Abstract
Lytic bone disease in myeloma is characterized by an increase in multinucleate osteoclasts in close proximity to tumor cells. However, the nature of osteoclast precursors and the mechanisms underlying multinuclearity are less understood. Here we show that culture of myeloma cell lines as well as primary myeloma cells with human dendritic cells (DCs) but not monocytes or macrophages leads to spontaneous cell-cell fusion, which then leads to the facile formation of multinucleate bone-resorbing giant cells. Osteoclastogenesis is cell contact dependent, leading to up-regulation of thrombospondin-1 (TSP-1) in DCs. Disruption of CD47–TSP-1 interaction by TSP-1–blocking antibodies or down-regulation of CD47 on tumor cells by RNA interference abrogates tumor-induced osteoclast formation. Blockade of CD47–TSP-1 interactions also inhibits receptor activator for nuclear factor κB ligand- and macrophage colony-stimulating factor–induced formation of osteoclasts from human monocytes. Further, TSP-1 blockade attenuates hypercalcemia induced by parathyroid hormone in vivo. These data point to a role for CD47–TSP-1 interactions in regulating cell-fusion events involved in human osteoclast formation. They also suggest that DCs, known to be enriched in myeloma tumors, may be direct precursors for tumor-associated osteoclasts. Disruption of CD47–TSP-1 interactions or preventing the recruitment of DCs to tumors may provide novel approaches to therapy of myeloma bone disease and osteoporosis.
Introduction
Multiple myeloma (MM) is a hematologic malignancy characterized by the accumulation of malignant plasma cells in the bone marrow. The development of progressive osteolytic bone disease is one of the earliest manifestations of myeloma and a major cause of disease-related morbidity.1 Bone destruction in myeloma is mediated by osteoclasts (OCLs) and further exacerbated by a decrease in bone-forming osteoblasts.2,3 Several factors secreted by myeloma cells (or surrounding stromal cells) have been implicated in promoting osteoclast formation (eg, receptor activator for nuclear factor κB ligand [RANKL], macrophage inflammatory protein 1α, tumor necrosis factor-α, interleukin [IL]–1, IL-6, lymphotoxin), or inhibiting osteoblast formation (Dickkopf-1, IL-3, transforming growth factor-β).2-9 A role for T cells in myeloma-associated osteoclastogenesis also has been implicated via the expression of RANKL by these cells.10 Bone destruction in MM typically is observed in close proximity to tumor cells. Contact-dependent mechanisms have been implicated in both myeloma bone disease and tumor growth.11,12 However, the nature of cell-associated factors promoting tumor-induced osteoclastogenesis remains to be fully clarified.
OCLs are generated by cell fusion events involving myeloid lineage precursors, leading to the formation of multinucleate giant cells.13 The mechanisms underlying osteoclastic cell fusion have been mostly studied with murine cells and have been less well characterized with human cells.14 The fusion machinery underlying osteoclastogenesis may in principle differ in physiologic and pathologic settings, although several molecules such as dendritic cell–specific transmembrane protein (DC-STAMP), signal regulatory protein (SIRP)α, CD47, and tetraspanins have been implicated in the fusion of murine/rat macrophages.14-16 Mechanisms underlying cell fusion in the setting of tumor-induced osteoclastogenesis have not been described.
Dendritic cells (DCs) are specialized antigen-presenting cells also derived from myeloid lineage precursors. DCs play a central role in the regulation of innate and adaptive immunity. Cells in the myelomonocytic lineage, including DCs, demonstrate high developmental and functional plasticity depending on the signals from the microenvironment.17 The authors of recent studies have described a novel pathway of OCL development from human DCs in culture in the presence of inflammatory cytokines,18 as well as from murine DCs in vitro and in the bone marrow microenvironment in vivo.19-22 DCs were also shown to be precursors for IL-17–dependent giant cell formation in Langerhans cell histiocytosis.23 The authors of other studies24-27 have shown that myeloma tumors in patients are extensively infiltrated by DCs. We and others24,28 have previously shown that the interaction of tumor cells and DCs promotes survival and clonogenicity of myeloma cells. However, the reverse, ie, the effect of myeloma cells on DCs, has been studied only in the context of their immunogenicity.29 Here we have examined the capacity of myeloma cells to induce the differentiation of DCs to OCLs.
Methods
Tumor cell lines and patient samples
The human MM cell lines ARK (gift from Dr J. Epstein, University of Arkansas), U266, and OPM-2 (from ATCC) were cultured in complete medium consisting of RPMI 1640 (Mediatech), 2 mmol/L l-glutamine, 20 μg/mL gentamicin sulfate (Mediatech), and 10% fetal bovine serum. Other tumor cell lines used were NCEB1 (mantle-cell lymphoma; gift from Dr Owen O'Connor, Memorial Sloan-Kettering Cancer Center) and MCF-7 (breast cancer; gift of Dr Livingston, Memorial Sloan-Kettering Cancer Center). Bone marrow specimens from myeloma patients were obtained after informed consent was obtained in accordance with the Declaration of Helsinki under a protocol approved by the institutional review board of Yale University.
Generation of human DCs
Peripheral blood buffy coats from healthy donors were purchased from New York Blood Center. Peripheral blood mononuclear cells were isolated by density gradient centrifugation (Ficoll-Paque plus; Amersham Biosciences). DCs were generated from purified blood monocytes as described.30 In brief, monocytes isolated by the use of CD14 microbeads (Miltenyi Biotech) were cultured in the presence of granulocyte monocyte colony stimulating factor (GM-CSF, 20 ng/mL; Immunex) and IL-4 (20 ng/mL; R&D Systems). DCs were typically used on day 5 or 6 of culture. For some experiments, DCs were matured by the use of inflammatory cytokines or lipopolysaccharide (LPS, 20 ng/mL; Sigma-Aldrich) as described.31
Methylcellulose cultures to study DC-tumor interactions
To assess the interactions between myeloid cells (monocytes/DCs) and tumor cells, tumor cells were mixed with purified CD14+ monocytes, monocyte-derived DCs (DCs), or GM-CSF–treated monocytes at a tumor/myeloid cell ratio of 1:100 before they were plated in methylcellulose containing 5% leukocyte-conditioned media (Methocult; StemCell Technologies Inc) as described.28 Cells were plated in 35-mm2 tissue culture dishes in quadruplicates and incubated at 37°C and 5% CO2. Cultures were monitored weekly and harvested periodically. The formation of multinucleate giant cells was monitored by immunofluorescence microscopy, as described in the section “Immunofluorescence microscopy.” Monocytes cultured with RANKL and M-CSF, as described in the section “Monocyte-derived OCLs,” were used as positive controls for the development of OCLs.
