Evans syndrome (ES) is a rare disease characterized by the simultaneous or sequential development of autoimmune hemolytic anemia (AIHA) and immune thrombocytopenia (ITP) and/or immune neutropenia. To better describe the characteristics and outcome of ES in adults, a survey was initiated in 2005. The data from 68 patients (60% of them women) fulfilling strict inclusion criteria for ES are reported. The mean age at time of ITP and/or AIHA onset was 52 plus or minus 33 years, both cytopenias occurred simultaneously in 37 cases (54.5%). ES was considered as “primary” in 34 patients (50%) but was associated with an underlying disorder in half of the cases, including mainly systemic lupus, lymphoproliferative disorders, and common variable immunodeficiency. All patients were given corticosteroids, but 50 of them (73%) required at least one “second-line” treatment, including splenectomy(n = 19) and rituximab (n = 11). At time of analysis, after a mean follow-up of 4.8 years, only 22 patients (32%) were in remission off treatment; 16 (24%) had died. In elderly patients, the risk of cardiovascular manifestations related to AIHA seems to be higher than the ITP-related risk of severe bleeding. In conclusion, ES is a potentially life-threatening condition that may be associated with other underlying autoimmune or lymphoproliferative disorders.
Introduction
Evans syndrome (ES), which was first described in 1951, is an autoimmune disorder characterized by the simultaneous or sequential development of autoimmune hemolytic anemia (AIHA) and immune thrombocytopenia (ITP) and/or immune neutropenia in the absence of any underlying cause.1,2 Although ES has been since its first description considered or defined as an “idiopathic” condition and thus mainly as a diagnosis of exclusion, ES may be associated with or show other diseases or conditions such as systemic lupus erythematosus (SLE),3 lymphoproliferative disorders,4,5 or primary immunodeficiencies.6 In childhood, ES may also show an autoimmune lymphoproliferative syndrome (ALPS), a disorder of disrupted lymphocyte homeostasis related to some mutations in the Fas apoptotic pathway.7 ES is a rare condition because it is diagnosed in only 0.8% to 3.7% of all patients with either ITP or AIHA at onset.8 Few and mainly pediatric data on ES are available in the literature9-11 ; therefore, the characteristics and outcome of adult's ES are poorly known. Moreover, because there have been no prospective or randomized controlled trials, the management of ES is based on empirical data, and it is mainly based on indirect evidence extrapolated from the “standard of care” in primary ITP or AIHA or both.
To better define the clinical spectrum of ES in adults and to describe the main characteristics and outcome of this rare condition, a survey was initiated in 2005 throughout the Working Group on Thrombocytopenias of the European Hematology Association. The data of 68 cases of ES included in this registry are reported here.
Methods
Patients
This retrospective cohort study was initiated in the department of Internal Medicine at Henri Mondor University Hospital (Créteil, France), a tertiary national referral center for adult autoimmune cytopenias. The study was then extended to 7 other centers in France (n = 3 departments of internal medicine) and Italy (n = 4 departments of hematology-oncology) throughout the Working Group on Thrombocytopenias of the European Hematology Association.
All consecutive patients with the diagnosis of ES in 1 of the 8 participating centers over a 17-year period and fulfilling the following eligibility criteria were included: age 16 years or more at diagnosis; ES defined by the occurrence (either simultaneously or sequentially within at most a 10-year period of time) of AIHA and ITP or autoimmune neutropenia. AIHA was defined by a hemoglobin (Hb) level of 11 g/dL or less at diagnosis with features of hemolysis (low haptoglobin level and/or elevated LDH and/or bilirubin levels) and a positive direct antiglobulin test (DAT) or when the DAT was negative, after the exclusion of any other cause of acquired or hereditary hemolytic anemia. ITP was defined according to American Society of Hematology criteria,12 and only patients with a platelet count below 100 × 109/L on 2 separate occasions were included. In case of concomitant active AIHA, the presence of a mild splenomegaly was not an exclusion criteria for ITP. Immune neutropenia was defined by a neutrophil count below 1.5 × 109/L on 2 separate occasions at least a week apart, without any other obvious cause and without any exposure to a myelotoxic drug. Exclusion criteria were the following: presence of numerous fragmented red cells (> 5%) on the blood smear with a negative DAT, suggesting a thrombotic thrombocytopenic purpura; a diagnosis of chronic cold agglutinin disease, any other cause of hereditary or acquired hemolytic anemia; drug-induced hemolytic anemia and/or thrombocytopenia. Beyond the DAT, the presence of other autoantibodies (eg, antinuclear or antiphospholipid antibodies) was not a criteria for exclusion, and the diagnosis of an associated autoimmune disease was assessed based on the usual classification criteria, namely the updated American College of Rheumatology revised classification criteria for SLE,13 the updated international criteria for definite primary antiphospholipid syndrome,14 and the European criteria for primary Sjögren syndrome.15 Patients fulfilling only 3 of the revised classification criteria for SLE from the American College of Rheumatology were defined as having an “incomplete” SLE.
To assess treatment efficacy, the following criteria were used. For AIHA, a complete response (CR) was defined by a Hb level of 12 g/dL or more in the absence of any transfusion without features of hemolysis (normal bilirubin and LDH levels ± normal haptoglobin level if performed). A partial response (PR) was defined by a Hb level of at least 10 g/dL with an increase of at least 2 g from baseline and a persistent hemolysis. For ITP, a CR was defined by a normal platelet count (ie, > 150 × 109/L) and a PR by a platelet count greater than 50 × 109/L with at least a 2-fold increase of the pretreatment count. The epidemiologic, clinical, and biologic data from every patient were collected by each investigator by using a standardized structured form and then reviewed and analyzed by the same investigator (M.M.).
Statistics
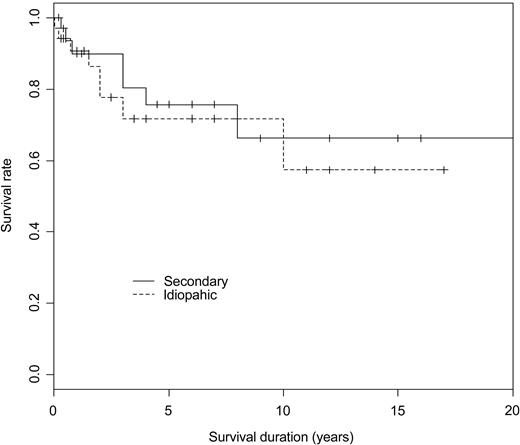
Descriptive statistics included mean (SD) or median (minimum-maximum) as appropriate for continuous variables and frequency (percentage) for categorical variables. Univariate analysis included the χ2 or Fisher exact test as appropriate to compare categorical variables and the nonparametric Wilcoxon test to compare continuous variables. The Kaplan and Meier curve was plotted to describe survival rate according to the type of ES (ie, primary vs secondary) and log-rank test compared both curves. Differences were considered as being significant when P values were less than .05.
The study was performed in accordance with the ethical standards of the Helsinki Declaration, including the provisions for patient informed consent, and it has been approved by the institutional review board of Henri Mondor Hospital.