To assess the need for cell contact between DCs and tumor cells, DCs were suspended in 2% Iscove modified Dulbecco medium (StemCell Technologies Inc) and were separated from tumor cells by a Transwell insert (Thermo Fisher Scientific). Controls inserts received 2% Iscove modified Dulbecco medium only. For some experiments, tumor cells and DCs were cultured in the presence or absence of either antithrombospondin-1 monoclonal antibody (anti-TSP-1 mAb clone C6.7; Thermo Fisher Scientific) or isotype control mAb. For some experiments, osteoprotegerin (OPG; 0.5 μg/mL; R&D Systems) was added to inhibit RANKL signaling.
Monocyte-derived OCLs
OCLs were generated from purified CD14+ monocytes separated from peripheral blood as described previously. Monocytes (0.5 × 106 cells/mL) were cultured in α-minimum essential medium (MEM) supplemented with 10% fetal bovine serum, gentamicin, RANKL (50 ng/mL; PeproTech), and M-CSF (25 ng/mL; PeproTech) in 24-well plate or on BioCoat osteologic discs (BD Biosciences) for 7 to 10 days. Cytokines were replenished every 3 days.
Immunofluorescence microscopy
Cytospins were made onto the poly-l-lysine–coated (Sigma) multiwell slides (Carlson Scientific Inc). Cells were fixed with acetone and stained with primary and secondary antibodies for 30 minutes at room temperature. Primary antibodies, anti-CD138 (fluorescein isothiocyanate [FITC]) or anti-CD11c (phycoerythrin; BD Biosciences), and the secondary antibody Alexa Fluor 488 goat anti–mouse IgG1 (Molecular Probes) were used at 1:30 and 1:200 dilution, respectively. 4′,6-Diamidino-2-phenylindole (DAPI) was used a nuclear stain. For some experiments, cells also were stained for the expression of TSP-1 on DCs by the use of anti–TSP-1 antibody (Thermo Fisher Scientific) followed by Alexa Fluor 568 goat anti–mouse IgG1 (Molecular Probes). Slides were evaluated for 2-color fluorescence microscopy by the use of an Olympus epifuorescence microscope with a motorized stage to allow 0.5-mm optical sections with a cooled charge-coupled device camera (Hammatsu) and Metamorph software (Universal Imaging Corp).
Tartarate-resistant acid phosphatase staining
Cytospins from tumor/myeloid cocultures from clonogenic assays were placed onto the poly-l-lysine–coated (Sigma-Aldrich) multiwell slides (Carlson Scientific Inc). Tartarate-resistant acid phosphatase (TRAP) activity of multinucleated giant cells (MGCs) observed in the cocultures was assessed by use of the leukocyte acid phosphatase kit (Sigma-Aldrich).
Bone resorption assays
To measure osteoclast-mediated bone resorption, cells harvested from clonogenic assays were cultured on BD Biocoat Osteologic discs (BD Biosciences). After an incubation of 2 weeks, osteologic discs were treated with bleach solution to remove cells and stained with Von Kossa staining kit (Diagnostic BioSystems) to enumerate resorption pits on each disc. Bone resorption was quantified by counting the resorption pits. Resorbed area was quantified by densitometry by the use of ImageJ software (W. S. Rasband, National Institutes of Health; http://rsb.info.nih.gov/ij/).
Dual immunofluorescence and XY FISH analysis
To evaluate whether the nuclei in multinucleate giant cells included those from tumor cells, DCs from female donors were cocultured with U266 cells (male origin). After an incubation of 2 weeks, cytospins were made onto the poly-l-lysine–coated (Sigma) multiwell slides (Carlson Scientific Inc). Cells were fixed with acetone and stained with CD11c (FITC). Fluorescence in situ hybridization (FISH) was performed by the use of plasmid DNA for the X centromere (pSV2X5)32 labeled with SpectrumGreen (Vysis; Abbott Laboratories) and for the Yqh region (pHY10)33 labeled with Spectrum Orange (Vysis) by nick translation. Cytospin slides were lightly treated with 20 μg/mL pepsin in 0.01 N HCl for 3 minutes at 37°C, rinsed with phosphate-buffered saline (PBS), incubated in PBS/MgCl2 for 5 minutes at room temperature, then fixed in 1% paraformaldehyde (in PBS/MgCl2) for 10 minutes, rinsed with PBS then briefly with pure water, and dehydrated through ethanols. Approximately 5 to 10 ng of labeled X&Y probes was mixed with hybridization buffer containing 50% formamide, 10% dextran sulfate, 1% sodium dodecyl sulfate, 1× Denhardt solution, 0.04 mol/L phosphate buffer, pH 7. Ten microliters of hybridization mix were then applied to the slides and a 22 × 22-mm coverslip was sealed in place with rubber cement. The slides were then heated to 73°C for 3 minutes on a Hybrite automated hybridizing station (Vysis; Abbott Laboratories) and then hybridized at 37°C overnight. The rubber cement was removed and the coverslips were soaked off in 2× saline sodium citrate (SCC) at 42°C. The slides were washed twice in 50% formamide in 1× SSC at 42°C for 5 minutes, then rinsed twice in 2× SSC, stained in 0.08 μg/mL DAPI solution, rinsed, and mounted in Vectashield antifade (Vector Laboratories). The slides were analyzed by use of a Zeiss Axioplan fluorescence microscope equipped with Isis imaging software (MetaSystems).
Flow cytometry for evaluation of tumor cells
Tumor cells were stained with anti-CD138 and anti-CD47 (BD Biosciences) antibodies and were analyzed with FACSCalibur. Data were analyzed by the use of FlowJo software (TreeStar).
Evaluation of TSP-1 expression by TaqMan
RNA was extracted from cells by the use of the RNeasy Mini Kit (QIAGEN). TSP-1 expression was quantified by use of the Assays-on-Demand primer-probes from Applied Biosystems. Reverse transcription polymerase chain reaction (RT-PCR) was performed by the use of EZ PCR Core Reagents (Applied Biosystems) according to the manufacturer's directions. The samples were amplified and quantified on an Applied Biosystems PRISM 7700 by use of the following thermal cycler conditions: 2 minutes at 50°C; 30 minutes at 60°C; 5 minutes at 95°C; and 40 cycles of 15 seconds at 95°C followed by 60 seconds at 60°C. The glyceraldehyde-3-phosphate dehydrogenase, a housekeeping gene, was used to normalize each sample. The data were analyzed, and samples were quantified by the software provided with the Applied Biosystems PRISM 7700.