Results
Characteristics of patients and associated diseases
The main characteristics of 68 patients, 41 women (60%) and 27 men, fulfilling the inclusion criteria have been collected and analyzed and are shown in Table 1. Five other cases were excluded from the analysis because of incomplete data (n = 3) or because the delay between cytopenias exceeded 10 years (n = 2 cases). Neither the prevalence nor the incidence of ES could be estimated given the retrospective design of the study, but, based on a cohort of approximately 500 cases of immune thrombocytopenia seen in our department (the Department of Internal Medicine at Henri Mondor Tertiary Referral Center) over a 15-year period, ES represented nearly 5% of all ITP cases. The mean age of the patients at time of analysis was 56.4 plus or minus 20.5 years, the mean age at time of ITP and AIHA diagnosis was identical (52 ± 33 years). Both cytopenias occurred simultaneously in 37 cases (54.5%), ITP preceded the onset of AIHA in 20 cases (29.5%), whereas AIHA was the first manifestation in only 11 patients (16%). When they occurred sequentially, the mean delay between both cytopenias was 4.2 plus or minus 3.5 years. A concurrent autoimmune neutropenia was observed in only 10 patients (14.7%) at the time of diagnosis of ES. ES was considered as idiopathic in 34 patients (50%) and was associated with an underlying disorder or condition in 34 cases. The various disorders or conditions associated with ES (ie, “secondary” ES) are shown in Table 2. After ES was diagnosed, 5 patients were diagnosed with a solid tumor (prostate, breast, pancreas, esophagus, and uterus cancers) during follow-up, but none of these malignancies has been considered as being related to ES. Among secondary ES, the mean age of patients with an associated autoimmune disease or a primary immunodeficiency was lower than the patients with non-Hodgkin lymphoma (NHL) or other miscellaneous disorders, although this difference did not reach a significant level (41 ± 15.3 years vs 58 ± 22 years; P = .051). Forty-four patients (66%) were tested for the presence of hepatitis C virus, and only 1 had a positive polymerase chain reaction with no features of active hepatitis. Overall, a marrow aspirate or biopsy had been performed in 62 patients (91%) and showed no abnormalities (other than an increased number of megakaryocytes and/or erythroblasts) in 50 cases (83%). Abnormalities suggesting a myelodysplastic syndrome were observed in 7 cases (11.5%); an increased number of small lymphocytes were found in 3 patients with chronic lymphocytic leukemia, and the presence of abnormal T cells was observed in a patient with a T-cell lymphoma.
Main characteristics of the 68 patients
| Characteristics . | Value . |
|---|---|
| Sex ratio (females/males) | 41 (60%)/27 |
| Mean age at ITP/AIHA onset | 55 ± 33 y |
| Mean (median and range) platelet level (×109/L) at ITP onset and/mean lowest level during follow-up | 31 (median 12.5: 2 to 100)/18 ± 15 |
| Mean Hb level (g/dL) at AIHA onset and/mean lowest level during follow-up | 7.6 ± 1.9/ 6.3 ± 1.2 |
| DAT pattern | |
| IgG | 43% |
| IgG + C3d | 53% |
| Negative | 4%* |
| No. of patients (%) who got a transfusion of packed red cell units for AIHA and/mean no. of units per patient | N = 32 (47%)/3.7 |
| No. of patients (%) with ITP-related bleeding symptoms | N = 44 (64%) |
| No. with a life-threatening hemorrhage | N = 3 (4.4%) |
| Characteristics . | Value . |
|---|---|
| Sex ratio (females/males) | 41 (60%)/27 |
| Mean age at ITP/AIHA onset | 55 ± 33 y |
| Mean (median and range) platelet level (×109/L) at ITP onset and/mean lowest level during follow-up | 31 (median 12.5: 2 to 100)/18 ± 15 |
| Mean Hb level (g/dL) at AIHA onset and/mean lowest level during follow-up | 7.6 ± 1.9/ 6.3 ± 1.2 |
| DAT pattern | |
| IgG | 43% |
| IgG + C3d | 53% |
| Negative | 4%* |
| No. of patients (%) who got a transfusion of packed red cell units for AIHA and/mean no. of units per patient | N = 32 (47%)/3.7 |
| No. of patients (%) with ITP-related bleeding symptoms | N = 44 (64%) |
| No. with a life-threatening hemorrhage | N = 3 (4.4%) |
DAT indicates direct antiglobulin test.
The 3 patients with a negative DAT had a simultaneous thrombocytopenia with a normal marrow and neither features of TTP on the smear nor any other cause of hemolytic anemia.