Inhibition of CD47 on tumor cells by RNA interference
Myeloma cells (U266) were harvested, washed with Opti-MEM I (Invitrogen), and resuspended in Opti-MEM I at a concentration of 2.5 × 107 cells/mL. A total of 4 × 106 tumor cells were electroporated with 20 μg of CD47 siRNA (siGENOME SMARTpool; Thermo Fisher Scientific) or nontargeting siRNA (siCONTROL Nontargeted siRNA; Thermo Fisher Scientific) in a 4-mm electroporation cuvette by use of the ECM830 Square Wave pulse of 500 V for 0.5 ms. The electroporated tumor cells were immediately resuspended in complete medium (RPMI 10% fetal calf serum). Inhibition of CD47 protein was examined by flow cytometry at various time points.
Effect of TSP-1 blockade on PTH-induced hypercalcemia in vivo
Six-week-old CD1 female mice were allowed to acclimate in the Yale animal care facility. After 1 week, animals were infused for 5 days with human (1-34)parathyroid hormone [(1-34)PTH] at 15 pmol/hour via a subcutaneous Alzett minipump implanted in the interscapular region. Animals were injected intraperitoneally with 0.3 mg of TSP-1 antibody (Thermo Scientific; mouse monoclonal; clone C6.7) or isotype control antibody at the time of minipump implantation and 2.5 days later. On day 5 after infusion, the animals were killed by anesthesia followed by terminal cardiac bleeding to collect blood for the measurement of serum calcium. Yale University provided Institutional Animal Care and Use Committee approval for the use of mice in this study.
Statistical analysis
Data from different experimental groups were compared by use of the Student t test or Mann-Whitney U test and significance set at P less than .05.
Results
Myeloma-induced fusion of DCs to form MGCs
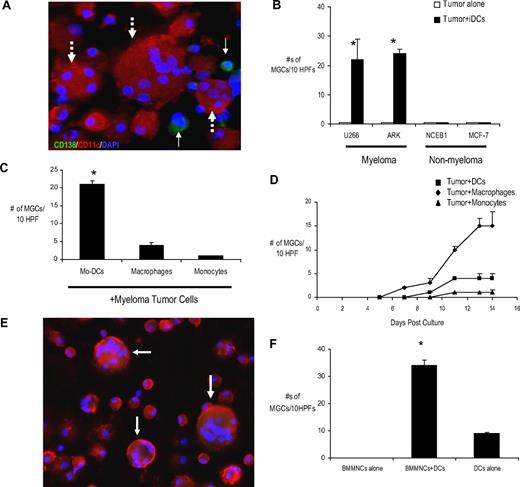
To study the effects of myeloma cells on DCs, tumor cells were cocultured with monocyte-derived DCs in methylcellulose cultures. The phenotype of DCs used in these experiments was CD14−, HLA-DR+, CD80lo, CD86lo, and CD83−, consistent with immature DCs (iDCs), as previously described (data not shown).34 After 12 to 14 days, cells were harvested and analyzed. Surprisingly, coculture of myeloma cells with iDCs led to facile formation of MGCs without the need to add RANKL or M-CSF to these cultures (Figure 1A). The formation of MGCs was observed only with myeloma cell lines but not lymphoma or breast cancer cells tested (Figure 1B). Myeloma-induced formation of MGC was also specific for DCs because it was not observed in cocultures with purified CD14 monocytes or monocytes treated with GM-CSF (Figure 1C). The formation of MGCs was rapid and could be observed as early as 7 to 8 days of culture (Figure 1D). Induction of MGC formation was proportional to the ratio of DC/tumor cells in these cocultures (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). To extend these observations to primary tumor cells, we obtained bone-marrow cells from myeloma patients and cocultured primary tumor cells with DCs. As with MM cell lines, cocultures of tumor cells from MM patients with DCs also led to the formation of MGCs (Figure 1E-F). Therefore, myeloma cells induce cell fusion in human DCs, leading to formation of MGCs in vitro.
Myeloma promotes MGC formation by inducing cell fusion in human dendritic cells. (A) Immunofluorescense analysis of MGCs in tumor-DC cocultures. Myeloma cells (U266) were plated with or without monocyte-derived DCs at a tumor/DC ratio of 1:100 in methylcellulose cultures. Cells were harvested for cytospins after an incubation of 2 weeks. Slides were acetone-fixed and stained for CD138 (green), CD11c (red), and DAPI (blue). MGCs were counted in 10 random high-power fields. Shown is a representative area with 3 CD138−CD11c+ giant cells with varying numbers of nuclei (dotted arrows) and CD138+ tumor cells (thin arrows). Original magnification, ×40. (B) Myeloma (U266 and ARK), breast cancer (MCF-7) or mantle cell lymphoma cell line (NCEB1) were plated with and without DCs at a ratio of 1:100 in methylcellulose cultures. Cells were harvested for cytospins after an incubation of 2 weeks. Slides were acetone-fixed and stained for CD138, CD11c, and DAPI as described in panel A. The numbers of MGCs were counted in 10 random high-power fields (HPF). Results are the mean ± SEM of 3 separate experiments. *P < .05. (C) Myeloma cells (U266) were plated with either monocytes (Monocytes), GM-CSF–treated monocytes (Macrophages), or immature DCs (Mo-DCs) cultured from the same donor at a tumor/myeloid cell ratio of 1:100 in methylcellulose cultures. Cells were harvested for cytospins after 2 weeks. Slides were acetone-fixed and stained as described previously. Numbers of MGCs were counted in 10 random HPF. Results are the mean ± SEM of 3 separate experiments for the cell line. *P < .05. (D) Kinetics of MGC formation in the experimental conditions in panel C were monitored by microscopy. *P < .05. (E) Bone marrow mononuclear cells (BMMNCs) from a myeloma patient were cocultured with DCs in methylcellulose cultures at a tumor/DC ratio of 1:100. Cytospins were made after an incubation of 2 weeks. Slides were acetone-fixed and stained for CD138, CD11c, and DAPI and counted for MGCs (arrows) as described previously. Data are representative of similar experiments on 3 separate patients. Original magnification, ×20. (F) Enumeration of MGCs in BMMNC + DC cocultures from a representative myeloma patient is shown. Data are representative of similar experiments on 3 different patients. BMMNCs alone and DCs alone were used as controls.