Secondary cases of Evans syndrome, n = 34
| Secondary cases . | n . |
|---|---|
| Autoimmune diseases | |
| Systemic lupus erythematosus | 7 |
| “Incomplete” lupus | 3 |
| Primary antiphospholipid syndrome | 2 |
| Sjögren syndrome | 2 |
| Immunodeficiencies | |
| Common variable immunodeficiency | 4 |
| IgA deficiency | 2 |
| Lymphomas | |
| B-cell non-Hodgkin malignant lymphoma | 2 |
| Chronic lymphocytic leukemia | 3 |
| T-cell non-Hodgkin lymphoma | 1 |
| Miscellaneous | |
| Chronic myelomonocytic leukemia | 1 |
| Unclassified lymphoproliferative disorder* | 3 |
| MGUS | 1 |
| Hepatitis C | 1 |
| Congenital asplenia | 1 |
| Idiopathic CD4 lymphocytopenia | 1 |
| Secondary cases . | n . |
|---|---|
| Autoimmune diseases | |
| Systemic lupus erythematosus | 7 |
| “Incomplete” lupus | 3 |
| Primary antiphospholipid syndrome | 2 |
| Sjögren syndrome | 2 |
| Immunodeficiencies | |
| Common variable immunodeficiency | 4 |
| IgA deficiency | 2 |
| Lymphomas | |
| B-cell non-Hodgkin malignant lymphoma | 2 |
| Chronic lymphocytic leukemia | 3 |
| T-cell non-Hodgkin lymphoma | 1 |
| Miscellaneous | |
| Chronic myelomonocytic leukemia | 1 |
| Unclassified lymphoproliferative disorder* | 3 |
| MGUS | 1 |
| Hepatitis C | 1 |
| Congenital asplenia | 1 |
| Idiopathic CD4 lymphocytopenia | 1 |
MGUS indicates monoclonal gammopathy of unknown significance.
Lymphadenopathy, splenomegaly + polyclonal hypergammaglobulinemia (n = 3) with an excess of large granular lymphocytes in 1 case. No features suggesting an ALPS (no excess of CD3+ C α/β+ CD4−/CD8− T cells in peripheral blood, no Fas, Fas-L, or caspase 8 genes mutations) has been found.
Complete data about the blood smear performed at time of AIHA onset were available in 57 patients. A significant spherocytosis was noted in 21 cases (37%), and the presence of a few to a moderate number of fragmented red cells (schistocytes) was mentioned in 10 cases (17.5%), all patients having a strongly positive DAT. A search for antiplatelet antibodies was performed in only 34 patients, 24 (70%) of whom had positive circulating and/or fixed IgG antiplatelet antibodies (APA) detected by immunofluorescence tests. When the patients with positive APA were tested for the presence of more specific antibodies by a monoclonal antibody immunization platelet assay, anti-IIbIIIa APA were predominantly found (60% of the cases) in combination with anti-IbIX in 2 patients.
Management
First-line treatments.
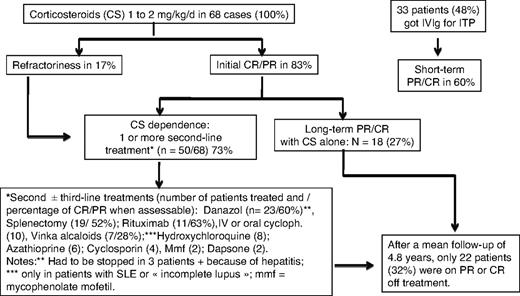
All 68 patients received at least one course of corticosteroids (prednisone or prednisolone) for the management of AIHA and/or ITP. Sixty-four patients (94%) were given corticosteroids at an initial dose varying from 1 to 2 mg/kg per day for the management of AIHA leading to an initial response rate in 53 cases (83%). Half of the responders had a CR, the other half had a PR, whereas 11 patients (17%) were considered as nonresponders. For the management of ITP, corticosteroids have been used in 54 patients (79%) with an overall initial response rate of 82% (CR in two-thirds, PR in one-third of the cases). Thirty-three patients (48%) were given intravenous immunoglobulin, almost exclusively for the management of ITP, leading to an initial (short-term) PR or CR in only 20 (60%) of the 33 cases.
Other treatment lines.
In total, 50 (73%) of the 68 patients received at least one “second-line” therapy, and 19 patients (28%) underwent splenectomy. Other second- or third-line therapies included danazol (n = 23 patients), oral or intravenous (intravenous pulses) cyclophosphamide (n = 10), rituximab (n = 11), hydroxychloroquine (n = 8 patients with SLE or “incomplete” lupus), vinka-alkaloids (n = 7), cyclosporine (n = 4), azathioprine (n = 6), mycophenolate (n = 2), and dapsone (for ITP, n = 2). Three other patients were given intravenous cyclophosphamide for the management of lupus nephritis, and 1 received oral cyclophosphamide for an underlying T-cell lymphoma.