Myeloma promotes MGC formation by inducing cell fusion in human dendritic cells. (A) Immunofluorescense analysis of MGCs in tumor-DC cocultures. Myeloma cells (U266) were plated with or without monocyte-derived DCs at a tumor/DC ratio of 1:100 in methylcellulose cultures. Cells were harvested for cytospins after an incubation of 2 weeks. Slides were acetone-fixed and stained for CD138 (green), CD11c (red), and DAPI (blue). MGCs were counted in 10 random high-power fields. Shown is a representative area with 3 CD138−CD11c+ giant cells with varying numbers of nuclei (dotted arrows) and CD138+ tumor cells (thin arrows). Original magnification, ×40. (B) Myeloma (U266 and ARK), breast cancer (MCF-7) or mantle cell lymphoma cell line (NCEB1) were plated with and without DCs at a ratio of 1:100 in methylcellulose cultures. Cells were harvested for cytospins after an incubation of 2 weeks. Slides were acetone-fixed and stained for CD138, CD11c, and DAPI as described in panel A. The numbers of MGCs were counted in 10 random high-power fields (HPF). Results are the mean ± SEM of 3 separate experiments. *P < .05. (C) Myeloma cells (U266) were plated with either monocytes (Monocytes), GM-CSF–treated monocytes (Macrophages), or immature DCs (Mo-DCs) cultured from the same donor at a tumor/myeloid cell ratio of 1:100 in methylcellulose cultures. Cells were harvested for cytospins after 2 weeks. Slides were acetone-fixed and stained as described previously. Numbers of MGCs were counted in 10 random HPF. Results are the mean ± SEM of 3 separate experiments for the cell line. *P < .05. (D) Kinetics of MGC formation in the experimental conditions in panel C were monitored by microscopy. *P < .05. (E) Bone marrow mononuclear cells (BMMNCs) from a myeloma patient were cocultured with DCs in methylcellulose cultures at a tumor/DC ratio of 1:100. Cytospins were made after an incubation of 2 weeks. Slides were acetone-fixed and stained for CD138, CD11c, and DAPI and counted for MGCs (arrows) as described previously. Data are representative of similar experiments on 3 separate patients. Original magnification, ×20. (F) Enumeration of MGCs in BMMNC + DC cocultures from a representative myeloma patient is shown. Data are representative of similar experiments on 3 different patients. BMMNCs alone and DCs alone were used as controls.
Phenotypic and functional characterization of myeloma-induced MGCs
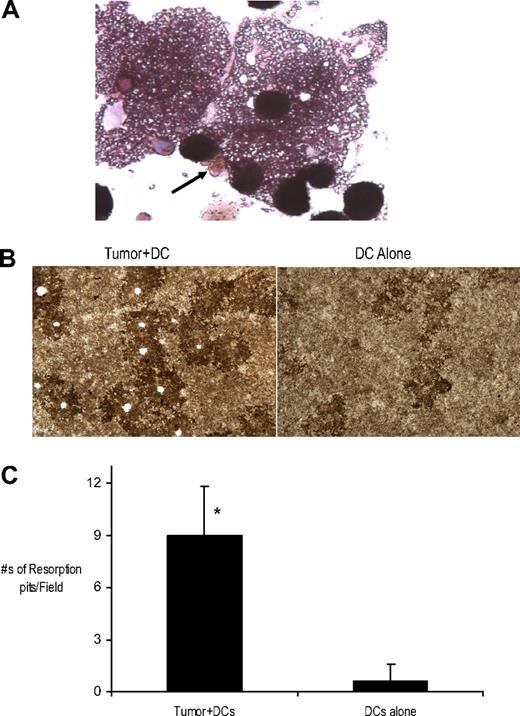
Myeloma-induced MGCs stained positive for TRAP (Figure 2A). Importantly, when these cells were placed on osteologic discs, they efficiently resorbed hydroxyapatite (Figure 2B). Greater resorption was observed with cocultures of DCs and tumor cells versus DCs alone (Figure 2C). Thus, MGCs induced by myeloma cells bear functional and phenotypic similarity to OCLs.
Characterization of MGCs present in myeloma-DC cocultures. (A) Tumor cells were cocultured with immature DCs at a tumor/DC ratio of 1:100 in methylcellulose cultures. Cells were harvested for cytospins after an incubation of 2 weeks and stained for TRAP activity Shown is a representative TRAP + MGC (↑). Original magnification, ×40. (B-C) Bone resorption activity of MGCs present in tumor-DC cocultures. Cells from the methylcellulose cultures of tumor + DCs or DCs alone were cultured on Biocoat osteologic discs for 2 weeks. (B) Appearance of resorbed bone (pits) after Von Kossa staining is shown. Original magnification, ×10. (C) Quantification of resorbed osteologic discs or pit area by light microscopy. *P < .05.
Characterization of MGCs present in myeloma-DC cocultures. (A) Tumor cells were cocultured with immature DCs at a tumor/DC ratio of 1:100 in methylcellulose cultures. Cells were harvested for cytospins after an incubation of 2 weeks and stained for TRAP activity Shown is a representative TRAP + MGC (↑). Original magnification, ×40. (B-C) Bone resorption activity of MGCs present in tumor-DC cocultures. Cells from the methylcellulose cultures of tumor + DCs or DCs alone were cultured on Biocoat osteologic discs for 2 weeks. (B) Appearance of resorbed bone (pits) after Von Kossa staining is shown. Original magnification, ×10. (C) Quantification of resorbed osteologic discs or pit area by light microscopy. *P < .05.