Splenectomy.
Nineteen patients, 11 with primary ES and 8 with secondary ES, underwent splenectomy (Table 3). The main reason for splenectomy was chronic severe thrombocytopenia in 11 cases, persisting AIHA in 6 cases, and both cytopenias in 2 patients. An initial response (assessed at least 1 month after the procedure) was achieved in 15 (78%) of 19 cases, including 10 CR and 5 PR. After a mean follow-up of 8 plus or minus 4.9 years after splenectomy, 5 of the initial responders had relapsed (from 2 months to 9 years after the procedure); therefore, the long-term response rate to splenectomy was 52% (10 of 19). No significant difference was observed between primary and secondary cases of ES. One patient died of sepsis on day 21 after the procedure, none of the patients had any documented venous thrombosis after splenectomy.
Comparisons of the main characteristics of primary and secondary cases
| . | Primary ES, n = 34 . | Secondary ES, n = 34 . | P . |
|---|---|---|---|
| Mean age, y | 62 ± 21.5 | 53.5 ± 17 | .07 |
| Mean age at diagnosis, y | 57 ± 23 | 48 ± 19 | .08 |
| Sex ratio | .4 | ||
| Females | 19 (56%) | 22 (65%) | |
| Males | 15 (44%) | 12 (35%) | |
| Occurrence of ITP and AIHA | .3 | ||
| Simultaneously | 23 (68%) | 19 (56%) | |
| Sequentially | 11 (32%) | 15 (44%) | |
| Mean lowest Hb level, g/dL | 6.9 ± 1.7 | 6.1 ± 2.0 | .07 |
| Median lowest platelet level (×109/L) | 8 (1-100) | 6 (1-90) | .5 |
| Need for transfusion | 18 (53%) | 15 (44%) | .5 |
| Splenectomy | 11 (32%) | 8 (24%) | .5 |
| Initial response | 8 (72%) | 7 (87%) | .6 |
| Deaths | 9 (26%) | 7 (20.5%) | .4 |
| . | Primary ES, n = 34 . | Secondary ES, n = 34 . | P . |
|---|---|---|---|
| Mean age, y | 62 ± 21.5 | 53.5 ± 17 | .07 |
| Mean age at diagnosis, y | 57 ± 23 | 48 ± 19 | .08 |
| Sex ratio | .4 | ||
| Females | 19 (56%) | 22 (65%) | |
| Males | 15 (44%) | 12 (35%) | |
| Occurrence of ITP and AIHA | .3 | ||
| Simultaneously | 23 (68%) | 19 (56%) | |
| Sequentially | 11 (32%) | 15 (44%) | |
| Mean lowest Hb level, g/dL | 6.9 ± 1.7 | 6.1 ± 2.0 | .07 |
| Median lowest platelet level (×109/L) | 8 (1-100) | 6 (1-90) | .5 |
| Need for transfusion | 18 (53%) | 15 (44%) | .5 |
| Splenectomy | 11 (32%) | 8 (24%) | .5 |
| Initial response | 8 (72%) | 7 (87%) | .6 |
| Deaths | 9 (26%) | 7 (20.5%) | .4 |
Rituximab.
Eleven patients received at least one course of rituximab for the treatment of ES, among whom 5 had previously undergone splenectomy. The main indication for using rituximab was chronic severe corticosteroid-dependent and/or refractory ITP in 7 cases, severe or relapsing AIHA in 3 cases, and the need to pursue long-term therapy with corticosteroids to maintain the a PR of both AIHA and ITP in 1 case. The initial response rate to rituximab was 82% (5 CR and 4 PR). One patient with an unclassified lymphoproliferative disease (see Table 1) and a severe thrombocytopenia did not respond to rituximab and eventually achieved a CR after a subsequent splenectomy, one patient with primary ES and a severe AIHA did not respond to rituximab. After a mean follow-up of 12 months after the first rituximab infusion, 2 of the 9 initial responders had a relapse of ITP and AIHA, respectively, yielding a long-term response rate (CR and PR) of 7 (64%) of 11. Among the patients who achieved a lasting remission after rituximab, the longest follow-up at the time of analysis was 42 months.