Mechanism of MGC formation
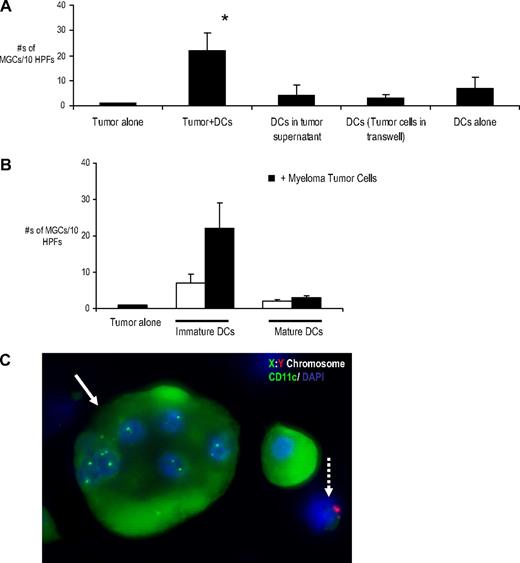
Myeloma-mediated formation of MGCs required cell-cell contact between tumor cells and DCs because we did not observe any MGCs when DCs and myeloma cells were separated by a Transwell or when DCs were cultured in the presence of supernatant from myeloma cells (Figure 3A). Myeloma cells fixed with paraformaldehyde before the addition to DCs could also induce the formation of MGCs, further indicating that this formation was not related to active secretion of cytokines from live myeloma cells (supplemental Figure 2). The capacity of DCs to undergo cell fusion is linked to their maturation status and primarily observed with immature DCs, as recently described for murine DCs.35 Activation of DCs with LPS led to marked inhibition of MGC formation (Figure 3B). To better understand the nature of fusing cells, we performed combined FISH and immunofluorescence analysis to test for heterotypic cell fusion between myeloma cells and iDCs. In these studies, DCs from a female donor were cocultured with male tumor cells (U266 cells), and XY FISH analysis along with immunofluorescence staining for CD11c and DAPI was performed to track tumor cell nuclei in the MGCs. MGCs were found to contain DC-derived nuclei, but we were unable to document tumor cell nuclei in the nuclear pool of MGCs (Figure 3C). Thus MM-induced formation of MGC is dependent on cell-cell contact between DCs and tumor cells and predominantly involves homotypic fusion between DCs.
Mechanism of myeloma-mediated MGC formation. (A) Cell-cell contact dependency. Myeloma cells (U266) were cultured in methylcellulose cultures with DCs as in Figure 1. DCs were either plated with U266 or separated from tumor cells by a Transwell insert. In addition, DCs also were cultured in the presence of supernatant from the myeloma cell line (U266). Cells were harvested onto slides after an incubation of 2 weeks to be stained (CD138, CD11c, and DAPI) and MGC enumeration. MGCs were counted in 10 random high-power fields (HPFs). Data are representative of 3 different experiments. *P < .05. (B) Myeloma cells (U266) were cultured with immature DCs or LPS-matured DCs in methylcellulose cultures. Immature DCs alone and mature DCs alone were used as the controls. Cells were harvested for cytospins after an incubation of 2 weeks. Slides were acetone-fixed and stained for CD138, CD11c, and DAPI. MGCs were counted in 10 random HPFs. Results are the mean ± SEM of 3 separate experiments. *P < .05. (C) Combined immunofluorescence and FISH analysis of MGCs. U266 cells (male origin) were cocultured with iDCs (female origin) in methylcellulose cultures as described previously. After 2 weeks, cells were harvested onto cytospins and stained for CD11c (green) and X (green):Y(Red) chromosome. Shown is a representative CD11c+ MGCs with 6 nuclei, all with XX chromosomes (arrow). Note U266 cell with Y (red) chromosome (dotted arrow). Original magnification, ×100.
Mechanism of myeloma-mediated MGC formation. (A) Cell-cell contact dependency. Myeloma cells (U266) were cultured in methylcellulose cultures with DCs as in Figure 1. DCs were either plated with U266 or separated from tumor cells by a Transwell insert. In addition, DCs also were cultured in the presence of supernatant from the myeloma cell line (U266). Cells were harvested onto slides after an incubation of 2 weeks to be stained (CD138, CD11c, and DAPI) and MGC enumeration. MGCs were counted in 10 random high-power fields (HPFs). Data are representative of 3 different experiments. *P < .05. (B) Myeloma cells (U266) were cultured with immature DCs or LPS-matured DCs in methylcellulose cultures. Immature DCs alone and mature DCs alone were used as the controls. Cells were harvested for cytospins after an incubation of 2 weeks. Slides were acetone-fixed and stained for CD138, CD11c, and DAPI. MGCs were counted in 10 random HPFs. Results are the mean ± SEM of 3 separate experiments. *P < .05. (C) Combined immunofluorescence and FISH analysis of MGCs. U266 cells (male origin) were cocultured with iDCs (female origin) in methylcellulose cultures as described previously. After 2 weeks, cells were harvested onto cytospins and stained for CD11c (green) and X (green):Y(Red) chromosome. Shown is a representative CD11c+ MGCs with 6 nuclei, all with XX chromosomes (arrow). Note U266 cell with Y (red) chromosome (dotted arrow). Original magnification, ×100.
Mechanisms of cell fusion
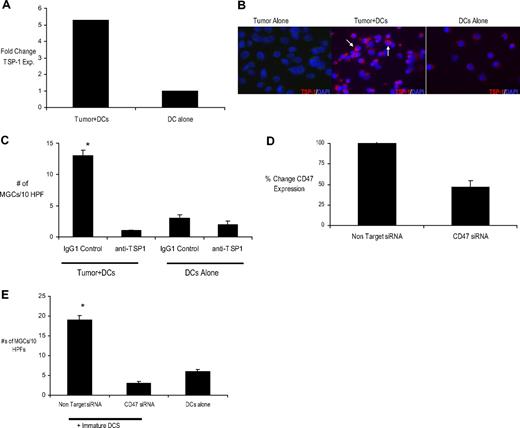
To identify components of cellular machinery involved in tumor cell fusion, we cocultured DCs with tumor cells and reisolated DCs by flow sorting CD11c+ population after overnight coculture. We then compared changes in gene expression in DCs cocultured with tumor cells versus DCs alone by the use of microarray analysis. These pilot experiments identified TSP-1 as one of the most significantly up-regulated genes in DCs after cocultured with tumor cells (data not shown). The induction of TSP-1 in DCs cocultured with MM cells was confirmed by the use of real-time RT-PCR (Figure 4A). This finding was also confirmed at the protein level by immunofluorescence microscopy (Figure 4B). Thus, interactions between tumor cells and DCs lead to up-regulation of TSP-1 on DCs. TSP-1 is a multifunctional matrix protein produced by variety of cell types and mediates cell-cell and cell-matrix interactions36 as well as bone resorption by OCLs in vitro.37 TSP-1 has several known ligands. We hypothesized that the interaction of TSP1 with CD47 may be critical for tumor-induced cell fusion. The expression of CD47 on myeloma cells was confirmed by flow cytometry (data not shown). Therefore, we tested the ability of anti-TSP-1 antibody (clone C6.7) that specifically blocks the binding of C-terminal domain of TSP-1 to integrin-associated protein/CD47) to block MGC formation. Addition of anti-TSP-1 antibody to DC-myeloma cocultures led to significant reduction in the formation of MGCs (Figure 4C). Anti-TSP-1 antibody could also inhibit the formation of OCLs in tumor-DC cocultures even when osteoclastogenic cytokines (RANKL and M-CSF) were added to these cultures (supplemental Figure 3). Together these data suggest a dominant role for TSP-1–CD47 interactions in myeloma-induced differentiation of DCs into OCLs.
Mechanism of myeloma-mediated cell fusion of DCs to OCLs. (A) Myeloma cells (U266) and DCs were cocultured overnight at a tumor/DC ratio of 1:2. After 24 hours, CD11c+ DCs were sorted by flow cytometry to > 99% purity. The expression of TSP-1 mRNA was analyzed by real-time RT-PCR (TaqMan) and normalized to the expression of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase. (B) Immunofluorescence analysis of TSP-1 in DCs cultured overnight either alone or with tumor cells at a tumor/DC ratio of 1:2. Tumor cells alone were used as control. Acetone-fixed cells on poly-l-lysine–coated slides were stained with anti–TSP-1 mab followed by Alexa Flour 568 as a secondary antibody. DAPI was used as a nuclear stain. Note intense staining of TSP-1 in DCs that had been cocultured with tumor cells (arrows). Original magnification, ×10. (C) Myeloma cells (U266) and immature DCs were cultured in methylcellulose cultures with and without TSP-1 antibody (to block TSP-1 and CD47/integrin-associated protein interaction) for 2 weeks. MGCs were counted as described previously. *P < .05. (D) Tumor cells (U266) were electroporated with 20 μg of CD47 siRNA (CD47) or nontargeting siRNA (control). The tumor cells were harvested at 48 hours after electroporation and were phenotypically characterized for CD138 (FITC) and CD47 (phycoerythrin) by flow cytometry. Shown is percent reduction in mean fluorescence intensity of CD47 expression on tumor cells electroporated with CD47 siRNA compared with nontargeting siRNA 48 hours after electroporation. (E) Myeloma cells (U266) electroporated with either CD47 siRNA or nontargeting control siRNA were cocultured with monocyte-derived DCs at a tumor/DC ratio of 1:100 in clonogenic assays. After an incubation of 2 weeks, cells were harvested onto poly-l-lysine–coated slides and were stained for enumeration of MGCs as described previously. *P < .05.
Mechanism of myeloma-mediated cell fusion of DCs to OCLs. (A) Myeloma cells (U266) and DCs were cocultured overnight at a tumor/DC ratio of 1:2. After 24 hours, CD11c+ DCs were sorted by flow cytometry to > 99% purity. The expression of TSP-1 mRNA was analyzed by real-time RT-PCR (TaqMan) and normalized to the expression of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase. (B) Immunofluorescence analysis of TSP-1 in DCs cultured overnight either alone or with tumor cells at a tumor/DC ratio of 1:2. Tumor cells alone were used as control. Acetone-fixed cells on poly-l-lysine–coated slides were stained with anti–TSP-1 mab followed by Alexa Flour 568 as a secondary antibody. DAPI was used as a nuclear stain. Note intense staining of TSP-1 in DCs that had been cocultured with tumor cells (arrows). Original magnification, ×10. (C) Myeloma cells (U266) and immature DCs were cultured in methylcellulose cultures with and without TSP-1 antibody (to block TSP-1 and CD47/integrin-associated protein interaction) for 2 weeks. MGCs were counted as described previously. *P < .05. (D) Tumor cells (U266) were electroporated with 20 μg of CD47 siRNA (CD47) or nontargeting siRNA (control). The tumor cells were harvested at 48 hours after electroporation and were phenotypically characterized for CD138 (FITC) and CD47 (phycoerythrin) by flow cytometry. Shown is percent reduction in mean fluorescence intensity of CD47 expression on tumor cells electroporated with CD47 siRNA compared with nontargeting siRNA 48 hours after electroporation. (E) Myeloma cells (U266) electroporated with either CD47 siRNA or nontargeting control siRNA were cocultured with monocyte-derived DCs at a tumor/DC ratio of 1:100 in clonogenic assays. After an incubation of 2 weeks, cells were harvested onto poly-l-lysine–coated slides and were stained for enumeration of MGCs as described previously. *P < .05.
Role of CD47 expression on tumor cells
Transient induction of CD47 on fusing cells has been implicated in cell fusion events in murine macrophages.16 Therefore, to directly evaluate the role of CD47 expression on tumor cells, we inhibited the expression of CD47 in tumor cells (U266) by the use of RNA interference. The expression of CD47 was inhibited in tumor cells treated with CD47 siRNA (Figure 4D). Down-regulation of CD47 did not have an effect on growth or survival of tumor cells (data not shown). However, culture of these cells with human DCs led to marked reduction in the tumor-induced formation of MGCs compared with controls (Figure 4E). These studies demonstrate that even partial inhibition of CD47 on tumor cells is sufficient to markedly inhibit tumor-induced formation of MGCs. Together these data point to a dominant role for CD47–TSP-1 interactions in myeloma-induced formation of OCLs in culture, with the expression of CD47 on MM cells being critically important.