Follow-up, outcome, and mortality
The mean follow-up from the diagnosis of ES was 4.8 years. At time of analysis (status at last visit), 22 patients (32%) were in remission (CR or PR) off treatment, 38 (56%) patients were on PR or CR on treatment (ie, prednisone at 0.1-1 mg/kg per day in combination with an immunosuppressor in 32 cases, or on immunosuppressor alone in 4 cases), whereas 8 patients (12%) still had an active disease while on treatment. Among the 34 patients with an initial diagnosis of idiopathic or primary ES, 2 patients subsequently developed a MDS, and 1 patient was diagnosed with a centroblastic stage IV B-cell lymphoma 8 years after the onset of ES (Figure 1). Among the 28 patients aged older than 60 years at AIHA diagnosis, a cardiovascular manifestation occurred in 6 patients (21.5%), including 1 myocardial infarction, 4 acute coronary syndromes, and 1 stroke. Sixteen patients (24%), 10 women (62.5%), and 6 men (mean age, 72.2 ± 20.5 years) had died at the time of analysis. None of the deaths was due to a hemorrhage, 3 patients died of an underlying cancer that was presumably unrelated to ES (Figure 2). The causes of deaths are summarized in Table 4.
Kaplan and Meier survival curves according to the type of ES (idiopathic vs secondary) in 68 patients with ES (P = .6, log-rank test).
Kaplan and Meier survival curves according to the type of ES (idiopathic vs secondary) in 68 patients with ES (P = .6, log-rank test).
Causes of deaths, n = 16
| Causes of death . | n . |
|---|---|
| Infection | 7 |
| Septic shock (n = 6) due to Staphylococcus aureus (1), Pseudomonas aeruginosa (1),* unknown bacteria (4)†; one patient with a prior history of stroke had a fatal aspiration pneumonia | |
| Cancer | 3 |
| Site of cancer: uterus (1), pancreas (1), esophagus (1) | |
| Cardiovascular | 3 |
| Stroke (2), myocardial infarction (1) | |
| Myelodysplastic syndrome (RAEB) | 1 |
| B-cell non-Hodgkin lymphoma | 1 |
| Unknown | 1 |
| Causes of death . | n . |
|---|---|
| Infection | 7 |
| Septic shock (n = 6) due to Staphylococcus aureus (1), Pseudomonas aeruginosa (1),* unknown bacteria (4)†; one patient with a prior history of stroke had a fatal aspiration pneumonia | |
| Cancer | 3 |
| Site of cancer: uterus (1), pancreas (1), esophagus (1) | |
| Cardiovascular | 3 |
| Stroke (2), myocardial infarction (1) | |
| Myelodysplastic syndrome (RAEB) | 1 |
| B-cell non-Hodgkin lymphoma | 1 |
| Unknown | 1 |
RAEB indicates refractory anemia with excess of blasts.
A concomitant hemorrhagic stroke was suspected at time of death.
Two of these patients had undergone a splenectomy 3 weeks and 2 years prior to death, respectively.
Discussion
Since its first description in the early 1950s, ES has long been considered as a rather incidental and “anecdotal” combination of ITP and AIHA and/or autoimmune neutropenia in the absence of any underlying cause. More recently, the spectrum of the disease has broadened, especially in children, and there is increasing evidence to suggest that ES reflects a state of profound immune dysregulation as opposed to a coincidental combination of immune cytopenias.16 To better define the clinical spectrum of adult ES, we have analyzed retrospectively the data from 68 adults with a well-defined ES seen in the departments of internal medicine or hematology of 8 major institutions in France and Italy. Despite the possible bias because of the retrospective design of the study and because the number of patients remains too small to point out many statistically significant differences between subgroups, this analysis gives a new insight into adult ES.
The diagnosis of ES still implies the exclusion of other conditions and especially thrombotic thrombocytopenic purpura, another severe condition that also requires prompt management. To avoid any detrimental misdiagnosis, the careful analysis of the peripheral blood smear and the DAT is of paramount importance. However, in clinical practice, true cases of ES may well show or precede a variety of underlying diseases or conditions which may influence both the management and outcome. For this reason, we believe that ES should no longer be considered only as an idiopathic condition but rather be classified as primary (or idiopathic) or secondary (ie, associated with an underlying disease) syndrome; a terminology currently used and accepted in the setting of ITP17 and AIHA.18 The high rate (50%) and the variety of associated conditions or diseases found in the present series strongly support the need for such a classification. As expected, ES was predominantly associated with SLE (or incomplete lupus) or to a lesser extent common variable immunodeficiency in younger patients (ie, age younger than 45 years), whereas NHL was the most commonly associated disease in patients aged older than 50 years. Interestingly, a stage IV B-cell NHL occurred 8 years after the onset of ES in 1 patient. This observation and the high number of associated NHLs or nonmalignant atypical lymphoproliferative disorders strongly suggest that patients with ES may be at higher risk of developing lymphoma, as previously observed in the setting of isolated wAIHAs (AIHA due to warm antibodies).19,20 There is no consensus about which laboratory tests and radiologic procedures should be systematically performed in patients with ES to look for an underlying disease. On the basis of this analysis and on our previous experience on isolated wAIHAs,21 we suggest that a minimal workup, including a chest and abdominal computed tomography scan should be performed in every patient diagnosed with ES (see Table 5 for proposals). To avoid a diagnostic bias, we arbitrarily considered 10 years as being the maximum delay between both cytopenias to retain the diagnosis of ES. That ITP and AIHA occurred sequentially in 39% of the cases reported here (mean delay between both cytopenias of 4.2 ± 3.4 years) suggests that both in primary or secondary ES, the disruption of self-tolerance may be a multiple step process and that being treated for one immune cytopenia does not necessarily prevent the occurrence of a second cytopenia. Three patients with an atypical lymphoproliferative syndrome (including 1 patient with a major hypergammaglobulinemia) have been screened for the presence of circulating CD3+ α/β+ CD4−/CD8− T cells and for abnormalities in the Fas-dependent apoptotic pathway, but no features of ALPS have been found in any of them. That some adults with ES and nonmalignant diffuse lymphadenopathy and/or a high level of gammaglobulins may have an underlying ALPS, as previously shown in children,7 cannot be ruled out at this stage, but will need a more systematic screening of consecutive patients with such a phenotype.22
Proposals for a minimal work-up in adults with a newly diagnosed ES
| Proposals . |
|---|
|
| Proposals . |
|---|
|
HCV indicates hepatitis C virus; and HBV, hepatitis B virus.
To be considered especially in patients with a history of venous and/or arterial thrombosis and/or in women with recurrent pregnancy loss.
To prevent viral reactivation in HBV carriers prior to corticosteroid therapy and especially if rituximab is considered.
To be considered in patients with features suggesting a lymphoproliferative disorder (monoclonal gammopathy).