Role of RANK-RANKL pathway in myeloma-induced DC fusion
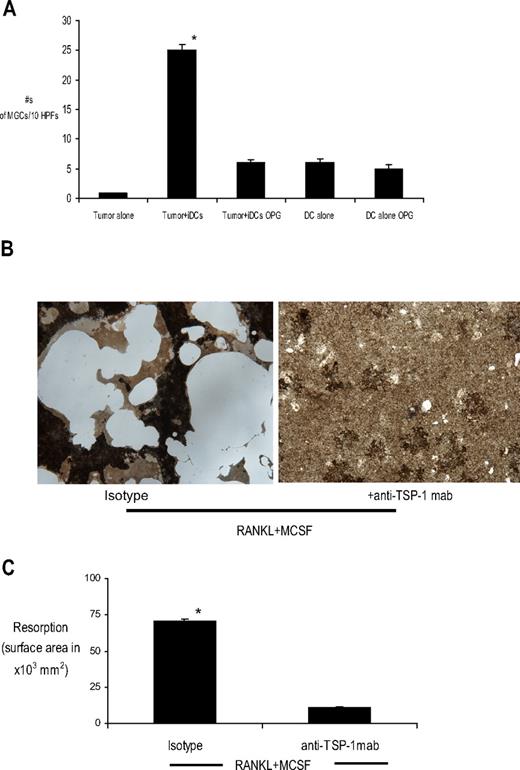
RANK-RANKL interactions play a critical role in osteoclastogenesis. Data regarding the expression of RANKL by myeloma cells are controversial. The results of some studies38-40 have suggested that MM cells express RANKL at low levels, although others have suggested that these signals are primarily derived from the stroma.40 MM-induced formation of OCLs in our studies is unlikely to be simply delivery of the RANKL signal from MM because the addition of exogenous soluble RANKL to iDCs did not lead to the formation of MGCs (data not shown). To further evaluate the role for RANKL in myeloma-induced transdifferentiation of DCs to OCLs, we cocultured DCs and myeloma cells in the presence of OPG, an inhibitor of RANK-RANKL pathway. Treatment with OPG led to significant reduction in myeloma-induced MGC formation compared with tumor DC cocultures without OPG (Figure 5A), suggesting a role of RANK-RANKL pathway in myeloma-induced DC fusion as well. Together these data suggest that MM-induced OCL formation depends on RANK-RANKL interactions but is not likely to be simply caused by the RANKL signal from myeloma cells.
Role of RANK-RANKL pathway. (A) Tumor cells were cocultured with immature DCs at a tumor/DC ratio of 1:100 in methylcellulose cultures with or without OPG (0.5 μg/mL). Cultures of DCs alone with or without OPG were used as controls. Cells were harvested for cytospins onto poly-l-lysine–coated slides after an incubation of 2 weeks. Cells were stained for CD11c, CD138, and DAPI as described previously for enumeration of MGCs. *P < .05. (B) Von Kossa staining of monocyte-derived OCLs cultured in the presence of RANKL and M-CSF with or without TSP-1 antibody over BioCoat osteologic discs. Appearance of resorbed bone (pits) is shown. Original magnification, ×10. (C) Quantitative analysis of resorbed bone area mediated by monocyte-derived OCLs cultured with or without anti–TSP-1 mab on osteologic discs. *P < .05.
Role of RANK-RANKL pathway. (A) Tumor cells were cocultured with immature DCs at a tumor/DC ratio of 1:100 in methylcellulose cultures with or without OPG (0.5 μg/mL). Cultures of DCs alone with or without OPG were used as controls. Cells were harvested for cytospins onto poly-l-lysine–coated slides after an incubation of 2 weeks. Cells were stained for CD11c, CD138, and DAPI as described previously for enumeration of MGCs. *P < .05. (B) Von Kossa staining of monocyte-derived OCLs cultured in the presence of RANKL and M-CSF with or without TSP-1 antibody over BioCoat osteologic discs. Appearance of resorbed bone (pits) is shown. Original magnification, ×10. (C) Quantitative analysis of resorbed bone area mediated by monocyte-derived OCLs cultured with or without anti–TSP-1 mab on osteologic discs. *P < .05.
Because MM-induced OCL formation depended on both CD47–TSP-1 and RANK-RANKL interactions, we analyzed whether CD47–TSP-1 interactions also regulate RANKL-dependent human OCL formation in other settings. Culture of human monocytes in the presence of RANKL and M-CSF leads to the formation of OCLs in culture. To evaluate the role of CD47–TSP-1 interactions in this system, monocytes were cultured in the presence of anti-TSP-1 antibody and osteoclastogenic cytokines. The addition of anti-TSP1 antibody led to marked inhibition of RANKL + M-CSF–induced formation of bone-resorbing OCLs (Figure 5B-C). These data suggest that CD47–TSP-1 interactions are critical for RANKL-induced human OCL formation in culture.
Effect of TSP-1 blockade on PTH-mediated hypercalcemia in vivo
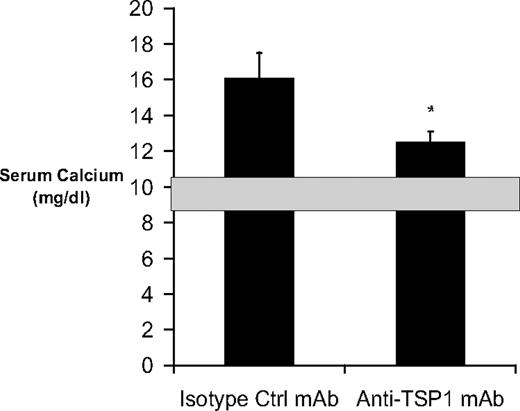
Given our in vitro findings that the CD47–TSP-1 interaction is functionally important in settings where RANKL-induced bone resorption occurs, we sought to address this relationship in vivo by using a model of RANKL-induced bone resorption and hypercalcemia. It is well established that the RANK-RANKL interaction plays a central role in mediating PTH-induced hypercalcemia and bone resorption.41 Injection of anti-TSP-1 mAb but not isotype control mAb led to clear inhibition of PTH-induced hypercalcemia (Figure 6). Therefore, TSP-1 blockade may have a significant effect on osteolysis and hypercalcemia in vivo.
Effect of TSP-1 blockade on PTH-induced hypercalcemia in vivo. CD1 female mice were infused with human (1-34)PTH at 15 pmoles/hr for 5 days via Alzett minipump implanted subcutaneously in the interscapular region. Animals were injected intraperitoneally with 0.3 mg of anti–TSP-1 antibody or isotype control at the time of minipump implantation and 2.5 days later. On day 5 after infusion, the levels of serum calcium were measured. Shaded area represents the normal range for serum calcium in CD1 mice. Data represent mean ± SEM of 6 animals per group. *P < .05.
Effect of TSP-1 blockade on PTH-induced hypercalcemia in vivo. CD1 female mice were infused with human (1-34)PTH at 15 pmoles/hr for 5 days via Alzett minipump implanted subcutaneously in the interscapular region. Animals were injected intraperitoneally with 0.3 mg of anti–TSP-1 antibody or isotype control at the time of minipump implantation and 2.5 days later. On day 5 after infusion, the levels of serum calcium were measured. Shaded area represents the normal range for serum calcium in CD1 mice. Data represent mean ± SEM of 6 animals per group. *P < .05.
Discussion
Here we have shown that myeloma cells induce surprisingly rapid cell-cell fusion in human DCs, leading to the formation of bone-resorbing OCLs. The authors of recent studies18-22 have shown that both human and murine DCs can act as direct precursors for OCLs in the presence of osteoclastogenic cytokines (RANKL and MCSF) in an inflammatory environment. Interestingly, myeloma-induced differentiation of DCs into OCLs in culture occurs without the need to add RANKL or M-CSF to these cultures. Coculture of human myeloma cells with CD14+ monocytes under identical conditions did not lead to the formation of giant cells. Together these data suggest that DCs may be the preferred precursors for myeloma induced osteoclastogenesis. This is particularly of interest in view of previous studies in which the authors showed marked enrichment of DCs within myeloma lesions, in close proximity to tumor cells.24-27
Myeloma-induced formation of OCLs required direct cell-cell contact with tumor cells, which is consistent with the clinical observation that lytic lesions are found in close proximity to tumor cells. These studies also identified a dominant role for CD47–TSP-1 interactions in myeloma-induced OCL formation. Interestingly, gene expression profiling studies of purified tumor cells in myeloma also have identified CD47 as one of the major differentially up-regulated genes in myeloma compared with normal plasma cells or to cells isolated from patients with monoclonal gammopathy of undetermined significance.42 Importantly, the authors of a recent study43 found increased expression of CD47 on tumor cells and of TSP-1 in the bone marrow microenvironment in myeloma and correlated this with lytic bone disease in patients. Tumor cells in MM expressed 10-fold greater levels of CD47 compared with those in monoclonal gammopathy of undetermined significance, a preneoplastic state lacking lytic bone lesions. Notably, in contrast to the observed increase in thrombospondin-1, an increase in transcripts for SIRPα, another receptor for CD47 was not observed.43 Our findings provide a possible mechanistic basis for these clinical correlations.
Both CD47 and TSP-1 have been implicated in regulating osteoclastogenesis in mice. Mice lacking CD47 have increased bone mass and deficient OCLs.44 In rat alveolar macrophages, transient induction of CD47 and its interaction with SIRPα was implicated as a part of the fusion machinery.16 Our data suggest that in the case of MM-induced OCL formation, the CD47 signal may be delivered “in trans” (ie, by a nonfusing cell), involves TSP-1 instead of SIRPα as a binding partner, and that this interaction is essential for OCL formation. Although myeloma cells promoted DC fusion, we were unable to show that tumor nuclei themselves were a part of multinucleate cells. However, these studies do not exclude such a possibility. The presence of tumor nuclei in a proportion of myeloma-associated OCLs was indeed suggested in a recent study,45 although such data need confirmation.
Blockade of CD47–TSP-1 interaction led to marked inhibition of RANKL-driven osteoclastogenesis and bone resorption when human monocytes were used as a source of OCL precursors. Therefore, the potential importance of CD47–TSP-1 axis in human osteoclastogenesis is not restricted to myeloma. Further studies are needed to evaluate the cross-talk between RANKL and CD47–TSP-1 mediated signaling. However these data suggest that CD47–TSP1-mediated signals either act downstream of and/or regulate RANKL-mediated signals that drive OCL formation. CD47 may also work in part by regulating the activation of integrin αvβ3.46 It will also be important to clarify the cross-talk with other molecules such as TRAIL (ie, tumor necrosis factor–related apoptosis-inducing ligand), which may also regulate RANKL-OPG signaling, particularly in the setting of cancer.47
The finding that DCs may act as precursors for cancer-associated OCLs also has potential implications for tumor immunology. Immunologic properties of DC-derived OCLs and subsequent T-cell activation also may regulate osteoclastogenesis itself.30 CD47 has been implicated as a marker of immune self and provides a “do-not-eat-me” signal.48-50 Overexpression of CD47 on tumor cells has therefore been postulated to inhibit innate resistance against tumors. Our data suggest that this molecule may deliver yet another signal to mediate cell fusion events in the microenvironment, which can have major effects on tumorigenesis.
Our data have several potential clinical implications. If DCs are major precursors for MM-associated OCLs, then preventing their recruitment to tumor lesions (such as by targeting chemokines/receptors) could be an effective approach for MM bone disease. Drugs that interfere with tumor-DC interactions (such as bortezomib)51 may also target OCL formation. The capacity of human DCs to fuse into OCLs also seems to depend on their activation status; therefore, approaches that regulate DC activation (eg, Toll-like receptor agonists) could also interdict OCL formation. Analogous to these findings, immature murine DCs recently were shown to be more likely than mature DCs to form OCLs.35 Finally, these data suggest that targeting CD47–TSP-1 interactions (eg, via TSP-1–blocking antibodies) may have a major effect on MM-induced OCL formation. In view of the capacity to regulate RANKL-driven human OCL formation, such therapies may have potential utility in other settings where increased bone resorption occurs, such as osteoporosis. In this regard, our finding that TSP-1 blockade leads to the attenuation of PTH-induced hypercalcemia in vivo is of interest. TSP-1 blockade may therefore be a novel approach for treating osteoporosis and malignancy associated lytic bone disease. Inhibition of PTH-induced bone resorption in vivo by TSP-1 blockade as shown here also supports combination therapies to enhance the anabolic effect of PTH on the bone.
In summary, these data demonstrate that interactions between myeloma cells and DCs are bidirectional and tumor cells can have major effects on DC biology. These studies emphasize the need to better understand the contributions of tumor infiltrating DCs to myeloma biology, both in terms of their protumor versus antitumor functions, and possible contributions to cell fusion events and differentiation to other cell types in the tumor microenvironment.52
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank clinical colleagues at St Vincent's Cancer Center for help with patient samples; Margaret Leversha and Lei Zhang in the molecular cytogenetics core at Memorial Sloan-Kettering Cancer Center for help with FISH analyses; and Marcus Bosenberg of Yale University for help with microscopy.
This work is supported in part by funds from the National Institutes of Health (CA106802, CA109465, and CA135110, to M.D.; and DE12459, DK45228, and AR46032 to K.I.).
National Institutes of Health
Authorship
Contribution: A.K. performed the study, analyzed data, and wrote the manuscript; M.V.D. designed the study, analyzed data, wrote, and revised the manuscript; S.R. performed some experiments; B.-H.S. performed in vivo experiments; and K.I. designed and analyzed data from in vivo experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Madhav V. Dhodapkar, MD, Section of Hematology, Yale University, 333 Cedar St, Box 208021, New Haven, CT 06510; e-mail: madhav.dhodapkar@yale.edu.