Regarding demographics, both the mean age at time of diagnosis of ES (52 years) and the sex ratio (60% of females) were in keeping with those usually observed in adult primary ITP and wAIHA seen separately.18,23 The severity and outcome of thrombocytopenia and hemolytic anemia could be strikingly different even in the single patient. ITP was symptomatic in two-thirds of the cases at onset and lead to a life-threatening hemorrhage in 3 patients, including a spontaneous intracranial hemorrhage in 2 patients (3%) younger than 50 years. In the absence of a common scale for rating bleeding manifestations and of a control group of patients with primary ITP, we can only speculate from this data that immune thrombocytopenia occurring in the setting of ES could more often lead to severe bleeding manifestation. More obvious, compared with the data previously reported in children,7,24,25 was the increased risk of cardiovascular complication (acute coronary syndrome or stroke) related to AIHA in patients aged 60 or older. This finding emphasizes that in this subgroup of patients, the risk of AIHA and AHAI-related complications is probably higher than the thrombocytopenia-related risk of severe bleeding and that red cell transfusion should not be delayed. Corticosteroids are the mainstay of therapy, and the typical course of therapy was a dose of prednisone at 1 to 2 mg/kg per day tapered over a few weeks in case of isolated thrombocytopenia or many months for the management of wAIHA. As commonly observed in primary ITP as well as in wAIHA, the large majority (∼ 80%) of patients did respond initially to corticosteroids, but eventually up to two-third were given at least one second-line treatment, mostly to spare corticosteroids. Danazol was frequently used as a corticosteroid-sparing agent (n = 22) and has been considered to be “effective” in nearly two-thirds of the patients. Its mechanism of action is still unknown, and, to avoid toxicity, the minimal effective dose (from 200 mg to a maximum of 400 mg per day) should be considered.26,27 For patients not responding to corticosteroids or who are corticosteroids dependent (ie, requiring a daily dose of prednisone ≥ 15 mg to maintain a remission), splenectomy may remain the preferred option. Indeed, the long-term response rate to splenectomy was 52% in the present series. However, patients who received a splenectomy have a lifelong increased risk of severe sepsis, and vaccination against Streptococcus pneumoniae, meningococcus, and Haemophilus is mandatory. Note, in our series an 80-year-old woman with ES who had been previously treated with corticosteroids and azathioprine died of sepsis 3 weeks after the procedure. In the absence of any reliable preoperative parameter predictive of a good response, the risk/benefit ratio of splenectomy must be carefully weighed in every patient. In patients who are reluctant to undergo splenectomy28 and/or in those with an underlying condition (SLE, common variable immunodeficiency, chronic lymphocytic leukemia) or comorbidities that may increase the risk of infections, or may not be improved by the procedure, other therapeutic options must be considered. Among these options, rituximab has already shown its efficacy in primary ITP,29 wAIHA,30 and, in a lesser extent, ES.31-34 In the present series, the initial response rate after rituximab treatment was as high as that previously observed in wAIHA but the follow-up was too short to draw definite conclusions. However, on the basis of the published data and on our own experience and given the relatively good safety profile of the drug, we believe that rituximab is now worth considering before splenectomy and/or the use of immunosuppressors in patients who have a chronic and corticosteroid-dependent ES. For patients who are nonresponsive to rituximab and/or splenectomy, the choice of a specific immunosuppressant (intravenous cyclophosphamide, cyclosporin, or azathioprine) is still based on personal and/or center experience. Although cyclosporin A35,36 and more recently mycophenolate mofetil37,38 have shown some efficacy in a small series of patients with refractory immune cytopenias, including a few cases of ES, the choice must rely mainly on the patient's profile (age, sex, comorbidities, and disease severity).
In conclusion, ES is more than a coincidental combination of immune cytopenias but rather a chronic state of profound dysregulation of the immune system that may be associated with or show other autoimmune or lymphoproliferative disorders as well as primary immunodeficiencies. The distinction between primary and secondary ES is important in the diagnosis because it may influence the management of the disease even if corticosteroids remain the cornerstone of treatment. Pooling more clinical and immunologic data in the future, throughout international working groups and networks will be helpful to learn more about ES pathophysiology and for refining therapeutic strategies.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.M. initiated the study and the survey, performed the data analysis, and wrote the manuscript; V.C., J.-C.P., L.C., G.E., F.Z., M.R., E.A., P.B., and B.G. included patients in the survey; A.-S.M. participated in the analysis of the data; A.D. performed statistics and reviewed the manuscript; and F.R. allowed the extension of the survey to Italy and participated in the writing of the manuscript.
Conflict-of-interest disclosure: M.M., F.Z., P.B., and B.G. have received research support for clinical studies from Roche; M.M., B.G. and F.R. have received fees from Amgen and GlaxoSmithKline for their participation in scientific advisory boards. The remaining authors declare no competing financial interests.
Correspondence: Marc Michel, Service de Médecine Interne, CHU Hôpital Henri-Mondor, Assistance Publique Hôpitaux de Paris, Université Paris 12, 51 Av du Mal de Lattre de Tassigny, 94010 Créteil cedex, France; e-mail: marc.michel@hmn.aphp.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal